The Role of Perivascular Adipose Tissue in the Pathogenesis of Endothelial Dysfunction in Cardiovascular Diseases and Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Endothelial Dysfunction (ED)
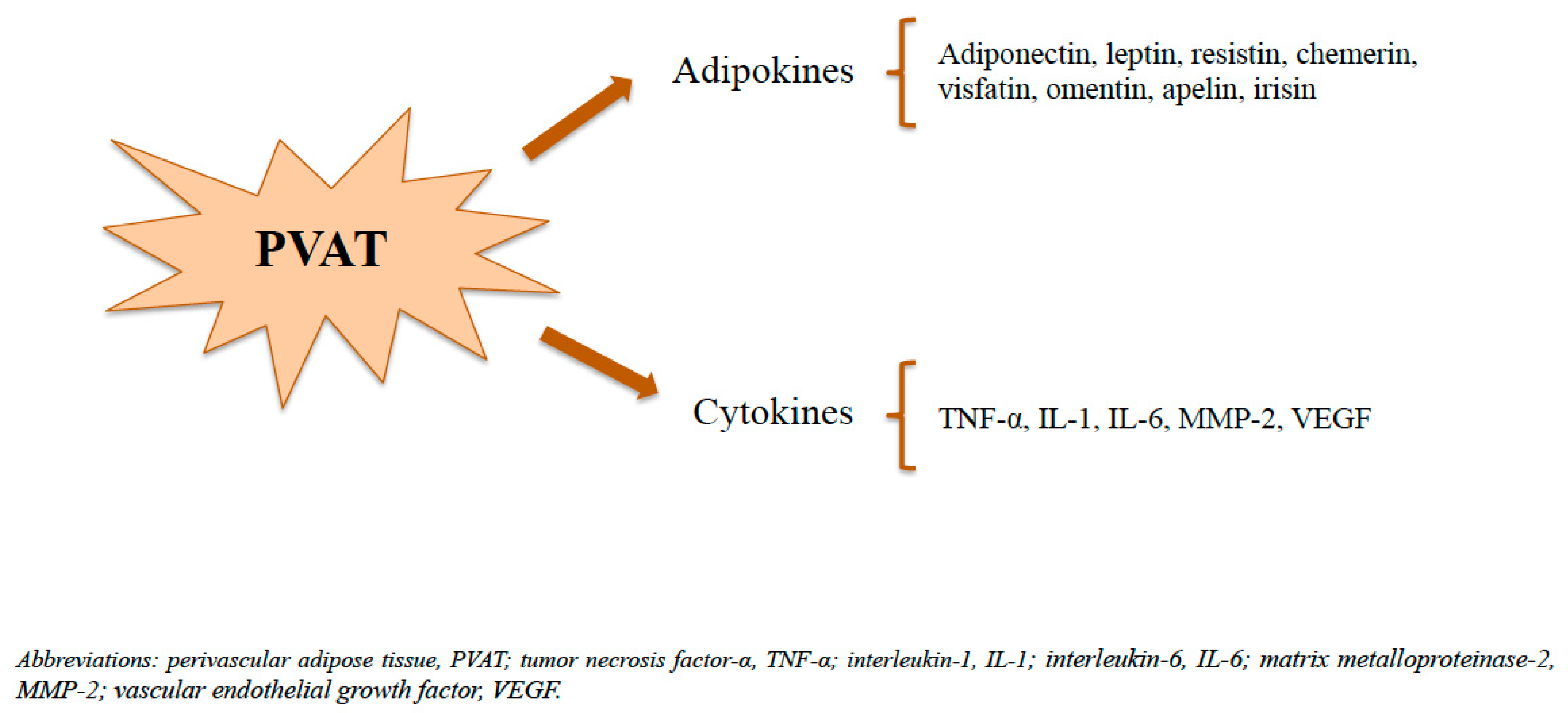
3. Perivascular Adipose Tissue (PVAT)
3.1. PVAT Modulation of Endothelial Dysfunction
3.2. PVAT Regulation of Insulin Sensitivity
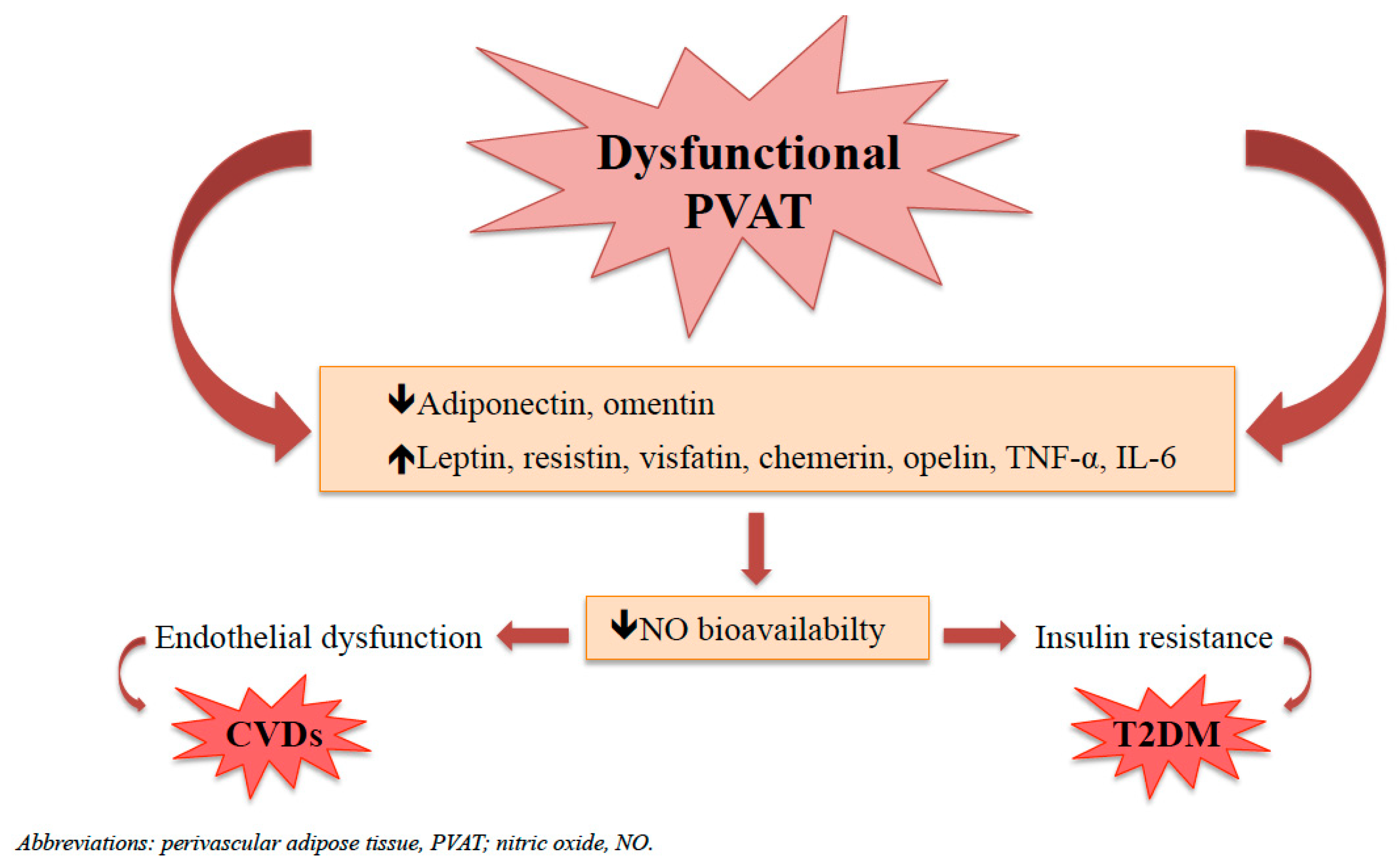
4. Dysfunctional PVAT and Its Implication in CVDs and T2DM
4.1. Dysfunctional PVAT in the Pathogenesis of Atherosclerosis
4.2. Dysfunctional PVAT Inducing Insulin Resistance
5. PVAT: New Therapeutic Target?
5.1. Statins
5.2. Antidiabetic Drugs
5.3. Pharmacological Modulation of AMP-Activated Protein Kinase (AMPK)
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budreviciute, A.; Damiati, S. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef] [PubMed]
- Huijts, T.; Stornes, P. Prevalence of physical and mental non-communicable diseases in Europe: Findings from the European Social Survey (2014) special module on the social determinants of health. Eur. J. Public Health 2017, 27 (Suppl. S1), 8–13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases (NCD). 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 29 June 2023).
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- WHO Regional Office for Europe. Monitoring Noncommunicable Disease Commitments in Europe 2021: Are We on Track to Reach Targets 10 Years after the Moscow Declaration and First United Nations High-Level Meeting? WHO Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Piepoli, M.F.; Hoes, A.W. ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Roth, G.A.; Mensah, G.A. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S144–S175. [Google Scholar] [CrossRef] [PubMed]
- Busebee, B.; Ghusn, W. Obesity: A review of pathophysiology and classification. Mayo Clin. Proc. 2023. ahead of print. [Google Scholar] [CrossRef]
- Zhang, X.; Ha, S. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin. Cancer Biol. 2023, 92, 16–27. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Nagueh, S.F. Heart failure with preserved ejection fraction: Insights into diagnosis and pathophysiology. Cardiovasc. Res. 2021, 117, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, F.; Iantorno, M. Vasodilator responses and endothelin-dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E787–E792. [Google Scholar] [CrossRef]
- Li, M.; Qian, M. Adipose tissue-endothelial cell interactions in obesity-induced endothelial dysfunction. Front. Cardiovasc. Med. 2021, 8, 681581. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Little, P.J.; Askew, C.D. Endothelial dysfunction and cardiovascular disease: History and analysis of the clinical utility of the relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Gokce, N. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sinning, C. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.; Eringa, E.C. Perivascular fat and the microcirculation: Relevance to insulin resistance, diabetes, and cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2012, 6, 80–90. [Google Scholar] [CrossRef]
- Eringa, E.C.; Bakker, W. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascul. Pharmacol. 2012, 56, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.I.; Serne, E.H. Perivascular adipose tissue and its role in type 2 diabetes and cardiovascular disease. Curr. Diabetes Rep. 2011, 11, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lastra, G.; Manrique, C. Perivascular adipose tissue, inflammation and insulin resistance: Link to vascular dysfunction and cardiovascular disease. Horm. Mol. Biol. Clin. Investig. 2015, 22, 19–26. [Google Scholar] [CrossRef]
- Szasz, T.; Bomfim, G.F. The influence of perivascular adipose tissue on vascular homeostasis. Vasc. Health Risk Manag. 2013, 9, 105–116. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Eringa, E. “Vasocrine” signalling from perivascular fat: A mechanism linking insulin resistance to vascular disease. Lancet 2005, 365, 1817–1820. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P. Endothelial Cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Poredos, P.; Poredos, A.V. Endothelial dysfunction and its clinical implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.M. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Higashi, Y.; Maruhashi, T. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Brown, N.K.; Zhou, Z. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef]
- Zaborska, K.E.; Wareing, M. Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. Br. J. Pharmacol. 2017, 174, 3388–3397. [Google Scholar] [CrossRef]
- Adachi, Y.; Ueda, K. Perivascular adipose tissue in vascular pathologies-a novel therapeutic target for atherosclerotic disease? Front. Cardiovasc. Med. 2023, 10, 1151717. [Google Scholar] [CrossRef] [PubMed]
- Deja, M.A.; Malinowski, M. Perivascular tissue mediated relaxation—A novel player in human vascular tone regulation. J. Physiol. Pharmacol. 2015, 66, 841–846. [Google Scholar] [PubMed]
- Li, X.; Ma, Z. Regional heterogeneity of perivascular adipose tissue: Morphology, origin, and secretome. Front. Pharmacol. 2021, 12, 697720. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alfonso, M.S.; Somoza, B. Role of perivascular adipose tissue in health and disease. Compr. Physiol. 2017, 8, 23–59. [Google Scholar]
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, S.; Stümer, J.; Pfeifer, A. PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front. Physiol. 2018, 9, 70. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Hillock-Watling, C.; Gotlieb, A.I. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc. Pathol. 2022, 61, 107459. [Google Scholar] [CrossRef]
- Costa, R.M.; Neves, K.B. perivascular adipose tissue as a relevant fat depot for cardiovascular risk in obesity. Front. Physiol. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Siegel-Axel, D.I.; Häring, H.U. Perivascular adipose tissue: An unique fat compartment relevant for the cardiometabolic syndrome. Rev. Endocr. Metab. Disord. 2016, 17, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.X.; Ruan, C.C. Perivascular adipose tissue-derived stromal cells contribute to vascular remodeling during aging. Aging Cell 2019, 18, e12969. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Laaksonen, T. Angiogenic effects and crosstalk of adipose-derived mesenchymal stem/stromal cells and their extracellular vesicles with endothelial cells. Int. J. Mol. Sci. 2021, 22, 10890. [Google Scholar] [CrossRef]
- Szasz, T.; Webb, R.C. Perivascular adipose tissue: More than just structural support. Clin. Sci. 2012, 122, 1–12. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Somoza, B. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef]
- Cheng, C.K.; Ding, H. Perivascular adipose tissue: Fine-tuner of vascular redox status and inflammation. Redox Biol. 2023, 62, 102683. [Google Scholar] [CrossRef]
- Rajsheker, S.; Manka, D. Crosstalk between perivascular adipose tissue and blood vessels. Curr. Opin. Pharmacol. 2010, 10, 191–196. [Google Scholar] [CrossRef]
- Ahmed, A.; Bibi, A. Perivascular adipose tissue and vascular smooth muscle tone: Friends or foes? Cells 2023, 12, 1196. [Google Scholar] [CrossRef]
- Cheng, C.K.; Bakar, H.A. Perivascular adipose tissue: The sixth man of the cardiovascular system. Cardiovasc. Drugs Ther. 2018, 32, 481–502. [Google Scholar] [CrossRef]
- Li, X.; Ballantyne, L.L. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef] [PubMed]
- Soltis, E.E.; Cassis, L.A. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens. A 1991, 13, 277–296. [Google Scholar] [CrossRef]
- Löhn, M.; Dubrovska, G. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002, 16, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Gollasch, M.; Dubrovska, G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol. Sci. 2004, 25, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Gollasch, M. Vasodilator signals from perivascular adipose tissue. Br. J. Pharmacol. 2012, 165, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Gollasch, M. Adipose-vascular coupling and potential therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 417–436. [Google Scholar] [CrossRef]
- Bełtowski, J. Endogenous hydrogen sulfide in perivascular adipose tissue: Role in the regulation of vascular tone in physiology and pathology. Can. J. Physiol. Pharmacol. 2013, 91, 889–898. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y. Endothelial nitric oxide synthase in the perivascular adipose tissue. Biomedicines 2022, 10, 1754. [Google Scholar] [CrossRef]
- Gao, Y.J.; Lu, C. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 2007, 151, 323–331. [Google Scholar] [CrossRef]
- Chang, L.; Garcia-Barrio, M.T. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1094–1109. [Google Scholar] [CrossRef]
- Lee, R.M.; Lu, C. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J. Hypertens. 2009, 27, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Sowka, A.; Dobrzyn, P. Role of perivascular adipose tissue-derived adiponectin in vascular homeostasis. Cells 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Lam, K.S. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 2007, 56, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 168–178. [Google Scholar] [CrossRef]
- Procopio, C.; Andreozzi, F. Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5′-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein. Endocrinology 2009, 150, 3584–3593. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Duranti, E. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: Role of perivascular adipose tissue. Eur. Heart J. 2015, 36, 784–794. [Google Scholar] [CrossRef]
- Virdis, A.; Colucci, R. Microvascular endothelial dysfunction in human obesity: Role of TNF-α. J. Clin. Endocrinol. Metab. 2019, 104, 341–348. [Google Scholar] [CrossRef]
- Virdis, A.; Santini, F. Vascular generation of tumor necrosis factor-α reduces nitric oxide availability in small arteries from visceral fat of obese patients. J. Am. Coll. Cardiol. 2011, 58, 238–247. [Google Scholar] [CrossRef]
- Saura, M.; Zaragoza, C. Stat3 mediates interleukin-6 [correction of interelukin-6] inhibition of human endothelial nitric-oxide synthase expression. J. Biol. Chem. 2006, 281, 30057–30062. [Google Scholar] [CrossRef]
- Hung, M.J.; Cherng, W.J. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J. Hypertens. 2010, 28, 940–951. [Google Scholar] [CrossRef]
- Didion, S.P. Cellular and oxidative mechanisms associated with interleukin-6 signaling in the vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef] [PubMed]
- Icli, B.; Feinberg, M.W. MicroRNAs in dysfunctional adipose tissue: Cardiovascular implications. Cardiovasc. Res. 2017, 113, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, J. MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ. Res. 2016, 118, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Rami, A.Z.A.; Hamid, A.A. Exploring the relationship of perivascular adipose tissue inflammation and the development of vascular pathologies. Mediat. Inflamm. 2022, 2022, 2734321. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Gunnarsson, R. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef]
- Williams, I.M.; Wasserman, D.H. Capillary endothelial insulin transport: The rate-limiting step for insulin-stimulated glucose uptake. Endocrinology 2022, 163, bqab252. [Google Scholar] [CrossRef]
- Keske, M.A.; Premilovac, D. Muscle microvascular blood flow responses in insulin resistance and ageing. J. Physiol. 2016, 594, 2223–2231. [Google Scholar] [CrossRef]
- Pepe, G.J.; Albrecht, E.D. Microvascular skeletal-muscle crosstalk in health and disease. Int. J. Mol. Sci. 2023, 24, 10425. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, N. The role of endothelial insulin signaling in the regulation of glucose metabolism. Rev. Endocr. Metab. Disord. 2013, 14, 207–216. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, N. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011, 13, 294–307. [Google Scholar] [CrossRef]
- Eringa, E.C.; Bakker, W. Regulation of vascular function and insulin sensitivity by adipose tissue: Focus on perivascular adipose tissue. Microcirculation 2007, 14, 389–402. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C. Adipose “talks” to distant organs to regulate insulin sensitivity and vascular function. Obesity 2010, 18, 2071–2076. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell. Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, Z. Adiponectin and insulin cross talk: The microvascular connection. Trends Cardiovasc. Med. 2014, 24, 319–324. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell. Biol. 2006, 7, 85–96. [Google Scholar]
- Zhao, L.; Chai, W. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circ. Res. 2013, 112, 1263–1271. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, Z. Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J. Physiol. 2015, 593, 4067–4979. [Google Scholar] [CrossRef]
- Meijer, R.I.; Bakker, W. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 2013, 62, 590–598. [Google Scholar] [CrossRef]
- Turaihi, A.H.; Serné, E.H. Perivascular adipose tissue controls insulin-stimulated perfusion, mitochondrial protein expression, and glucose uptake in muscle through adipomuscular arterioles. Diabetes 2020, 69, 603–613. [Google Scholar] [CrossRef]
- Dominici, F.P.; Burghi, V. Modulation of the action of insulin by angiotensin-(1-7). Clin. Sci. 2014, 126, 613–630. [Google Scholar]
- Fu, Z.; Zhao, L. Angiotensin-(1-7) recruits muscle microvasculature and enhances insulin’s metabolic action via mas receptor. Hypertension 2014, 63, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, F.; Tesauro, M. Favorable vascular actions of angiotensin-(1-7) in human obesity. Hypertension 2018, 71, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Dray, C.; Knauf, C. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008, 8, 437–445. [Google Scholar]
- Chen, Y.; Qin, Z. Role of inflammation in vascular disease-related perivascular adipose tissue dysfunction. Front. Endocrinol. 2021, 12, 710842. [Google Scholar]
- Qi, X.Y.; Qu, S.L. Perivascular adipose tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar]
- Saxton, S.N.; Clark, B.J. Mechanistic links between obesity, diabetes, and blood pressure: Role of perivascular adipose tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wang, B. Hypoxia in adipose tissue: A basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 2008, 100, 227–235. [Google Scholar] [CrossRef]
- Greenstein, A.S.; Khavandi, K. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef]
- Victorio, J.A.; Davel, A.P. Perivascular adipose tissue oxidative stress on the pathophysiology of cardiometabolic diseases. Curr. Hypertens. Rev. 2020, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y. Perivascular adipose tissue as a target for antioxidant therapy for cardiovascular complications. Antioxidants 2020, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Clozel, M. Mechanisms of ET-1-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 2007, 50, 621–628. [Google Scholar]
- Zhang, H.; Park, Y. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Wang, B.; Jenkins, J.R. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: Integrated response to TNF-alpha. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E731–E740. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M. Leptin stimulates both endothelin-1 and nitric oxide activity in lean subjects but not in patients with obesity-related metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 1235–1241. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar]
- Libby, P.; Buring, J.E. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Sorop, O.; Olver, T.D. The microcirculation: A key player in obesity-associated cardiovascular disease. Cardiovasc. Res. 2017, 113, 1035–1045. [Google Scholar]
- Shah, S.J.; Lam, C.S.P. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Agabiti-Rosei, C. Microcirculation in hypertension: A therapeutic target to prevent cardiovascular disease? J. Clin. Med. 2023, 12, 4892. [Google Scholar] [CrossRef]
- Sinha, A.; Rahman, H. Coronary microvascular dysfunction and heart failure with preserved ejection fraction: What are the mechanistic links? Curr. Opin. Cardiol. 2023, 38, 521–526. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Migliaro, S. Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction. Front. Physiol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, C.; Paini, A. Modulation of vascular reactivity by perivascular adipose tissue (PVAT). Curr. Hypertens. Rep. 2018, 20, 44. [Google Scholar] [CrossRef]
- Hu, H.; Garcia-Barrio, M. Roles of perivascular adipose tissue in hypertension and atherosclerosis. Antioxid. Redox Signal. 2021, 34, 736–749. [Google Scholar] [CrossRef]
- Schulman, I.H.; Zhou, M.S. Vascular insulin resistance: A potential link between cardiovascular and metabolic diseases. Curr. Hypertens. Rep. 2009, 11, 48–55. [Google Scholar] [CrossRef]
- da Costa, R.M.; Neves, K.B. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef]
- Eringa, E.C.; Stehouwer, C.D. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-alpha dependence on c-Jun N-terminal kinase. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 274–280. [Google Scholar] [CrossRef]
- Shemyakin, A.; Salehzadeh, F. Endothelin-1 reduces glucose uptake in human skeletal muscle in vivo and in vitro. Diabetes 2011, 60, 2061–2067. [Google Scholar] [CrossRef]
- Aghamohammadzadeh, R.; Greenstein, A.S. Effects of bariatric surgery on human small artery function: Evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J. Am. Coll. Cardiol. 2013, 62, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bussey, C.E.; Withers, S.B. Obesity-related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Saxton, S.N.; Toms, L.K. Restoring perivascular adipose tissue function in obesity using exercise. Cardiovasc. Drugs Ther. 2021, 35, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Channon, K.M. Statins: Pleiotropic regulators of cardiovascular redox state. Antioxid. Redox Signal. 2014, 20, 1195–1197. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M. Statins as anti-inflammatory agents in atherogenesis: Molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 2012, 18, 1519–1530. [Google Scholar] [CrossRef]
- Davignon, J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004, 109 (Suppl. S1), III39–III43. [Google Scholar] [CrossRef]
- Bellia, A.; Rizza, S. Early vascular and metabolic effects of rosuvastatin compared with simvastatin in patients with type 2 diabetes. Atherosclerosis 2010, 210, 199–201. [Google Scholar] [CrossRef]
- Bellia, A.; Rizza, S. Deterioration of glucose homeostasis in type 2 diabetic patients one year after beginning of statins therapy. Atherosclerosis 2012, 223, 197–203. [Google Scholar] [CrossRef]
- Zeng, Z.H.; Zhang, Z.H. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin. Exp. Hypertens. 2009, 31, 355–363. [Google Scholar] [CrossRef]
- Bełtowski, J.; Jamroz-Wiśniewska, A. Modulation of H2S metabolism by statins: A new aspect of cardiovascular pharmacology. Antioxid. Redox Signal. 2012, 17, 81–94. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012, 33, 187–215. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Obata, A. Unexpected pleiotropic effects of SGLT2 inhibitors: Pearls and pitfalls of this novel antidiabetic class. Int. J. Mol. Sci. 2021, 22, 3062. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hirai, T. Effects of SGLT2 inhibitors on atherosclerosis: Lessons from cardiovascular clinical outcomes in type 2 diabetic patients and basic researches. J. Clin. Med. 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Z. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef]
- Elrakaybi, A.; Laubner, K. Cardiovascular protection by SGLT2 inhibitors—Do anti-inflammatory mechanisms play a role? Mol. Metab. 2022, 64, 101549. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Q. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway. J. Int. Med. Res. 2021, 49, 300060521992981. [Google Scholar] [CrossRef]
- Han, F.; Hou, N. Liraglutide improves vascular dysfunction by regulating a cAMP-independent PKA-AMPK pathway in perivascular adipose tissue in obese mice. Biomed. Pharmacother. 2019, 120, 109537. [Google Scholar] [CrossRef]
- Ganbaatar, B.; Fukuda, D. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur. J. Pharmacol. 2020, 875, 173040. [Google Scholar] [CrossRef]
- Almabrouk, T.A.; Ewart, M.A. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br. J. Pharmacol. 2014, 171, 595–617. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xiao, N. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol. Res. 2014, 89, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, L. Methotrexate improves perivascular adipose tissue/endothelial dysfunction via activation of AMPK/eNOS pathway. Mol. Med. Rep. 2017, 15, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, K. Calycosin directly improves perivascular adipose tissue dysfunction by upregulating the adiponectin/AMPK/eNOS pathway in obese mice. Food Funct. 2018, 9, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X. Diosgenin regulates adipokine expression in perivascular adipose tissue and ameliorates endothelial dysfunction via regulation of AMPK. J. Steroid Biochem. Mol. Biol. 2016, 155 (Pt A), 155–165. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur. J. Nutr. 2018, 57, 1563–1575. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, A.; Cardillo, C.; Della Morte, D.; Tesauro, M. The Role of Perivascular Adipose Tissue in the Pathogenesis of Endothelial Dysfunction in Cardiovascular Diseases and Type 2 Diabetes Mellitus. Biomedicines 2023, 11, 3006. https://doi.org/10.3390/biomedicines11113006
Valentini A, Cardillo C, Della Morte D, Tesauro M. The Role of Perivascular Adipose Tissue in the Pathogenesis of Endothelial Dysfunction in Cardiovascular Diseases and Type 2 Diabetes Mellitus. Biomedicines. 2023; 11(11):3006. https://doi.org/10.3390/biomedicines11113006
Chicago/Turabian StyleValentini, Alessia, Carmine Cardillo, David Della Morte, and Manfredi Tesauro. 2023. "The Role of Perivascular Adipose Tissue in the Pathogenesis of Endothelial Dysfunction in Cardiovascular Diseases and Type 2 Diabetes Mellitus" Biomedicines 11, no. 11: 3006. https://doi.org/10.3390/biomedicines11113006
APA StyleValentini, A., Cardillo, C., Della Morte, D., & Tesauro, M. (2023). The Role of Perivascular Adipose Tissue in the Pathogenesis of Endothelial Dysfunction in Cardiovascular Diseases and Type 2 Diabetes Mellitus. Biomedicines, 11(11), 3006. https://doi.org/10.3390/biomedicines11113006






