Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Epidemiology of PCOS
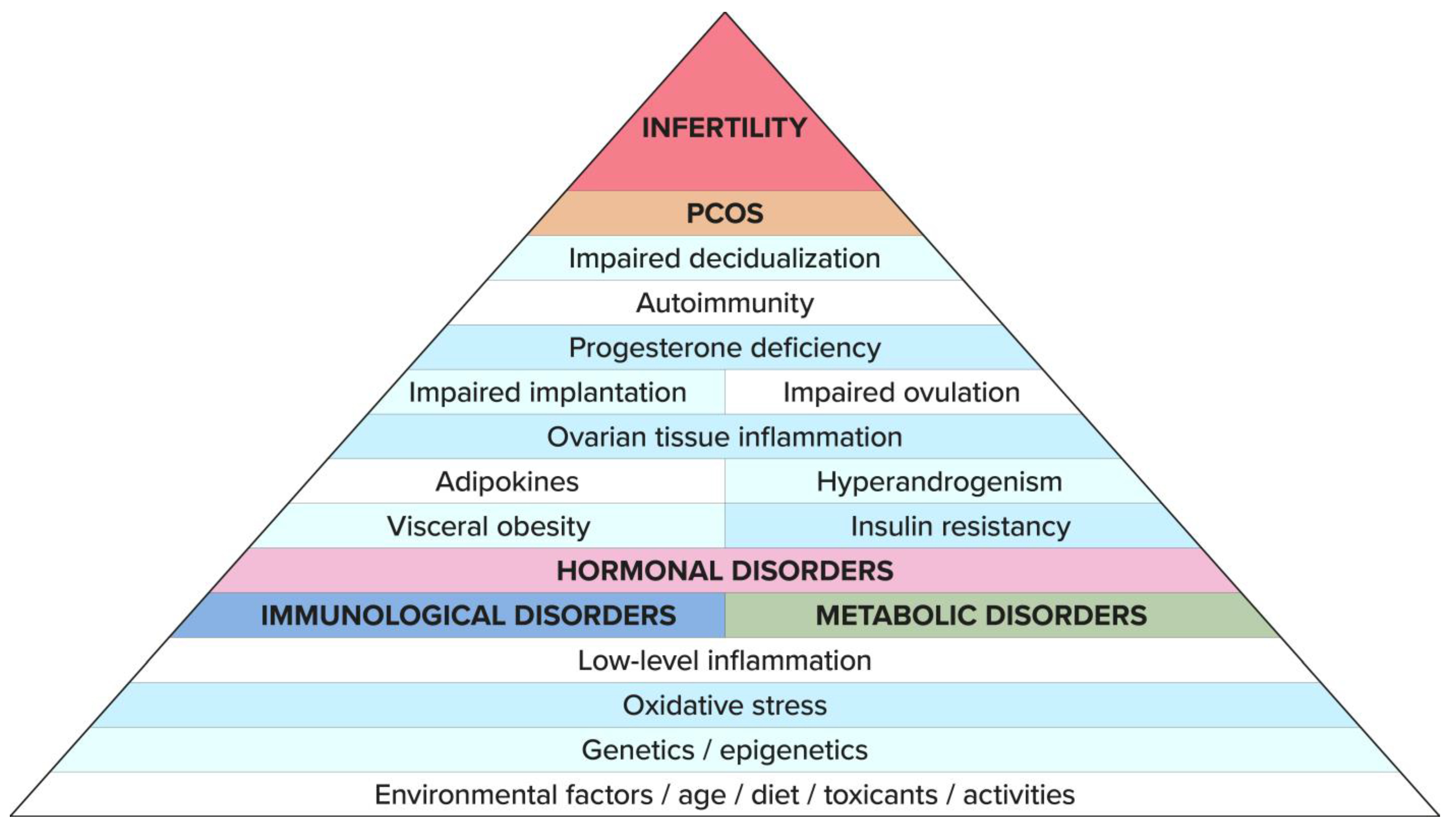
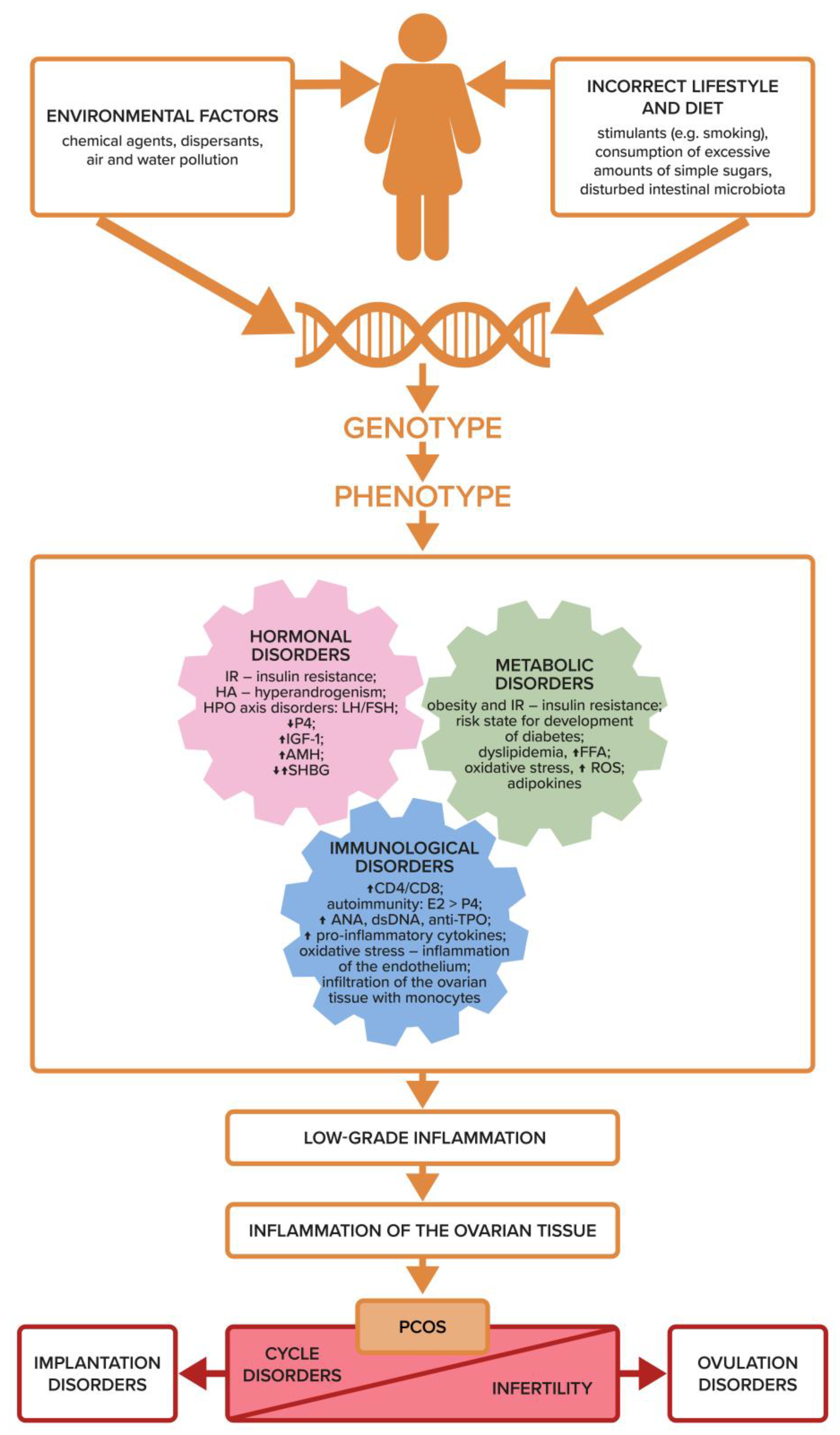
3. Etiology of PCOS and Infertility
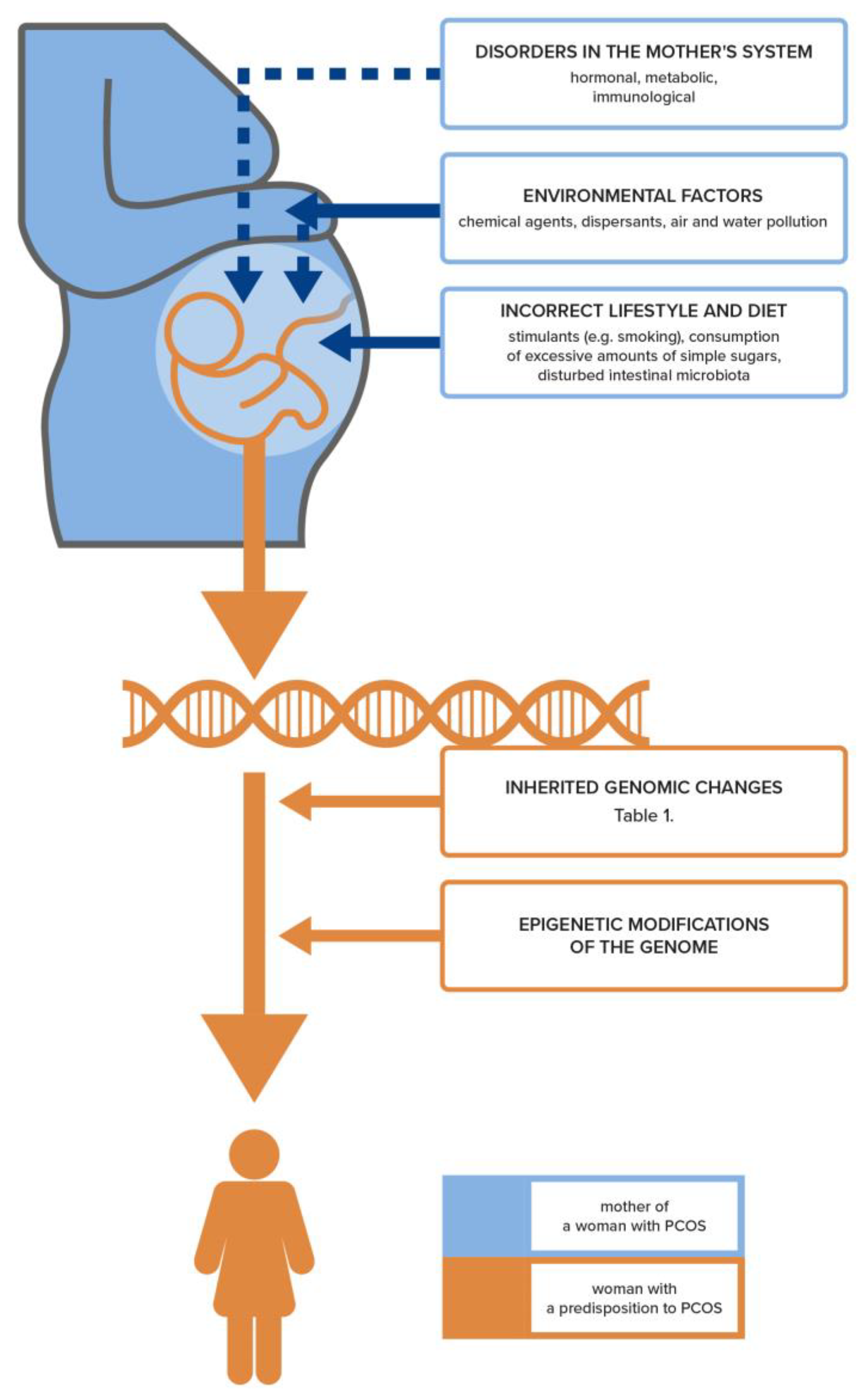
4. Genetic and Epigenetic Basis for the Development of PCOS and Infertility
5. The Role of Adipose Tissue in PCOS-Related Infertility
6. Adipokines
6.1. Chemerin
6.2. Leptin
6.3. Follistatin and Visfatin
6.4. Adiponectin
6.5. Omentin
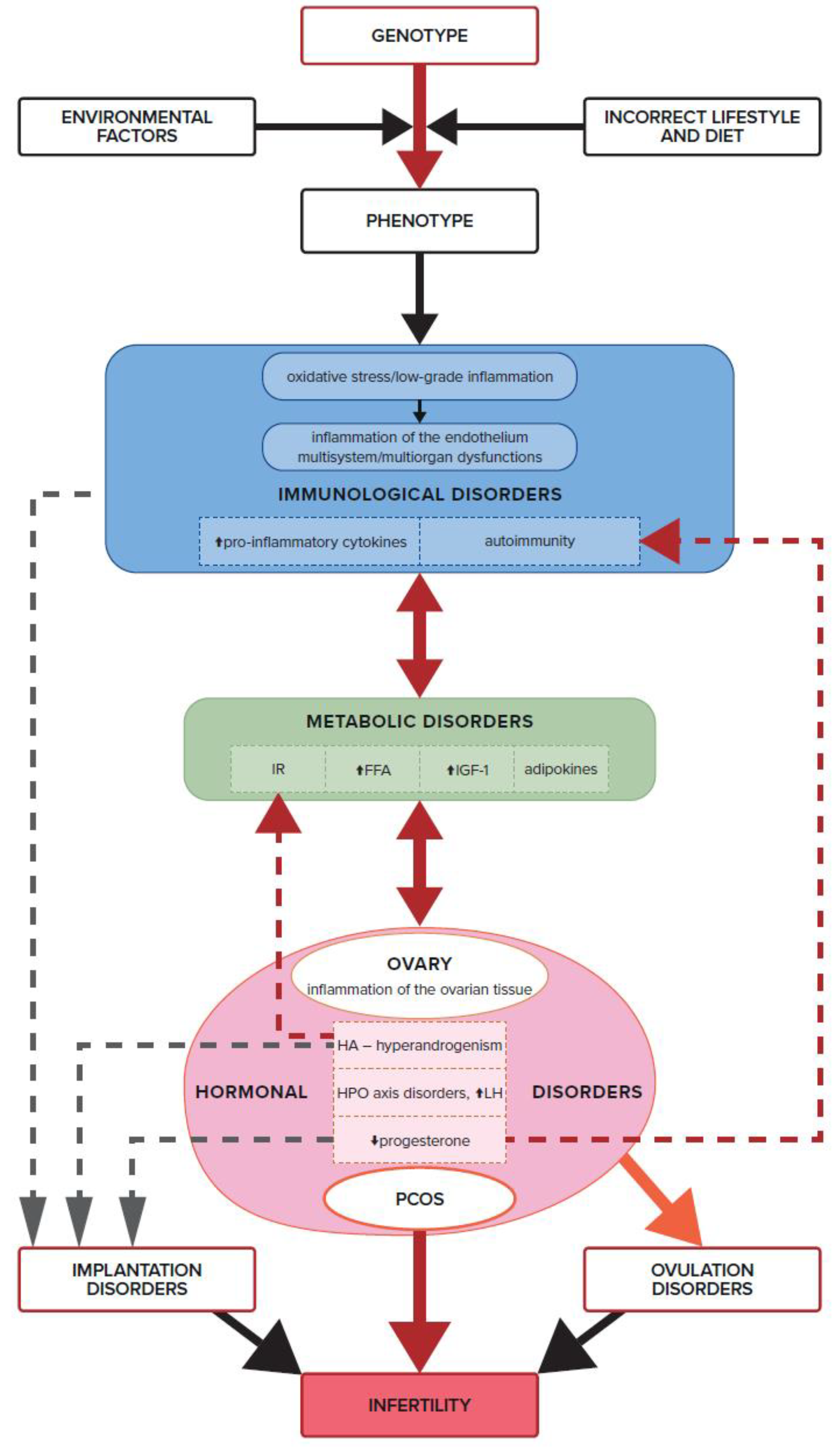
7. Insulin Resistance, IGF-1, Androgens, and Ovulation Disorders
8. Dysfunction of the Immune System, Inflammation, and Ovulation Disorders
9. Progesterone Deficiency in PCOS and Immune Infertility
10. Autoimmune Disorders in PCOS
11. Summary
12. Conclusions
- Regardless of the presence of obesity and its obvious impact on the IR, a number of scientific studies suggest that women with PCOS exhibit an intrinsic form of IR that is unique to the disorder and contributes to infertility at the level of both ovulatory and implantation disorders.
- IR contributes to the development of the immunological processes that trigger inflammation, which has a negative impact on the processes taking place within the ovary. IR induces the overproduction of androgens in the ovaries through IGF-1 receptors, leading to hyperandrogenism and its aftermath.
- The influence of obesity on fertility may be associated with an increased leptin concentration, hypoadiponectinemia, a high concentration of triglycerides and free fatty acids in ovarian follicular fluid, and also the presence of oxidative stress and inflammatory mediators. This leads to abnormal ovulation and infertility.
- In recent reports, chronic, low-grade inflammation—regardless of the starting point—appears to be the most crucial factor contributing to the pathogenesis of PCOS and infertility.
- Oxidative stress present in the ovarian cells of PCOS patients has a negative impact on fertility, regardless of how the abnormal redox balance is triggered. It also points to a direct relationship between IR and immune system dysfunction by triggering oxidative stress and inflammation in the endothelium and, thus, also in the ovarian tissue.
- The effect of progesterone on the immune processes associated with ovulation, implantation, and pregnancy development confirms the key role of this immunomodulating hormone in reproduction. A chronic progesterone deficiency in women with PCOS, as well as hormonal and immunological imbalances, are unquestionably two of the most important causes of infertility in this syndrome.
- The balance between the effects of estrogen and progesterone in a woman’s body not only guarantees the proper hormonal balance that is conducive to maintaining fertility but also allows the inhibition of autoimmune reactions, also after supplementation in PCOS.
- The treatment of infertility in PCOS should be carried out by interdisciplinary teams of specialists so that patients are correctly diagnosed and the complex therapy successfully restores health and fertility.
Author Contributions
Funding
Conflicts of Interest
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Inhorn, M.; Patrizio, P. Infertility around the Globe: New Thinking on Gender, Reproductive Technologies and Global Movements in the 21st Century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Ombelet, W.; Cooke, I.; Dyer, S.; Serour, G.; Devroey, P. Infertility and the Provision of Infertility Medical Services in Developing Countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Masud, J.; Islam, Y.N.; Haque, F.K.M. An Update on Polycystic Ovary Syndrome: A Review of the Current State of Knowledge in Diagnosis, Genetic Etiology, and Emerging Treatment Options. Womens Heal. 2022, 18, 17455057221117966. [Google Scholar] [CrossRef]
- Lorzadeh, N.; Kazemirad, N.; Kazemirad, Y. Human Immunodeficiency: Extragonadal Comorbidities of Infertility in Women. Immunity Inflamm. Dis. 2020, 8, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Ahmed, I.; Ahmed, S.A.F. Evaluation of Causes of Female Infertility Using Ultrasonography in Najran, Saudi Arabia. J. Reproduct. Health 2022, 26, 90–95. [Google Scholar]
- Kanwal, H.I.; Shahid, M.; Bacha, R. Sonographic Evaluation of Various Causes of Female Infertility: A Literature Review. J. Diagnostic Med. Sonogr. 2022, 38, 155–159. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Carson-Chahhoud, K.; Sullman, M.J.M.; Collins, G.S.; Kolahi, A.-A.; Avery, J. Prevalence, Incidence and Years Lived with Disability Due to Polycystic Ovary Syndrome in 204 Countries and Territories, 1990–2019. Hum. Reprod. 2022, 37, 1919–1931. [Google Scholar] [CrossRef]
- Ganie, M.; Vasudevan, V.; Wani, I.; Baba, M.; Arif, T.; Rashid, A. Epidemiology, Pathogenesis, Genetics & Management of Polycystic Ovary Syndrome in India. Indian J. Med. Res. 2019, 150, 333. [Google Scholar] [CrossRef]
- Rudnicka, E.; Duszewska, A.M.; Kucharski, M.; Tyczyński, P.; Smolarczyk, R. Oxidative Stress and Reproductive Function: Oxidative Stress in Polycystic Ovary Syndrome. Reproduction 2022, 164, F145–F154. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Padmanabhan, V.; Chazenbalk, G.D.; Abbott, D.H. Polycystic Ovary Syndrome as a Plausible Evolutionary Outcome of Metabolic Adaptation. Reprod. Biol. Endocrinol. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- de Melo, G.B.; Soares, J.F.; Costa, T.C.L.; Benevides, R.O.A.; Vale, C.C.; Paes, A.M. de A.; Gaspar, R.S. Early Exposure to High-Sucrose Diet Leads to Deteriorated Ovarian Health. Front. Endocrinol. 2021, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Vurbic, D.; Harder, V.S.; Redner, R.R.; Lopez, A.A.; Phillips, J.K. Co-Occurring Obesity and Smoking among U.S. Women of Reproductive Age: Associations with Educational Attainment and Health Biomarkers and Outcomes. Prev. Med. 2015, 80, 60–66. [Google Scholar] [CrossRef]
- Fauser, B.; Tarlatzis, B.; Rebar, R.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.E. Consensus on Women’s Health Aspects of Polycystic Ovary Syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef]
- Kakoly, N.; Khomami, M.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; Ranasinha, S.; Teede, H.J.; Moran, L.J. Ethnicity, Obesity and the Prevalence of Impaired Glucose Tolerance and Type 2 Diabetes in PCOS: A Systematic Review and Meta-Regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef]
- Lentscher, J.; Slocum, B.; Torrealday, S. Polycystic Ovarian Syndrome and Fertility. Clin. Obstet. Gynecol. 2021, 64, 65–75. [Google Scholar] [CrossRef]
- Petríková, J.; Lazúrová, I.; Yehuda, S. Polycystic ovary syndrome and autoimmunity. Eur. J. Intern. Med. 2010, 21, 369–371. [Google Scholar] [CrossRef]
- Chen, P.; Jia, R.; Liu, Y.; Cao, M.; Zhou, L.; Zhao, Z. Progress of Adipokines in the Female Reproductive System: A Focus on Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 936. [Google Scholar] [CrossRef]
- Shan, H.; Luo, R.; Guo, X.; Li, R.; Ye, Z.; Peng, T.; Liu, F.; Yang, Z. Abnormal Endometrial Receptivity and Oxidative Stress in Polycystic Ovary Syndrome. Front. Pharmacol. 2022, 13, 904942. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S.; Almunawwarrah, A.; Arabia, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of Polycystic Ovary Syndrome in a Dutch Twin-Family Study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Hiam, D.; Moreno-Asso, A.; Teede, H.J.; Laven, J.S.E.; Stepto, N.K.; Moran, L.J.; Gibson-Helm, M. The Genetics of Polycystic Ovary Syndrome: An Overview of Candidate Gene Systematic Reviews and Genome-Wide Association Studies. J. Clin. Med. 2019, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Deng, Q. Epigenetic Inheritance of Polycystic Ovary Syndrome—Challenges and Opportunities for Treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Does a Male Polycystic Ovarian Syndrome Equivalent Exist? J. Endocrinol. Investig. 2018, 41, 49–57. [Google Scholar] [CrossRef]
- Zhu, J.; Pujol-Gualdo, N.; Wittemans, L.B.; Lindgren, C.M.; Laisk, T.; Hirschhorn, J.N.; Chan, Y.M. Evidence from Men for Ovary-Independent Effects of Genetic Risk Factors for Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e1577–e1587. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, Hormonal and Metabolic Aspects of PCOS: An Update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.; Oberfield, S.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.-P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, P.; Shetty, P.K.; Manjeera, L.; Patil, P. Hormonal, Genetic, Epigenetic and Environmental Aspects of Polycystic Ovarian Syndrome. Gene Rep. 2022, 29, 101698. [Google Scholar] [CrossRef]
- Zou, J.; Li, Y.; Liao, N.; Liu, J.; Zhang, Q.; Luo, M.; Xiao, J.; Chen, Y.; Wang, M.; Chen, K.; et al. Identification of Key Genes Associated with Polycystic Ovary Syndrome (PCOS) and Ovarian Cancer Using an Integrated Bioinformatics Analysis. J. Ovarian Res. 2022, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-Scale Genome-Wide Meta-Analysis of Polycystic Ovary Syndrome Suggests Shared Genetic Architecture for Different Diagnosis Criteria. PLOS Genet. 2018, 14, e1007813, Correction in PLOS Genet. 2019, 15, e1008517. [Google Scholar] [CrossRef]
- Kumar, R.; Minerva, S.; Shah, R.; Bhat, A.; Verma, S.; Chander, G.; Bhat, G.R.; Thapa, N.; Bhat, A.; Wakhloo, A.; et al. Role of Genetic, Environmental, and Hormonal Factors in the Progression of PCOS: A Review. J. Reprod. Healthc. Med. 2022, 3, 3. [Google Scholar] [CrossRef]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Binti Osman, M.; Sakinah, M.; Nordin, N.; Abdul Hamid, H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes 2022, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic Ovary Syndrome (PCOS) and Genetic Predisposition: A Review Article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef]
- Nautiyal, H.; Imam, S.; Alshehri, S.; Ghoneim, M.; Afzal, M.; Alzarea, S.I.; Güven, E.; Al-Abbasi, F.A.; Kazmi, I. Polycystic Ovarian Syndrome: A Complex Disease with a Genetics Approach. Biomedicines 2022, 10, 540. [Google Scholar] [CrossRef]
- Harada, M. Pathophysiology of Polycystic Ovary Syndrome Revisited: Current Understanding and Perspectives Regarding Future Research. Reprod. Med. Biol. 2022, 21, e12487. [Google Scholar] [CrossRef]
- Gorsic, L.; Kosova, G.; Werstein, B.; Sisk, R.; Legro, R.S.; Hayes, M.G.; Teixeira, J.M.; Dunaif, A.; Urbanek, M. Pathogenic Anti-Müllerian Hormone Variants in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 2862–2872. [Google Scholar] [CrossRef]
- Franks, S.; Stark, J.; Hardy, K. Follicle Dynamics and Anovulation in Polycystic Ovary Syndrome. Hum. Reproduct Update 2008, 14, 367–378. [Google Scholar] [CrossRef]
- La Marca, A.; Sighinolfi, G.; Radi, D.; Argento, C.; Baraldi, E.; Artenisio, A.C. Anti-Müllerian Hormone (AMH) as a Predictive Marker in Assisted Reproductive Technology (ART). Hum. Reproduct Update 2010, 16, 113–130. [Google Scholar] [CrossRef]
- Nguyen, M.; Krishnan, S.; Phatak, S.; Karakas, S.E. Anti-Mullerian Hormone-Based Phenotyping Identifies Subgroups of Women with Polycystic Ovary Syndrome with Differing Clinical and Biochemical Characteristics. Diagnostics 2023, 13, 500. [Google Scholar] [CrossRef]
- Abutorabi, E.; Rashidi, B.; Irani, S.; Haghollahi, F.; Bagheri, M. Investigation of the FSHR, CYP11, and INSR Mutations and Polymorphisms in Iranian Infertile Women with Polycystic Ovary Syndrome (PCOS). Rep. Biochem. Mol. Biol. 2021, 9, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Ozcan, S.S.; Aran, T.; Kara, O.; Yilmaz, N. Evaluation of Endometrial Receptivity by Measuring HOXA-10, HOXA-11, and Leukemia Inhibitory Factor Expression in Patients with Polycystic Ovary Syndrome. Gynecol. Minim. Invasive Ther. 2019, 8, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hardt, J.; Kim, J.J. Global Analysis of Genes Regulated by HOXA10 in Decidualization Reveals a Role in Cell Proliferation. MHR Basic Sci. Reprod. Med. 2008, 14, 357–366. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef]
- Torres-Carrillo, N.M.; Torres-Carrillo, N.; Vázquez-Del Mercado, M.; Delgado-Rizo, V.; Oregón-Romero, E.; Parra-Rojas, I.; Muñoz-Valle, J.F. The −844 G/A PAI-1 Polymorphism Is Associated with MRNA Expression in Rheumatoid Arthritis. Rheumatol. Int. 2008, 28, 355–360. [Google Scholar] [CrossRef]
- Polat, S.; Şimşek, Y. Plasminogenactivator Inhibitor-1 Polymorphism and Risk of Polycystic Ovary Syndrome in Turkish Women. Meta Gene 2021, 30, 100959. [Google Scholar] [CrossRef]
- SM, B.; A, R.; S, M.; C, M.; MA, R.; AH, J.; SR, V.; IA, G.; JJ, R.; M, B.; et al. American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Venous Thromboembolism in the Context of Pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef]
- Zhai, J.; Li, Z.; Zhou, Y.; Yang, X. The role of plasminogen activator inhibitor-1 in gynecological and obstetrical diseases: An update review. J. Reprod. Immunol. 2022, 150, 103490. [Google Scholar] [CrossRef]
- Klinger, K.W.; Winqvist, R.; Riccio, A.; Andreasen, P.A.; Sartorio, R.; Nielsen, L.S.; Stuart, N.; Stanislovitis, P.; Watkins, P.; Douglas, R. Plasminogen Activator Inhibitor Type 1 Gene Is Located at Region Q21.3-Q22 of Chromosome 7 and Genetically Linked with Cystic Fibrosis. Proc. Natl. Acad. Sci. 1987, 84, 8548–8552. [Google Scholar] [CrossRef] [PubMed]
- Hoirisch-Clapauch, S.; Brenner, B. The Role of the Fibrinolytic System in Female Reproductive Disorders and Depression. Thromb. Update 2020, 1, 100004. [Google Scholar] [CrossRef]
- Piquette, G.; Simón, C.; El Danasouri, I.; Frances, A.; Polan, M.L. Gene Regulation of Interleukin-1β, Interleukin-1 Receptor Type I, and Plasminogen Activator Inhibitor-1 and-2 in Human Granulosa-Luteal Cells. Fertil. Steril. 1994, 62, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Devin, J.; Johnson, J.; Eren, M.; Gleaves, L.A.; Bradham, W.S.; Bloodworth, J.R.; Vaughan, D.E. Transgenic Overexpression of Plasminogen Activator Inhibitor-1 Promotes the Development of Polycystic Ovarian Changes in Female Mice. J. Mol. Endocrinol. 2007, 39, 9–16. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Liu, X.-M.; Nin, L.-F.; Shi, L.; Chen, S.-R. Serine protease and ovarian paracrine factors in regulation of ovulation. Front. Biosci. 2013, 18, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Dina, C.; Durand, E.; Froguel, P. PAI-1 Polymorphisms Modulate Phenotypes Associated with the Metabolic Syndrome in Obese and Diabetic Caucasian Population. Diabetologia 2003, 46, 1284–1290. [Google Scholar] [CrossRef]
- Yılmaz, M.; Bukan, N.; Ersoy, R.; Karakoç, A.; Yetkin, I.; Ayvaz, G.; Cakır, N.; Arslan, M. Glucose Intolerance, Insulin Resistance and Cardiovascular Risk Factors in First Degree Relatives of Women with Polycystic Ovary Syndrome. Hum. Reproduct. 2005, 20, 2414–2420. [Google Scholar] [CrossRef]
- Vázquez-Martínez, E.; Gómez-Viais, Y.I.; García-Gómez, E.; Reyes-Mayoral, C.; Reyes-Muñoz, E.; Camacho-Arroyo, I.; Cerbón, M.A. DNA Methylation in the Pathogenesis of Polycystic Ovary Syndrome. Reproduction 2019, 158, R27–R40. [Google Scholar] [CrossRef]
- Kokosar, M.; Benrick, A.; Perfilyev, A.; Fornes, R.; Nilsson, E.; Maliqueo, M.; Behre, C.J.; Sazonova, A.; Ohlsson, C.; Ling, C.; et al. Epigenetic and Transcriptional Alterations in Human Adipose Tissue of Polycystic Ovary Syndrome. Sci. Rep. 2016, 6, 22883. [Google Scholar] [CrossRef]
- Kokosar, M.; Benrick, A.; Perfilyev, A.; Nilsson, E.; Källman, T.; Ohlsson, C.; Ling, C.; Stener-Victorin, E. A Single Bout of Electroacupuncture Remodels Epigenetic and Transcriptional Changes in Adipose Tissue in Polycystic Ovary Syndrome. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Zhong, T.; Men, Y.; Lu, L.; Geng, T.; Zhou, J.; Mitsuhashi, A.; Shozu, M.; Maihle, N.J.; Carmichael, G.G.; Taylor, H.S.; et al. Metformin Alters DNA Methylation Genome-Wide via the H19/SAHH Axis. Oncogene 2017, 36, 2345–2354. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, J.; Xi, M.; Jia, Y.; Wong, Y.; Zhao, J.; Ding, L.; Zhang, J.; Wen, A. Pharmacogenetic Variation and Metformin Response. Curr. Drug Metab. 2013, 14, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.; Ehrmann, D.A. Metformin Therapy for the Reproductive and Metabolic Consequences of Polycystic Ovary Syndrome. Diabetologia 2017, 60, 1656–1661. [Google Scholar] [CrossRef]
- Rababa’h, A.M.; Matani, B.R.; Yehya, A. An update of polycystic ovary syndrome: Causes and therapeutics options. Heliyon 2022, 8, e11010. [Google Scholar] [CrossRef]
- Yildiz, B.; Bozdag, G.; Otegen, U.; Harmanci, A.; Boynukalin, K.; Vural, Z.; Kirazli, S.; Yarali, H. Visfatin and retinol-binding protein 4 concentrations in lean, glucose-tolerant women with PCOS. Reprod. Biomed. Online 2010, 20, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The Endocrine Adipose Organ. Rev. Endocr. Metab. Disord. 2022, 23, 1–4. [Google Scholar] [CrossRef]
- Steiner, B.M.; Berry, D.C. The Regulation of Adipose Tissue Health by Estrogens. Front. Endocrinol. 2022, 13, 889923. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.; San Millán, J.L. Abdominal Adiposity and the Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2007, 18, 266–272. [Google Scholar] [CrossRef]
- Pereira, S.; Solange, S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease. J. Innate Immun. 2022, 14, 4–30. [Google Scholar] [CrossRef]
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.; Chura, L.; Liang, X.; Lane, M.; Norman, R.J.; Robker, R.L. Exposure to Lipid-Rich Follicular Fluid Is Associated with Endoplasmic Reticulum Stress and Impaired Oocyte Maturation in Cumulus-Oocyte Complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef]
- Bausenwein, J.; Serke, H.; Eberle, K.; Hirrlinger, J.; Jogschies, P.; Hmeidan, F.A.; Blumenauer, V.; Spanel-Borowski, K. Elevated Levels of Oxidized Low-Density Lipoprotein and of Catalase Activity in Follicular Fluid of Obese Women. Mol. Hum. Reprod. 2009, 16, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Cramer, D.W.; Liberman, R.F.; Barbieri, R.L. Predictive Value of Serum and Follicular Fluid Leptin Concentrations during Assisted Reproductive Cycles in Normal Women and in Women with the Polycystic Ovarian Syndrome. Hum. Reprod. 2000, 15, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Norman, R.J.; Davies, M.J.; Moran, L.J. The Effect of Obesity on Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Obes. Rev. 2013, 14, 95–109. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Sun, X.; Wang, X.; Wang, H.; Chen, X. Circulating Adipokine Levels in Nonobese Women With Polycystic Ovary Syndrome and in Nonobese Control Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 11, 537809. [Google Scholar] [CrossRef]
- Michalakis, K.; Segars, J.H. The Role of Adiponectin in Reproduction: From Polycystic Ovary Syndrome to Assisted Reproduction. Fertil. Steril. 2010, 94, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Nikołajuk, A.; Karczewska-Kupczewska, M.; Kowalska, I.; Otziomek, E.; Górska, M.; Strączkowski, M. Relationships between Serum Adiponectin and Soluble TNF-α Receptors and Glucose and Lipid Oxidation in Lean and Obese Subjects. Acta Diabetol. 2012, 49, 17–24. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Toosy, S.; Sodi, R.; Pappachan, J.M. Lean polycystic ovary syndrome (PCOS): An evidence-based practical approach. J. Diabetes Metab. Disord. 2018, 17, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J. Lean Women with Polycystic Ovary Syndrome Respond to Insulin Reduction with Decreases in Ovarian P450c17α Activity and Serum Androgens 1. J. Clin. Endocrinol. Metab. 1997, 82, 4075–4079. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Zhang, L.; Wei, W.; Liu, L.; Li, B.; Zhang, L.; Zhang, Y.; Hui, Y.; Lei, Y. Circulating Chemerin Levels in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Gynecol. Endocrinol. 2022, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Q.; Wang, W.; Qi, J.; He, Y.; Wang, Y.; Lu, Y.; Wu, H.; Ding, Y.; Sun, Y. Elevated chemerin induces insulin resistance in human granulosa-lutein cells from polycystic ovary syndrome patients. FASEB J. 2018, 33, 11303–11313. [Google Scholar] [CrossRef]
- Lima, P.; Nivet, A.; Wang, Q.; Chen, Y.A.; Leader, A.; Cheung, A.; Tzeng, C.R.; Tsang, B.K. Polycystic Ovary Syndrome: Possible Involvement of Androgen-Induced, Chemerin-Mediated Ovarian Recruitment of Monocytes/Macrophages. Biol. Reprod. 2018, 99, 838–852. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Huang, C.; Ge, L.; Xue, L.; Xiao, Z.; Xiao, T.; Zhao, H.; Ren, P.; Zhang, J.V. Chemerin: A Functional Adipokine in Reproductive Health and Diseases. Biomedicines 2022, 10, 1910. [Google Scholar] [CrossRef]
- Luo, X.; Gong, Y.; Cai, L.; Zhang, L.; Dong, X. Chemerin Regulates Autophagy to Participate in Polycystic Ovary Syndrome. J. Int. Med. Res. 2021, 49, 030006052110583. [Google Scholar] [CrossRef]
- Boucsein, A.; Kamstra, K.; Tups, A. Central Signalling Cross-talk between Insulin and Leptin in Glucose and Energy Homeostasis. J. Neuroendocrinol. 2021, 33, e12944. [Google Scholar] [CrossRef]
- Odle, A.K.; Akhter, N.; Syed, M.M.; Allensworth-James, M.L.; Beneš, H.; Melgar Castillo, A.I.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.V. Leptin Regulation of Gonadotrope Gonadotropin-Releasing Hormone Receptors As a Metabolic Checkpoint and Gateway to Reproductive Competence. Front. Endocrinol. 2018, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, A.; Hernández-Coronado, C.G.; Rosales-Torres, A.M.; Hernández-Medrano, J.H. Leptin regulates neuropeptides associated with food intake and GnRH secretion. Ann. Endocrinologie 2019, 80, 38–46. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Pérez, A.P.; Santamaría-López, E.; Prados, N.; Fernández-Sánchez, M.; Sánchez-Margalet, V. Sam68 mediates leptin signaling and action in human granulosa cells: Possible role in leptin resistance in PCOS. Endocr. Connect. 2020, 9, 479–488. [Google Scholar] [CrossRef]
- Kucera, R.; Babuska, V.; Ulcova-Gallova, Z.; Kulda, V.; Topolcan, O. Follicular Fluid Levels of Anti-Müllerian Hormone, Insulin-like Growth Factor 1 and Leptin in Women with Fertility Disorders. Syst. Biol. Reprod. Med. 2018, 64, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S.; Reddy, S.T.; Singh, R. Novel Roles of Follistatin/Myostatin in Transforming Growth Factor-β Signaling and Adipose Browning: Potential for Therapeutic Intervention in Obesity Related Metabolic Disorders. Front. Endocrinol. 2021, 12, 653179. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chang, D.-M.; Lin, K.-C.; Shin, S.-J.; Lee, Y.-J. Visfatin in Overweight/Obesity, Type 2 Diabetes Mellitus, Insulin Resistance, Metabolic Syndrome and Cardiovascular Diseases: A Meta-Analysis and Systemic Review. Diabetes. Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef]
- Cekmez, F.; Cekmez, Y.; Pirgon, O.; Canpolat, F.E.; Aydinoz, S.; Ipcioglu, O.M.; Karademir, F. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur. Cytokine Netw. 2011, 22, 32–37. [Google Scholar] [CrossRef]
- Dİkmen, E.; Tarkun, İ.; Cantürk, Z.; Çetİnarslan, B. Plasma Visfatin Level in Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2011, 27, 475–479. [Google Scholar] [CrossRef]
- Jongwutiwes, T.; Lertvikool, S.; Leelaphiwat, S.; Rattanasiri, S.; Jultanmas, R.; Weerakiet, S. Serum Visfatin in Asian Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2009, 25, 536–542. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and Polycystic Ovary Syndrome. Clin. Endocrinol. 2021, 95, 531–541. [Google Scholar] [CrossRef]
- Lee, W.; Wu, C.; Lin, H.; Lee, I.; Wu, C.M.; Tseng, J.J.; Chou, M.M.; Sheu, W.H. Visfatin-Induced Expression of Inflammatory Mediators in Human Endothelial Cells through the NF-ΚB Pathway. Int. J. Obes. 2009, 33, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, K.; Michalska-Jakubus, M.; Potembska, E.; Kowal, M.; Pietrzak, A.; Krasowska, D. Visfatin and Chemerin Levels Correspond with Inflammation and Might Reflect the Bridge between Metabolism, Inflammation and Fibrosis in Patients with Systemic. Adv. Dermatol. Allergol. Postępy Dermatol. I Alergol. 2019, 36, 551–565. [Google Scholar] [CrossRef]
- Ezzati-Mobaser, S.; Malekpour-Dehkordi, Z.; Nourbakhsh, M.; Tavakoli-Yaraki, M.; Ahmadpour, F.; Golpour, P.; Nourbakhsh, M. The Up-Regulation of Markers of Adipose Tissue Fibrosis by Visfatin in Pre-Adipocytes as Well as Obese Children and Adolescents. Cytokine 2020, 134, 155193. [Google Scholar] [CrossRef]
- Buechler, C.; Krautbauer, S.; Eisinger, K. Adipose tissue fibrosis. World J. Diabetes 2015, 6, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Vionnet, N.; Dupont, S.; Gallina, S.; Francke, S.; Dotte, S.; De Matos, F.; Durand, E.; Leprêtre, F.; Lecoeur, C.; Gallina, P.; et al. Genomewide Search for Type 2 Diabetes–Susceptibility Genes in French Whites: Evidence for a Novel Susceptibility Locus for Early-Onset Diabetes On. Am. J. Hum. Genet. 2000, 67, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Funcke, J.; Scherer, P.E. Beyond Adiponectin and Leptin: Adipose Tissue-Derived Mediators of Inter-Organ Communication. J. Lipid Res. 2019, 60, 1648–1697. [Google Scholar] [CrossRef]
- Laplante, M.; Sell, H.; MacNaul, K.; Richard, D.; Berger, J.P.; Deshaies, Y. PPAR-γ Activation Mediates Adipose Depot− Specific Effects on Gene Expression and Lipoprotein Lipase Activity: Mechanisms for Modulation of Postprandial Lipemia. Am. Diabetes Assoc. 2003, 52, 291–299. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens Decrease Plasma Adiponectin, an Insulin-Sensitizing Adipocyte-Derived Protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef]
- Toulis, K.; Goulis, D.; Farmakiotis, D. Adiponectin Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and a Meta-Analysis. Hum. Reprod. Update 2009, 15, 297–307. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D. PPAR-γ and Wnt Regulate the Differentiation of MSCs into Adipocytes and Osteoblasts Respectively. Curr. Stem Cell Res. Ther. 2018, 13, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.S.A.; Approbato, M.S.; Maia, M.C.S.; Ferreira, E.A.B.F.E.; Zanluchi, N. Ovulatory Status of Overweight Women without Polycystic Ovary Syndrome. JBRA Assist. Reprod. 2019, 23, 2–6. [Google Scholar] [CrossRef]
- Carmina, E.; Bucchieri, S.; Mansueto, P.; Rini, G.; Ferin, M.; Lobo, R.A. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil. Steril. 2009, 91, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Liao, G.; Zhang, W.; Xu, D.; Lu, J.; Tang, M.; Yan, Y.; Hong, C.; Wang, Y. Effects of Exogenous Adiponectin Supplementation in Early Pregnant PCOS Mice on the Metabolic Syndrome of Adult Female Offspring. J. Ovarian Res. 2021, 14, 15. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Yu, J.; Zeng, Z.-G.; Liu, Y.; Liu, J.-Y.; Xu, J.-X. Circulating Omentin-1 Levels in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Gynecol. Endocrinol. 2017, 33, 244–249. [Google Scholar] [CrossRef]
- Mahde, A.; Shaker, M.; Al-Mashhadani, Z. Study of Omentin1 and Other Adipokines and Hormones in PCOS Patients. Oman Med J. 2009, 24, 108–118. [Google Scholar] [PubMed]
- Orlik, B.; Madej, P.; Owczarek, A.; Skałba, P.; Chudek, J.; Olszanecka-Glinianowicz, M. Plasma Omentin and Adiponectin Levels as Markers of Adipose Tissue Dysfunction in Normal Weight and Obese Women with Polycystic Ovary Syndrome. Clin. Endocrinol. 2014, 81, 529–535. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guérif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef]
- Xing, C.; Li, C.; He, B. Insulin Sensitizers for Improving the Endocrine and Metabolic Profile in Overweight Women with PCOS. J. Clin. Endocrinol. Metab. 2020, 105, 2950–2963. [Google Scholar] [CrossRef]
- Siemienowicz, K.J.; Coukan, F.; Franks, S.; Rae, M.T.; Duncan, W.C. Aberrant subcutaneous adipogenesis precedes adult metabolic dysfunction in an ovine model of polycystic ovary syndrome (PCOS). Mol. Cell. Endocrinol. 2021, 519, 111042. [Google Scholar] [CrossRef]
- Barber, T.M.; McCarthy, M.I.; Wass, J.A.H.; Franks, S. Obesity and Polycystic Ovary Syndrome. Clin. Endocrinol. 2006, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, B.; Jiang, X.; Li, Z.; Zhao, S.; Cui, L.; Chen, Z.-J. Metabolic Disturbances in Non-Obese Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Fertil. Steril. 2019, 111, 168–177. [Google Scholar] [CrossRef]
- Shoelson, S.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Alesi, S.; Villani, A.; Mantzioris, E.; Takele, W.W.; Cowan, S.; Moran, L.J.; Mousa, A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients 2022, 14, 3914. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxidative Med. Cell. Longev. 2015, 2016, 1–14. [Google Scholar] [CrossRef]
- Snider, A.; Wood, J.R. Obesity Induces Ovarian Inflammation and Reduces Oocyte Quality. Reproduction 2019, 158, R79–R90. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Xia, B.; Zhang, L.; Zhang, C.; Leng, J. Mitochondria-Targeted Antioxidant Therapy for an Animal Model of PCOS-IR. Int. J. Mol. Med. 2018, 43, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Turner, N. Mitochondrial Dysfunction and Insulin Resistance: An Update. Endocr. Connect. 2015, 4, R1. [Google Scholar] [CrossRef]
- Shukla, P.; Mukherjee, S. Mitochondrial Dysfunction: An Emerging Link in the Pathophysiology of Polycystic Ovary Syndrome. Mitochondrion 2020, 52, 24–39. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; Bañuls, C.; Sanchez-Serrano, M.; Sola, E.; Gomez, M.; Hernandez-Mijares, A. Mitochondrial Complex I Impairment in Leukocytes from Polycystic Ovary Syndrome Patients with Insulin Resistance. J. Clin. Endocrinol. Metab. 2009, 94, 3505–3512. [Google Scholar] [CrossRef]
- Victor, V.; Banuls, C.; Bellod, L.; Rovira, S.; Gomez, M.; Rocha, M. Polycystic Ovary Syndrome Induces Leukocyte. In Endothelial Cell Interactions; Bioscientifica: Bristol, UK, 2012. [Google Scholar]
- Naigaonkar, A.; Dadachanji, R.; Hinduja, I.; Mukherjee, S. Altered redox status may contribute to aberrant folliculogenesis and poor reproductive outcomes in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2021, 38, 2609–2623. [Google Scholar] [CrossRef]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive Oxygen Species Are Indispensable in Ovulation. Proc. Natl. Acad. Sci. 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Turan, V.; Sezer, E.D.; Zeybek, B.; Sendag, F. Infertility and the Presence of Insulin Resistance Are Associated with Increased Oxidative Stress in Young, Non-obese Turkish Women with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2015, 28, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Moti, M.; Amini, L.; Ardakani, S.S.M.; Kamalzadeh, S.; Masoomikarimi, M.; Jafarisani, M. Oxidative stress and anti-oxidant defense system in Iranian women with polycystic ovary syndrome. Iran. J. Reprod. Med. 2015, 13, 373–378. [Google Scholar]
- Özer, A.; Bakacak, M.; Kiran, H.; Ercan, O.; Kostu, B.; Pektas, M.K.; Kilinç, M.; Aslan, F. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol. Pol. 2016, 87, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-Z.; Li, W.; Cheng, Y.; Zhang, M.; Niu, X.-C.; Gao, Q.-W.; Lu, Y.; Tian, T.; Du, S.; Mi, Y.; et al. The Cytoprotective Role of Omentin against Oxidative Stress-Induced PC12 Apoptosis. Artif. Cells, Nanomed. Biotechnol. 2021, 49, 483–492. [Google Scholar] [CrossRef]
- Magoffin, D.A. Ovarian Theca Cell. Int. J. Biochem. Cell Biol. 2005, 37, 1344–1349. [Google Scholar] [CrossRef]
- Afradiasbagharani, P.; Hosseini, E.; Allahveisi, A.; Bazrafkan, M. The Insulin-like Growth Factor and Its Players: Their Functions, Significance, and Consequences in All Aspects of Ovarian Physiology. Middle East Fertil. Soc. J. 2022, 27, 27. [Google Scholar] [CrossRef]
- Dri, M.; Klinger, F.G.; De Felici, M. The ovarian reserve as target of insulin/IGF and ROS in metabolic disorder-dependent ovarian dysfunctions. Reprod. Fertil. 2021, 2, R103–R112. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, A.; Chalid, M.T.; Farid, R.B.; Nusratuddin, N. The correlation between insulin-like growth factor binding protein 1 (IGFBP-1) and homeostasis model assessment of insulin resistance (HOMA-IR) in polycystic ovarian syndrome with insulin resistance. Int. J. Reprod. Biomed. 2018, 16, 679–682. [Google Scholar]
- Kristiansen, S.B.; Endoh, A.; Casson, P.R.; Buster, J.E.; Hornsby, P.J. Induction of steroidogenic enzyme genes by insulin and IGF-I in cultured adult human adrenocortical cells. Steroids 1997, 62, 258–265. [Google Scholar] [CrossRef]
- Baptiste, C.G.; Battista, M.-C.; Trottier, A.; Baillargeon, J.-P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010, 122, 42–52. [Google Scholar] [CrossRef]
- Silva, J.R.V.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Orisaka, M.; Tajima, K.; Hattori, K.; Kotsuji, F. Luteinizing Hormone-Induced Akt Phosphorylation and Androgen Production Are Modulated by MAP Kinase in Bovine Theca Cells. J. Ovarian Res. 2009, 2, 17. [Google Scholar] [CrossRef]
- Briden, L.; Shirin, S.; Prior, J.C. The central role of ovulatory disturbances in the etiology of androgenic polycystic ovary syndrome (PCOS)—Evidence for treatment with cyclic progesterone. Drug Discov. Today Dis. Model. 2020, 32, 71–82. [Google Scholar] [CrossRef]
- Rudnicka, E.; Kunicki, M.; Suchta, K.; Machura, P.; Grymowicz, M.; Smolarczyk, R.; Khraibi, A.A. Inflammatory Markers in Women with Polycystic Ovary Syndrome. BioMed Res. Int. 2020, 2020, 4092470. [Google Scholar] [CrossRef] [PubMed]
- Gallinelli, A.; Ciaccio, I.; Giannella, L.; Salvatori, M.; Marsella, T.; Volpe, A. Correlations between concentrations of interleukin-12 and interleukin-13 and lymphocyte subsets in the follicular fluid of women with and without polycystic ovary syndrome. Fertil. Steril. 2003, 79, 1365–1372. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced Ovarian Glyoxalase-I Activity by Dietary Glycotoxins and Androgen Excess: A Causative Link to Polycystic Ovarian Syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef]
- Merhi, Z. Advanced Glycation End Products and Their Relevance in Female Reproduction. Hum. Reprod. 2014, 29, 135–145. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Kalofoutis, A.; Creatsas, G. Increased Levels of Serum Advanced Glycation End-Products in Women with Polycystic Ovary Syndrome. Clin. Endocrinol. 2005, 62, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mouanness, M.; Nava, H.; Dagher, C.; Merhi, Z. Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review. Nutrients 2022, 14, 3578. [Google Scholar] [CrossRef]
- Spanel-Borowski, K. Ovulation as Danger Signaling Event of Innate Immunity. Mol. Cell. Endocrinol. 2011, 333, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Lin, P.-C.; Zhou, S.; Barakat, R.; Bashir, S.T.; Choi, J.M.; Cacioppo, J.A.; Oakley, O.R.; Duffy, D.M.; Lydon, J.P.; et al. Progesterone Receptor Serves the Ovary as a Trigger of Ovulation and a Terminator of Inflammation. Cell Rep. 2020, 31, 107496. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Witchel, S.; Oberfield, S.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.; Ko, C.; Jo, M.; Brannstrom, M.; E Curry, T. Ovulation: Parallels with Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil. Steril. 2011, 95, 1048–1058.e2. [Google Scholar] [CrossRef]
- Wu, R.; Fujii, S.; Ryan, N.; Van der Hoek, K.H.; Jasper, M.J.; Sini, I.; Robertson, S.A.; Robker, R.L.; Norman, R.J. Ovarian Leukocyte Distribution and Cytokine/Chemokine MRNA Expression in Follicular Fluid Cells in Women with Polycystic Ovary Syndrome. Hum. Reprod. 2007, 22, 527–535. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Mao, X.; Lei, H.; Dong, B.; Guo, D.; Zheng, B.; Sun, P. Peripheral Blood Inflammatory-Immune Cells as a Predictor of Infertility in Women with Polycystic Ovary Syndrome. J. Inflamm. Res. 2020, 13, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Y.; Lin, X.; Gao, Y.; Zhu, Y. Prognositic Value of CD73-Adenosinergic Pathway in Solid Tumor: A Meta-Analysis and Systematic Review. Oncotarget 2017, 8, 57327–57336. [Google Scholar] [CrossRef]
- Połeć, A.; Raki, M.; Åbyholm, T.; Tanbo, T.G.; Fedorcsák, P. Interaction between Granulosa-Lutein Cells and Monocytes Regulates Secretion of Angiogenic Factors in Vitro. Hum. Reprod. 2011, 26, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in Ovarian Folliculogenesis, Oocyte Maturation and Luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Enskog, A. Leukocyte networks and ovulation. J. Reprod. Immunol. 2002, 57, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Park, J.; Moon, W.; Dam, P.T.; Cho, M.-K.; Chun, S.-Y. Cumulus Cell-Expressed Type I Interferons Induce Cumulus Expansion in Mice. Biol. Reprod. 2015, 92, 20. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in Ovarian Granulosa Cells Are Essential for Female Fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef]
- Połeć, A.; Tanbo, T.; Fedorcsák, P. ORIGINAL ARTICLE: Cellular Interaction Regulates Interleukin-8 Secretion by Granulosa-Lutein Cells and Monocytes/Macrophages. Am. J. Reprod. Immunol. 2008, 61, 85–94. [Google Scholar] [CrossRef]
- Qin, L.; Xu, W.; Li, X.; Meng, W.; Hu, L.; Luo, Z.; Wang, Y.; Luo, S.; Li, S. Differential Expression Profile of Immunological Cytokines in Local Ovary in Patients with Polycystic Ovarian Syndrome: Analysis by Flow Cytometry. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 136–141. [Google Scholar] [CrossRef]
- Prior, J.C. Women’s Reproductive System as Balanced Estradiol and Progesterone Actions—A Revolutionary, Paradigm-Shifting Concept in Women’s Health. Drug Discov. Today Dis. Model. 2020, 32, 31–40. [Google Scholar] [CrossRef]
- Nagy, B.; Szekeres-Barthó, J.; Kovács, G.L.; Sulyok, E.; Farkas, B.; Várnagy, A.; Vértes, V.; Kovács, K.; Bódis, J. Key to Life: Physiological Role and Clinical Implications of Progesterone. Int. J. Mol. Sci. 2021, 22, 11039. [Google Scholar] [CrossRef]
- Kicińska, A.M.; Stachowska, A.; Kajdy, A.; Wierzba, T.H.; Maksym, R.B. Successful Implementation of Menstrual Cycle Biomarkers in the Treatment of Infertility in Polycystic Ovary Syndrome—Case Report. Healthcare 2023, 11, 616. [Google Scholar] [CrossRef]
- Moore, A.M.; Prescott, M.; Marshall, C.J.; Yip, S.H.; Campbell, R.E. Enhancement of a Robust Arcuate GABAergic Input to Gonadotropin-Releasing Hormone Neurons in a Model of Polycystic Ovarian Syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 596–601. [Google Scholar] [CrossRef]
- Robker, R.L.; Russell, D.L.; Espey, L.L.; Lydon, J.P.; O’Malley, B.W.; Richards, J.S. Progesterone-Regulated Genes in the Ovulation Process: ADAMTS-1 and Cathepsin L Proteases. Proc. Natl. Acad. Sci. USA 2000, 97, 4689–4694. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yu, E.; Rabello, G.; Merlo, S.; Zemmar, A.; Walton, K.D.; Moreno, H.; Moreira, J.E.; Sugimori, M.; Llinás, R.R. Enhanced Synaptic Transmission at the Squid Giant Synapse by Artificial Seawater Based on Physically Modified Saline. Front. Synaptic Neurosci. 2014, 6, 2. [Google Scholar] [CrossRef]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Piette, P. The History of Natural Progesterone, the Never-Ending Story. Climacteric 2018, 21, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Engler, J.B.; Kursawe, N.; Solano, M.E.; Patas, K.; Wehrmann, S.; Heckmann, N.; Lühder, F.; Reichardt, H.M.; Arck, P.C.; Gold, S.M.; et al. Glucocorticoid Receptor in T Cells Mediates Protection from Autoimmunity in Pregnancy. Proc. Natl. Acad. Sci. USA 2017, 114, E181–E190. [Google Scholar] [CrossRef] [PubMed]
- Brazdova, A.; Senechal, H.; Peltre, G.; Poncet, P. Immune Aspects of Female Infertility. Int. J. Fertil. Steril. 2016, 10, 1–10. [Google Scholar] [PubMed]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T Cells in Embryo Implantation and the Immune Response to Pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef]
- Arruvito, L.; Giulianelli, S.; Flores, A.C.; Paladino, N.; Barboza, M.; Lanari, C.; Fainboim, L. NK Cells Expressing a Progesterone Receptor Are Susceptible to Progesterone-Induced Apoptosis. J. Immunol. 2008, 180, 5746–5753. [Google Scholar] [CrossRef]
- Polgar, B.; Kispal, G.; Lachmann, M.; Paar, G.; Nagy, E.; Csere, P.; Miko, E.; Szereday, L.; Varga, P.; Szekeres-Bartho, J. Molecular Cloning and Immunologic Characterization of a Novel CDNA Coding for Progesterone-Induced Blocking Factor. J. Immunol. 2003, 171, 5956–5963. [Google Scholar] [CrossRef]
- Cutolo, M.; Straub, R.H. Sex Steroids and Autoimmune Rheumatic Diseases: State of the Art. Nat. Rev. Rheumatol. 2020, 16, 628–644. [Google Scholar] [CrossRef]
- McGlade, E.A.; Miyamoto, A.; Winuthayanon, W. Progesterone and Inflammatory Response in the Oviduct during Physiological and Pathological Conditions. Cells 2022, 11, 1075. [Google Scholar] [CrossRef]
- Chabrolle, C.; Tosca, L.; Ramé, C.; Lecomte, P.; Royère, D.; Dupont, J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil. Steril. 2009, 92, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Kronzer, V.L.; Bridges, S.L.; Davis, J.M. Why Women Have More Autoimmune Diseases than Men: An Evolutionary Perspective. Evol. Appl. 2021, 14, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Mobeen, H.; Afzal, N.; Kashif, M. Polycystic Ovary Syndrome May Be an Autoimmune Disorder. Scientifica 2016, 2016, 4071735. [Google Scholar] [CrossRef]
- Eaton, S.; Sethi, J.K. Immunometabolic Links between Estrogen, Adipose Tissue and Female Reproductive Metabolism. Biology 2019, 8, 8. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Shoenfeld, Y. The Role of Gender and Organ Specific Autoimmunity. Autoimmun. Rev. 2012, 11, A377–A385. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Capellino, S.; Villaggio, B.; Montagna, P.; Seriolo, B.; Straub, R.H. Sex Hormones Influence on the Immune System: Basic and Clinical Aspects in Autoimmunity. Lupus 2004, 13, 635–638. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, L.; Peng, Y.; Li, Y.; Liu, R.; Yin, C. Immune Regulation in Polycystic Ovary Syndrome. Clin. Chim. Acta 2022, 531, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Beecham, A.; Cabral, D.; Yanuck, D.; Slifer, S.; Wang, L.; Blanton, S.H.; Sacco, R.L.; Juo, S.-H.H.; Rundek, T. Carotid Plaque and Candidate Genes Related to Inflammation and Endothelial Function in Hispanics From Northern Manhattan. Stroke 2011, 42, 889–896. [Google Scholar] [CrossRef]
- Papadimitraki, E.D.; Bertsias, G.K.; Boumpas, D.T. Toll like Receptors and Autoimmunity: A Critical Appraisal. J. Autoimmun. 2007, 29, 310–318. [Google Scholar] [CrossRef]
- Makled, A.K.; Fathi, H.M.; Gomaa, M.F.; Bakr, R.M. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2015, 20, 86–90. [Google Scholar] [CrossRef]
- Hefler-Frischmuth, K.; Walch, K.; Hefler, L.; Tempfer, C.; Grimm, C. Serologic Markers of Autoimmunity in Women with Recurrent Pregnancy Loss. Am. J. Reprod. Immunol. 2017, 77, e12635. [Google Scholar] [CrossRef] [PubMed]
- Haller-Kikkatalo, K.; Salumets, A.; Uibo, R. Review on Autoimmune Reactions in Female Infertility: Antibodies to Follicle Stimulating Hormone. J. Immunol. Res. 2012, 2012, 762541. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Blank, M. Autoantibodies Associated with Reproductive Failure. Lupus 2004, 13, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Pisklakova, A.; Nagrani, M.; Mohanachandran, S.; Abraham, J. ODP220 Latent Autoimmune Diabetes of Adults and PCOS-an Unusual Combination. J. Endocr. Soc. 2022, 6, A324. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Adjunct Therapy for Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2010, 6, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Codner, E.; Soto, N.; Lopez, P.; Trejo, L.; AÁvila, A.; Eyzaguirre, F.C.; IÍniguez, G.; Cassorla, F. Diagnostic Criteria for Polycystic Ovary Syndrome and Ovarian Morphology in Women with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Janssen, O.; Mehlmauer, N.; Hahn, S.; Öffner, A.H.; Gärtner, R. High Prevalence of Autoimmune Thyroiditis in Patients with Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2004, 150, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Hepşen, S.; Karaköse, M.; Çakal, E.; Öztekin, S.; Ünsal, İ.; Akhanlı, P.; Uçan, B.; Özbek, M. The Assessment of Thyroid Autoantibody Levels in Euthyroid Patients with Polycystic Ovary Syndrome. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D. Recurrent Pregnancy Loss in Patients with Thyroid Dysfunction. Indian J. Endocrinol. Metab. 2012, 16, S350. [Google Scholar] [CrossRef]
- Ramos-Leví, A.; Marazuela, M. Pathogenesis of Thyroid Autoimmune Disease: The Role of Cellular Mechanisms. Endocrinol. Nutr. 2016, 63, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, S.; Fan, Z. Pathogenesis Markers of Hashimoto’s Disease—A Mini Review. Front. Biosci. 2022, 27, 297. [Google Scholar] [CrossRef]
- Arduc, A.; Aycicek Dogan, B.; Bilmez, S.; Imga Nasiroglu, N.; Tuna, M.M.; Isik, S.; Berker, D.; Guler, S. High Prevalence of Hashimoto’s Thyroiditis in Patients with Polycystic Ovary Syndrome: Does the Imbalance between Estradiol and Progesterone Play a Role? Endocr. Res. 2015, 40, 204–210. [Google Scholar] [CrossRef]
- Lee, C.E.; Lee, N.K.; Lee, C.S.; Byeon, S.H.; Kim, S.S.; Lee, S.W.; Kim, Y.J. Association between Polycystic Ovary Syndrome and Non-Infectious Uveitis. Sci. Rep. 2023, 13, 277. [Google Scholar] [CrossRef]
- Levinson, W. Review of Medical Microbiology and Immunology; McGraw-Hill Medical: New York, NY, USA, 2014. [Google Scholar]
- Baser, H.; Can, U.; Baser, S.; Yerlikaya, F.H.; Aslan, U.; Hidayetoglu, B.T. Assesment of Oxidative Status and Its Association with Thyroid Autoantibodies in Patients with Euthyroid Autoimmune Thyroiditis. Endocrine 2015, 48, 916–923. [Google Scholar] [CrossRef]
- Öztürk, U.; Vural, P.; Özderya, A.; Karadağ, B.; Doğru-Abbasoğlu, S.; Uysal, M. Oxidative stress parameters in serum and low density lipoproteins of Hashimoto’s thyroiditis patients with subclinical and overt hypothyroidism. Int. Immunopharmacol. 2012, 14, 349–352. [Google Scholar] [CrossRef]
| Gens Involves in the Pathophysiology of PCOS | ||||
|---|---|---|---|---|
| Steroidogenesis | Insulin Secretion and Action | Effect of Steroid Hormones | Gonadotropin Regulation | Others |
| CYP21, CYP11a, CYP19, CYP17 | IRS group, INSR, CAPN10, FTO | AR, SHBG, DENND1A | FSHR, LHCGR, AMH, HOXA group, BMP | PAI-1 |
| Hyperandrogenism | Diabetes, obesity Oxidative stress | Hyperandrogenism | Infertility | Infertility |
| Ovulation and Implantation Disorders | ||||
| Infertility/Cycle Disorders | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicińska, A.M.; Maksym, R.B.; Zabielska-Kaczorowska, M.A.; Stachowska, A.; Babińska, A. Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome. Biomedicines 2023, 11, 1567. https://doi.org/10.3390/biomedicines11061567
Kicińska AM, Maksym RB, Zabielska-Kaczorowska MA, Stachowska A, Babińska A. Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome. Biomedicines. 2023; 11(6):1567. https://doi.org/10.3390/biomedicines11061567
Chicago/Turabian StyleKicińska, Aleksandra Maria, Radoslaw B. Maksym, Magdalena A. Zabielska-Kaczorowska, Aneta Stachowska, and Anna Babińska. 2023. "Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome" Biomedicines 11, no. 6: 1567. https://doi.org/10.3390/biomedicines11061567
APA StyleKicińska, A. M., Maksym, R. B., Zabielska-Kaczorowska, M. A., Stachowska, A., & Babińska, A. (2023). Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome. Biomedicines, 11(6), 1567. https://doi.org/10.3390/biomedicines11061567







