Mannose-Binding Lectin Reduces Oxidized Low-Density Lipoprotein Induced Vascular Endothelial Cells Injury by Inhibiting LOX1-ox-LDL Binding and Modulating Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnosis Criteria and Sample Collection
2.2. Cells Culture
2.3. Cell Transfection
2.4. Cells Viability Assay
2.5. Cell Apoptosis Assay
2.6. DCs Maturation Assay
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot
2.10. Immunofluorescence
2.11. Co-Immunoprecipitation (Co-IP) Analysis
2.12. Animals and Ex Vivo Experiment
2.13. Statistical Analysis
3. Results
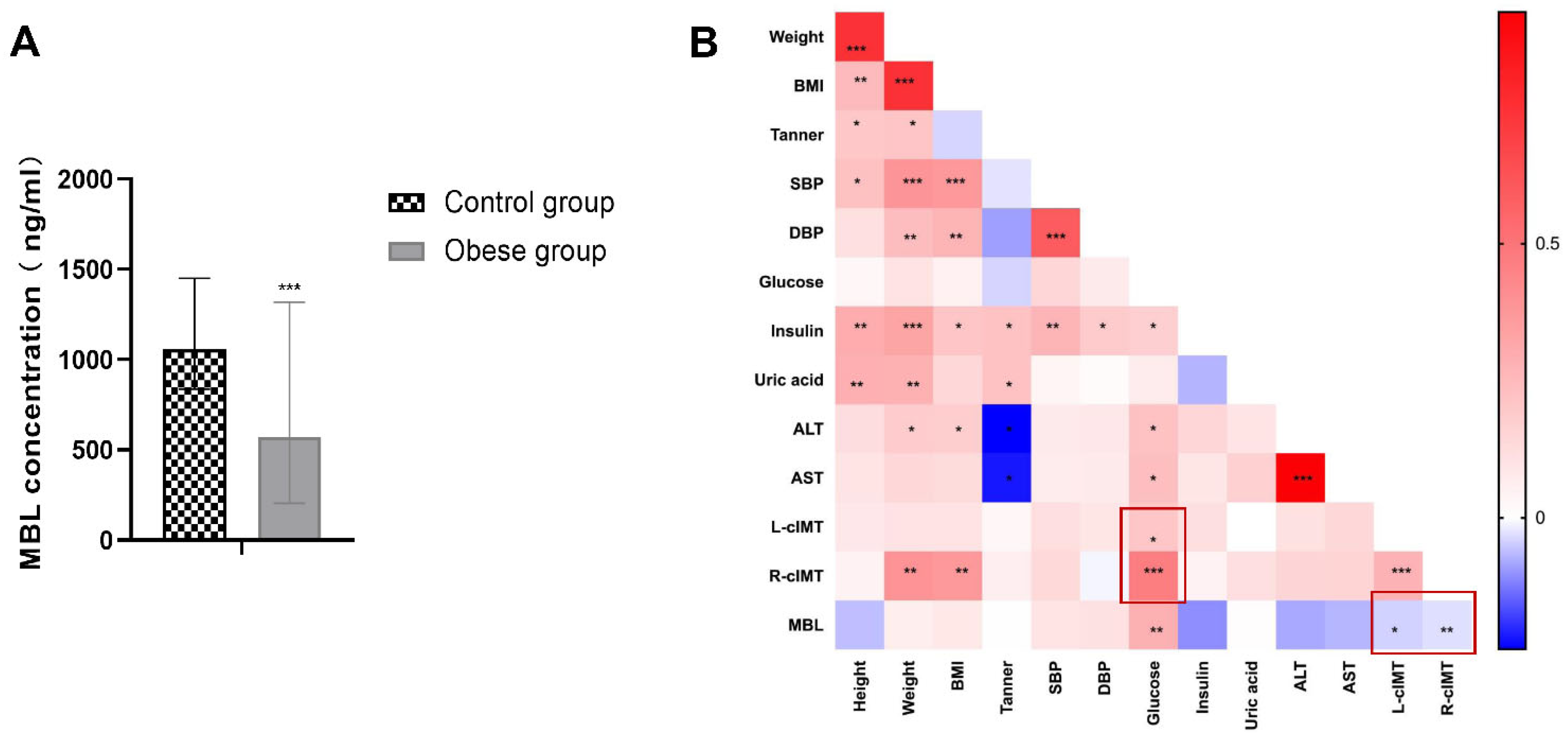
3.1. Serum MBL Level Decreased in Children with Obesity and Negatively Correlated with cIMT
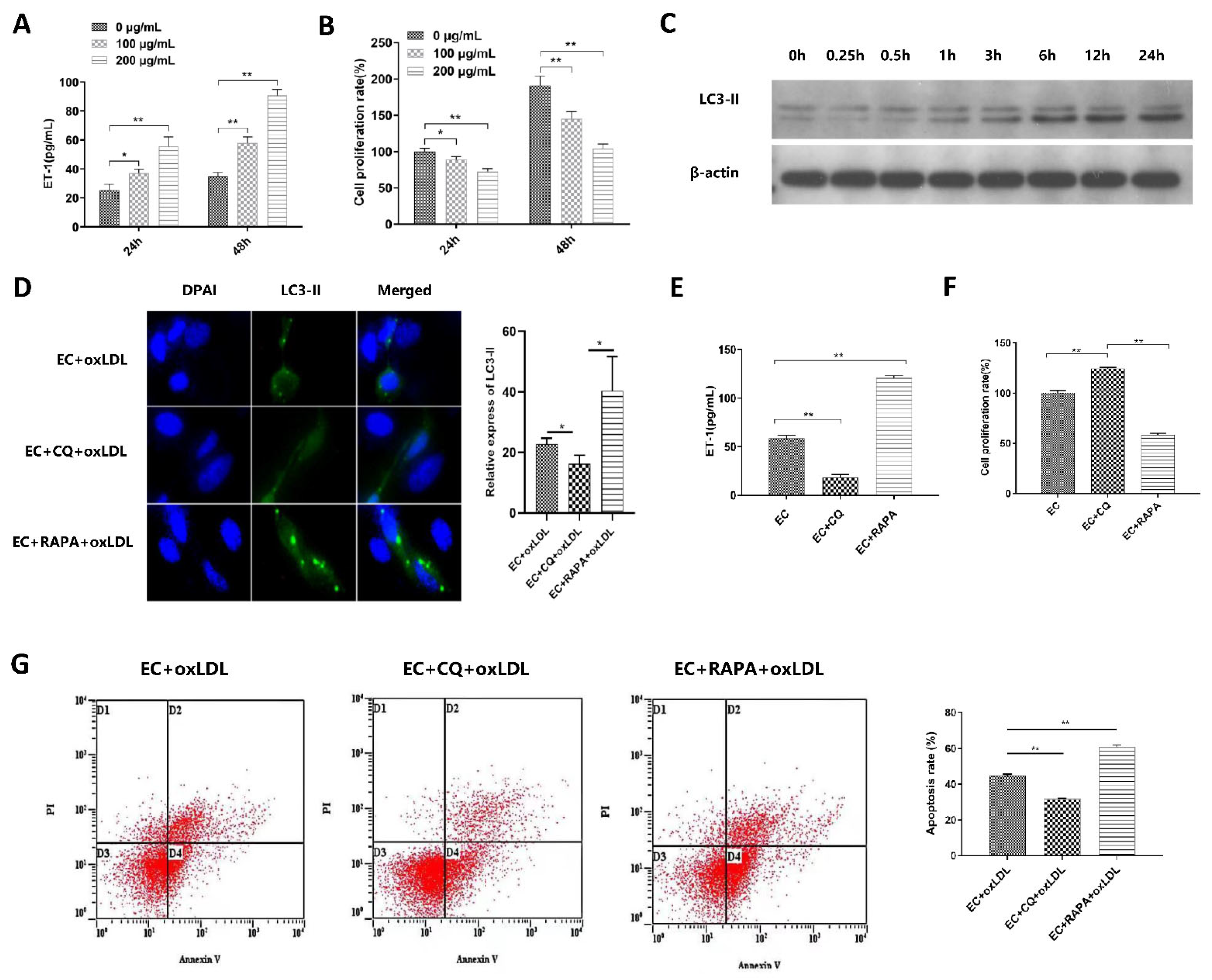
3.2. Autophagy Is Involved in ox-LDL-Induced EC Injury, Proliferation, and Apoptosis
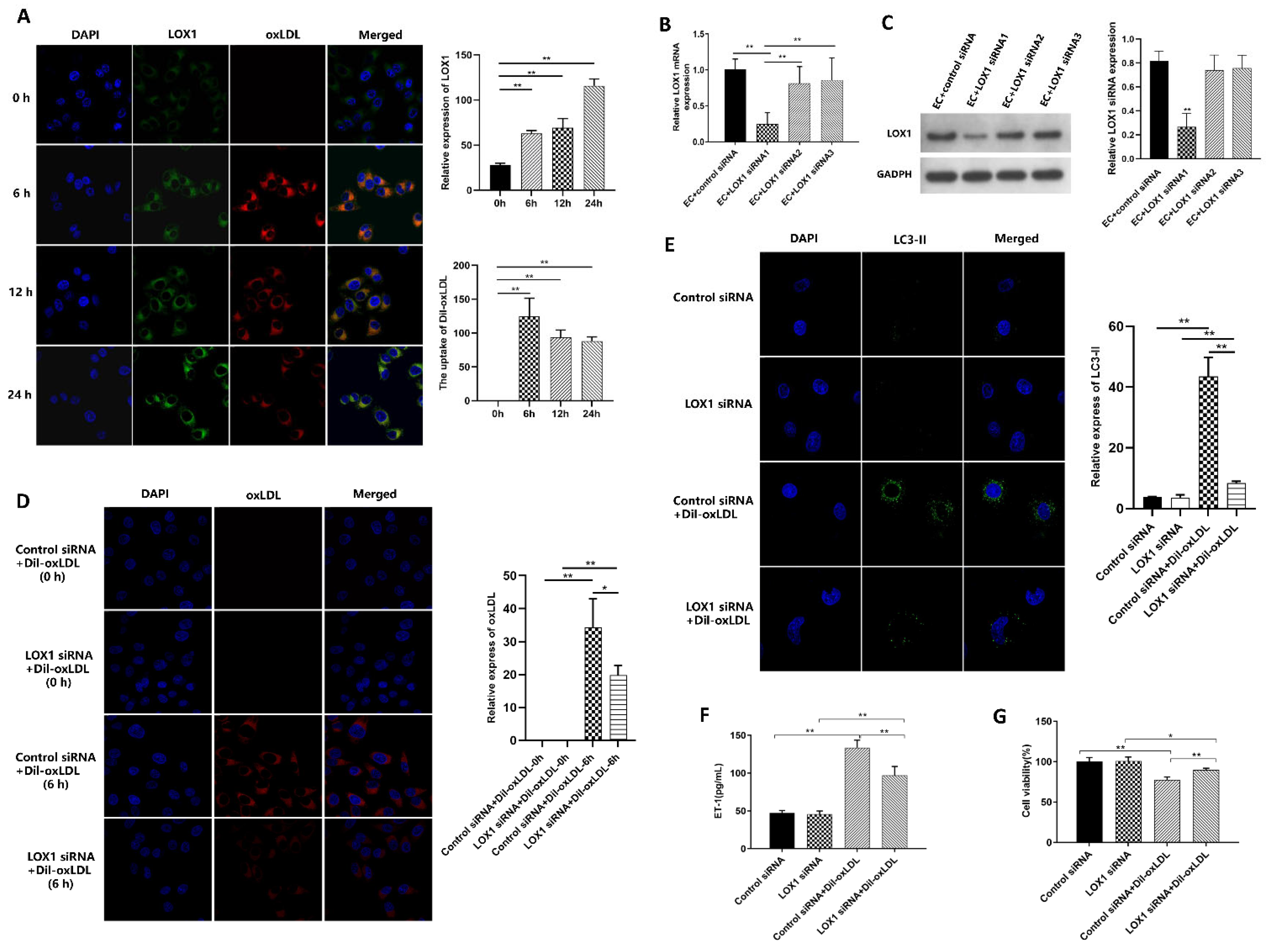
3.3. Ox-LDL-Induced EC Autophagy and Injury via LOX1 Pathway
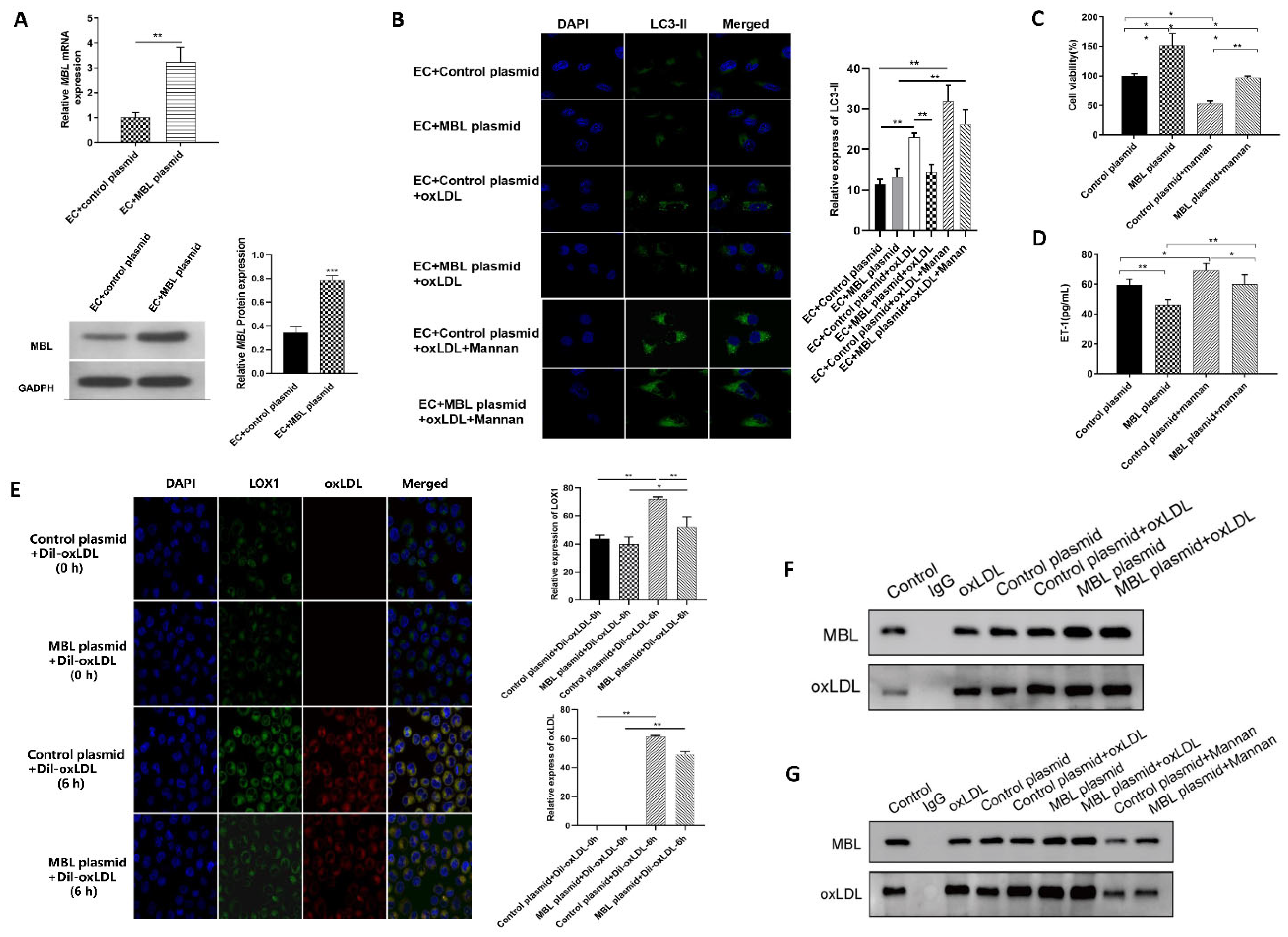
3.4. MBL Over-Expression In Vitro May Block the Binding of LOX1 and ox-LDL, Further Inhibiting ox-LDL-Induced EC Autophagy and Injury
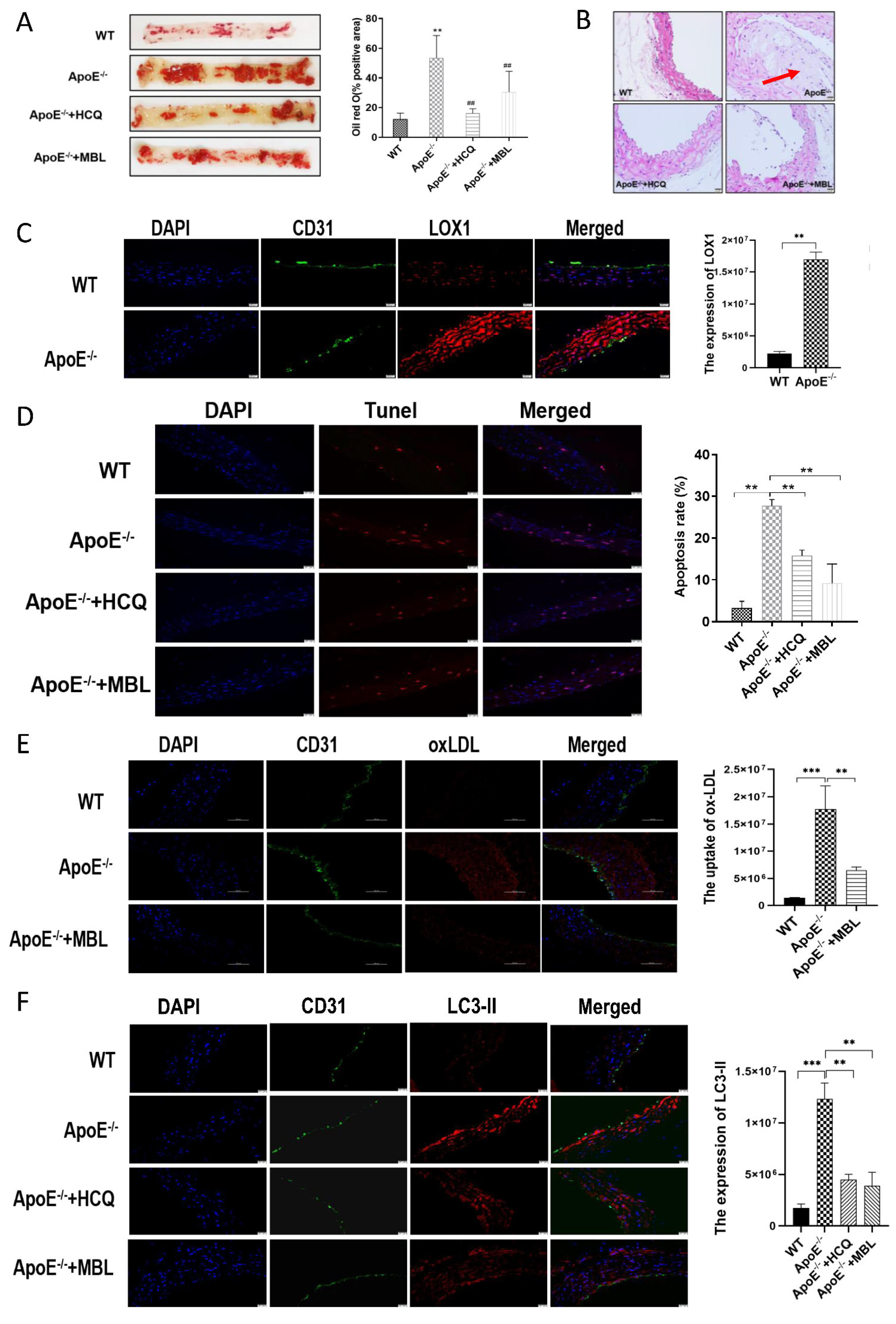
3.5. MBL Alleviates Atherosclerotic Plaque Formation by Modulating Autophagy and Competitively Inhibiting LOX1-ox-LDL Binding
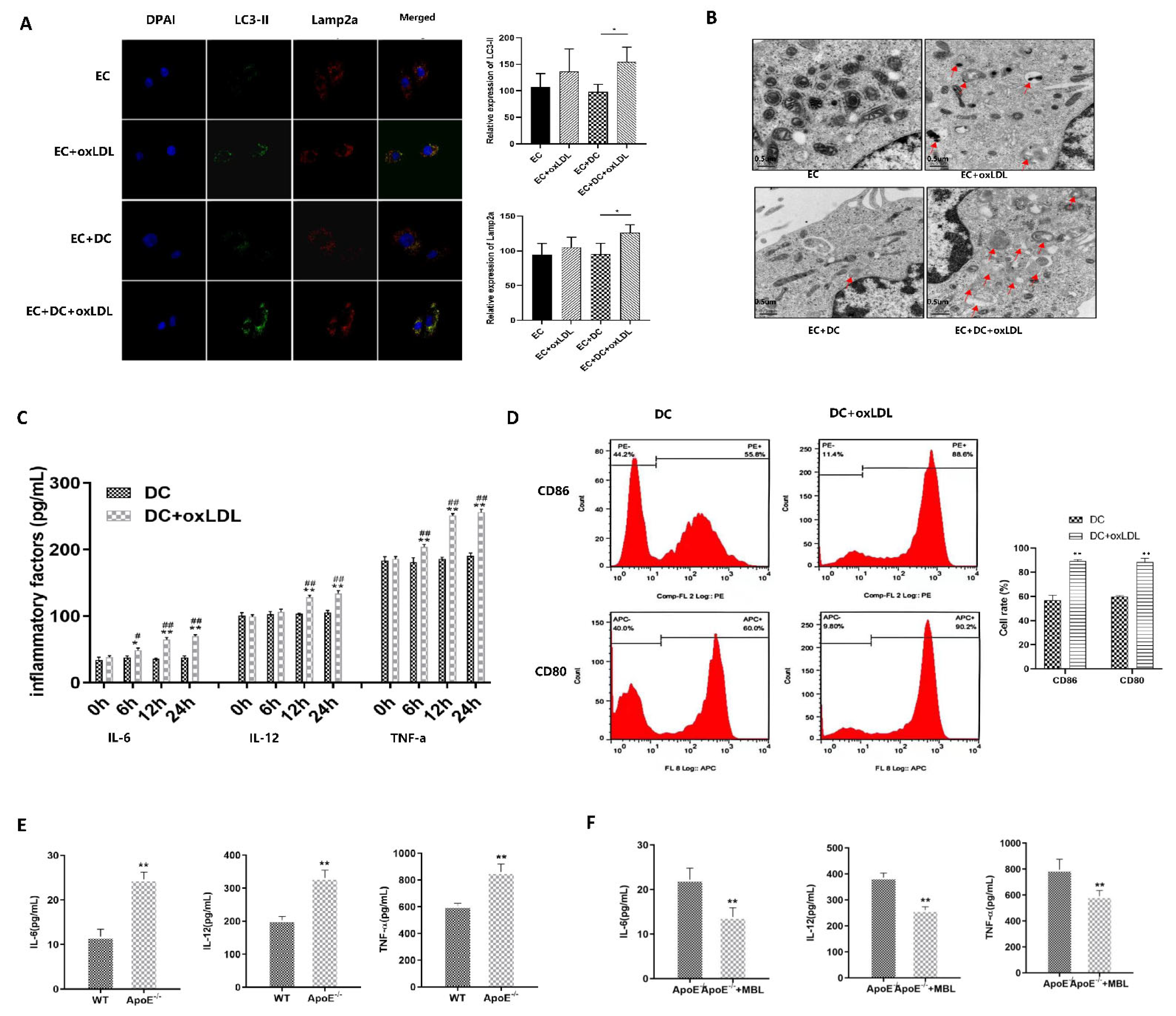
3.6. MBL Alleviates ox-LDL-Induced ECs Injury and Atherosclerotic Plaque Formation via Modulating DCs Matsuration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Rockberg, J.; Jorgensen, L.; Taylor, B.; Sobocki, P.; Johansson, G. Risk of mortality and recurrent cardiovascular events in patients with acute coronary syndromes on high intensity statintreatment. Prev. Med. Rep. 2017, 6, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.X.; Mallat, Z. Targeting the Immune System in Atherosclerosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1691–1706. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.R.; Tang, X.; Lin, C.P.; Yan, D.; Xue, S.; Qian, R.Z.; Guo, D.Q. Identification of potential therapeutic targets for atherosclerosis by analysing the gene signature related to different immune cells and immune regulators in atheromatous plaques. BMC Med. Genom. 2021, 14, 145. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016, 37, 2406–2413. [Google Scholar] [CrossRef]
- IL6R Genetics Consortium Emerging Risk Factors Collaboration; Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Nickel, T.; Schmauss, D.; Hanssen, H.; Sicic, Z.; Krebs, B.; Jankl, S.; Summo, C.; Fraunberger, P.; Walli, A.K.; Pfeiler, S.; et al. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis 2009, 205, 442–450. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Sun, C.; Chen, Z.; Fan, Y.; Deng, X.; Wang, X.; Mehta, J.L. Concentration polarization of ox-LDL activates autophagy and apoptosis via regulating LOX-1 expression. Sci. Rep. 2013, 3, 2091. [Google Scholar] [CrossRef]
- Mollace, V.; Gliozzi, M.; Musolino, V.; Carresi, C.; Muscoli, S.; Mollace, R.; Tavernese, A.; Gratteri, S.; Palma, E.; Morabito, C.; et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int. J. Cardiol. 2015, 184, 152–158. [Google Scholar] [CrossRef]
- Neglia, L.; Fumagalli, S.; Orsini, F.; Zanetti, A.; Perego, C.; De Simoni, M.G. Mannose-binding lectin has a direct deleterious effect on ischemic brain microvascular endothelial cells. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2020, 40, 1608–1620. [Google Scholar] [CrossRef]
- Cai, K.; Ma, Y.; Wang, J.; Nie, W.; Guo, J.; Zhang, M.; Yang, Y.; Chen, J.; Han, F. Mannose-binding lectin activation is associated with the progression of diabetic nephropathy in type 2 diabetes mellitus patients. Ann. Transl. Med. 2020, 8, 1399. [Google Scholar] [CrossRef]
- Hokazono, K.; Belizario, F.S.; Portugal, V.; Messias-Reason, I.; Nisihara, R. Mannose Binding Lectin and Pentraxin 3 in Patients with Diabetic Retinopathy. Arch. Med. Res. 2018, 49, 123–129. [Google Scholar] [CrossRef]
- Vengen, I.T.; Madsen, H.O.; Garred, P.; Platou, C.; Vatten, L.; Videm, V. Mannose-binding lectin deficiency is associated with myocardial infarction: The HUNT2 study in Norway. PLoS ONE 2012, 7, e42113. [Google Scholar] [CrossRef]
- Orsatti, C.L.; Nahas, E.A.; Nahas-Neto, J.; Orsatti, F.L.; Linhares, I.M.; Witkin, S.S. Mannose-binding lectin gene polymorphism and risk factors for cardiovascular disease in postmenopausal women. Mol. Immunol. 2014, 61, 23–27. [Google Scholar] [CrossRef]
- Gedebjerg, A.; Bjerre, M.; Kjaergaard, A.D.; Steffensen, R.; Nielsen, J.S.; Rungby, J.; Friborg, S.G.; Brandslund, I.; Thiel, S.; Beck-Nielsen, H.; et al. Mannose-Binding Lectin and Risk of Cardiovascular Events and Mortality in Type 2 Diabetes: A Danish Cohort Study. Diabetes Care 2020, 43, 2190–2198. [Google Scholar] [CrossRef]
- Kaplar, M.; Sweni, S.; Kulcsar, J.; Cogoi, B.; Esze, R.; Somodi, S.; Papp, M.; Olah, L.; Magyar, M.T.; Szabo, K.; et al. Mannose-Binding Lectin Levels and Carotid Intima-Media Thickness in Type 2 Diabetic Patients. J. Diabetes Res. 2016, 2016, 8132925. [Google Scholar] [CrossRef]
- Fraser, D.A.; Tenner, A.J. Innate immune proteins C1q and mannan-binding lectin enhance clearance of atherogenic lipoproteins by human monocytes and macrophages. J. Immunol. 2010, 185, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.B.; Rahman, M.J.; Tarbell, K.V. Flow cytometric gating for spleen monocyte and DC subsets: Differences in autoimmune NOD mice and with acute inflammation. J. Immunol. Methods 2016, 432, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, T.R.; Jensen, L.; Hansen, A.; Dani, R.; Jensenius, J.C.; Dobo, J.; Gal, P.; Thiel, S. Oligomerization of Mannan-binding Lectin Dictates Binding Properties and Complement Activation. Scand. J. Immunol. 2016, 84, 12–19. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, J.K.; van Golde, L.M.; Batenburg, J.J. Collectins: Players of the innate immune system. Eur. J. Biochem. 2004, 271, 1229–1249. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Takahashi, K.; Shi, L.; Savill, J.; Ezekowitz, R.A. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 2005, 174, 3220–3226. [Google Scholar] [CrossRef]
- Hertle, E.; Arts, I.C.; van der Kallen, C.J.; Feskens, E.J.; Schalkwijk, C.G.; Hoffmann-Petersen, I.T.; Thiel, S.; Stehouwer, C.D.; van Greevenbroek, M.M. Distinct Longitudinal Associations of MBL, MASP-1, MASP-2, MASP-3, and MAp44 With Endothelial Dysfunction and Intima-Media Thickness: The Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) Study. Arter. Thromb. Vasc. Biol. 2016, 36, 1278–1285. [Google Scholar] [CrossRef]
- Caribé, P.M.V.; Villar, C.C.; Romito, G.A.; Takada, J.Y.; Pacanaro, A.P.; Strunz, C.M.C.; César, L.A.M.; Mansur, A.P. Prospective, case-controlled study evaluating serum concentration of sirtuin-1 and mannose-binding lectin in patients with and without periodontal and coronary artery disease. Ther. Adv. Chronic. Dis. 2020, 11, 2040622320919621. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Liao, X.; Sluimer, J.C.; Wang, Y.; Subramanian, M.; Brown, K.; Pattison, J.S.; Robbins, J.; Martinez, J.; Tabas, I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012, 15, 545–553. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Y.; Tong, T.; Shi, J.; Zhang, Y.; Yang, Y.; Liu, C. Autophagy in atherosclerosis: A phenomenon found in human carotid atherosclerotic plaques. Chin. Med. J. 2015, 128, 69–74. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhang, Y.H.; Li, R.B.; Zhou, L.Y.; An, T.; Zhang, R.C.; Zhai, M.; Huang, Y.; Yan, K.W.; Dong, Y.H.; et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat. Commun. 2018, 9, 29. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Li, Z.; Wang, M. Regulation and mechanism of mannan-binding lectin (MBL) on adipogenic differ entiation of 3T3-L1 preadipocytes. Chin. J. Microbiol. Immunol. 2020, 10, 270–279. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yang, C.; Mu, Y.; Wang, Y.; Zhang, W.; Yang, Y.; Chen, C.; Song, S.; Shen, Z.; et al. Mannan-Binding Lectin Suppresses Peptidoglycan-Induced TLR2 Activation and Inflammatory Responses. Mediat. Inflamm. 2019, 2019, 1349784. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, H.J.; Zhang, L.Y.; Lu, X.; Zuo, D.M.; Shan, G.Q.; Xu, T.Y.; Chen, Z.L. Mannan-binding lectin at supraphysiological concentrations inhibits differentiation of dendritic cells from human CD14+ monocytes. Microbiol. Immunol. 2015, 59, 724–734. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Chen, Y.; Zhang, L.; Lu, X.; Chen, Z. Mannan-binding lectin regulates dendritic cell maturation and cytokine production induced by lipopolysaccharide. BMC Immunol. 2011, 12, 1. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Clemente, J.C.; Giannarelli, C. Physical Activity, Immune System, and the Microbiome in Cardiovascular Disease. Front. Physiol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Wu, M.; Wang, F.; Yang, J.; Li, P.; Yan, D.; Yang, Y.; Zhang, W.; Ren, J.; Zhang, Z.; Wang, M. The responses of the gut microbiota to MBL deficiency. Mol. Immunol. 2020, 122, 99–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Chen, X.; Zhang, L.; Yuan, J.; Lin, H.; Zhu, M.; Xu, X.; Dong, G.; Fu, J.; Wu, W. Mannose-Binding Lectin Reduces Oxidized Low-Density Lipoprotein Induced Vascular Endothelial Cells Injury by Inhibiting LOX1-ox-LDL Binding and Modulating Autophagy. Biomedicines 2023, 11, 1743. https://doi.org/10.3390/biomedicines11061743
Zhou X, Chen X, Zhang L, Yuan J, Lin H, Zhu M, Xu X, Dong G, Fu J, Wu W. Mannose-Binding Lectin Reduces Oxidized Low-Density Lipoprotein Induced Vascular Endothelial Cells Injury by Inhibiting LOX1-ox-LDL Binding and Modulating Autophagy. Biomedicines. 2023; 11(6):1743. https://doi.org/10.3390/biomedicines11061743
Chicago/Turabian StyleZhou, Xuelian, Xuefeng Chen, Li Zhang, Jinna Yuan, Hu Lin, Mingqiang Zhu, Xiaoqin Xu, Guanping Dong, Junfen Fu, and Wei Wu. 2023. "Mannose-Binding Lectin Reduces Oxidized Low-Density Lipoprotein Induced Vascular Endothelial Cells Injury by Inhibiting LOX1-ox-LDL Binding and Modulating Autophagy" Biomedicines 11, no. 6: 1743. https://doi.org/10.3390/biomedicines11061743
APA StyleZhou, X., Chen, X., Zhang, L., Yuan, J., Lin, H., Zhu, M., Xu, X., Dong, G., Fu, J., & Wu, W. (2023). Mannose-Binding Lectin Reduces Oxidized Low-Density Lipoprotein Induced Vascular Endothelial Cells Injury by Inhibiting LOX1-ox-LDL Binding and Modulating Autophagy. Biomedicines, 11(6), 1743. https://doi.org/10.3390/biomedicines11061743





