SDF-1α-Releasing Microspheres Effectively Extend Stem Cell Homing after Myocardial Infarction
Abstract
1. Introduction
2. Methods
2.1. Animal Studies
2.2. Animal Experimental Design
2.3. Microspheres with SDF-1α
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. RNA Isolation and Quantitative RT-PCR
2.6. Statistical Analysis
3. Results
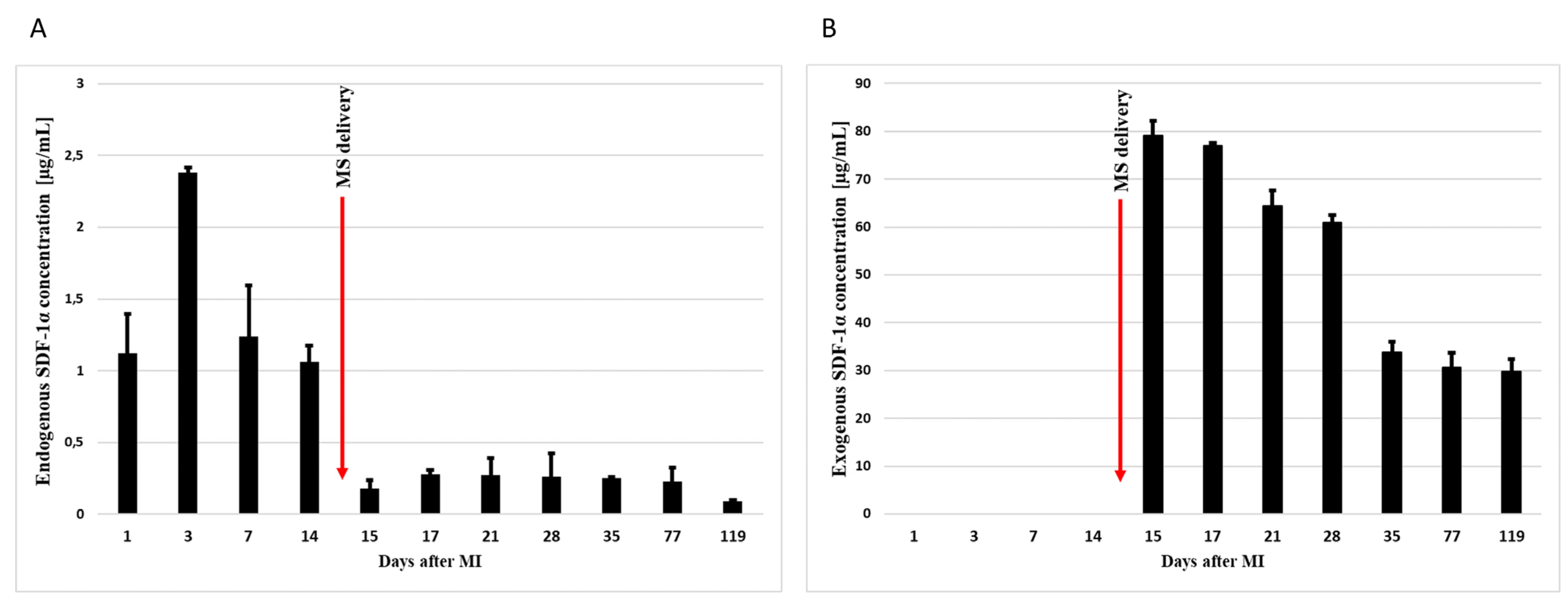
3.1. Intrapericardial Administration of Microspheres Increases the Concentration of SDF-1α in the Myocardium
3.2. SDF-1α-Releasing Microspheres Affect Gene Expression in the Heart after MI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Vilela, E.M.; Fontes-Carvalho, R. Inflammation and ischemic heart disease: The next therapeutic target? Rev. Port. Cardiol. Engl. Ed. 2021, 40, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.G.S.J.; Sharpe, N. Left Ventricular Remodeling After Myocardial Infarction Pathophysiology and Therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Doppler, S.A.; Deutsch, M.A.; Lange, R.; Krane, M. Cardiac regeneration: Current therapies—Future concepts. J. Thorac. Dis. 2013, 5, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Broughton, K.M.; Wang, B.J.; Firouzi, F.; Khalafalla, F.; Dimmeler, S.; Fernandez-Aviles, F.; Sussman, M.A. Mechanisms of Cardiac Repair and Regeneration. Circ. Res. 2018, 122, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522. [Google Scholar] [CrossRef]
- Rheault-Henry, M.; White, I.; Grover, D.; Atoui, R. Stem cell therapy for heart failure: Medical breakthrough, or dead end? World J. Stem Cells 2021, 13, 236–259. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Zbinden, S.; Epstein, S.E.; Murry, C.E. Cell-based therapy for myocardial ischemia and infarction: Pathophysiological mechanisms. Annu. Rev. Pathol. 2007, 2, 307–339. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, S.K.; Muhlstedt, S.; Ozcelik, C.; Bader, M. SDF-1α as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol. Ther. 2011, 129, 97–108. [Google Scholar] [CrossRef]
- Kucia, M.; Dawn, B.; Hunt, G.; Guo, Y.; Wysoczynski, M.; Majka, M.; Ratajczak, J.; Rezzoug, F.; Ildstad, S.T.; Bolli, R.; et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ. Res. 2004, 95, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Askari, A.T.; Unzek, S.; Popovic, Z.B.; Goldman, C.K.; Forudi, F.; Kiedrowski, M.; Rovner, A.; Ellis, S.G.; Thomas, J.D.; DiCorleto, P.E.; et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003, 362, 697–703. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Gupta, S.K. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 α mRNA is selectively induced in rat model of myocardial infarction. Inflammation 2001, 25, 293–300. [Google Scholar] [CrossRef]
- Zalewski, J.; Claus, P.; Bogaert, J.; Driessche, N.V.; Driesen, R.B.; Galan, D.T.; Sipido, K.R.; Buszman, P.; Milewski, K.; Van de Werf, F. Cyclosporine A reduces microvascular obstruction and preserves left ventricular function deterioration following myocardial ischemia and reperfusion. Basic Res. Cardiol. 2015, 110, 18. [Google Scholar] [CrossRef]
- Dębiński, M.; Buszman, P.P.; Milewski, K.; Wojakowski, W.; Jackiewicz, W.; Pająk, J.; Szurlej, D.; Fryc-Stanek, J.; Wiernek, S.; Jelonek, M.; et al. Intracoronary adiponectin at reperfusion reduces infarct size in a porcine myocardial infarction model. Int. J. Mol. Med. 2011, 27, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Buszman, P.P.; Wojakowski, W.; Milewski, K.; Dębiński, M.; Pająk, J.; Aboodi, M.S.; Jackiewicz, W.; Kawka, M.; Bochenek, A.; Prats, J.; et al. Controlled reperfusion with intravenous bivalirudin and intracoronary abciximab combination therapy in the porcine myocardial infarction model. Thromb Res. 2012, 130, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Konarski, Ł.; Dębiński, M.; Kolarczyk-Haczyk, A.; Jelonek, M.; Kondys, M.; Buszman, P. Aborted myocardial infarction in patients with ST-segment elevation myocardial infarction treated with mechanical reperfusion. Kardiol. Pol. 2021, 79, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bajdak-Rusinek, K.; Fus-Kujawa, A.; Jelonek, K.; Musiał-Kulik, M.; Buszman, P.P.; Żyła-Uklejewicz, D.; Sekowska, A.W.; Kasperczyk, J.; Buszman, P.E. Controlled Release of Encapsuled Stromal-Derived Factor 1α Improves Bone Marrow Mesenchymal Stromal Cells Migration. Bioengineering 2022, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Gelfand, E.V.; Cannon, C.P. Myocardial infarction: Contemporary management strategies. J. Intern. Med. 2007, 262, 59–77. [Google Scholar] [CrossRef]
- Segers, V.F.; Lee, R. Stem-cell therapy for cardiac disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Jankowski, K.; Reca, R.; Wysoczynski, M.; Bandura, L.; Allendorf, D.J.; Zhang, J.; Ratajczak, J.; Ratajczak, M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Wojakowski, W.; Reca, R.; Machalinski, B.; Gozdzik, J.; Majka, M.; Baran, J.; Ratajczak, J.; Ratajczak, M.Z. The migration of bone marrow-derived nonhematopoietic tissue-committed stem cells is regulated in an SDF-1-, HGF-, and LIF dependent manner. Arch. Immunol. Ther. Exp. 2006, 54, 121–135. [Google Scholar] [CrossRef]
- Chirica, A.; Terzic, A.; Park, S.; Ikeda, Y.; Faustino, R.; Nelson, T.J. SDF-1-enhanced cardiogenesis requires CXCR4 induction in pluripotent stem cells. J. Cardiovasc. Transl. Res. 2010, 3, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Peura, M.; Bizik, J.; Salmenpera, P.; Noro, A.; Korhonen, M.; Pätilä, T.; Vento, A.; Vaheri, A.; Alitalo, R.; Vuola, J.; et al. Bone marrow mesenchymal stem cells undergo nemesis and induce keratinocyte wound healing utilizing the HGF/c-Met/PI3K pathway. Wound Repair Regen. 2009, 17, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Rosenberg, M.; Kiessling, F.; Eckstein, V.; Heger, T.; Krebs, J.; Ho, A.D.; Katus, H.A.; Frey, N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc. Res. 2008, 77, 143–150. [Google Scholar] [CrossRef]
- Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Mesenchymal stem cells and neovascularization: Role of platelet-derived growth factor receptors. J. Cell. Mol. Med. 2007, 11, 1012–1030. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Enhancing the Migration Ability of Mesenchymal Stromal Cells by Targeting the SDF-1/CXCR4 Axis. BioMed Res. Int. 2013, 2013, 561098. [Google Scholar] [CrossRef]
- Wojakowski, W.; Tendera, M.; Michalowska, A.; Majka, M.; Kucia, M.; Maślankiewicz, K.; Wyderka, R.; Ochała, A.; Ratajczak, M.Z. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation 2004, 110, 3213–3220. [Google Scholar] [CrossRef]
- Hu, X.; Dai, S.; Wu, W.; Tan, W.; Zhu, X.; Mu, J.; Guo, Y.; Bolli, R.; Rokosh, G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: Role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation 2007, 116, 654–663. [Google Scholar] [CrossRef]
- Musialek, P.; Tekieli, L.; Kostkiewicz, M.; Miszalski-Jamka, T.; Klimeczek, P.; Mazur, W.; Szot, W.; Majka, M.; Banys, R.P.; Jarocha, D.; et al. Infarct size determines myocardial uptake of CD34+ cells in the peri-infarct zone: Results from a study of (99m)Tc-extametazime-labeled cell visualization integrated with cardiac magnetic resonance infarct imaging. Circ. Cardiovasc. Imaging 2013, 6, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dill, T.; Schachinger, V.; Rolf, A.; Möllmann, S.; Thiele, H.; Tillmanns, H.; Assmus, B.; Dimmeler, S.; Zeiher, A.M.; Hamm, C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: Results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIRAMI) cardiac magnetic resonance imaging substudy. Am. Heart J. 2009, 157, 541–547. [Google Scholar] [PubMed]
- Segers, V.F.; Tokunou, T.; Higgins, L.J.; MacGillivray, C.; Gannon, J.; Lee, R.T. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation 2007, 116, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Nakamura, Y.; Wang, X.; Hu, Q.; Suggs, L.J.; Zhang, J. Controlled release of stromal cell-derived factor-1 α in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007, 13, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mal, N.; Kiedrowski, M.; Chacko, M.; Askari, A.T.; Popovic, Z.B.; Koc, O.N.; Penn, M.S. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kim, C.H.; Abdel-Latif, A.; Schneider, G.; Kucia, M.; Morris, A.J.; Laughlin, M.J.; Ratajczak, J. A novel perspective on stem cell homing and mobilization: Review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, T.; Li, Q.; Chen, B.; Hou, X.; Xiao, Z.; Dai, J. Controlled Release of Collagen-Binding SDF-1α Improves Cardiac Function after Myocardial Infarction by Recruiting Endogenous Stem Cells. Sci. Rep. 2016, 6, 26683. [Google Scholar] [CrossRef]
- Mansor, M.H.; Najberg, M.; Contini, A.; Alvarez-Lorenzo, C.; Garcion, E.; Jérôme, C.; Boury, F. Development of a non-toxic and non-denaturing formulation process for encapsulation of SDF-1α into PLGA/PEG-PLGA nanoparticles to achieve sus-tained release. Eur. J. Pharm. Biopharm. 2018, 125, 38–50. [Google Scholar] [CrossRef]
- Oleszko-Torbus, N.; Bochenek, M.; Utrata-Wesołek, A.; Hajduk, B.; Kubacki, J.; Jałowiecki, Ł.; Płaza, G.; Kowalczuk, A.; Mendrek, B. Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility-Interactions with Water and Use for Cell Culture. Materials 2020, 13, 2702. [Google Scholar] [CrossRef]
- Ramos, T.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 2. [Google Scholar] [CrossRef]
- Louka, D.A.; Holwell, N.; Thomas, B.H.; Chen, F.; Amsden, B.G. Highly Bioactive SDF-1α Delivery from Low-Melting-Point, Biodegradable Polymer Microspheres. ACS Biomater. Sci. Eng. 2018, 4, 3747–3758. [Google Scholar] [CrossRef]
- Abbott, J.D.; Huang, Y.; Liu, D.; Hickey, R.; Krause, D.; Giordano, F. Stromal Cell–Derived Factor-1α Plays a Critical Role in Stem Cell Recruitment to the Heart After Myocardial Infarction but Is Not Sufficient to Induce Homing in the Absence of Injury. Circulation 2004, 110, 3300–3305. [Google Scholar] [CrossRef]
- Vermeulen, M.; Le Pesteur, F.; Gagnerault, M.C.; Mary, J.Y.; Sainteny, F.; Lepault, F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood 1998, 92, 894–900. [Google Scholar] [CrossRef]
- Frenette, P.S.; Subbarao, S.; Mazo, I.B.; von Andrian, U.H.; Wagner, D.D. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc. Natl. Acad. Sci. USA 1998, 95, 14423–14428. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Grabovsky, V.; Habler, L.; Sandbank, J.; Arenzana-Seisdedos, F.; Petit, I.; Ben-Hur, H.; Lapidot, T.; Alon, R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34_ cells on vascular endothelium under shear flow. J. Clin. Investig. 1999, 104, 1199–1211. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef] [PubMed]
- Zamproni, L.N.; Mundim, M.V.; Porcionatto, M.A.; des Rieux, A. Injection of SDF-1 loaded nanoparticles following traumatic brain injury stimulates neural stem cell recruitment. Int. J. Pharm. 2017, 519, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, T.; Huang, S.; Suen, C.W.; Cheng, X.; Li, J.; Hou, H.; She, G.; Zhang, H.; Wang, H.; et al. Sustained Release SDF-1α/TGF-β1-Loaded Silk Fibroin-Porous Gelatin Scaffold Promotes Cartilage Repair. ACS Appl. Mater. Interfaces 2019, 11, 14608–14618. [Google Scholar] [CrossRef]
- Holloway, J.L.; Ma, H.; Rai, R.; Hankenson, K.D.; Burdick, J.A. Synergistic Effects of SDF-1α and BMP-2 Delivery from Proteolytically Degradable Hyaluronic Acid Hydrogels for Bone Repair. Macromol. Biosci. 2015, 15, 1218–1223. [Google Scholar] [CrossRef]


 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | Study Group | ||||||||||
| Animals number | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 |
| Days after MI | 24 h | 72 h | 7 d | 14 d | 15 d | 17 d | 21 d | 28 d | 35 d | 77 d | 119 d |
| Days after MS delivery | 24 h | 72 h | 7 d | 14 d | 3 wks | 9 wks | 15 wks | ||||
| ELISA test | x | x | x | x | x | x | x | x | x | x | x |
| RT-PCR | x | x | x | x | x | x | x | x | x | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajdak-Rusinek, K.; Fus-Kujawa, A.; Buszman, P.; Żyła-Uklejewicz, D.; Jelonek, K.; Musiał-Kulik, M.; Fernandez, C.; Michalak, M.; George, K.; Kasperczyk, J.; et al. SDF-1α-Releasing Microspheres Effectively Extend Stem Cell Homing after Myocardial Infarction. Biomedicines 2023, 11, 343. https://doi.org/10.3390/biomedicines11020343
Bajdak-Rusinek K, Fus-Kujawa A, Buszman P, Żyła-Uklejewicz D, Jelonek K, Musiał-Kulik M, Fernandez C, Michalak M, George K, Kasperczyk J, et al. SDF-1α-Releasing Microspheres Effectively Extend Stem Cell Homing after Myocardial Infarction. Biomedicines. 2023; 11(2):343. https://doi.org/10.3390/biomedicines11020343
Chicago/Turabian StyleBajdak-Rusinek, Karolina, Agnieszka Fus-Kujawa, Piotr Buszman, Dorota Żyła-Uklejewicz, Katarzyna Jelonek, Monika Musiał-Kulik, Carlos Fernandez, Magdalena Michalak, Kurian George, Janusz Kasperczyk, and et al. 2023. "SDF-1α-Releasing Microspheres Effectively Extend Stem Cell Homing after Myocardial Infarction" Biomedicines 11, no. 2: 343. https://doi.org/10.3390/biomedicines11020343
APA StyleBajdak-Rusinek, K., Fus-Kujawa, A., Buszman, P., Żyła-Uklejewicz, D., Jelonek, K., Musiał-Kulik, M., Fernandez, C., Michalak, M., George, K., Kasperczyk, J., & Buszman, P. (2023). SDF-1α-Releasing Microspheres Effectively Extend Stem Cell Homing after Myocardial Infarction. Biomedicines, 11(2), 343. https://doi.org/10.3390/biomedicines11020343








