New Insights into the Use of Liraglutide—Impact on Cardiovascular Risk and Microvascular Outcomes
Abstract
1. Introduction
2. General Information about the Group of GLP-1 Receptor Agonists with Special Emphasis on Liraglutide
3. Safety and Efficacy of Liraglutide in the Treatment of Type 2 Diabetes
4. Molecular Effects of Liraglutide Treatment—Effects on Oxidative Stress and Endothelial Dysfunction
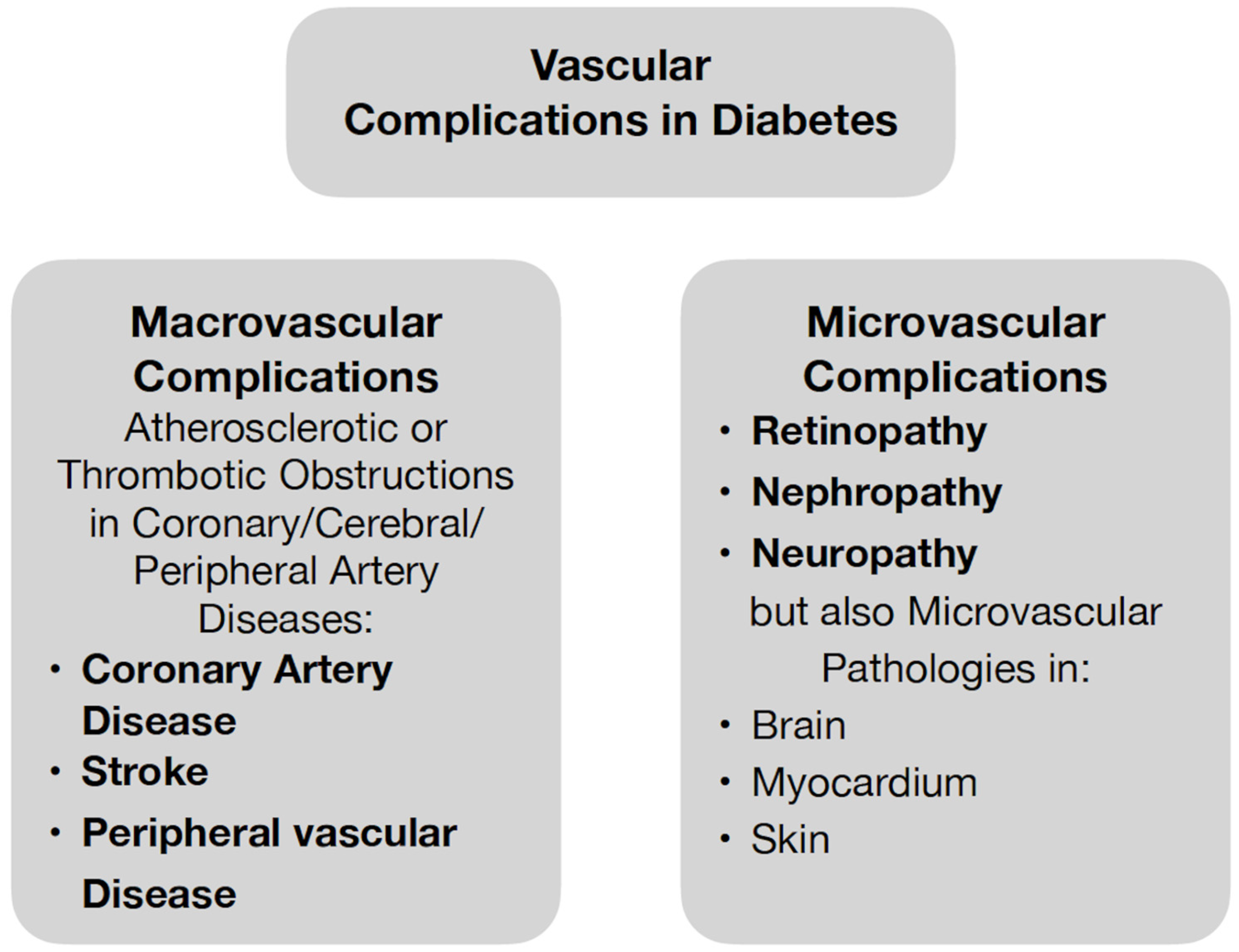
5. Microvascular Outcomes of Liraglutide Treatment
6. Cardiovascular Outcomes of Liraglutide Treatment
7. The Use of Liraglutide in the Treatment of Obesity—Results
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sapra, A.; Bhandari, P. Diabetes Mellitus; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Heart in diabetes: A microvascular disease. Diabetes Care 2011, 34 (Suppl. S2), S145–S149. [Google Scholar] [CrossRef]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Glucagon-Like Peptide-1 (GLP-1) Analogues. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Anawalt, B.; Blackman, M.R.; Boyce, A.; Chrousos, G.; Corpas, E.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; Hofland, J.; et al. Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Latif, W.; Lambrinos, K.J.; Rodriguez, R. Compare and Contrast the Glucagon-Like Peptide-1 Receptor Agonists (GLP1RAs); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wanner, C.; Lachin, J.M.; Inzucchi, S.E.; Fitchett, D.; Mattheus, M.; George, J.; Woerle, H.J.; Broedl, U.C.; von Eynatten, M.; Zinman, B.; et al. Empagliflozin and Clinical Outcomes in Patients with Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease. Circulation 2018, 137, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.E.; Fonseca, V.; Mosenzon, O.; Raz, I.; Goldman, B.; Idorn, T.; von Scholten, B.J.; Poulter, N.R. Effects of Liraglutide Versus Placebo on Cardiovascular Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Circulation 2018, 138, 2908–2918. [Google Scholar] [CrossRef]
- Verma, S.; Wanner, C.; Zwiener, I.; Ofstad, A.P.; George, J.T.; Fitchett, D.; Zinman, B.; EMPA-REG OUTCOME Investigators. Influence of Microvascular Disease on Cardiovascular Events in Type 2 Diabetes. J. Am. Coll. Cardiol. 2019, 73, 2780–2782. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Zheng, Y.; Li, J.; Wang, Y.; Lin, Y.; He, Z.; Zhao, W.; Chen, C.; Qiu, K.; et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Duan, C.M.; Wan, T.F.; Wang, Y.; Yang, Q.W. Cardiovascular outcomes of liraglutide in patients with type 2 diabetes: A systematic review and meta-analysis. Medicine 2019, 98, e17860. [Google Scholar] [CrossRef]
- Tsapas, A.; Avgerinos, I.; Karagiannis, T.; Malandris, K.; Manolopoulos, A.; Andreadis, P.; Liakos, A.; Matthews, D.R.; Bekiari, E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann. Intern. Med. 2020, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Taheri, S.; Saffaei, A.; Amani, B.; Akbarzadeh, A.; Peiravian, F.; Yousefi, N. Efficacy and Safety of Dulaglutide Compared to Liraglutide: A Systematic Review and Meta-analysis in Patients with Type 2 Diabetes Mellitus. Iran J. Pharm. Res. 2019, 18, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Miyoshi, H.; Bosch Traberg, H.; Divyalasya, T.V.S.; Nishijima, K.; Terauchi, Y. A randomized trial to investigate the efficacy and safety of once-daily liraglutide 1.8 mg in Japanese adults with type 2 diabetes exhibiting an inadequate response to liraglutide 0.9 mg. J. Diabetes Investig. 2022, 13, 1321–1329. [Google Scholar] [CrossRef]
- Wang, L.; Xin, Q.; Wang, Y.; Chen, Z.; Yuan, R.; Miao, Y.; Zhang, G.; Cong, W. Efficacy and safety of liraglutide in type 2 diabetes mellitus patients complicated with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2021, 171, 105765. [Google Scholar] [CrossRef] [PubMed]
- Capehorn, M.S.; Catarig, A.M.; Furberg, J.K.; Janez, A.; Price, H.C.; Tadayon, S.; Vergès, B.; Marre, M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020, 46, 100–109. [Google Scholar] [CrossRef]
- Rubanyi, G.M. The role of endothelium in cardiovascular homeostasis and diseases. J. Cardiovasc. Pharmacol. 1993, 22 (Suppl. S4), S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Liu, F.Q.; Wang, J.; Wang, X.P.; Hou, X.G.; Sun, Y.; Qin, W.D.; Wei, S.J.; Zhang, Y.; Chen, L.; et al. Hyperglycemia induces apoptosis of pancreatic islet endothelial cells via reactive nitrogen species-mediated Jun N-terminal kinase activation. Biochim. Biophys. Acta 2011, 1813, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.F.; Hull, R.L. The islet endothelial cell: A novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia 2017, 60, 952–959. [Google Scholar] [CrossRef]
- Le, Y.; Wei, R.; Yang, K.; Lang, S.; Gu, L.; Liu, J.; Hong, T.; Yang, J. Liraglutide ameliorates palmitate-induced oxidative injury in islet microvascular endothelial cells through GLP-1 receptor/PKA and GTPCH1/eNOS signaling pathways. Peptides 2020, 124, 170212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Wu, W.; Shi, C.; Hu, S.; Yin, T.; Ma, Q.; Han, T.; Zhang, Y.; Tian, F.; et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic. Biol. Med. 2016, 95, 278–292. [Google Scholar] [CrossRef]
- Rizzo, M.; Abate, N.; Chandalia, M.; Rizvi, A.A.; Giglio, R.V.; Nikolic, D.; Marino Gammazza, A.; Barbagallo, I.; Isenovic, E.R.; Banach, M.; et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: A prospective pilot study. J. Clin. Endocrinol. Metab. 2015, 100, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Nikolic, D.; Patti, A.M.; Mannina, C.; Montalto, G.; McAdams, B.S.; Rizvi, A.A.; Cosentino, F. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 2814–2821. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Beckman, J.A.; Nian, H.; Garner, E.M.; Mayfield, D.; Devin, J.K.; Koethe, J.R.; Brown, J.D.; Cahill, K.N.; Yu, C.; et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: A randomized controlled trial. Diabetes Obes. Metab. 2023, 25, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef]
- Guyton, J.; Jeon, M.; Brooks, A. Glucagon-like peptide 1 receptor agonists in type 1 diabetes mellitus. Am. J. Health Syst. Pharm. 2019, 76, 1739–1748. [Google Scholar] [CrossRef]
- Verma, S.; Bain, S.C.; Honoré, J.B.; FE Mann, J.; ANauck, M.; EPratley, R.; Rasmussen, S.; Sejersten Ripa, M.; Zinman, B.; Buse, J.B. Impact of microvascular disease on cardiovascular outcomes in type 2 diabetes: Results from the LEADER and SUSTAIN 6 clinical trials. Diabetes Obes. Metab. 2020, 22, 2193–2198. [Google Scholar] [CrossRef]
- GRADE Study Research Group; Nathan, D.M.; Lachin, J.M.; Bebu, I.; Burch, H.B.; Buse, J.B.; Cherrington, A.L.; Fortmann, S.P.; Green, J.B.; Kahn, S.E.; et al. Glycemia Reduction in Type 2 Diabetes—Microvascular and Cardiovascular Outcomes. N. Engl. J. Med. 2022, 387, 1075–1088. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Suhrs, H.E.; Raft, K.F.; Bové, K.; Madsbad, S.; Holst, J.J.; Zander, M.; Prescott, E. Effect of liraglutide on body weight and microvascular function in non-diabetic overweight women with coronary microvascular dysfunction. Int. J. Cardiol. 2019, 283, 28–34. [Google Scholar] [CrossRef]
- Faber, R.; Zander, M.; Pena, A.; Michelsen, M.M.; Mygind, N.D.; Prescott, E. Effect of the glucagon-like peptide-1 analogue liraglutide on coronary microvascular function in patients with type 2 diabetes—A randomized, single-blinded, cross-over pilot study. Cardiovasc. Diabetol. 2015, 14, 41. [Google Scholar] [CrossRef]
- von Scholten, B.J.; Persson, F.; Rosenlund, S.; Hovind, P.; Faber, J.; Hansen, T.W.; Rossing, P. The effect of liraglutide on renal function: A randomized clinical trial. Diabetes Obes. Metab. 2017, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Nreu, B.; Scatena, A.; Andreozzi, F.; Sesti, G.; Mannucci, E.; Monami, M. Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A meta-analysis of randomized controlled trials. Acta Diabetol. 2017, 54, 933–941, Erratum in Acta Diabetol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cha, A.S.; Chen, Y.; Fazioli, K.; Rivara, M.B.; Devine, E.B. Microvascular Benefits of New Antidiabetic Agents: A Systematic Review and Network Meta-Analysis of Kidney Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, 1225–1234. [Google Scholar] [CrossRef]
- Cianflone, D.; Rizvi, A.A.; Rizzo, M. Microvascular and macrovascular effects of liraglutide. Int. J. Cardiol. 2019, 286, 17–18. [Google Scholar] [CrossRef]
- Coppola, A.; Marfella, R.; Coppola, L.; Tagliamonte, E.; Fontana, D.; Liguori, E.; Cirillo, T.; Cafiero, M.; Natale, S.; Astarita, C. Effect of weight loss on coronary circulation and adiponectin levels in obese women. Int. J. Cardiol. 2009, 134, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Nerla, R.; Tarzia, P.; Sestito, A.; Di Monaco, A.; Infusino, F.; Matera, D.; Greco, F.; Tacchino, R.M.; Lanza, G.A.; Crea, F. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Tarzia, P.; Lanza, G.A.; Sestito, A.; Villano, A.; Russo, G.; Figliozzi, S.; Lamendola, P.; De Vita, A.; Crea, F. Long-term effects of bariatric surgery on peripheral endothelial function and coronary microvascular function. Obes. Res. Clin. Pract. 2017, 11, 114–117. [Google Scholar] [CrossRef]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Diamant, M.; Serné, E.H.; van Raalte, D.H. GLP-1-Based Therapies Have No Microvascular Effects in Type 2 Diabetes Mellitus: An Acute and 12-Week Randomized, Double-Blind, Placebo-Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2125–2132. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785, Erratum in: Lancet Diabetes Endocrinol. 2020, 8, e2. [Google Scholar] [CrossRef]
- Bethel, M.A.; Patel, R.A.; Merrill, P.; Lokhnygina, Y.; Buse, J.B.; Mentz, R.J.; Pagidipati, N.J.; Chan, J.C.; Gustavson, S.M.; Iqbal, N.; et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 105–113. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Bain, S.C.; Franek, E.; Jodar-Gimeno, E.; Nauck, M.A.; Pratley, R.; Réa, R.R.; Kerr Saraiva, J.F.; Rasmussen, S.; Tornøe, K.; et al. Effect of Liraglutide on Cardiovascular Outcomes in Elderly Patients: A Post Hoc Analysis of a Randomized Controlled Trial. Ann. Intern. Med. 2019, 170, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Bain, S.C.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Pratley, R.E.; Linder, M.; Monk Fries, T.; et al. Cardiovascular Risk Reduction With Liraglutide: An Exploratory Mediation Analysis of the LEADER Trial. Diabetes Care 2020, 43, 1546–1552. [Google Scholar] [CrossRef]
- Verma, S.; Poulter, N.R.; Bhatt, D.L.; Bain, S.C.; Buse, J.B.; Leiter, L.A.; Nauck, M.A.; Pratley, R.E.; Zinman, B.; Ørsted, D.D.; et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus With or Without History of Myocardial Infarction or Stroke. Circulation 2018, 138, 2884–2894. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Tornøe, K.; Rasmussen, S.; Treppendahl, M.B.; Marso, S.P.; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Cardiovascular outcomes in patients who experienced a myocardial infarction while treated with liraglutide versus placebo in the LEADER trial. Diab. Vasc. Dis. Res. 2018, 15, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Bizino, M.B.; Jazet, I.M.; Westenberg, J.J.M.; van Eyk, H.J.; Paiman, E.H.M.; Smit, J.W.A.; Lamb, H.J. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: Randomized placebo-controlled trial. Cardiovasc. Diabetol. 2019, 18, 55, Erratum in: Cardiovasc. Diabetol. 2019, 18, 101. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Ren, Y.; Bai, J.; Zhang, G.; Cui, Y. The efficacy and safety of liraglutide in the obese, non-diabetic individuals: A systematic review and meta-analysis. Afr. Health Sci. 2019, 19, 2591–2599. [Google Scholar] [CrossRef]
- Konwar, M.; Bose, D.; Jaiswal, S.K.; Maurya, M.K.; Ravi, R. Efficacy and Safety of Liraglutide 3.0 mg in Patients with Overweight and Obese with or without Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1201977. [Google Scholar] [CrossRef]
- Garvey, W.T.; Birkenfeld, A.L.; Dicker, D.; Mingrone, G.; Pedersen, S.D.; Satylganova, A.; Skovgaard, D.; Sugimoto, D.; Jensen, C.; Mosenzon, O. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care 2020, 43, 1085–1093. [Google Scholar] [CrossRef]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S.; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, L.; Guo, J.; Fan, Y.; Pan, Q.; Li, T.; Sun, X.; Li, P. Degree of obesity and gastrointestinal adverse reactions influence the weight loss effect of liraglutide in overweight or obese patients with type 2 diabetes. Ther. Adv. Chronic Dis. 2023, 14, 20406223231161516. [Google Scholar] [CrossRef] [PubMed]

| Long-Acting | Short-Acting |
|---|---|
| Liraglutide | Exenatide (administered twice a day) |
| Exenatide (administered once a week) | Lixisenatide |
| Dulaglutide | |
| Semaglutide |
| Authors | Htike et al. [7] | Taheri et al. [17] | Seino et al. [18] | Wang et al. [19] |
| Year | 2017 | 2019 | 2022 | 2021 |
| All patients | 14,464 | 1433 | 466 | 1557 |
| Study design | Systematic review | Systematic review and metaanalysis | RCT | Meta-analysis |
| Patient characteristics | Obese or overweight patients with metabolic syndrome aged 35 and over | Patients with T2DM | Patients with T2DM | Patients with T2DM and CAD |
| Duration | 40 weeks | 26–52 weeks | 26 weeks | 8–26 weeks |
| Safety and efficacy in lowering blood glucose levels | GLP-1R analogues are effective drugs in glycemic control. A higher incidence of gastrointestinal adverse events has been demonstrated | Liraglutide and Dulaglutide effectively reduced HbA1c levels, with Dulaglutide showing greater efficacy in this regard. Dulaglutide was more likely to cause gastrointestinal adverse effects | Increasing the dose of liraglutide from 0.6 mg to 1.8 mg once a day was more effective in lowering HbA1c levels. Both doses were well tolerated by patients. | The inclusion of liraglutide in therapy significantly improved indicators of glycemic control including lowered HbA1c, FBG. It probably did not increase the risk of side effects. |
| Authors | Rizzo et al. [26] | Mashayekhi et al. [28] |
| Year | 2015 | 2023 |
| All patients | 20 | 88 |
| Study design | clinical trial | RCT |
| Patient characteristics |
|
|
| Aim of the study | evaluation of the effects of liraglutide on oxidative stress, HO-1, and ghrelin levels | evaluation of the effects of GLP-1R agonists on vascular endothelial function, fibrinolysis, and inflammation |
| Duration | 2 months | 14 weeks |
| Treatment with liraglutide | initial dose 0.6 mg per day, after two weeks 1.2 mg per day | initial dose 0.6 mg per day, after one week 1.2 mg per day, after two weeks 1.8 mg per day |
| Effect of liraglutide on oxidative stress/inflammation/endothelial dysfunction | decrease in serum levels of lipid hydroperoxides and HO-1 | reduction in chemokine MCP-1 levels |
| Study | GRADE | LEADER |
| Authors | GRADE Study Research Group [33] | Marso et al. [34] |
| Year | 2022 | 2016 |
| All patients | 5047 | 9340 |
| Study design | RCT | RCT |
| Patient characteristics |
|
|
| Aim of the study | Comparison of glucose-lowering drugs in relation to microvascular and CVD outcomes. | To investigate the CV effect of liraglutide therapy. |
| Duration | 5.0 years | 3.8 years |
| Treatment with liraglutide | 1.8 mg per day | 1.8 mg per day |
| Microvascular outcome | no significant differences in the incidence of microvascular complications between the glargine, glimepiride, liraglutide, and sitagliptin groups | lower risk of microvascular events |
| Authors | Suhrs et al. [35] | Faber et al. [36] |
| Year | 2019 | 2015 |
| All patients | 29 | 20 |
| Study design | RCT | RCT |
| Patient characteristics |
|
|
| Aim of the study | Verifying whether liraglutide ameliorates CMD and symptoms through weight loss. | To examine the short-term effects of GLP-1 therapy on coronary microcirculation. |
| Duration | control period: 5 weeks intervention period: 12 weeks | treatment: 10 weeks wash-out: 2 weeks |
| Treatment with liraglutide | 3 mg per day | initial dose 0.6 mg per day, after two weeks 1.2 mg per day |
| Microvascular outcome | the lack of improvement in coronary microvascular function | no significant effect on microvascular function |
| Authors | Marso et al. [34] | Gilbert et al. [47] | Verma et al. [49] | Nauck et al. [50] |
| Year | 2016 | 2019 | 2018 | 2018 |
| All patients | 9340 | 9340 | 9340 | 9340 |
| Study design | RCT | RCT | RCT | RCT |
| Patient characteristics | Patients with T2DM and high CV risk | Patients aged 75 years or older, patients aged 60–74 years with risk factors for CVD, and patients younger than 60 years but with CVD | Patients with or without T2DM and MI/stroke | Patients with T2DM and high CV risk |
| Duration | 3.8 years | 3.8 years | 3.8 years | 3.8 years |
| Cardiovascular outcome | There was a lower rate of death from CV causes and death from any cause among individuals using liraglutide. | Elderly patients aged 75 years or older benefited more from liraglutide treatment in reducing MACE and death from any cause compared to younger patients | Patients on liraglutide benefited on all CV endpoints compared to placebo, but in patients with CV risk factors only, liraglutide had a neutral effect on outcomes. | Patients with a history of MI had a 7 times greater risk of hospitalization for heart failure or risk of death from CV causes than patients without a history of MI. |
| Authors | Zhang et al. [52] | The SCALE study [53] | Konwar et al. [54] | Kelly et al. [55] | Zhou et al. [56] |
| Year | 2019 | 2020 | 2022 | 2020 | 2022 |
| All patients | 4754 | 396 | 6867 | 251 | 90 |
| Study design | Meta-analysis | RCT | Systematic review and meta-analysis | RCT | RCT |
| Patient characteristics | Obese patients without T2DM | Overweight or obese patients with T2DM using basal insulin | Overweight or obese patients with or without diabetes | Adolescents with obesity | Overweight or obese patients with T2DM |
| Aim of the study | Safety and efficacy of liraglutide in obese patients without diabetes mellitus | Evaluation of the efficacy and safety of incorporating liraglutide into insulin therapy in obese or overweight patients with T2DM | Evaluation of the efficacy and safety of liraglutide in obese or overweight patients with or without diabetes | Safety and efficacy of liraglutide in obese adolescents | Identification of factors having the greatest impact on weight loss in patients using liraglutide |
| Duration | 14–55 weeks | 52 weeks | 12–56 weeks | 56 weeks | 52 weeks |
| Treatment with liraglutide | 3.0 mg once a day | 3.0 mg once a day | 3.0 mg once a day | 3.0 mg once a day | 1.2 mg or 1.8 mg once a day |
| Impact on body weight | Weight reduction | Weight reduction | Weight reduction | Weight reduction | Weight reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. New Insights into the Use of Liraglutide—Impact on Cardiovascular Risk and Microvascular Outcomes. Biomedicines 2023, 11, 1159. https://doi.org/10.3390/biomedicines11041159
Wronka M, Krzemińska J, Młynarska E, Rysz J, Franczyk B. New Insights into the Use of Liraglutide—Impact on Cardiovascular Risk and Microvascular Outcomes. Biomedicines. 2023; 11(4):1159. https://doi.org/10.3390/biomedicines11041159
Chicago/Turabian StyleWronka, Magdalena, Julia Krzemińska, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2023. "New Insights into the Use of Liraglutide—Impact on Cardiovascular Risk and Microvascular Outcomes" Biomedicines 11, no. 4: 1159. https://doi.org/10.3390/biomedicines11041159
APA StyleWronka, M., Krzemińska, J., Młynarska, E., Rysz, J., & Franczyk, B. (2023). New Insights into the Use of Liraglutide—Impact on Cardiovascular Risk and Microvascular Outcomes. Biomedicines, 11(4), 1159. https://doi.org/10.3390/biomedicines11041159