Immediate Effects of TECAR Therapy on Gastrocnemius and Quadriceps Muscles with Spastic Hypertonia in Chronic Stroke Survivors: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Participants
2.4. Randomization
2.5. Intervention
2.6. Outcomes
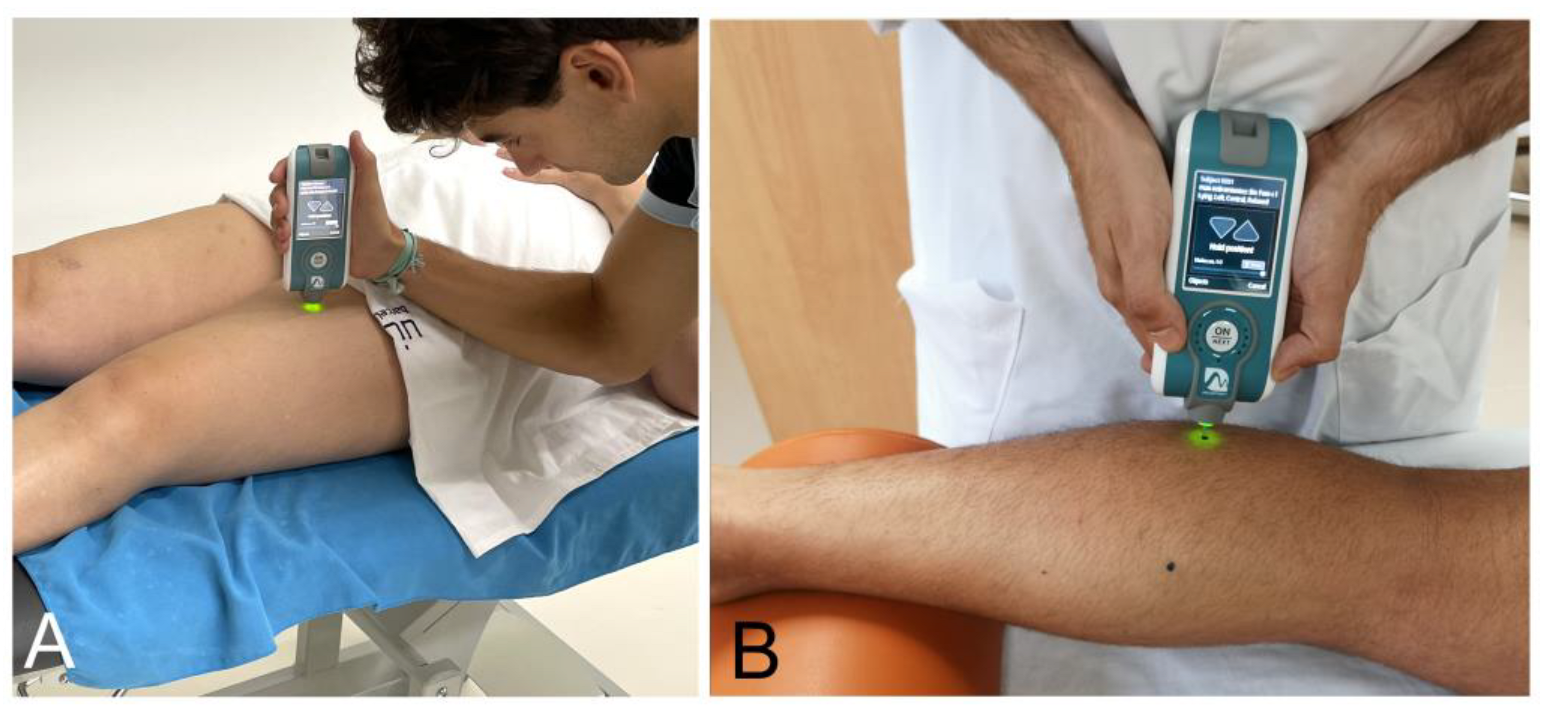
2.6.1. Neuromuscular Properties (Myotonometry)
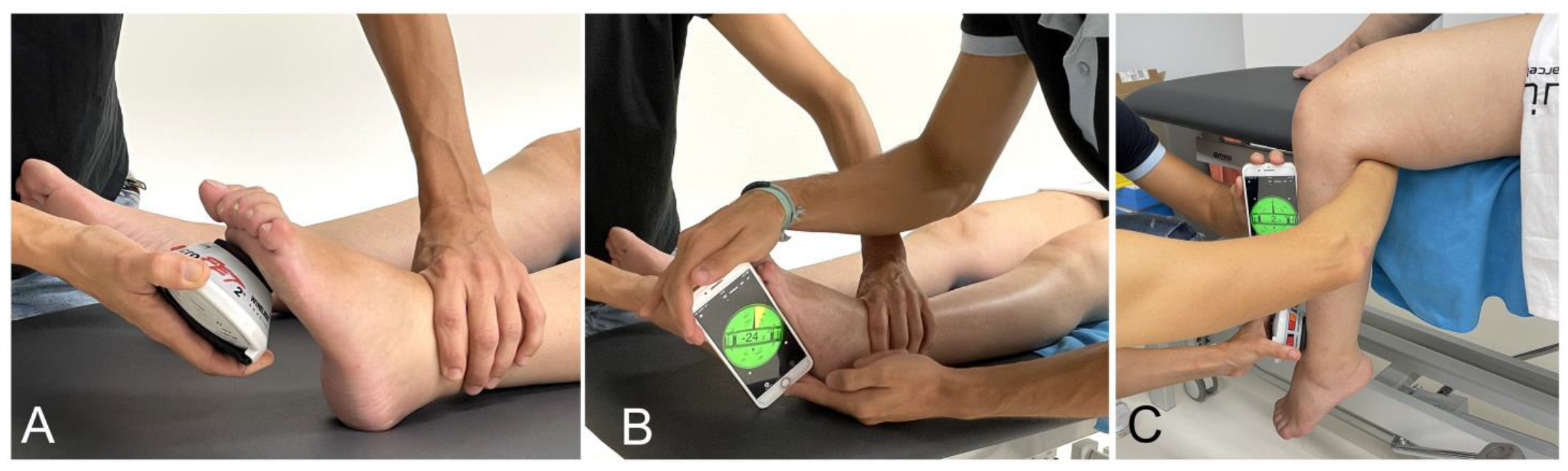
2.6.2. Passive Range of Motion (PROM)
2.6.3. Modified Ashworth Scale (MAS)
2.6.4. Degrees of Modified Ashworth Scale
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef]
- van den Noort, J.C.; Bar-On, L.; Aertbeliën, E.; Bonikowski, M.; Braendvik, S.M.; Broström, E.W.; Buizer, A.I.; Burridge, J.H.; van Campenhout, A.; Dan, B.; et al. European Consensus on the Concepts and Measurement of the Pathophysiological Neuromuscular Responses to Passive Muscle Stretch. Eur. J. Neurol. 2017, 24, 981-e38. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E.; Rymer, W.Z. A New Definition of Poststroke Spasticity and the Interference of Spasticity With Motor Recovery From Acute to Chronic Stages. Neurorehabil. Neural Repair 2021, 35, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-Stroke Spasticity: Predictors of Early Development and Considerations for Therapeutic Intervention. PM&R 2015, 7, 60–67. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Useros-Olmo, A.I.; Almazán-Polo, J.; Martín-Sevilla, M.; Romero-Morales, C.; Sanz-Corbalán, I.; Rodríguez-Sanz, D.; López-López, D. Quantitative Ultrasound Imaging Pixel Analysis of the Intrinsic Plantar Muscle Tissue between Hemiparesis and Contralateral Feet in Post-Stroke Patients. Int. J. Environ. Res. Public Health 2018, 15, 2519. [Google Scholar] [CrossRef]
- Pradines, M.; Ghedira, M.; Portero, R.; Masson, I.; Marciniak, C.; Hicklin, D.; Hutin, E.; Portero, P.; Gracies, J.-M.; Bayle, N. Ultrasound Structural Changes in Triceps Surae After a 1-Year Daily Self-Stretch Program: A Prospective Randomized Controlled Trial in Chronic Hemiparesis. Neurorehabil. Neural Repair 2019, 33, 245–259. [Google Scholar] [CrossRef]
- Stecco, A.; Stecco, C.; Raghavan, P. Peripheral Mechanisms Contributing to Spasticity and Implications for Treatment. Curr. Phys. Med. Rehabil. Rep. 2014, 2, 121–127. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Hu, G.-C. Post-Stroke Spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. Int. J. Gerontol. 2018, 12, 280–284. [Google Scholar] [CrossRef]
- Dorňák, T.; Justanová, M.; Konvalinková, R.; Říha, M.; Mužík, J.; Hoskovcová, M.; Srp, M.; Navrátilová, D.; Otruba, P.; Gál, O.; et al. Prevalence and Evolution of Spasticity in Patients Suffering from First-Ever Stroke with Carotid Origin: A Prospective, Longitudinal Study. Eur. J. Neurol. 2019, 26, 880–886. [Google Scholar] [CrossRef]
- Pundik, S.; McCabe, J.; Skelly, M.; Tatsuoka, C.; Daly, J.J. Association of Spasticity and Motor Dysfunction in Chronic Stroke. Ann. Phys. Rehabil. Med. 2019, 62, 397–402. [Google Scholar] [CrossRef]
- Sunnerhagen, K.S.; Opheim, A.; Alt Murphy, M. Onset, Time Course and Prediction of Spasticity after Stroke or Traumatic Brain Injury. Ann. Phys. Rehabil. Med. 2019, 62, 431–434. [Google Scholar] [CrossRef]
- Deltombe, T.; Wautier, D.; De Cloedt, P.; Fostier, M.; Gustin, T. Assessment and Treatment of Spastic Equinovarus Foot after Stroke: Guidance from the Mont-Godinne Interdisciplinary Group. J. Rehabil. Med. 2017, 49, 461–468. [Google Scholar] [CrossRef]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Poststroke Spasticity: Sequelae and Burden on Stroke Survivors and Caregivers. Neurology 2013, 80, S45–S52. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Calvo-Sanz, J.; Serra-Llobet, P.; Alcoba-Kait, J.; González-Rueda, V.; Rodríguez-Rubio, P.R. The Effectiveness of Massage Therapy for Improving Sequelae in Post-Stroke Survivors. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4424. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, S.J. Effects of Joint Mobilization and Stretching on the Range of Motion for Ankle Joint and Spatiotemporal Gait Variables in Stroke Patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104933. [Google Scholar] [CrossRef] [PubMed]
- Martins, A. The Role of Spasticity in Functional Neurorehabilitation-Part II: Non-Pharmacological and Pharmacological Management: A Multidisciplinary Approach. Arch. Med. 2016, 8, 3. [Google Scholar]
- van den Dolder, P.A.; Ferreira, P.H.; Refshauge, K.M. Effectiveness of Soft Tissue Massage and Exercise for the Treatment of Non-Specific Shoulder Pain: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2014, 48, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Wachi, M.; Jiroumaru, T.; Satonaka, A.; Ikeya, M.; Noguchi, S.; Suzuki, M.; Hyodo, Y.; Oka, Y.; Fujikawa, T. Effects of Capacitive and Resistive Electric Transfer Therapy on Pain and Lumbar Muscle Stiffness and Activity in Patients with Chronic Low Back Pain. J. Phys. Ther. Sci. 2022, 34, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Sonoda, T.; Tashiro, Y.; Suzuki, Y.; Kajiwara, Y.; Zeidan, H.; Nakayama, Y.; Kawagoe, M.; Shimoura, K.; Tatsumi, M.; et al. Effect of Capacitive and Resistive Electric Transfer on Changes in Muscle Flexibility and Lumbopelvic Alignment after Fatiguing Exercise. J. Phys. Ther. Sci. 2018, 30, 719–725. [Google Scholar] [CrossRef]
- Tashiro, Y.; Hasegawa, S.; Yokota, Y.; Nishiguchi, S.; Fukutani, N.; Shirooka, H.; Tasaka, S.; Matsushita, T.; Matsubara, K.; Nakayama, Y.; et al. Effect of Capacitive and Resistive Electric Transfer on Haemoglobin Saturation and Tissue Temperature. Int. J. Hyperth. 2017, 33, 696–702. [Google Scholar] [CrossRef] [PubMed]
- López-de-Celis, C.; Hidalgo-García, C.; Pérez-Bellmunt, A.; Fanlo-Mazas, P.; González-Rueda, V.; Tricás-Moreno, J.M.; Ortiz, S.; Rodríguez-Sanz, J. Thermal and Non-Thermal Effects off Capacitive-Resistive Electric Transfer Application on the Achilles Tendon and Musculotendinous Junction of the Gastrocnemius Muscle: A Cadaveric Study. BMC Musculoskelet. Disord. 2020, 21, 46. [Google Scholar] [CrossRef]
- Schillebeeckx, F.; DE Groef, A.; DE Beukelaer, N.; Desloovere, K.; Verheyden, G.; Peers, K. Muscle and Tendon Properties of the Spastic Lower Leg after Stroke Defined by Ultrasonography: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2021, 57, 495–510. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Bensmail, D.; Yelnik, A. Non-Pharmacological Interventions for Spasticity in Adults: An Overview of Systematic Reviews. Ann. Phys. Rehabil. Med. 2019, 62, 265–273. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, H.; Wang, Y.; Cong, D. Non-pharmacological intervention for rehabilitation of post-stroke spasticity: A protocol for systematic review and network meta-analysis. Medicine 2021, 100, e25788. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, S.H.; Yoo, J.-I.; Lee, S.-U. Ultrasonographic Evaluation for the Effect of Extracorporeal Shock Wave Therapy on Gastrocnemius Muscle Spasticity in Patients With Chronic Stroke. PM&R 2019, 11, 363–371. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Arab, T.K.; Jalaie, S. The Interrater and Intrarater Reliability of the Modified Ashworth Scale in the Assessment of Muscle Spasticity: Limb and Muscle Group Effect. NeuroRehabilitation 2008, 23, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Chen, C.-Y.; Chen, H.-C.; Wu, C.-Y.; Lin, K.-C.; Hsieh, Y.-W.; Shen, I.-H. Responsiveness and Minimal Clinically Important Difference of Modified Ashworth Scale in Patients with Stroke. Eur. J. Phys. Rehabil. Med. 2019, 55, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, J.; Ovcar Stante, K.; Rakusa, M. The Validity of the Montreal Cognitive Assessment (MoCA) for the Screening of Vascular Cognitive Impairment after Ischemic Stroke. Acta Neurol. Belg. 2020, 120, 681–685. [Google Scholar] [CrossRef]
- Chuang, L.; Wu, C.; Lin, K. Reliability, Validity, and Responsiveness of Myotonometric Measurement of Muscle Tone, Elasticity, and Stiffness in Patients With Stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef]
- Garcia-Bernal, M.I.; Heredia-Rizo, A.M.; Gonzalez-Garcia, P.; Cortés-Vega, M.D.; Casuso-Holgado, M.J. Validity and Reliability of Myotonometry for Assessing Muscle Viscoelastic Properties in Patients with Stroke: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 5062. [Google Scholar] [CrossRef]
- Meseguer-Henarejos, A.-B.; Sánchez-Meca, J.; López-Pina, J.-A.; Carles-Hernández, R. Inter- and Intra-Rater Reliability of the Modified Ashworth Scale: A Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 576–590. [Google Scholar] [CrossRef]
- Craven, B.C.; Morris, A.R. Modified Ashworth Scale Reliability for Measurement of Lower Extremity Spasticity among Patients with SCI. Spinal Cord 2010, 48, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, T.; Goljar Kregar, N.; Puh, U. Reliability of the Modified Ashworth Scale After Stroke for 13 Muscle Groups. Arch. Phys. Med. Rehabil. 2023, 104, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.A.; Katalinic, O.M.; Herbert, R.D.; Moseley, A.M.; Lannin, N.A.; Schurr, K. Stretch for the Treatment and Prevention of Contracture: An Abridged Republication of a Cochrane Systematic Review. J. Physiother. 2017, 63, 67–75. [Google Scholar] [CrossRef]
- Jiménez-Del-Barrio, S.; Cadellans-Arróniz, A.; Ceballos-Laita, L.; Estébanez-de-Miguel, E.; López-de-Celis, C.; Bueno-Gracia, E.; Pérez-Bellmunt, A. The Effectiveness of Manual Therapy on Pain, Physical Function, and Nerve Conduction Studies in Carpal Tunnel Syndrome Patients: A Systematic Review and Meta-Analysis. Int. Orthop. 2022, 46, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Szabo, D.A.; Neagu, N.; Teodorescu, S.; Predescu, C.; Sopa, I.S.; Panait, L. TECAR Therapy Associated with High-Intensity Laser Therapy (Hilt) and Manual Therapy in the Treatment of Muscle Disorders: A Literature Review on the Theorised Effects Supporting Their Use. J. Clin. Med. 2022, 11, 6149. [Google Scholar] [CrossRef]
- Bingöl, H.; Yilmaz, Ö. EXERCISE THERAPY AND REHABILITATION Effects of Functional Massage on Spasticity and Motor Functions in Children with Cerebral Palsy: A Randomized Controlled Study. J. Exerc. Ther. Rehabil. 2018, 5, 135–142. [Google Scholar]
- Thanakiatpinyo, T.; Suwannatrai, S.; Suwannatrai, U.; Khumkaew, P.; Wiwattamongkol, D.; Vannabhum, M.; Pianmanakit, S.; Kuptniratsaikul, V. The Efficacy of Traditional Thai Massage in Decreasing Spasticity in Elderly Stroke Patients. Clin. Interv. Aging 2014, 9, 1311–1319. [Google Scholar] [CrossRef]
- Moyer, C.A.; Rounds, J.; Hannum, J.W. A Meta-Analysis of Massage Therapy Research. Psychol. Bull. 2004, 130, 3–18. [Google Scholar] [CrossRef]
- García-Bernal, M.I.; González-García, P.; Madeleine, P.; Casuso-Holgado, M.J.; Heredia-Rizo, A.M. Characterization of the Structural and Mechanical Changes of the Biceps Brachii and Gastrocnemius Muscles in the Subacute and Chronic Stage after Stroke. Int. J. Environ. Res. Public Health 2023, 20, 1405. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Gallone, M.F.; Covelli, I.; Tafuri, S.; Moretti, B. Short Term Efficacy of Capacitive-Resistive Diathermy Therapy in Patients with Low Back Pain: A Prospective Randomized Controlled Trial. J. Biol. Regul. Homeost. Agents 2017, 31, 509–515. [Google Scholar] [PubMed]
- Kulig, K.; Chang, Y.-J.; Winiarski, S.; Bashford, G.R. Ultrasound-Based Tendon Micromorphology Predicts Mechanical Characteristics of Degenerated Tendons. Ultrasound Med. Biol. 2016, 42, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.W.; Barrance, P.J.; Buchanan, T.S.; Higginson, J.S. Paretic Muscle Atrophy and Non-Contractile Tissue Content in Individual Muscles of the Post-Stroke Lower Extremity. J. Biomech. 2011, 44, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Hunnicutt, J.L.; Gregory, C.M. Skeletal Muscle Changes Following Stroke: A Systematic Review and Comparison to Healthy Individuals. Top. Stroke Rehabil. 2017, 24, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Pignataro, A.; Piran, G.; Duso, M.; Mimche, P.; Ermani, M.; Del Felice, A. Short-Wave Diathermy in the Clinical Management of Musculoskeletal Disorders: A Pilot Observational Study. Int. J. Biometeorol. 2020, 64, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Pezzi, L.; Centra, M.A.; Porreca, A.; Barbato, C.; Bellomo, R.G.; Saggini, R. Effects of Capacitive and Resistive Electric Transfer Therapy in Patients with Painful Shoulder Impingement Syndrome: A Comparative Study. J. Int. Med. Res. 2020, 48, 300060519883090. [Google Scholar] [CrossRef]
- Akazawa, N.; Harada, K.; Okawa, N.; Tamura, K.; Moriyama, H. Muscle Mass and Intramuscular Fat of the Quadriceps Are Related to Muscle Strength in Non-Ambulatory Chronic Stroke Survivors: A Cross-Sectional Study. PLoS ONE 2018, 13, e0201789. [Google Scholar] [CrossRef]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]
- Bavikatte, G.; Subramanian, G.; Ashford, S.; Allison, R.; Hicklin, D. Early Identification, Intervention and Management of Post-Stroke Spasticity: Expert Consensus Recommendations. J. Cent. Nerv. Syst. Dis. 2021, 13, 117957352110365. [Google Scholar] [CrossRef]
- Ferry, B.; Compagnat, M.; Yonneau, J.; Bensoussan, L.; Moucheboeuf, G.; Muller, F.; Laborde, B.; Jossart, A.; David, R.; Magne, J.; et al. Awakening the Control of the Ankle Dorsiflexors in the Post-Stroke Hemiplegic Subject to Improve Walking Activity and Social Participation: The WAKE (Walking Ankle IsoKinetic Exercise) Randomised, Controlled Trial. Trials 2022, 23, 661. [Google Scholar] [CrossRef]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early Development of Spasticity Following Stroke: A Prospective, Observational Trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
| Experimental Group | Control Group | |
|---|---|---|
| Mean ± SD n (%) | Mean ± SD n (%) | |
| Sex | ||
| Women | 7 (38.9%) | 7 (38.9%) |
| Men | 11 (61.1%) | 11 (61.1%) |
| Age (years) | 58.8 ± 11.9 | 58.3 ± 11.0 |
| Weight (kg) | 75.3 ± 14.6 | 76.6 ± 17.8 |
| Height (cm) | 169.4 ± 9.8 | 170.6 ± 7.0 |
| BMI | 26.1 ± 4.1 | 26.3 ± 5.6 |
| Type of stroke | ||
| Hemorrhagic | 9 (50%) | 6 (33.3%) |
| Ischemic | 9 (50%) | 12 (55.7%) |
| Time onset (years) | 6.4 ± 3.1 | 11.1 ± 8.4 |
| Affected side | ||
| Right | 14 (77.8%) | 10 (55.6%) |
| Left | 4 (22.2%) | 8 (44.4%) |
| Tobacco/alcohol use | ||
| None | 14 (77.8%) | 11 (61.1%) |
| Tobacco | 1 (5.6%) | 4 (22.2%) |
| Alcohol | 3 (16.7%) | 3 (16.7%) |
| Physiotherapy (days/week) | 1.6 ± 1.0 | 2.2 ± 2.5 |
| T0 | T1 | T2 | T0–T1 | T0–T2 | T1–T2 | ||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | p | p | p | ||
| Experimental Group | |||||||
| MAS Hip flexion | 0 Normal | 10 (55.6%) | 11 (61.1%) | 11 (61.1%) | 0.368 | 0.368 | 1.000 |
| 1 Light tone | 5 (27.8%) | 5 (27.8%) | 5 (27.8%) | ||||
| 1+ Light tone plus | 3 (16.7%) | 2 (11.1%) | 2 (11.1%) | ||||
| 2 Pronounced tone | - | - | |||||
| MAS Knee flexion | 0 Normal | 4 (22.2%) | 8 (44.4%) | 8 (44.4%) | 0.136 | 0.199 | 0.607 |
| 1 Light tone | 8 (44.4%) | 6 (33.3%) | 5 (27.8%) | ||||
| 1+ Light tone plus | 2 (11.1%) | 3 (16.7%) | 3 (16.7%) | ||||
| 2 Pronounced tone | 4 (22.2%) | 1 (5.6%) | 2 (11.1%) | ||||
| MAS Ankle dorsiflexion | 0 Normal | - | - | - | 0.046 | 0.019 | 0.261 |
| 1 Light tone | 1 (5.6%) | 5 (27.8%) | 5 (27.8%) | ||||
| 1+ Light tone plus | 4 (22.2%) | 6 (33.3%) | 7 (38.9%) | ||||
| 2 Pronounced tone | 13 (72.2%) | 7 (38.9%) | 6 (33.3%) | ||||
| Control Group | |||||||
| MAS Hip flexion | 0 Normal | 11 (61.1%) | 11 (61.1%) | 11 (61.1%) | 1.000 | 1.000 | 0.317 |
| 1 Light tone | 5 (27.8%) | 6 (33.3%) | 5 (27.8%) | ||||
| 1+ Light tone plus | 1 (5.6%) | 1 (5.6%) | 2 (11.1%) | ||||
| 2 Pronounced tone | 1 (5.6%) | - | - | ||||
| MAS Knee flexion | 0 Normal | 6 (33.3%) | 6 (33.3%) | 6 (33.3%) | 1.000 | 1.000 | 1.000 |
| 1 Light tone | 7 (38.9%9 | 7 (38.9%) | 7 (38.9%) | ||||
| 1+ Light tone plus | 3 (16.7%) | 3 (16.7%) | 3 (16.7%) | ||||
| 2 Pronounced tone | 2 (11.1%) | 2 (11.1%) | 2 (11.1%) | ||||
| MAS Ankle dorsiflexion | 0 Normal | 3 (16.7%) | 3 (16.7%) | 4 (22.2%) | 0.368 | 0.261 | 0.368 |
| 1 Light tone | 4 (22.2%) | 5 (27.8%) | 5 (27.8%) | ||||
| 1+ Light tone plus | 3 (16.7%) | 3 (16.7%) | 2 (11.1%) | ||||
| 2 Pronounced tone | 8 (44.4%) | 7 (38.9%) | 7 (38.9%) | ||||
| Difference T1–T0 | Difference T2–T0 | Difference T2–T1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Experimental Group | Control Group | Experimental Group | Control Group | Experimental Group | Control Group | |||
| Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | |
| MAS Hip (°) | 5.3 ± 9.4 | 3.2 ± 6.0 | 0.443 | 8.0 ± 8.4 | 1.6 ± 6.5 | 0.022 | 2.7 ± 8.6 | −1.7 ± 6.9 | 0.542 |
| MAS Knee (°) | 11.3 ± 12.7 | 3.3 ± 6.2 | 0.016 | 11.9 ± 12.4 | 1.6 ± 4.1 | 0.000 | 0.7 ± 5.7 | −1.8 ± 5.1 | 0.239 |
| MAS Ankle (°) | 2.2 ± 4.5 | 1.7 ± 3.2 | 0.501 | 3.4 ± 3.7 | 1.1 ± 2.7 | 0.011 | 1.2 ± 3.7 | −0.6 ± 3.5 | 0.134 |
| PROM—Ankle dorsiflexion (°) | 2.9 ± 4.2 | 0.4 ± 3.1 | 0.161 | 3.2 ± 4.7 | 0.3 ± 2.8 | 0.034 | 0.3 ± 1.6 | −0.1 ± 1.8 | 0.888 |
| PROM—Knee (°) | 2.5 ± 9.6 | 1.0 ± 3.0 | 0.012 | 2.9 ± 9.4 | 1.1 ± 3.0 | 0.019 | 0.4 ± 2.0 | 0.2 ± 0.9 | 0.323 |
| GM—Tone (Hz) | 4.1 ± 22.9 | −0.6 ± 2.7 | 0.388 | −1.5 ± 3.2 | −0.4 ± 2.0 | 0.186 | −5.7 ± 23.7 | 0.3 ± 3.1 | 0.300 |
| GM—Stiffness (N/m) | −36.8 ± 50.8 | −19.7 ± 50.5 | 0.317 | −28.5 ± 73.4 | −5.9 ± 52.9 | 0.297 | 8.3 ± 61.6 | 13.8 ± 65.6 | 0.799 |
| GM—Relaxation (m/s) | 2.6 ± 4.3 | 0.8 ± 4.2 | 0.212 | 1.9 ± 5.3 | 0.4 ± 4.2 | 0.344 | −0.7 ± 5.2 | −0.5 ± 4.3 | 0.863 |
| GL—Tone (Hz) | −1.2 ± 2.8 | −0.8 ± 3.6 | 0.664 | −1.6 ± 2.7 | −0.1 ± 3.8 | 0.198 | −0.3 ± 2.6 | 0.6 ± 3.7 | 0.373 |
| GL—Stiffness (N/m) | −20.3 ± 46.6 | −36.1 ± 102.8 | 0.556 | −26.2 ± 44.0 | −21.3 ± 132.7 | 0.884 | −5.9 ± 44.6 | 14.8 ± 80.2 | 0.346 |
| GL—Relaxation (m/s) | 2.2 ± 5.0 | 1.5 ± 4.7 | 0.661 | 2.5 ± 4.4 | −0.5 ± 5.6 | 0.084 | 0.3 ± 3.7 | −2.0 ± 4.7 | 0.118 |
| RF—Tone (Hz) | −0.2 ± 1.3 | 0.0 ± 1.8 | 0.682 | −0.2 ± 1.3 | 0.0 ± 1.2 | 0.638 | 0.0 ± 1.0 | 0.0 ± 1.6 | 0.957 |
| RF—Stiffness (N/m) | −4.3 ± 23.9 | 0.8 ± 53.5 | 0.710 | −7.6 ± 22.4 | −2.8 ± 28.0 | 0.568 | −3.3 ± 18.0 | −3.6 ± 45.8 | 0.979 |
| RF—Relaxation (m/s) | −2.5 ± 11.2 | −16.8 ± 70.3 | 0.401 | −1.8 ± 11.2 | −16.8 ± 69.9 | 0.376 | 0.7 ± 1.8 | 0.0 ± 2.4 | 0.332 |
| T0 | T1 | Difference T1–T0 | T2 | Difference T2–T0 | Difference T2–T1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | Mean | 95% CI | p | ŋ2 | Mean ± SD | Mean | 95% CI | p | ŋ2 | Mean | 95% CI | p | ŋ2 |
| Experimental Group | |||||||||||||||
| MAS Hip (°) | 97.6 ± 21.1 | 102.9 ± 18.2 | 5.3 | [0.62; 11.18] | 0.089 | 0.02 | 105.6 ± 18.2 | 8.0 | [2.73; 13.27] | 0.003 | 0.04 | 2.7 | [−2.66; 8.10] | 0.590 | 0.01 |
| MAS Knee (°) | 104.7 ± 16.1 | 115.9 ± 16.2 | 11.3 | [3.31; 19.25] | 0.005 | 0.11 | 116.6 ± 14.8 | 11.9 | [4.20; 19.69] | 0.002 | 0.13 | 0.7 | [−2.88; 4.21] | 1.000 | 0.00 |
| MAS Ankle (°) | 18.8 ± 5.6 | 20.9 ± 7.3 | 2.2 | [−0.66; 4.99] | 0.173 | 0.03 | 22.2 ± 6.8 | 3.4 | [1.07; 5.71] | 0.004 | 0.07 | 1.2 | [−1.08; 3.52] | 0.528 | 0.01 |
| PROM—Ankle dorsiflexion (°) | 14.0 ± 6.9 | 16.8 ± 8.7 | 2.9 | [0.27; 5.48] | 0.028 | 0.03 | 17.1 ± 8.8 | 3.2 | [0.22; 6.09] | 0.033 | 0.04 | 0.3 | [−0.73; 1.28] | 1.000 | 0.00 |
| PROM—Knee (°) | 28.1 ± 15.8 | 30.6 ± 12.6 | 2.5 | [−3.49; 8.39] | 0.850 | 0.01 | 31.1 ± 12.6 | 2.9 | [−2.99; 8.82] | 0.621 | 0.01 | 0.4 | [−0.84; 1.67] | 1.000 | 0.00 |
| GM—Tone (Hz) | 17.8 ± 3.7 | 21.9 ± 22.6 | 4.1 | [−10.19; 18.45] | 1.000 | 0.02 | 16.3 ± 3.8 | −1.5 | [−3.54; 0.45] | 0.165 | 0.04 | −5.7 | [−20.52; 9.18] | 0.975 | 0.03 |
| GM—Stiffness (N/m) | 323.4 ± 71.3 | 286.6 ± 46.6 | −36.8 | [−68.62; −5.02] | 0.021 | 0.09 | 294.9 ± 67.9 | −28.5 | [−74.42; 17.43] | 0.354 | 0.04 | 8.3 | [−30.19; 46.84] | 1.000 | 0.00 |
| GM—Relaxation (m/s) | 18.4 ± 4.6 | 21.1 ± 6.0 | 2.6 | [−0.05; 5.32] | 0.056 | 0.08 | 20.3 ± 6.1 | 1.9 | [−1.40; 5.21] | 0.434 | 0.03 | −0.7 | [−3.97; 2.50] | 1.000 | 0.00 |
| GL—Tone (Hz) | 18.3 ± 4.2 | 17.1 ± 4.6 | −1.2 | [−3.00; 0.53] | 0.240 | 0.02 | 16.8 ± 4.6 | −1.6 | [−3.26; 0.13] | 0.075 | 0.03 | −0.3 | [−1.98; 1.36] | 1.000 | 0.00 |
| GL—Stiffness (N/m) | 352.5 ± 101.0 | 332.2 ± 95.9 | −20.2 | [−49.42; 8.88] | 0.247 | 0.01 | 326.3 ± 94.1 | −26.2 | [−53.68; 1.36] | 0.066 | 0.02 | −5.9 | [−33.82; 22.04] | 1.000 | 0.00 |
| GL—Relaxation (m/s) | 17.4 ± 5.6 | 19.6 ± 6.7 | 2.2 | [−0.86; 5.35] | 0.217 | 0.03 | 19.9 ± 6.6 | 2.5 | [−0.20; 5.24] | 0.075 | 0.04 | 0.3 | [−2.01; 2.57] | 1.000 | 0.00 |
| RF—Tone (Hz) | 14.5 ± 1.8 | 14.3 ± 1.6 | −0.2 | [−1.05; 0.58] | 1.000 | 0.00 | 14.3 ± 1.6 | −0.2 | [−0.99; 0.59] | 1.000 | 0.00 | 0.0 | [−0.61; 0.68] | 1.000 | 0.00 |
| RF—Stiffness (N/m) | 287.5 ± 36.8 | 283.2 ± 35.7 | −4.3 | [−19.29; 10.61] | 1.000 | 0.00 | 279.9 ± 38.9 | −7.6 | [−21.64; 6.34] | 0.495 | 0.01 | −3.3 | [−14.56; 7.95] | 1.000 | 0.00 |
| RF—Relaxation (m/s) | 23.9 ± 12.0 | 21.4 ± 3.7 | −2.5 | [−9.52; 4.49] | 1.000 | 0.02 | 22.1 ± 3.5 | −1.8 | [−0.46; 1.83] | 1.000 | 0.01 | 0.7 | [−0.46; 1.86] | 0.398 | 0.01 |
| Control Group | |||||||||||||||
| MAS Hip (°) | 100.4 ± 15.8 | 103.6 ± 16.0 | 3.2 | [−0.55; 6.99] | 0.110 | 0.01 | 101.9 ± 18.6 | 1.6 | [−2.49; 5.60] | 0.965 | 0.00 | −1.7 | [−5.96; 2.63] | 0.951 | 0.00 |
| MAS Knee (°) | 100.9 ± 22.1 | 104.3 ± 21.8 | 3.3 | [−0.56; 7.23] | 0.109 | 0.01 | 102.5 ± 22.0 | 1.6 | [−1.02; 4.13] | 0.383 | 0.00 | −1.8 | [−4.95; 1.40] | 0.466 | 0.00 |
| MAS Ankle (°) | 18.8 ± 6.4 | 20.5 ± 7.8 | 1.7 | [−0.35; 3.68] | 0.126 | 0.01 | 19.9 ± 7.2 | 1.1 | [−0.65; 2.77] | 0.359 | 0.01 | −0.6 | [−2.81; 1.59] | 1.000 | 0.00 |
| PROM—Ankle dorsiflexion (°) | 16.7 ± 5.7 | 17.1 ± 6.6 | 0.4 | [−1.58; 2.36] | 1.000 | 0.00 | 17.0 ± 6.5 | 0.3 | [−1.48; 2.03] | 1.000 | 0.00 | −0.1 | [−1.22; 1.00] | 1.000 | 0.00 |
| PROM—Knee (°) | 25.4 ± 16.7 | 26.3 ± 17.1 | 1.0 | [−0.93; 2.84] | 0.588 | 0.00 | 26.5 ± 16.8 | 1.1 | [−0.73; 2.98] | 0.380 | 0.00 | 0.2 | [−0.37; 0.70] | 1.000 | 0.00 |
| GM—Tone (Hz) | 17.2 ± 3.6 | 16.6 ± 3.1 | −0.6 | [−2.34; 1.09] | 1.000 | 0.01 | 16.9 ± 3.3 | −0.4 | [−1.59; 0.88] | 1.000 | 0.00 | 0.3 | [−1.65; 2.19] | 1.000 | 0.00 |
| GM—Stiffness (N/m) | 305.4 ± 81.0 | 285.8 ± 62.5 | −19.7 | [−51.27; 11.94] | 0.350 | 0.02 | 299.6 ± 72.4 | −5.9 | [−38.98; 27.20] | 1.000 | 0.00 | 13.8 | [−27.28; 54.83] | 1.000 | 0.01 |
| GM—Relaxation (m/s) | 18.3 ± 5.8 | 19.2 ± 5.0 | 0.8 | [−1.82; 3.47] | 1.000 | 0.01 | 18.7 ± 5.8 | 0.4 | [−2.27; 3.02] | 1.000 | 0.00 | −0.5 | [−3.17; 2.26] | 1.000 | 0.00 |
| GL—Tone (Hz) | 16.8 ± 4.2 | 16.1 ± 3.5 | −0.8 | [−3.00; 1.47] | 1.000 | 0.01 | 16.7 ± 3.3 | −0.1 | [−2.49; 2.25] | 1.000 | 0.00 | 0.6 | [−1.69; 2.98] | 1.000 | 0.01 |
| GL—Stiffness (N/m) | 326.7 ± 113.7 | 290.6 ± 57.7 | −36.1 | [−100.46; 28.24] | 0.464 | 0.04 | 305.4 ± 86.4 | −21.3 | [−104.37; 61.71] | 1.000 | 0.01 | 14.8 | [−35.40; 64.95] | 1.000 | 0.01 |
| GL—Relaxation (m/s) | 19.0 ± 6.0 | 20.6 ± 5.2 | 1.5 | [−1.42; 4.48] | 0.560 | 0.02 | 18.6 ± 4.3 | −0.5 | [−3.95; 3.04] | 1.000 | 0.00 | −2.0 | [−4.94; 0.97] | 0.973 | 0.04 |
| RF—Tone (Hz) | 14.0 ± 2.0 | 14.0 ± 2.9 | 0.0 | [−1.17; 1.13] | 1.000 | 0.00 | 14.0 ± 1.9 | −0.0 | [−0.76; 0.75] | 1.000 | 0.00 | 0.0 | [−0.99; 1.01] | 1.000 | 0.00 |
| RF—Stiffness (N/m) | 273.9 ± 44.1 | 274.7 ± 69.5 | 0.8 | [−32.65; 34.31] | 1.000 | 0.00 | 271.1 ± 44.4 | −2.8 | [−20.28; 14.72] | 1.000 | 0.00 | −3.6 | [−32.27; 25.05] | 1.000 | 0.00 |
| RF—Relaxation (m/s) | 39.2 ± 72.7 | 22.4 ± 5.0 | −16.8 | [−60.75; 27.18] | 0.975 | 0.03 | 22.4 ± 4.4 | −16.8 | [−60.56; 26.97] | 0.968 | 0.03 | −0.1 | [−1.49; 1.46] | 1.000 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rueda, L.; Cabanas-Valdés, R.; Salgueiro, C.; Rodríguez-Sanz, J.; Pérez-Bellmunt, A.; López-de-Celis, C. Immediate Effects of TECAR Therapy on Gastrocnemius and Quadriceps Muscles with Spastic Hypertonia in Chronic Stroke Survivors: A Randomized Controlled Trial. Biomedicines 2023, 11, 2973. https://doi.org/10.3390/biomedicines11112973
García-Rueda L, Cabanas-Valdés R, Salgueiro C, Rodríguez-Sanz J, Pérez-Bellmunt A, López-de-Celis C. Immediate Effects of TECAR Therapy on Gastrocnemius and Quadriceps Muscles with Spastic Hypertonia in Chronic Stroke Survivors: A Randomized Controlled Trial. Biomedicines. 2023; 11(11):2973. https://doi.org/10.3390/biomedicines11112973
Chicago/Turabian StyleGarcía-Rueda, Laura, Rosa Cabanas-Valdés, Carina Salgueiro, Jacobo Rodríguez-Sanz, Albert Pérez-Bellmunt, and Carlos López-de-Celis. 2023. "Immediate Effects of TECAR Therapy on Gastrocnemius and Quadriceps Muscles with Spastic Hypertonia in Chronic Stroke Survivors: A Randomized Controlled Trial" Biomedicines 11, no. 11: 2973. https://doi.org/10.3390/biomedicines11112973
APA StyleGarcía-Rueda, L., Cabanas-Valdés, R., Salgueiro, C., Rodríguez-Sanz, J., Pérez-Bellmunt, A., & López-de-Celis, C. (2023). Immediate Effects of TECAR Therapy on Gastrocnemius and Quadriceps Muscles with Spastic Hypertonia in Chronic Stroke Survivors: A Randomized Controlled Trial. Biomedicines, 11(11), 2973. https://doi.org/10.3390/biomedicines11112973








