Changes in Inner Retina Thickness and Macular Sensitivity in Patients with Type 2 Diabetes with Moderate Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
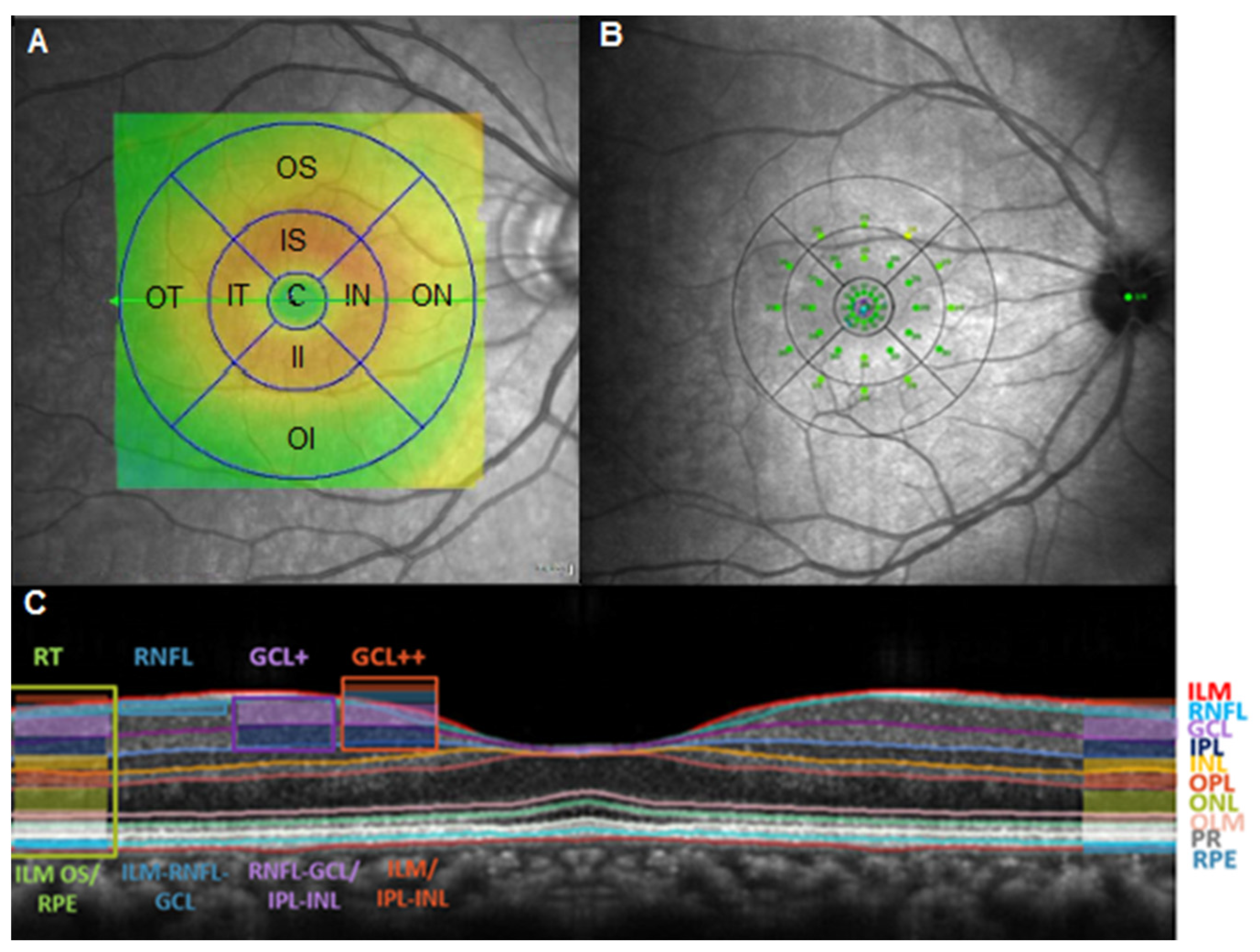
2.2. Study Protocol
2.3. Statistical Analysis
3. Results
3.1. Demographics
3.2. OCT: Total Retina and IRL Thickness Assessment
3.3. MAIA Retinal Sensitivity Assessment
3.4. Structural and Functional Correlations
3.5. RNFL and GCL Thickness Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef]
- Sohn, E.H.; Van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; Van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Fernández-Espinosa, G.; Boned-Murillo, A.; Orduna-Hospital, E.; Díaz-Barreda, M.D.; Sánchez-Cano, A.; Bielsa-Alonso, S.; Acha, J.; Pinilla, I. Retinal Vascularization Abnormalities Studied by Optical Coherence Tomography Angiography (OCTA) in Type 2 Diabetic Patients with Moderate Diabetic Retinopathy. Diagnostics 2022, 12, 379. [Google Scholar] [CrossRef]
- Fernández-Espinosa, G.; Orduna-Hospital, E.; Boned-Murillo, A.; Diaz-Barreda, M.D.; Sanchez-Cano, A.; Sopeña-Pinilla, M.; Pinilla, I. Choroidal and Retinal Thicknesses in Type 2 Diabetes Mellitus with Moderate Diabetic Retinopathy Measured by Swept Source OCT. Biomedicines 2022, 10, 2314. [Google Scholar] [CrossRef]
- McAnany, J.J.; Persidina, O.S.; Park, J.C. Clinical electroretinography in diabetic retinopathy: A review. Surv. Ophthalmol. 2022, 67, 712–722. [Google Scholar] [CrossRef]
- Rohrschneider, K.; Bültmann, S.; Springer, C. Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog. Retin. Eye Res. 2008, 27, 536–548. [Google Scholar] [CrossRef]
- Midena, E.; Vujosevic, S. Microperimetry in diabetic retinopathy. Saudi J. Ophthalmol. 2011, 25, 131. [Google Scholar] [CrossRef]
- Orduna-Hospital, E.; Otero-Rodríguez, J.; Perdices, L.; Sánchez-Cano, A.; Boned-Murillo, A.; Acha, J.; Pinilla, I. Microperimetry and Optical Coherence Tomography Changes in Type-1 Diabetes Mellitus without Retinopathy. Diagnostics 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 823–833. [Google Scholar]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902. [Google Scholar] [CrossRef]
- Chai, Q.; Yao, Y.; Guo, C.; Lu, H.; Ma, J. Structural and functional retinal changes in patients with type 2 diabetes without diabetic retinopathy. Ann. Med. 2022, 54, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, C.; Gong, Y.; Zhang, Y.; Jin, X.; Wei, S.; Zhang, M. Peripapillary Retinal Nerve Fiber Layer Changes in Preclinical Diabetic Retinopathy: A Meta-Analysis. PLoS ONE 2015, 10, e0125919. [Google Scholar] [CrossRef] [PubMed]
- Stem, M.S.; Gardner, T.W. Neurodegeneration in the Pathogenesis of Diabetic Retinopathy: Molecular Mechanisms and Therapeutic Implications. Curr. Med. Chem. 2013, 20, 3241. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic Retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Carpineto, P.; Toto, L.; Aloia, R.; Ciciarelli, V.; Borrelli, E.; Vitacolonna, E.; Di Nicola, M.; Di Antonio, L.; Mastropasqua, R. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye 2016, 30, 673. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Z.; Bao, T.; Wang, S.; Jiang, T.; Zhang, Y.; Li, Q.; Zhu, X. Evaluation of Early Retinal Nerve Injury in Type 2 Diabetes Patients Without Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 475672. [Google Scholar] [CrossRef]
- Mehboob, M.A.; Amin, Z.A.; Ul Islam, Q. Comparison of retinal nerve fiber layer thickness between normal population and patients with diabetes mellitus using optical coherence tomography. Pak. J. Med. Sci. 2019, 35, 29. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Pritchard, N.; Sampson, G.P.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Diagnostic capability of retinal thickness measures in diabetic peripheral neuropathy. J. Optom. 2017, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, R.; Sah, S.; Gupta, N. Study of Retinal Nerve Fibre Layer Thickness in Patients with Diabetes Mellitus Using Fourier Domain Optical Coherence Tomography. J. Clin. Diagn. Res. 2016, 10, NC05. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Guo, Z.; Wang, F.; Li, R.; Zhao, L.; Lin, R. Alterations in retinal nerve fiber layer thickness in early stages of diabetic retinopathy and potential risk factors. Curr. Eye Res. 2017, 43, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Zivadinov, R.; Mahfooz, N.; Carl, E.; Drake, A.; Schneider, J.; Teter, B.; Hussein, S.; Mehta, B.; Weiskopf, M.; et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J. Neuroinflamm. 2011, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Kardys, A.; Weinstock-Guttman, B.; Dillon, M.; Masud, M.W.; Weinstock, N.; Mahfooz, N.; Lang, J.K.; Weinstock, A.; Lincoff, N.; Zivadinov, R.; et al. Cholesterol affects retinal nerve fiber layer thickness in patients with multiple sclerosis with optic neuritis. Eur. J. Neurol. 2013, 20, 1264–1271. [Google Scholar] [CrossRef]
- Ebrahimi, K.B.; Handa, J.T. Lipids, Lipoproteins, and Age-Related Macular Degeneration. J. Lipids 2011, 2011, 802059. [Google Scholar] [CrossRef]
- Picconi, F.; Parravano, M.; Ylli, D.; Pasqualetti, P.; Coluzzi, S.; Giordani, I.; Malandrucco, I.; Lauro, D.; Scarinci, F.; Giorno, P.; et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: The role of glycemic variability. Acta Diabetol. 2017, 54, 489. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Tan, Y.; He, M.; Wang, L.; Li, Y.; Huang, W. Correlation between Renal Function and Peripapillary Choroidal Thickness in Treatment-Naïve Diabetic Eyes Using Swept-Source Optical Coherence Tomography. Curr. Eye Res. 2020, 45, 1526–1533. [Google Scholar] [CrossRef]
- Srivastav, K.; Saxena, S.; Mahdi, A.A.; Kruzliak, P.; Khanna, V.K. Increased serum urea and creatinine levels correlate with decreased retinal nerve fibre layer thickness in diabetic retinopathy. Biomarkers 2015, 20, 470–473. [Google Scholar] [CrossRef]
- Turpin, A.; Chen, S.; Sepulveda, J.A.; McKendrick, A.M. Customizing Structure–Function Displacements in the Macula for Individual Differences. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5984–5989. [Google Scholar] [CrossRef] [PubMed]
- Drasdo, N.; Millican, C.L.; Katholi, C.R.; Curcio, C.A. The Length of Henle Fibers in the Human Retina and a Model of Ganglion Receptive Field Density in the Visual Field. Vision Res. 2007, 47, 2901. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Chen, Y.F.; Liu, M.; Liu, K.; McAnany, J.J. Structural and functional abnormalities in early-stage diabetic retinopathy. Curr. Eye Res. 2020, 45, 975. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Plasencia, M.; Abreu-González, R.; Gómez-Culebras, M.A. Structure–Function Correlation Using OCT Angiography And Microperimetry In Diabetic Retinopathy. Clin. Ophthalmol. 2019, 13, 2181. [Google Scholar] [CrossRef]
- Taylor, L.J.; Josan, A.S.; Jolly, J.K.; Maclaren, R.E. Microperimetry as an Outcome Measure in RPGR-associated Retinitis Pigmentosa Clinical Trials. Transl. Vis. Sci. Technol. 2023, 12, 4. [Google Scholar] [CrossRef]



| Control Group | DM2 Group | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Age (years) | 60.79 | 8.62 | 64.06 | 11.98 | 0.082 |
| Sex (female-male %) | 39.7–60.3 | 20.4–79.6 | |||
| Time from diagnosis (years) | 2.50 | 2.88 | |||
| HbA1c (%) | 7.58 | 1.29 | |||
| BCVA (LogMAR) | 0.04 | 0.05 | 0.12 | 0.17 | <0.001 |
| SE (D) | 0.03 | 1.58 | 0.37 | 1.70 | 0.110 |
| AL (mm) | 23.73 | 1.46 | 23.23 | 0.84 | 0.080 |
| IOP (mmHg) | 15.30 | 2.89 | 14.76 | 2.49 | 0.676 |
| Disease progression time (years) | 2.50 | 2.88 | |||
| HbA1c (%) | 7.58 | 1.29 | |||
| Cholesterol (mg/dL) | 148.04 | 33.18 | |||
| HDL (mg/dL) | 47.83 | 15.21 | |||
| LDL (mg/dL) | 71.47 | 23.09 | |||
| TG (mg/dL) | 122.24 | 51.71 | |||
| GF (mL/min) | 73.57 | 20.52 | |||
| Creatine (mg/dL) | 1.05 | 0.49 | |||
| Retinal Sensitivity (dB) | |||
|---|---|---|---|
| Control | DM | Control vs. DM | |
| Media ± SD | Media ± SD | p | |
| Macular integrity | 64.84 ± 30.02 | 77.82 ± 28.04 | 0.005 |
| Average threshold | 26.69 ± 2.26 | 24.45 ± 3.63 | <0.0001 |
| Fixation stability P1 | 88.25 ± 13.03 | 77.96 ± 26.02 | 0.162 |
| Fixation stability P2 | 96.78 ± 4.71 | 89.26 ± 17.31 | 0.016 |
| BCEA 63 area | 1.82 ± 1.93 | 4.33 ± 6.65 | 0.121 |
| BCEA 63 angle | 2.39 ± 62.12 | 9.20 ± 50.55 | 0.637 |
| BCEA 95 area | 45.35 ± 5.79 | 12.84 ± 20.03 | 0.142 |
| BCEA 95 angle | 2.39 ± 6.11 | 4.64 ± 50.25 | 0.956 |
| Fixation loses (%) | 4.20 ± 10.71 | 7.34 ± 17.99 | 0.432 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boned-Murillo, A.; Fernández-Espinosa, G.; Orduna-Hospital, E.; Díaz-Barreda, M.D.; Sánchez-Cano, A.; Sopeña-Pinilla, M.; Bielsa-Alonso, S.; Pinilla, I. Changes in Inner Retina Thickness and Macular Sensitivity in Patients with Type 2 Diabetes with Moderate Diabetic Retinopathy. Biomedicines 2023, 11, 2972. https://doi.org/10.3390/biomedicines11112972
Boned-Murillo A, Fernández-Espinosa G, Orduna-Hospital E, Díaz-Barreda MD, Sánchez-Cano A, Sopeña-Pinilla M, Bielsa-Alonso S, Pinilla I. Changes in Inner Retina Thickness and Macular Sensitivity in Patients with Type 2 Diabetes with Moderate Diabetic Retinopathy. Biomedicines. 2023; 11(11):2972. https://doi.org/10.3390/biomedicines11112972
Chicago/Turabian StyleBoned-Murillo, Ana, Guisela Fernández-Espinosa, Elvira Orduna-Hospital, Maria Dolores Díaz-Barreda, Ana Sánchez-Cano, María Sopeña-Pinilla, Sofía Bielsa-Alonso, and Isabel Pinilla. 2023. "Changes in Inner Retina Thickness and Macular Sensitivity in Patients with Type 2 Diabetes with Moderate Diabetic Retinopathy" Biomedicines 11, no. 11: 2972. https://doi.org/10.3390/biomedicines11112972
APA StyleBoned-Murillo, A., Fernández-Espinosa, G., Orduna-Hospital, E., Díaz-Barreda, M. D., Sánchez-Cano, A., Sopeña-Pinilla, M., Bielsa-Alonso, S., & Pinilla, I. (2023). Changes in Inner Retina Thickness and Macular Sensitivity in Patients with Type 2 Diabetes with Moderate Diabetic Retinopathy. Biomedicines, 11(11), 2972. https://doi.org/10.3390/biomedicines11112972







