Advances in Recombinant Adeno-Associated Virus Vectors for Neurodegenerative Diseases
Abstract
:1. Introduction
2. Main Section
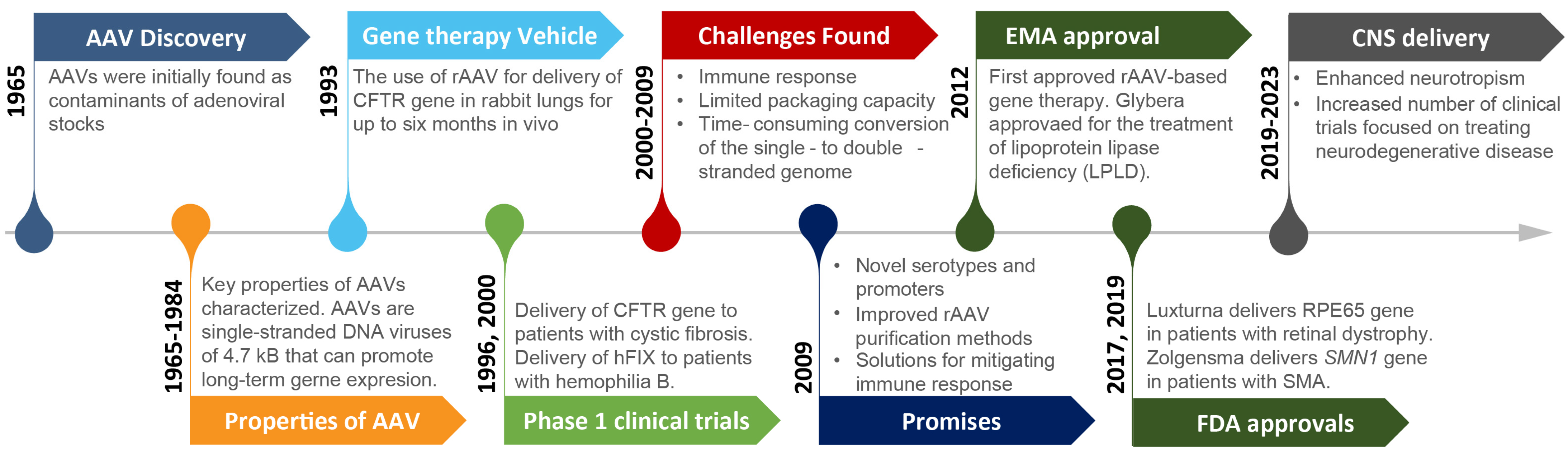
2.1. rAAV Vectors in the Clinic
2.2. rAAV Vectors for Neurodegenerative Diseases in Clinical Trials
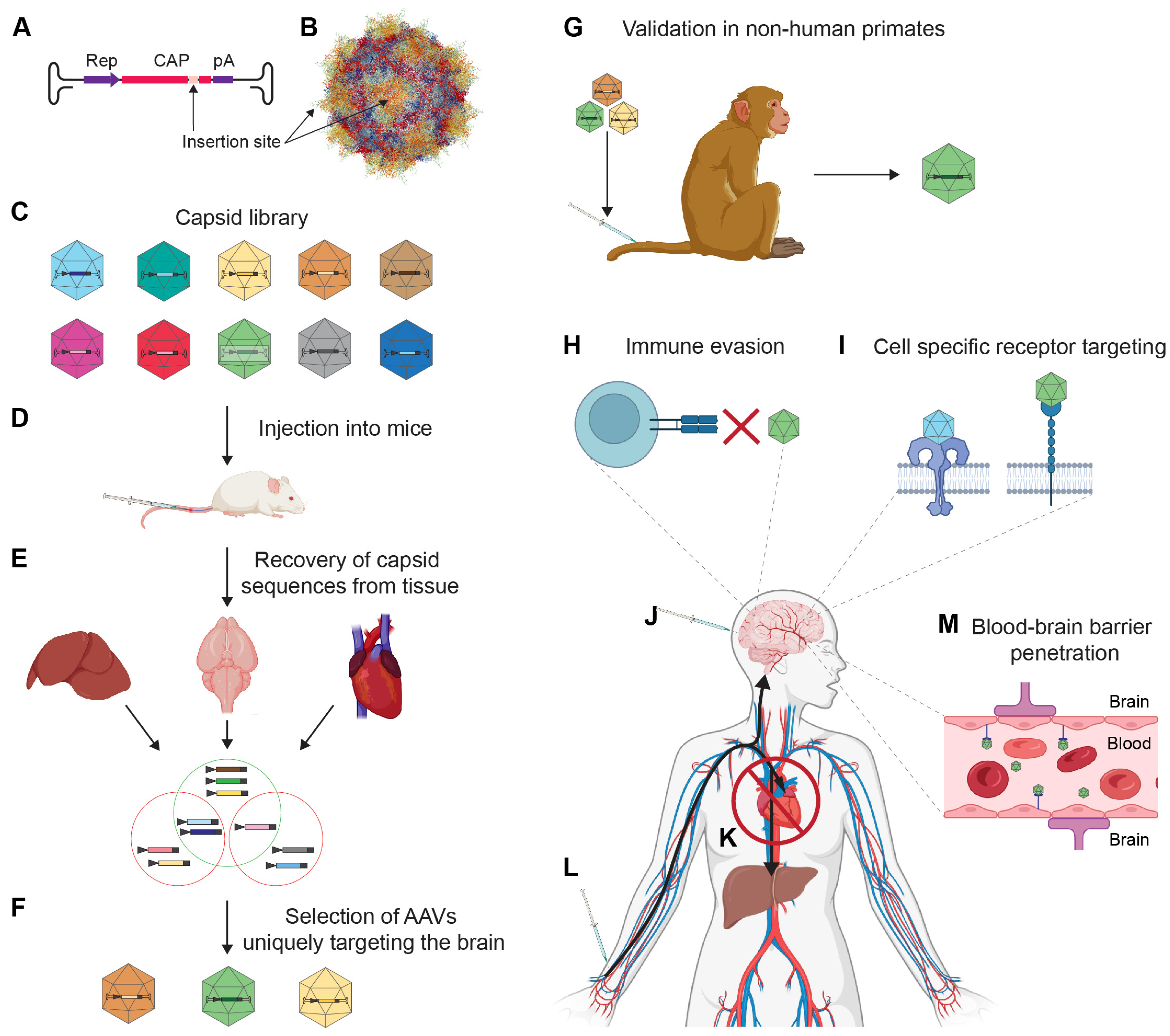
2.3. rAAV Capsids with Improved Tropism for Brain Indications
2.4. Altering the Expression Levels of Gene Products
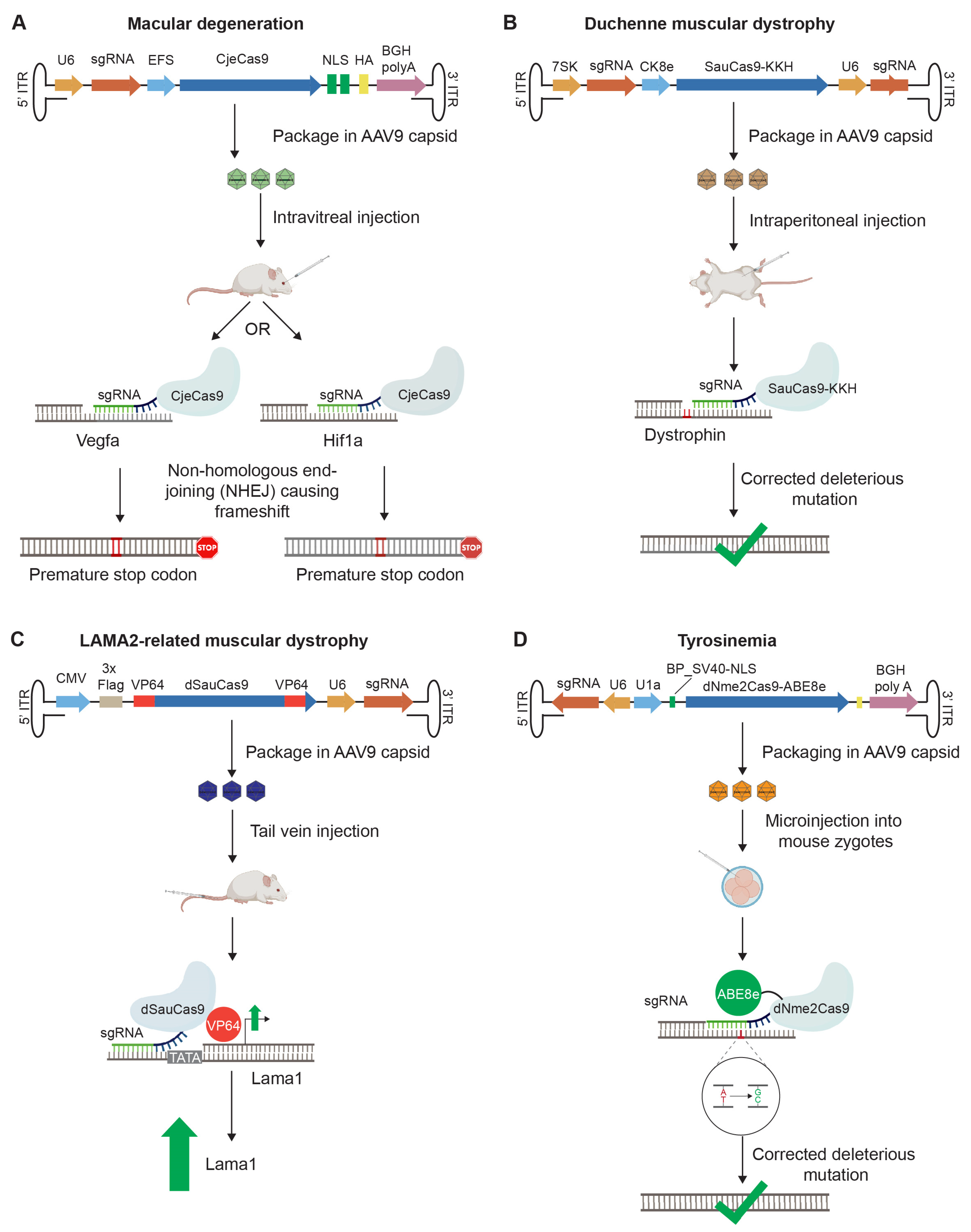
2.5. Gene Editing Approaches
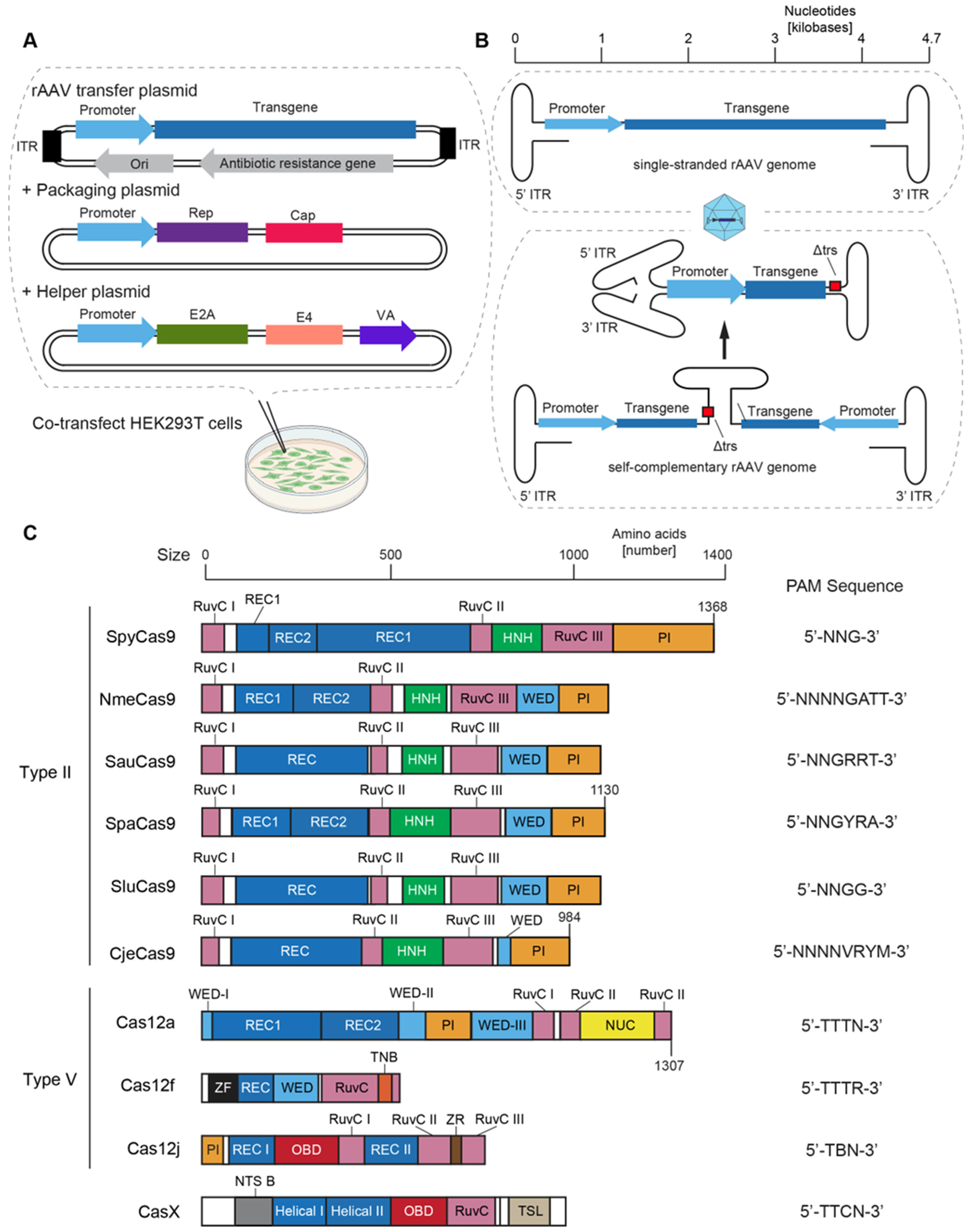
2.6. Payloads of rAAV Vectors
2.7. Optimization of Cas Enzymes for rAAV Gene Editing Vectors
2.8. All-in-One rAAV Vector Designs for In Vivo Gene Editing
2.9. Off-Target Gene Edits
2.10. rAAV Vector-Dependent Toxicity and Immune Responses in Humans
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burdett, T.; Nuseibeh, S. Changing trends in the development of AAV-based gene therapies: A meta-analysis of past and present therapies. Gene Ther. 2022, 30, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Serrano, A.; Rico, A.J.; Roda, E.; Honrubia, A.; Arrieta, S.; Ariznabarreta, G.; Chocarro, J.; Lorenzo-Ramos, E.; Pejenaute, A.; Vázquez, A.; et al. Adeno-Associated Viral Vectors as Versatile Tools for Neurological Disorders: Focus on Delivery Routes and Therapeutic Perspectives. Biomedicines 2022, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Martier, R.; Konstantinova, P. Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock. Front. Neurosci. 2020, 14, 580179. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022, 19, 176–190. [Google Scholar] [CrossRef]
- Moreno, A.M.; Fu, X.; Zhu, J.; Katrekar, D.; Shih, Y.-R.V.; Marlett, J.; Cabotaje, J.; Tat, J.; Naughton, J.; Lisowski, L.; et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. 2018, 26, 1818–1827. [Google Scholar] [CrossRef]
- Li, F.; Wing, K.; Wang, J.H.; Luu, C.D.; Bender, J.A.; Chen, J.; Wang, Q.; Lu, Q.; Nguyen Tran, M.T.; Young, K.M.; et al. Comparison of CRISPR/Cas Endonucleases for in vivo Retinal Gene Editing. Front. Cell Neurosci. 2020, 14, 570917. [Google Scholar] [CrossRef]
- György, B.; Nist-Lund, C.; Pan, B.; Asai, Y.; Karavitaki, K.D.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Solanes, P.; Spataro, S.; et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 2019, 25, 1123–1130. [Google Scholar] [CrossRef]
- Guan, L.; Han, Y.; Yang, C.; Lu, S.; Du, J.; Li, H.; Lin, J. CRISPR-Cas9-Mediated Gene Therapy in Neurological Disorders. Mol. Neurobiol. 2022, 59, 968–982. [Google Scholar] [CrossRef]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–756. [Google Scholar] [CrossRef]
- Daya, S.; Berns, K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008, 21, 583–593. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayandharan, G.R. Basic Biology of Adeno-Associated Virus (AAV) Vectors Used in Gene Therapy. Curr. Gene Ther. 2014, 14, 86–100. [Google Scholar] [CrossRef]
- Flotte, T.R.; Afione, S.A.; Conrad, C.; McGrath, S.A.; Solow, R.; Oka, H.; Zeitlin, P.L.; Guggino, W.B.; Carter, B.J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 1993, 90, 10613–10617. [Google Scholar] [CrossRef]
- Flotte, T.; Carter, B.; Conrad, C.; Guggino, W.; Reynolds, T.; Rosenstein, B.; Taylor, G.; Walden, S.; Wetzel, R. A Phase I Study of an Adeno-Associated Virus-CFTR Gene Vector in Adult CF Patients with Mild Lung Disease. Johns Hopkins Children’s Center, Baltimore, Maryland. Hum. Gene Ther. 1996, 7, 1145–1159. [Google Scholar] [CrossRef]
- Kay, M.A.; Manno, C.S.; Ragni, M.V.; Larson, P.J.; Couto, L.B.; McClelland, A.; Glader, B.; Chew, A.J.; J Tai, S.; Herzog, R.W.; et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000, 24, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.A.; Tedesco, N.; Leborgne, C.; Ronzitti, G. Overcoming the Challenges Imposed by Humoral Immunity to AAV Vectors to Achieve Safe and Efficient Gene Transfer in Seropositive Patients. Front. Immunol. 2022, 13, 857276. [Google Scholar] [CrossRef]
- Hamilton, B.A.; Wright, J.F. Challenges Posed by Immune Responses to AAV Vectors: Addressing Root Causes. Front. Immunol. 2021, 12, 675897. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Twisk, J.; Kwikkers, K.; Aronica, E.; Brisson, D.; Methot, J.; Petry, H.; Gaudet, D. Immune Responses to Intramuscular Administration of Alipogene Tiparvovec (AAV1-LPLS447X) in a Phase II Clinical Trial of Lipoprotein Lipase Deficiency Gene Therapy. Hum. Gene Ther. 2014, 25, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Smalley, E. First AAV gene therapy poised for landmark approval. Nat. Biotechnol. 2017, 35, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Urquhart, L. FDA new drug approvals in Q2 2019. Nat. Rev. Drug Dis. 2019, 18, 575–576. [Google Scholar] [CrossRef]
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Gene therapy successes point to better therapies. Proc. Natl. Acad. Sci. USA 2019, 116, 23866–23870. [Google Scholar] [CrossRef] [PubMed]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Reilly, A.; Chehade, L.; Kothary, R. Curing SMA: Are we there yet? Gene Ther. 2023, 30, 8–17. [Google Scholar] [CrossRef]
- Kuzmin, D.A.; Shutova, M.V.; Johnston, N.R.; Smith, O.P.; Fedorin, V.V.; Kukushkin, Y.S.; Loo, J.C.M. The clinical landscape for AAV gene therapies. Nat. Rev. Drug Disc. 2021, 20, 173–175. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Y.; Ju, D. Gene therapy for neurodegenerative disorders: Advances, insights and prospects. Acta Pharmaceut. Sin. B 2020, 10, 1347–1359. [Google Scholar] [CrossRef]
- Le Bras, A. Optimizing AAV delivery to the brain. Lab. Anim. 2022, 51, 45. [Google Scholar] [CrossRef]
- Taghian, T.; Marosfoi, M.G.; Puri, A.S.; Cataltepe, O.I.; King, R.M.; Diffie, E.B.; Maguire, A.S.; Martin, D.R.; Fernau, D.; Batista, A.R.; et al. A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna. Mol. Ther. 2020, 28, 411–421. [Google Scholar] [CrossRef]
- Fischell, J.M.; Fishman, P.S. A Multifaceted Approach to Optimizing AAV Delivery to the Brain for the Treatment of Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 747726. [Google Scholar]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Cagin, U. Targeting Age-Related Neurodegenerative Diseases by AAV-Mediated Gene Therapy. In Reviews on New Drug Targets in Age-Related Disorders: Part II; Guest, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 213–223. [Google Scholar]
- Mijanović, O.; Branković, A.; Borovjagin, A.; Butnaru, D.V.; Bezrukov, E.A.; Sukhanov, R.B.; Shpichka, A.; Timashev, P.; Ulasov, I. Battling Neurodegenerative Diseases with Adeno-Associated Virus-Based Approaches. Viruses 2020, 12, 460. [Google Scholar] [CrossRef]
- Strittmatter, W.J.; Saunders, A.M.; Schmechel, D.; Pericak-Vance, M.; Enghild, J.; Salvesen, G.S.; Roses, A.D. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 1977–1981. [Google Scholar] [CrossRef]
- Zhao, L.; Gottesdiener, A.J.; Parmar, M.; Li, M.; Kaminsky, S.M.; Chiuchiolo, M.J.; Sondhi, D.; Sullivan, P.M.; Holtzman, D.M.; Crystal, R.G.; et al. Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models. Neurobiol. Aging 2016, 44, 159–172. [Google Scholar] [CrossRef]
- Arboleda-Velasquez, J.F.; Lopera, F.; O’Hare, M.; Delgado-Tirado, S.; Marino, C.; Chmielewska, N.; Saez-Torres, K.L.; Amarnani, D.; Schultz, A.P.; Sperling, R.A.; et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: A case report. Nat. Med. 2019, 25, 1680–1683. [Google Scholar] [CrossRef]
- Mietzsch, M.; Yu, J.C.; His, J.; Chipman, P.; Broecker, F.; Fuming, Z.; Linhardt, R.J.; Seeberger, P.H.; Heilbronn, R.; McKenna, R.; et al. Structural Study of Aavrh.10 Receptor and Antibody Interactions. J. Virol. 2021, 95, e0124921. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.B.; Kaplitt, M.G.; De, B.P.; Chen, A.; Flagiello, T.; Salami, C.; Pey, E.; Zhao, L.; Ricart Arbona, R.J.; Monette, S.; et al. AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer’s Disease. Hum. Gene Ther. Clin. Dev. 2018, 29, 24–47. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, C.M.; Shan, L.; Zhang, Y.J.; Hoffer, B.J.; Leonard, S.; Troncoso, J.C.; Vonsatel, P.; Tomac, A.C. Gene expression patterns for GDNF and its receptors in the human putamen affected by Parkinson’s disease: A real-time PCR study. Mol. Cell Endocrinol. 2006, 252, 160–166. [Google Scholar] [CrossRef]
- Furr Stimming, E.; Sung, V.; Testa, C.; Testa, C.; Kostyk, S.; Ross, C.A.; Samii, A.; Geschwind, M.D.; Hall, D.; Dayalu, P.; et al. Interim results from cohort 1 of the double-blind, dose-escalation phase I/II clinical trial of amt-130 (HD-genetrx-1) for early-stage huntington’s disease (HD). In Proceedings of the European Huntington’s Disease Network (EHDN) Plenary Meeting, Bologna, Italy, 16–18 September 2022. [Google Scholar]
- Stanimirovic, D.B.; Sandhu, J.K.; Costain, W.J. Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood–Brain Barrier. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2018, 32, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yuan, J.; Yang, Y.; Yue, Y.; Hu, Z.; Fadera, S.; Chen, H. Incisionless targeted adeno-associated viral vector delivery to the brain by focused ultrasound-mediated intranasal administration. EBioMedicine 2022, 84, 104277. [Google Scholar] [CrossRef]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 2019, 101, 839–862. [Google Scholar] [CrossRef]
- Chandran, J.; Chowdhury, E.A.; Perkinton, M.; Jamier, T.; Sutton, D.; Wu, S.; Dobson, C.; Shah, D.K.; Chessell, I.; Meno-Tetang, G.M.L. Assessment of AAV9 distribution and transduction in rats after administration through Intrastriatal, Intracisterna magna and Lumbar Intrathecal routes. Gene Ther. 2023, 30, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Harashima, H. Current Status and Challenges Associated with CNS-Targeted Gene Delivery across the BBB. Pharmaceutics 2020, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Piguet, F.; Alves, S.; Cartier, N. Clinical Gene Therapy for Neurodegenerative Diseases: Past, Present, and Future. Hum. Gene Ther. 2017, 28, 988–1003. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004, 78, 6381–6388. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Jose, A.; Chipman, P.; Bhattacharya, N.; Daneshparvar, N.; McKenna, R.; Agbandje-McKenna, M. Completion of the AAV Structural Atlas: Serotype Capsid Structures Reveals Clade-Specific Features. Viruses 2021, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Cearley, C.N.; Wolfe, J.H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 2006, 13, 528–537. [Google Scholar] [CrossRef]
- Aschauer, D.F.; Kreuz, S.; Rumpel, S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS ONE 2013, 8, e76310. [Google Scholar] [CrossRef]
- Ballon, D.J.; Rosenberg, J.B.; Fung, E.K.; Nikolopoulou, A.; Kothari, P.; De, B.P.; He, B.; Chen, A.; Heier, L.A.; Sondhi, D.; et al. Quantitative Whole-Body Imaging of I-124-Labeled Adeno-Associated Viral Vector Biodistribution in Nonhuman Primates. Hum. Gene Ther. 2020, 31, 1237–1259. [Google Scholar] [CrossRef] [PubMed]
- Pupo, A.; Fernández, A.; Low, S.H.; François, A.; Suárez-Amarán, L.; Samulski, R.J. AAV vectors: The Rubik’s cube of human gene therapy. Mol. Ther. 2022, 30, 3515–3541. [Google Scholar] [CrossRef] [PubMed]
- Girod, A.; Ried, M.; Wobus, C.; Lahm, H.; Leike, K.; Kleinschmidt, J.; Deleage, G.; Hallek, M. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat. Med. 1999, 5, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Michelfelder, S.; Varadi, K.; Raupp, C.; Hunger, A.; Korbelin, J.; Pahrmann, C.; Schrepfer, S.; Muller, O.J.; Kleinschmidt, J.A.; Trepel, M. Peptide ligands incorporated into the threefold spike capsid domain to re-direct gene transduction of AAV8 and AAV9 in vivo. PLoS ONE 2011, 6, e23101. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Liu, Y.; Qu, Y.; Wang, K.; Zhang, Y.; Chang, Y.; Yang, Z.; Wan, J.; Liu, J.; et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates. Nat. Biomed. Eng. 2022, 6, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Fakhiri, J.; Grimm, D. Fantastic AAV Gene Therapy Vectors and How to Find Them-Random Diversification, Rational Design and Machine Learning. Pathogens 2022, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotech. 2016, 34, 204–209. [Google Scholar] [CrossRef]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.L.; Sanchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; Deverman, B.E.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Ojala, D.S.; Sun, S.; Santiago-Ortiz, J.L.; Shapiro, M.G.; Romero, P.A.; Schaffer, D.V. In Vivo Selection of a Computationally Designed SCHEMA AAV Library Yields a Novel Variant for Infection of Adult Neural Stem Cells in the SVZ. Mol. Ther. 2018, 26, 304–319. [Google Scholar] [CrossRef]
- Davidsson, M.; Wang, G.; Aldrin-Kirk, P.; Cardoso, T.; Nolbrant, S.; Hartnor, M.; Mudannayake, J.; Parmar, M.; Bjorklund, T. A systematic capsid evolution approach performed in vivo for the design of AAV vectors with tailored properties and tropism. Proc. Natl. Acad. Sci. USA 2019, 116, 27053–27062. [Google Scholar] [CrossRef]
- Hanlon, K.S.; Meltzer, J.C.; Buzhdygan, T.; Cheng, M.J.; Sena-Esteves, M.; Bennett, R.E.; Sullivan, T.P.; Razmpour, R.; Gong, Y.; Ng, C.; et al. Selection of an Efficient AAV Vector for Robust CNS Transgene Expression. Mol. Ther. Methods Clin. Dev. 2019, 15, 320–332. [Google Scholar] [CrossRef]
- Ravindra Kumar, S.; Miles, T.F.; Chen, X.; Brown, D.; Dobreva, T.; Huang, Q.; Ding, X.; Luo, Y.; Einarsson, P.H.; Greenbaum, A.; et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat. Methods 2020, 17, 541–550. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; Wang, W.; Child, M.A.; Ren, X.Q.; Huang, C.; Ren, A.Z.; Tocci, J.; Chen, Q.; Bittner, K.; Tyson, K.; et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol. Ther. Methods Clin. Dev. 2021, 20, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2022, 25, 106–115. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Im, S.K.; Fang, S. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, S.N.; Lock, J.L.; Schoderboeck, L.; Abraham, W.C.; Hughes, S.M. CNS Transduction Benefits of AAV-PHP.eB over AAV9 Are Dependent on Administration Route and Mouse Strain. Mol. Ther. Methods Clin. Dev. 2020, 19, 447–458. [Google Scholar] [CrossRef]
- Batista, A.R.; King, O.D.; Reardon, C.P.; Davis, C.; Shankaracharya; Philip, V.; Gray-Edwards, H.; Aronin, N.; Lutz, C.; Landers, J.; et al. Ly6a Differential Expression in Blood–Brain Barrier Is Responsible for Strain Specific Central Nervous System Transduction Profile of AAV-PHP.B. Hum. Gene Ther. 2019, 31, 90–102. [Google Scholar] [CrossRef]
- Beharry, A.; Gong, Y.; Kim, J.C.; Hanlon, K.S.; Nammour, J.; Hieber, K.; Eichler, F.; Cheng, M.; Stemmer-Rachamimov, A.; Stankovic, K.M.; et al. The AAV9 Variant Capsid AAV-F Mediates Widespread Transgene Expression in Nonhuman Primate Spinal Cord After Intrathecal Administration. Hum. Gene Ther. 2022, 33, 61–75. [Google Scholar] [CrossRef]
- Aldrin-Kirk, P.; Akerblom, M.; Cardoso, T.; Nolbrant, S.; Adler, A.F.; Liu, X.; Heuer, A.; Davidsson, M.; Parmar, M.; Bjorklund, T. A novel two-factor monosynaptic TRIO tracing method for assessment of circuit integration of hESC-derived dopamine transplants. Stem Cell Rep. 2022, 17, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.H.; Bashir, A.; Sinai, S.; Jain, N.K.; Ogden, P.J.; Riley, P.F.; Church, G.M.; Colwell, L.J.; Kelsic, E.D. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 2021, 39, 691–696. [Google Scholar] [CrossRef]
- Marques, A.D.; Kummer, M.; Kondratov, O.; Banerjee, A.; Moskalenko, O.; Zolotukhin, S. Applying machine learning to predict viral assembly for adeno-associated virus capsid libraries. Mol. Ther. Methods Clin. Dev. 2021, 20, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Brookes, D.H.; Busia, A.; Carneiro, A.; Fannjiang, C.; Popova, G.; Shin, D.; Donohue, K.C.; Chang, E.F.; Nowakowski, T.J. Optimal trade-off control in machine learning-based library design, with application to adeno-associated virus (AAV) for gene therapy. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wec, A.Z.; Lin, K.S.; Kwasnieski, J.C.; Sinai, S.; Gerold, J.; Kelsic, E.D. Overcoming Immunological Challenges Limiting Capsid-Mediated Gene Therapy with Machine Learning. Front. Immunol. 2021, 12, 674021. [Google Scholar] [CrossRef] [PubMed]
- Zinn, E.; Pacouret, S.; Khaychuk, V.; Turunen, H.T.; Carvalho, L.S.; Andres-Mateos, E.; Shah, S.; Shelke, R.; Maurer, A.C.; Plovie, E.; et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Rep. 2015, 12, 1056–1068. [Google Scholar] [CrossRef]
- Hudry, E.; Andres-Mateos, E.; Lerner, E.P.; Volak, A.; Cohen, O.; Hyman, B.T.; Maguire, C.A.; Vandenberghe, L.H. Efficient Gene Transfer to the Central Nervous System by Single-Stranded Anc80L65. Mol. Ther. Methods Clin. Dev. 2018, 10, 197–209. [Google Scholar] [CrossRef]
- Andres-Mateos, E.; Landegger, L.D.; Unzu, C.; Phillips, J.; Lin, B.M.; Dewyer, N.A.; Sanmiguel, J.; Nicolaou, F.; Valero, M.D.; Bourdeu, K.I.; et al. Choice of vector and surgical approach enables efficient cochlear gene transfer in nonhuman primate. Nat. Commun. 2022, 13, 1359. [Google Scholar] [CrossRef]
- Riyad, J.M.; Weber, T. Intracellular trafficking of adeno-associated virus (AAV) vectors: Challenges and future directions. Gene Ther. 2021, 28, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.L.; Chapman, M.S. Adeno-associated virus (AAV) cell entry: Structural insights. Trends Microbiol. 2022, 30, 432–451. [Google Scholar] [CrossRef]
- Hordeaux, J.; Yuan, Y.; Clark, P.M.; Wang, Q.; Martino, R.A.; Sims, J.J.; Bell, P.; Raymond, A.; Stanford, W.L.; Wilson, J.M. The GPI-Linked Protein LY6A Drives AAV-PHP.B Transport across the Blood-Brain Barrier. Mol. Ther. 2019, 27, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, R.; Li, H.; Yin, K.; Ma, X.; Lou, Z. Structural basis for the neurotropic AAV9 and the engineered AAVPHP.eB recognition with cellular receptors. Mol. Ther. Methods Clin. Dev. 2022, 26, 52–60. [Google Scholar] [CrossRef]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An essential receptor for adeno-associated virus infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, L.; Cui, M.; Sun, Z.; Hu, M.; Zhang, R.; Stuart, W.; Zhao, X.; Yang, Z.; Li, X.; et al. Adeno-associated virus 2 bound to its cellular receptor AAVR. Nat. Microbiol. 2019, 4, 675–682. [Google Scholar] [CrossRef]
- Jang, S.; Shen, H.K.; Ding, X.; Miles, T.F.; Gradinaru, V. Structural basis of receptor usage by the engineered capsid AAV-PHP.eB. Mol. Ther. Methods Clin. Dev. 2022, 26, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Meisen, W.H.; Nejad, Z.B.; Hardy, M.; Zhao, H.; Oliverio, O.; Wang, S.; Hale, C.; Ollmann, M.M.; Collins, P.J. Pooled Screens Identify GPR108 and TM9SF2 as Host Cell Factors Critical for AAV Transduction. Mol. Ther. Methods Clin. Dev. 2020, 17, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Hordeaux, J.; Wang, Q.; Katz, N.; Buza, E.L.; Bell, P.; Wilson, J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol. Ther. 2018, 26, 664–668. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, A.T.; Chan, K.Y.; Sorensen, H.; Barry, A.J.; Azari, B.; Zheng, Q.; Beddow, T.; Zhao, B.; Tobey, I.G.; et al. Targeting AAV vectors to the central nervous system by engineering capsid-receptor interactions that enable crossing of the blood-brain barrier. PLoS Biol. 2023, 21, e3002112. [Google Scholar] [CrossRef] [PubMed]
- Shmerling, M.; Chalik, M.; Smorodinsky, N.I.; Meeker, A.; Roy, S.; Sagi-Assif, O.; Meshel, T.; Danilevsky, A.; Shomron, N.; Levinger, S.; et al. LY6S, a New IFN-Inducible Human Member of the Ly6a Subfamily Expressed by Spleen Cells and Associated with Inflammation and Viral Resistance. ImmunoHorizons 2022, 6, 253–272. [Google Scholar] [CrossRef]
- Ghauri, M.S.; Ou, L. AAV Engineering for Improving Tropism to the Central Nervous System. Biology 2023, 12, 186. [Google Scholar] [CrossRef]
- Stanton, A.C.; Lagerborg, K.A.; Tellez, L.; Krunnfusz, A.; King, E.M.; Ye, S.; Solomon, I.H.; Tabebordbar, M.; Sabeti, P.C. Systemic administration of novel engineered AAV capsids facilitates enhanced transgene expression in the macaque CNS. Med 2023, 4, 31–50.e38. [Google Scholar] [CrossRef]
- Fischer, S.; Strobel, B.; Weinmann, J.; Gillardon, F. Two engineered AAV capsid variants for efficient transduction of human cortical neurons directly converted from iPSC. J. Neurosci. Methods 2022, 368, 109457. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Pekrun, K.; Khan, T.A.; Zhang, F.; Paşca, S.P.; Kay, M.A. Selection of rAAV vectors that cross the human blood-brain barrier and target the central nervous system using a transwell model. Mol. Ther. Methods Clin. Dev. 2022, 27, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Chuapoco, M.R.; Flytzanis, N.C.; Goeden, N.; Christopher Octeau, J.; Roxas, K.M.; Chan, K.Y.; Scherrer, J.; Winchester, J.; Blackburn, R.J.; Campos, L.J.; et al. Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nat. Nanotechnol. 2023. [CrossRef]
- Pipe, S.; Leebeek, F.W.G.; Ferreira, V.; Sawyer, E.K.; Pasi, J. Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene Transfer. Mol. Ther. Methods Clin. Dev. 2019, 15, 170–178. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, S.; Luo, Z.; Hu, Y.; Peng, X.; Lv, J.; Zhao, S.; Feng, J.; Huang, G.; Wan, Q.L.; et al. MiniCAFE, a CRISPR/Cas9-based compact and potent transcriptional activator, elicits gene expression in vivo. Nucleic Acids Res. 2021, 49, 4171–4185. [Google Scholar] [CrossRef]
- Lau, C.H.; Ho, J.W.; Lo, P.K.; Tin, C. Targeted Transgene Activation in the Brain Tissue by Systemic Delivery of Engineered AAV1 Expressing CRISPRa. Mol. Ther. Nucl. Acids 2019, 16, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef]
- Goedert, M.; Falcon, B.; Clavaguera, F.; Tolnay, M. Prion-like mechanisms in the pathogenesis of tauopathies and synucleinopathies. Curr. Neurol. Neurosci. Rep. 2014, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef]
- Boettcher, M.; McManus, M.T. Choosing the Right Tool for the Job: RNAi, TALEN, or CRISPR. Mol. Cell 2015, 58, 575–585. [Google Scholar] [CrossRef]
- Boudreau, R.L.; Rodríguez-Lebrón, E.; Davidson, B.L. RNAi medicine for the brain: Progresses and challenges. Hum. Mol. Genet. 2011, 20, R21–R27. [Google Scholar] [CrossRef]
- Iuchi, S. Three classes of C2H2 zinc finger proteins. Cell Mol. Life Sci. 2001, 58, 625–635. [Google Scholar] [CrossRef]
- Chandrasegaran, S.; Carroll, D. Origins of Programmable Nucleases for Genome Engineering. J. Mol. Biol. 2016, 428, 963–989. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161. [Google Scholar] [CrossRef]
- Paschon, D.E.; Lussier, S.; Wangzor, T.; Xia, D.F.; Li, P.W.; Hinkley, S.J.; Scarlott, N.A.; Lam, S.C.; Waite, A.J.; Truong, L.N.; et al. Diversifying the structure of zinc finger nucleases for high-precision genome editing. Nat. Commun. 2019, 10, 1133. [Google Scholar] [CrossRef]
- Zeitler, B.; Froelich, S.; Marlen, K.; Shivak, D.A.; Yu, Q.; Li, D.; Pearl, J.R.; Miller, J.C.; Zhang, L.; Paschon, D.E.; et al. Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington’s disease. Nat. Med. 2019, 25, 1131–1142. [Google Scholar] [CrossRef]
- Wegmann, S.; DeVos, S.L.; Zeitler, B.; Marlen, K.; Bennett, R.E.; Perez-Rando, M.; MacKenzie, D.; Yu, Q.; Commins, C.; Bannon, R.N.; et al. Persistent repression of tau in the brain using engineered zinc finger protein transcription factors. Sci. Adv. 2021, 7, eabe1611. [Google Scholar] [CrossRef]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, J.R.; Sheng, J.; Wang, M.C.; Bernardo-Colón, A.; Rex, T.S. Optimization of S. aureus dCas9 and CRISPRi Elements for a Single Adeno-Associated Virus that Targets an Endogenous Gene. Mol. Ther. Methods Clin. Dev. 2020, 19, 139–148. [Google Scholar] [CrossRef]
- Deutsch, M.; Günther, A.; Lerchundi, R.; Rose, C.R.; Balfanz, S.; Baumann, A. AAV-Mediated CRISPRi and RNAi Based Gene Silencing in Mouse Hippocampal Neurons. Cells 2021, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem. Biol. 2018, 13, 406–416. [Google Scholar] [CrossRef]
- Mohr, S.E.; Smith, J.A.; Shamu, C.E.; Neumuller, R.A.; Perrimon, N. RNAi screening comes of age: Improved techniques and complementary approaches. Nat. Rev. Mol. Cell Biol. 2014, 15, 591–600. [Google Scholar] [CrossRef]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucl. Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- Borel, F.; Kay, M.A.; Mueller, C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol. Ther. 2014, 22, 692–701. [Google Scholar] [CrossRef]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Giering, J.C.; Grimm, D.; Storm, T.A.; Kay, M.A. Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol. Ther. 2008, 16, 1630–1636. [Google Scholar] [CrossRef]
- Pfister, E.L.; Chase, K.O.; Sun, H.; Kennington, L.A.; Conroy, F.; Johnson, E.; Miller, R.; Borel, F.; Aronin, N.; Mueller, C. Safe and Efficient Silencing with a Pol II, but Not a Pol lII, Promoter Expressing an Artificial miRNA Targeting Human Huntingtin. Mol. Ther. Nucl. Acids 2017, 7, 324–334. [Google Scholar] [CrossRef]
- Keiser, M.S.; Ranum, P.T.; Yrigollen, C.M.; Carrell, E.M.; Smith, G.R.; Muehlmatt, A.L.; Chen, Y.H.; Stein, J.M.; Wolf, R.L.; Radaelli, E.; et al. Toxicity after AAV delivery of RNAi expression constructs into nonhuman primate brain. Nat. Med. 2021, 27, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Mao, Q.; Tai, P.W.L.; He, R.; Ai, J.; Su, Q.; Zhu, Y.; Ma, H.; Li, J.; Gong, S. Short DNA Hairpins Compromise Recombinant Adeno-Associated Virus Genome Homogeneity. Mol. Ther. 2017, 25, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Ashizawa, A.T. The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery. Biomedicines 2021, 9, 2021. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019, 70, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Mazur, C.; Powers, B.; Zasadny, K.; Sullivan, J.M.; Dimant, H.; Kamme, F.; Hesterman, J.; Matson, J.; Oestergaard, M.; Seaman, M.; et al. Brain pharmacology of intrathecal antisense oligonucleotides revealed through multimodal imaging. JCI Insight 2019, 4, e129240. [Google Scholar] [CrossRef] [PubMed]
- Monine, M.; Norris, D.; Wang, Y.; Nestorov, I. A physiologically-based pharmacokinetic model to describe antisense oligonucleotide distribution after intrathecal administration. J. Pharmacokin. Pharmacodyn. 2021, 48, 639–654. [Google Scholar] [CrossRef]
- Rook, M.E.; Southwell, A.L. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs 2022, 36, 105–119. [Google Scholar] [CrossRef]
- Moazami, M.P.; Wood, M.J.A. RNase-H-mediated silencing in the CNS proves predictably nontrivial. Med 2022, 3, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Hawellek, D.J.; Garces, P.; Meghdadi, A.H.; Waninger, S.; Smith, A.; Manchester, M.; Schobel, S.A.; Hipp, J.F. Changes in brain activity with tominersen in early-manifest Huntington’s disease. Brain Commun. 2022, 4, fcac149. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Juillerat, A.; Dubois, G.; Valton, J.; Thomas, S.; Stella, S.; Marechal, A.; Langevin, S.; Benomari, N.; Bertonati, C.; Silva, G.H.; et al. Comprehensive analysis of the specificity of transcription activator-like effector nucleases. Nucl. Acids Res. 2014, 42, 5390–5402. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Chapman, J.R.; Taylor, M.R.G.; Boulton, S.J. Playing the End Game: DNA Double-Strand Break Repair Pathway Choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Critchlow, S.E.; Jackson, S.P. DNA end-joining: From yeast to man. Trends Biochem. Sci. 1998, 23, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Krejci, L.; Altmannova, V.; Spirek, M.; Zhao, X. Homologous recombination and its regulation. Nucl. Acids Res. 2012, 40, 5795–5818. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zapata, D.; Tang, Y.; Teng, Y.; Li, Y. In vivo delivery of CRISPR-Cas9 genome editing components for therapeutic applications. Biomaterials 2022, 291, 121876. [Google Scholar] [CrossRef]
- Paquet, D.; Kwart, D.; Chen, A.; Sproul, A.; Jacob, S.; Teo, S.; Olsen, K.M.; Gregg, A.; Noggle, S.; Tessier-Lavigne, M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 2016, 533, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhong, X.; Li, Q.; Su, J.; Liu, Y.; Mo, L.; Deng, H.; Yang, Y. CRISPR-Cas9-Mediated In Vivo Gene Integration at the Albumin Locus Recovers Hemostasis in Neonatal and Adult Hemophilia B Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 520–531. [Google Scholar] [CrossRef]
- Fu, Y.W.; Dai, X.Y.; Wang, W.T.; Yang, Z.X.; Zhao, J.J.; Zhang, J.P.; Wen, W.; Zhang, F.; Oberg, K.C.; Zhang, L.; et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucl. Acids Res. 2021, 49, 969–985. [Google Scholar] [CrossRef]
- Sinha, S.; Villarreal, D.; Shim, E.Y.; Lee, S.E. Risky business: Microhomology-mediated end joining. Mut. Res. Fundament. Mol. Mech. Mutagen. 2016, 788, 17–24. [Google Scholar] [CrossRef]
- Aida, T.; Nakade, S.; Sakuma, T.; Izu, Y.; Oishi, A.; Mochida, K.; Ishikubo, H.; Usami, T.; Aizawa, H.; Yamamoto, T.; et al. Gene cassette knock-in in mammalian cells and zygotes by enhanced MMEJ. BMC Genom. 2016, 17, 979. [Google Scholar] [CrossRef]
- Newby, G.A.; Liu, D.R. In vivo somatic cell base editing and prime editing. Mol. Ther. 2021, 29, 3107–3124. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Xie, W.; Yu, H.; Lai, L.; Li, Z. Compact Cje3Cas9 for Efficient In Vivo Genome Editing and Adenine Base Editing. CRISPR J. 2022, 5, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ravendran, S.; Mikkelsen, N.S.; Haldrup, J.; Cai, H.; Ding, X.; Paludan, S.R.; Thomsen, M.K.; Mikkelsen, J.G.; Bak, R.O. A truncated reverse transcriptase enhances prime editing by split AAV vectors. Mol. Ther. 2022, 30, 2942–2951. [Google Scholar] [CrossRef] [PubMed]
- Earley, L.F.; Conatser, L.M.; Lue, V.M.; Dobbins, A.L.; Li, C.; Hirsch, M.L.; Samulski, R.J. Adeno-Associated Virus Serotype-Specific Inverted Terminal Repeat Sequence Role in Vector Transgene Expression. Hum. Gene Ther. 2020, 31, 151–162. [Google Scholar] [CrossRef]
- McCarty, D.M.; Monahan, P.E.; Samulski, R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001, 8, 1248–1254. [Google Scholar] [CrossRef]
- McCarty, D.M.; Fu, H.; Monahan, P.E.; Toulson, C.E.; Naik, P.; Samulski, R.J. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003, 10, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, H.I.; Li, J.; Sun, L.; Zhang, J.; Xiao, X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003, 10, 2105–2111. [Google Scholar] [CrossRef]
- Zhang, Y.; Nishiyama, T.; Li, H.; Huang, J.; Atmanli, A.; Sanchez-Ortiz, E.; Wang, Z.; Mireault, A.A.; Mammen, P.P.A.; Bassel-Duby, R.; et al. A consolidated AAV system for single-cut CRISPR correction of a common Duchenne muscular dystrophy mutation. Mol. Ther. Methods Clin. Dev. 2021, 22, 122–132. [Google Scholar] [CrossRef]
- Grieger, J.C.; Samulski, R.J. Packaging Capacity of Adeno-Associated Virus Serotypes: Impact of Larger Genomes on Infectivity and Postentry Steps. J. Virol. 2005, 79, 9933–9944. [Google Scholar] [CrossRef]
- Allocca, M.; Doria, M.; Petrillo, M.; Colella, P.; Garcia-Hoyos, M.; Gibbs, D.; Kim, S.R.; Maguire, A.; Rex, T.S.; Di Vicino, U.; et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J. Clin. Investig. 2008, 118, 1955–1964. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Krooss, S.A.; Dai, Z.; Schmidt, F.; Rovai, A.; Fakhiri, J.; Dhingra, A.; Yuan, Q.; Yang, T.; Balakrishnan, A.; Steinbrück, L.; et al. Ex Vivo/In vivo Gene Editing in Hepatocytes Using “All-in-One” CRISPR-Adeno-Associated Virus Vectors with a Self-Linearizing Repair Template. iScience 2020, 23, 100764. [Google Scholar] [CrossRef] [PubMed]
- Halbert, C.L.; Allen, J.M.; Miller, A.D. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat. Biotechnol. 2002, 20, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Yue, Y.; Liu, M.; Ghosh, A.; Engelhardt, J.F.; Chamberlain, J.S.; Duan, D. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 2005, 23, 1435–1439. [Google Scholar] [CrossRef]
- Ghosh, A.; Yue, Y.; Lai, Y.; Duan, D. A Hybrid Vector System Expands Adeno-associated Viral Vector Packaging Capacity in a Transgene-independent Manner. Mol. Ther. 2008, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Maclaren, R.E. Adeno-associated Virus (AAV) Dual Vector Strategies for Gene Therapy Encoding Large Transgenes. Yale J. Biol. Med. 2017, 90, 611–623. [Google Scholar]
- Yan, Z.; Zhang, Y.; Duan, D.; Engelhardt, J.F. Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 6716–6721. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Xiao, X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat. Med. 2000, 6, 599–602. [Google Scholar] [CrossRef]
- Friedland, A.E.; Baral, R.; Singhal, P.; Loveluck, K.; Shen, S.; Sanchez, M.; Marco, E.; Gotta, G.M.; Maeder, M.L.; Kennedy, E.M. Characterization of Staphylococcus aureus Cas9, a smaller Cas9 for all-in-one adeno-associated virus delivery and paired nickase applications. Genome Biol. 2015, 16, 257. [Google Scholar] [CrossRef]
- Fu, H.; Muenzer, J.; Kang, L.; Jennings, J.S.; Samulski, R.J.; McCarty, D.M. Optimization of AAV Serotype and Promoter for Increased Distribution of Expression in Both Somatic and Central Nervous Systems of Mice. Mol. Ther. 2005, 11, S332. [Google Scholar] [CrossRef]
- Domenger, C.; Grimm, D. Next-generation AAV vectors—Do not judge a virus (only) by its cover. Hum. Mol. Genet. 2019, 28, R3–R14. [Google Scholar] [CrossRef]
- Jackson, K.L.; Dayton, R.D.; Deverman, B.E.; Klein, R.L. Better Targeting, Better Efficiency for Wide-Scale Neuronal Transduction with the Synapsin Promoter and AAV-PHP.B. Front. Mol. Nurosci. 2016, 9, 116. [Google Scholar] [CrossRef]
- Klein, R.L.; Hamby, M.E.; Gong, Y.; Hirko, A.C.; Wang, S.; Hughes, J.A.; King, M.A.; Meyer, E.M. Dose and promoter effects of adeno-associated viral vector for green fluorescent protein expression in the rat brain. Exp. Neurol. 2002, 176, 66–74. [Google Scholar] [CrossRef]
- Finneran, D.J.; Njoku, I.P.; Flores-Pazarin, D.; Ranabothu, M.R.; Nash, K.R.; Morgan, D.; Gordon, M.N. Toward Development of Neuron Specific Transduction After Systemic Delivery of Viral Vectors. Front. Neurol. 2021, 12, 685802. [Google Scholar] [CrossRef]
- Radhiyanti, P.T.; Konno, A.; Matsuzaki, Y.; Hirai, H. Comparative study of neuron-specific promoters in mouse brain transduced by intravenously administered AAV-PHP.eB. Neurosci. Lett. 2021, 756, 135956. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.J.; Foti, S.B.; Schwartz, J.W.; Bachaboina, L.; Taylor-Blake, B.; Coleman, J.; Ehlers, M.D.; Zylka, M.J.; McCown, T.J.; Samulski, R.J. Optimizing Promoters for Recombinant Adeno-Associated Virus-Mediated Gene Expression in the Peripheral and Central Nervous System Using Self-Complementary Vectors. Hum. Gene Ther. 2011, 22, 1143–1153. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Rahman, K.; Smith, Y.; Galvan, A. Characterization of the GfaABC1D promoter to selectively target astrocytes in the rhesus macaque brain. J. Neurosci. Methods 2022, 372, 109530. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Hilton, S.; Carnicer-Lombarte, A.; Hobo, B.; Verhaagen, J.; Fawcett, J.W. Optimization of adeno-associated viral vector-mediated transduction of the corticospinal tract: Comparison of four promoters. Gene Ther. 2021, 28, 56–74. [Google Scholar] [CrossRef]
- Ibraheim, R.; Song, C.Q.; Mir, A.; Amrani, N.; Xue, W.; Sontheimer, E.J. All-in-one adeno-associated virus delivery and genome editing by Neisseria meningitidis Cas9 in vivo. Genome Biol. 2018, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.E.; Peddle, C.F.; Stevanovic, M.; Barnard, A.R.; McClements, M.E.; MacLaren, R.E. Promoter Orientation within an AAV-CRISPR Vector Affects Cas9 Expression and Gene Editing Efficiency. CRISPR J. 2020, 3, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.K.; Samulski, R.J.; McCown, T.J. AAV Capsid-Promoter Interactions Determine CNS Cell-Selective Gene Expression In Vivo. Mol. Ther. 2020, 28, 1373–1380. [Google Scholar] [CrossRef]
- Bohlen, M.O.; McCown, T.J.; Powell, S.K.; El-Nahal, H.G.; Daw, T.; Basso, M.A.; Sommer, M.A.; Samulski, R.J. Adeno-Associated Virus Capsid-Promoter Interactions in the Brain Translate from Rat to the Nonhuman Primate. Hum. Gene Ther. 2020, 31, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Fath, S.; Bauer, A.P.; Liss, M.; Spriestersbach, A.; Maertens, B.; Hahn, P.; Ludwig, C.; Schäfer, F.; Graf, M.; Wagner, R. Multiparameter RNA and codon optimization: A standardized tool to assess and enhance autologous mammalian gene expression. PLoS ONE 2011, 6, e17596. [Google Scholar] [CrossRef]
- Mordstein, C.; Cano, L.; Morales, A.C.; Young, B.; Ho, A.T.; Rice, A.M.; Liss, M.; Hurst, L.D.; Kudla, G. Transcription, mRNA Export, and Immune Evasion Shape the Codon Usage of Viruses. Genome Biol. Evol. 2021, 13, evab106. [Google Scholar] [CrossRef]
- Liss, M.; Daubert, D.; Brunner, K.; Kliche, K.; Hammes, U.; Leiherer, A.; Wagner, R. Embedding permanent watermarks in synthetic genes. PLoS ONE 2012, 7, e42465. [Google Scholar] [CrossRef]
- Hana, S.; Peterson, M.; McLaughlin, H.; Marshall, E.; Fabian, A.J.; McKissick, O.; Koszka, K.; Marsh, G.; Craft, M.; Xu, S.; et al. Highly efficient neuronal gene knockout in vivo by CRISPR-Cas9 via neonatal intracerebroventricular injection of AAV in mice. Gene Ther. 2021, 28, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, T.; Urbinati, F.; Perumbeti, A.; Jiang, G.; Zarzuela, A.; Chang, L.J.; Kohn, D.B.; Malik, P. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007, 14, 1298–1304. [Google Scholar] [CrossRef]
- Zhang, H.; Bamidele, N.; Liu, P.; Ojelabi, O.; Gao, X.D.; Rodriguez, T.; Cheng, H.; Kelly, K.; Watts, J.K.; Xie, J.; et al. Adenine Base Editing In Vivo with a Single Adeno-Associated Virus Vector. GEN Biotechnol. 2022, 1, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kim, V.N. A tale of non-canonical tails: Gene regulation by post-transcriptional RNA tailing. Nat. Rev. Mol. Cell Biol. 2020, 21, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Dastjerdi, A.; Newman, A.; Burgio, G. The Expanding Class 2 CRISPR Toolbox: Diversity, Applicability, and Targeting Drawbacks. BioDrugs 2019, 33, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Cong, L.; Yan, W.X.; Ran, F.A.; Zetsche, B.; Li, Y.; Kurabayashi, A.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Staphylococcus aureus Cas9. Cell 2015, 162, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, J.; Cheng, Z.; Amrani, N.; Liu, C.; Wang, K.; Ibraheim, R.; Edraki, A.; Huang, X. Structures of Neisseria meningitidis Cas9 Complexes in Catalytically Poised and Anti-CRISPR-Inhibited States. Mol. Cell 2019, 76, 938–952.e935. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Amrani, N.; Gao, X.D.; Liu, P.; Edraki, A.; Mir, A.; Ibraheim, R.; Gupta, A.; Sasaki, K.E.; Wu, T.; Donohoue, P.D.; et al. NmeCas9 is an intrinsically high-fidelity genome-editing platform. Genome Biol. 2018, 19, 214. [Google Scholar] [CrossRef]
- Bolukbasi, M.F.; Gupta, A.; Wolfe, S.A. Creating and evaluating accurate CRISPR-Cas9 scalpels for genomic surgery. Nat. Methods 2016, 13, 41–50. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Joung, J.K. Defining and improving the genome-wide specificities of CRISPR–Cas9 nucleases. Nat. Rev. Genet. 2016, 17, 300–312. [Google Scholar] [CrossRef]
- Tycko, J.; Myer, V.E.; Hsu, P.D. Methods for Optimizing CRISPR-Cas9 Genome Editing Specificity. Mol. Cell 2016, 63, 355–370. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, Y.; Gootenberg, J.S.; Hirano, H.; Ran, F.A.; Nakane, T.; Ishitani, R.; Zhang, F.; Nishimasu, H.; Nureki, O. Crystal Structure of the Minimal Cas9 from Campylobacter jejuni Reveals the Molecular Diversity in the CRISPR-Cas9 Systems. Mol. Cell 2017, 65, 1109–1121.e1103. [Google Scholar] [CrossRef]
- Kim, E.; Koo, T.; Park, S.W.; Kim, D.; Kim, K.; Cho, H.Y.; Song, D.W.; Lee, K.J.; Jung, M.H.; Kim, S.; et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017, 8, 14500. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Xie, W.; Song, Y.; Li, J.; Lai, L.; Li, Z. Versatile and efficient in vivo genome editing with compact Streptococcus pasteurianus Cas9. Mol. Ther. 2022, 30, 256–267. [Google Scholar] [CrossRef]
- Sun, H.; Fu, S.; Cui, S.; Yin, X.; Sun, X.; Qi, X.; Cui, K.; Wang, J.; Ma, L.; Liu, F.Y.; et al. Development of a CRISPR-SaCas9 system for projection- and function-specific gene editing in the rat brain. Sci. Adv. 2020, 6, eaay6687. [Google Scholar] [CrossRef]
- Edraki, A.; Mir, A.; Ibraheim, R.; Gainetdinov, I.; Yoon, Y.; Song, C.Q.; Cao, Y.; Gallant, J.; Xue, W.; Rivera-Pérez, J.A.; et al. A Compact, High-Accuracy Cas9 with a Dinucleotide PAM for In Vivo Genome Editing. Mol. Cell 2019, 73, 714–726.e714. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.H.; Ye, S.; Lim, S.; Jo, A.; Lee, H.; Hong, F.; Lee, S.E.; Oh, S.J.; Kim, N.R.; Kim, K.; et al. CRISPR-Cas9 Gene Editing Protects from the A53T-SNCA Overexpression-Induced Pathology of Parkinson’s Disease In Vivo. CRISPR J. 2022, 5, 95–108. [Google Scholar] [CrossRef]
- Ibraheim, R.; Tai, P.W.L.; Mir, A.; Javeed, N.; Wang, J.; Rodríguez, T.C.; Namkung, S.; Nelson, S.; Khokhar, E.S.; Mintzer, E.; et al. Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo. Nat. Commun. 2021, 12, 6267. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Koo, T.; Cho, C.S.; Kim, J.H.; Kim, J.S.; Kim, J.H. Long-Term Effects of In Vivo Genome Editing in the Mouse Retina Using Campylobacter jejuni Cas9 Expressed via Adeno-Associated Virus. Mol. Ther. 2019, 27, 130–136. [Google Scholar] [CrossRef]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Gupta, A.; Bednarski, C.; Gehrig-Giannini, S.; Richter, F.; Pitzler, C.; Gamalinda, M.; Galonska, C.; Takeuchi, R.; Wang, K.; et al. Improved CRISPR genome editing using small highly active and specific engineered RNA-guided nucleases. Nat. Commun. 2021, 12, 4219. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V.; et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef]
- Takeda, S.N.; Nakagawa, R.; Okazaki, S.; Hirano, H.; Kobayashi, K.; Kusakizako, T.; Nishizawa, T.; Yamashita, K.; Nishimasu, H.; Nureki, O. Structure of the miniature type V-F CRISPR-Cas effector enzyme. Mol. Cell 2021, 81, 558–570.e553. [Google Scholar] [CrossRef]
- Pausch, P.; Al-Shayeb, B.; Bisom-Rapp, E.; Tsuchida, C.A.; Li, Z.; Cress, B.F.; Knott, G.J.; Jacobsen, S.E.; Banfield, J.F.; Doudna, J.A. CRISPR-CasPhi from huge phages is a hypercompact genome editor. Science 2020, 369, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Yin, J.; Yuan, S.; Ou, L.; Liu, M.; Zhang, W.; Hu, J. Comprehensive assessment of miniature CRISPR-Cas12f nucleases for gene disruption. Nat. Commun. 2022, 13, 5623. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.; Li, J.; Patel, A.; Liu, S.; Xu, Q.; Hilton, I.B. Quantification of Genome Editing and Transcriptional Control Capabilities Reveals Hierarchies among Diverse CRISPR/Cas Systems in Human Cells. ACS Synth. Biol. 2022, 11, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Orlova, N.; Oakes, B.L.; Ma, E.; Spinner, H.B.; Baney, K.L.M.; Chuck, J.; Tan, D.; Knott, G.J.; Harrington, L.B.; et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature 2019, 566, 218–223. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Topkar, V.V.; Zheng, Z.; Joung, J.K. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 2015, 33, 1293–1298. [Google Scholar] [CrossRef]
- Nakagawa, R.; Ishiguro, S.; Okazaki, S.; Mori, H.; Tanaka, M.; Aburatani, H.; Yachie, N.; Nishimasu, H.; Nureki, O. Engineered Campylobacter jejuni Cas9 variant with enhanced activity and broader targeting range. Commun. Biol. 2022, 5, 211. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, C.; Wang, S.; Gao, S.; Wei, J.; Li, M.; Hou, L.; Mao, H.; Wei, Y.; Qi, T.; et al. Discovery and engineering of small SlugCas9 with broad targeting range and high specificity and activity. Nucleic Acids Re.s 2021, 49, 4008–4019. [Google Scholar] [CrossRef]
- Xu, X.; Chemparathy, A.; Zeng, L.; Kempton, H.R.; Shang, S.; Nakamura, M.; Qi, L.S. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol. Cell 2021, 81, 4333–4345.e4334. [Google Scholar] [CrossRef]
- Pausch, P.; Soczek, K.M.; Herbst, D.A.; Tsuchida, C.A.; Al-Shayeb, B.; Banfield, J.F.; Nogales, E.; Doudna, J.A. DNA interference states of the hypercompact CRISPR-CasPhi effector. Nat. Struct. Mol. Biol. 2021, 28, 652–661. [Google Scholar] [CrossRef]
- Tsuchida, C.A.; Zhang, S.; Doost, M.S.; Zhao, Y.; Wang, J.; O’Brien, E.; Fang, H.; Li, C.P.; Li, D.; Hai, Z.Y.; et al. Chimeric CRISPR-CasX enzymes and guide RNAs for improved genome editing activity. Mol. Cell 2022, 82, 1199–1209 e1196. [Google Scholar] [CrossRef]
- Saha, C.; Mohanraju, P.; Stubbs, A.; Dugar, G.; Hoogstrate, Y.; Kremers, G.J.; van Cappellen, W.A.; Horst-Kreft, D.; Laffeber, C.; Lebbink, J.H.G.; et al. Guide-free Cas9 from pathogenic Campylobacter jejuni bacteria causes severe damage to DNA. Sci. Adv. 2020, 6, eaaz4849. [Google Scholar] [CrossRef]
- Koo, T.; Lu-Nguyen, N.B.; Malerba, A.; Kim, E.; Kim, D.; Cappellari, O.; Cho, H.Y.; Dickson, G.; Popplewell, L.; Kim, J.S. Functional Rescue of Dystrophin Deficiency in Mice Caused by Frameshift Mutations Using Campylobacter jejuni Cas9. Mol. Ther. 2018, 26, 1529–1538. [Google Scholar] [CrossRef]
- Hanson, B.; Wood, M.J.A.; Roberts, T.C. Molecular correction of Duchenne muscular dystrophy by splice modulation and gene editing. RNA Biol. 2021, 18, 1048–1062. [Google Scholar] [CrossRef]
- Nelson, C.E.; Wu, Y.; Gemberling, M.P.; Oliver, M.L.; Waller, M.A.; Bohning, J.D.; Robinson-Hamm, J.N.; Bulaklak, K.; Castellanos Rivera, R.M.; Collier, J.H.; et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 427–432. [Google Scholar] [CrossRef]
- Min, Y.L.; Li, H.; Rodriguez-Caycedo, C.; Mireault, A.A.; Huang, J.; Shelton, J.M.; McAnally, J.R.; Amoasii, L.; Mammen, P.P.A.; Bassel-Duby, R.; et al. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci. Adv. 2019, 5, eaav4324. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, J.; Liu, Y.; Jin, X.; Zhong, X.; Mo, L.; Wang, Q.; Deng, H.; Yang, Y. In vivo PCSK9 gene editing using an all-in-one self-cleavage AAV-CRISPR system. Mol. Ther. Methods Clin. Dev. 2021, 20, 652–659. [Google Scholar] [CrossRef]
- Kemaladewi, D.U.; Bassi, P.S.; Erwood, S.; Al-Basha, D.; Gawlik, K.I.; Lindsay, K.; Hyatt, E.; Kember, R.; Place, K.M.; Marks, R.M.; et al. A mutation-independent approach for muscular dystrophy via upregulation of a modifier gene. Nature 2019, 572, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Zhou, Q.; Gao, Q.; Li, J.; Huang, W.; Liu, Y. Multiplexed promoterless gene expression with CRISPReader. Genome Biol. 2019, 20, 113. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Cai, Z. Synthesizing AND gate minigene circuits based on CRISPReader for identification of bladder cancer cells. Nat. Commun. 2020, 11, 5486. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R.; Wang, X.; Witte, I.P.; Huang, T.P.; Levy, J.M.; Raguram, A.; Banskota, S.; Seidah, N.G.; Musunuru, K.; Liu, D.R. Efficient in vivo base editing via single adeno-associated viruses with size-optimized genomes encoding compact adenine base editors. Nat. Biomed. Eng. 2022, 6, 1272–1283. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther. Nucl. Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Piao, X.; Meng, D.; Zhang, X.; Song, Q.; Lv, H.; Jia, Y. Dual-gRNA approach with limited off-target effect corrects C9ORF72 repeat expansion in vivo. Sci. Rep. 2022, 12, 5672. [Google Scholar] [CrossRef]
- Alipanahi, R.; Safari, L.; Khanteymoori, A. CRISPR genome editing using computational approaches: A survey. Front. Bioinf. 2022, 2, 1001131. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, J.; Kim, J.-S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3, expanding the CRISPR web toolbox beyond genome editing. Nucl. Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef] [PubMed]
- Cradick, T.J.; Qiu, P.; Lee, C.M.; Fine, E.J.; Bao, G. COSMID: A Web-based Tool for Identifying and Validating CRISPR/Cas Off-target Sites. Mol. Ther. Nucleic Acids 2014, 3, e214. [Google Scholar] [CrossRef]
- Li, B.; Chen, P.B.; Diao, Y. CRISPR-SE: A brute force search engine for CRISPR design. NAR Genom. Bioinform. 2021, 3, lqab013. [Google Scholar] [CrossRef]
- Chuai, G.; Ma, H.; Yan, J.; Chen, M.; Hong, N.; Xue, D.; Zhou, C.; Zhu, C.; Chen, K.; Duan, B.; et al. DeepCRISPR: Optimized CRISPR guide RNA design by deep learning. Genome Biol. 2018, 19, 80. [Google Scholar] [CrossRef]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef]
- Atkins, A.; Chung, C.-H.; Allen, A.G.; Dampier, W.; Gurrola, T.E.; Sariyer, I.K.; Nonnemacher, M.R.; Wigdahl, B. Off-Target Analysis in Gene Editing and Applications for Clinical Translation of CRISPR/Cas9 in HIV-1 Therapy. Front. Genome Ed. 2021, 3, 673022. [Google Scholar] [CrossRef]
- Cameron, P.; Fuller, C.K.; Donohoue, P.D.; Jones, B.N.; Thompson, M.S.; Carter, M.M.; Gradia, S.; Vidal, B.; Garner, E.; Slorach, E.M.; et al. Mapping the genomic landscape of CRISPR–Cas9 cleavage. Nat. Methods 2017, 14, 600–606. [Google Scholar] [CrossRef]
- Giannoukos, G.; Ciulla, D.M.; Marco, E.; Abdulkerim, H.S.; Barrera, L.A.; Bothmer, A.; Dhanapal, V.; Gloskowski, S.W.; Jayaram, H.; Maeder, M.L.; et al. UDiTaS™, a genome editing detection method for indels and genome rearrangements. BMC Genom. 2018, 19, 212. [Google Scholar] [CrossRef]
- Pawluk, A.; Davidson, A.R.; Maxwell, K.L. Anti-CRISPR: Discovery, mechanism and function. Nat. Rev. Microbiol. 2018, 16, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Pu, Q.; Wu, Q.; Zhou, C.; Wang, B.; Schettler, J.; Wang, Z.; Qin, S.; Gao, P.; Li, R.; et al. High-throughput screen reveals sRNAs regulating crRNA biogenesis by targeting CRISPR leader to repress Rho termination. Nat. Commun. 2019, 10, 3728. [Google Scholar] [CrossRef] [PubMed]
- Workman, R.E.; Pammi, T.; Nguyen, B.T.K.; Graeff, L.W.; Smith, E.; Sebald, S.M.; Stoltzfus, M.J.; Euler, C.W.; Modell, J.W. A natural single-guide RNA repurposes Cas9 to autoregulate CRISPR-Cas expression. Cell 2021, 184, 675–688 e619. [Google Scholar] [CrossRef]
- Shivram, H.; Cress, B.F.; Knott, G.J.; Doudna, J.A. Controlling and enhancing CRISPR systems. Nat. Chem. Biol. 2021, 17, 10–19. [Google Scholar] [CrossRef]
- Klamroth, R.; Hayes, G.; Andreeva, T.; Gregg, K.; Suzuki, T.; Mitha, I.H.; Hardesty, B.; Shima, M.; Pollock, T.; Slev, P.; et al. Global Seroprevalence of Pre-existing Immunity Against AAV5 and Other AAV Serotypes in People with Hemophilia A. Hum. Gene Ther. 2022, 33, 432–441. [Google Scholar] [CrossRef]
- Vandamme, C.; Adjali, O.; Mingozzi, F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum. Gene Ther. 2017, 28, 1061–1074. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Investig. 2009, 119, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Kurupati, R.K.; Li, Y.; Kuranda, K.; Zhou, X.; Mingozzi, F.; High, K.A.; Ertl, H.C.J. The Effect of CpG Sequences on Capsid-Specific CD8(+) T Cell Responses to AAV Vector Gene Transfer. Mol. Ther. 2020, 28, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, X.; Lin, J.; Ou, L. A versatile toolkit for overcoming AAV immunity. Front. Immunol. 2022, 13, 991832. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Shelke, R.; Guan, S.; Somanathan, S. Higher Seroprevalence of Anti-Adeno-Associated Viral Vector Neutralizing Antibodies Among Racial Minorities in the United States. Hum. Gene Ther. 2022, 33, 442–450. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Chang, R.Y.K.; Chan, H.K. Inhalation delivery technology for genome-editing of respiratory diseases. Adv. Drug Deliv. Rev. 2021, 168, 217–228. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jaffar Ali, D.; Xu, H.; Kumaravel, S.; Si, K.; Li, Y.; Sun, B.; Ma, J.; Xiao, Z. Epithelial cell -derived microvesicles: A safe delivery platform of CRISPR/Cas9 conferring synergistic anti-tumor effect with sorafenib. Exp. Cell Res. 2020, 392, 112040. [Google Scholar] [CrossRef]
- Ferdosi, S.R.; Ewaisha, R.; Moghadam, F.; Krishna, S.; Park, J.G.; Ebrahimkhani, M.R.; Kiani, S.; Anderson, K.S. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat. Commun. 2019, 10, 1842. [Google Scholar] [CrossRef]
- Wassmer, S.J.; Carvalho, L.S.; György, B.; Vandenberghe, L.H.; Maguire, C.A. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep. 2017, 7, 45329. [Google Scholar] [CrossRef]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef]
- Orefice, N.S.; Souchet, B.; Braudeau, J.; Alves, S.; Piguet, F.; Collaud, F.; Ronzitti, G.; Tada, S.; Hantraye, P.; Mingozzi, F.; et al. Real-Time Monitoring of Exosome Enveloped-AAV Spreading by Endomicroscopy Approach: A New Tool for Gene Delivery in the Brain. Mol. Ther. Methods Clin. Dev. 2019, 14, 237–251. [Google Scholar] [CrossRef]
- Mietzsch, M.; Hull, J.A.; Makal, V.E.; Jimenez Ybargollin, A.; Yu, J.C.; McKissock, K.; Bennett, A.; Penzes, J.; Lins-Austin, B.; Yu, Q.; et al. Characterization of the Serpentine Adeno-Associated Virus (SAAV) Capsid Structure: Receptor Interactions and Antigenicity. J. Virol. 2022, 96, e0033522. [Google Scholar] [CrossRef]
- Goedeker, N.L.; Dharia, S.D.; Griffin, D.A.; Coy, J.; Truesdale, T.; Parikh, R.; Whitehouse, K.; Santra, S.; Asher, D.R.; Zaidman, C.M. Evaluation of rAAVrh74 gene therapy vector seroprevalence by measurement of total binding antibodies in patients with Duchenne muscular dystrophy. Ther. Adv. Neurol. Dis. 2023, 16, 17562864221149781. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, A.T.; Chan, K.Y.; Sorensen, H.; Barry, A.J.; Azari, B.; Beddow, T.; Zheng, Q.; Zhao, B.; Tobey, I.G.; et al. Targeting AAV vectors to the CNS via de novo engineered capsid-receptor interactions. bioRxiv 2022. [Google Scholar] [CrossRef]
- Brimble, M.A.; Cheng, P.H.; Winston, S.M.; Reeves, I.L.; Souquette, A.; Spence, Y.; Zhou, J.; Wang, Y.D.; Morton, C.L.; Valentine, M.; et al. Preventing packaging of translatable P5-associated DNA contaminants in recombinant AAV vector preps. Mol. Ther. Methods Clin. Dev. 2022, 24, 280–291. [Google Scholar] [CrossRef]
- Faust, S.M.; Bell, P.; Cutler, B.J.; Ashley, S.N.; Zhu, Y.; Rabinowitz, J.E.; Wilson, J.M. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Investig. 2013, 123, 2994–3001. [Google Scholar] [CrossRef]
- Elangkovan, N.; Dickson, G. Gene Therapy for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, S303–S316. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.F. Codon Modification and PAMPs in Clinical AAV Vectors: The Tortoise or the Hare? Mol. Ther. 2020, 28, 701–703. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Ann. Rev. Immun. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Limon, J.J.; So, L.; Jellbauer, S.; Chiu, H.; Corado, J.; Sykes, S.M.; Raffatellu, M.; Fruman, D.A. mTOR kinase inhibitors promote antibody class switching via mTORC2 inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, E5076–E5085. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Yang, Y. The role of natural regulatory T cells in infection. Immun. Res. 2011, 49, 124–134. [Google Scholar] [CrossRef]
- Velazquez, V.M.; Meadows, A.S.; Pineda, R.J.; Camboni, M.; McCarty, D.M.; Fu, H. Effective Depletion of Pre-existing Anti-AAV Antibodies Requires Broad Immune Targeting. Mol. Ther. Methods Clin. Dev. 2017, 4, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Kuranda, K.; Quinn, W.; Chekaoui, A.; Ambrose, R.; Hasanpourghai, M.; Novikov, M.; Newman, D.; Cole, C.; Zhou, X.; et al. The Effect of Rapamycin and Ibrutinib on Antibody Responses to Adeno-Associated Virus Vector-Mediated Gene Transfer. Hum. Gene Ther. 2022, 33, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Sands, E.; Kivitz, A.; DeHaan, W.; Leung, S.S.; Johnston, L.; Kishimoto, T.K. Tolerogenic nanoparticles mitigate the formation of anti-drug antibodies against pegylated uricase in patients with hyperuricemia. Nat. Commun. 2022, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Ragni, M.V.; Rasko, J.E.J.; Raffini, L.J.; Samelson-Jones, B.J.; Ozelo, M.; Hazbon, M.; Runowski, A.R.; Wellman, J.A.; Wachtel, K.; et al. Long-Term Follow-Up of the First in Human Intravascular Delivery of AAV for Gene Transfer: AAV2-hFIX16 for Severe Hemophilia B. Mol. Ther. 2020, 28, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic | Specificity | Trial Benchmarks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease 1 | Gene (Name) | Protein Function and Therapeutic Rationale | Mechanism/Approach | Delivery | Serotype | Promoter 2 | Cumulative Duration | Most Advanced Phase | Sponsor | Trial ID 3 |
| AD | APOE2 (LX1001) | Apolipoprotein E ε2 is an isoform of APOE that is reduced in neurons in AD and confers protection. | EGE/AE | Cisterna magna | AAVrh.10h | CAG | 2019–2023 | I | Lexeo Therapeutics | NCT05400330 |
| hTERT (AAV-hTERT) | Human telomerase reverse transcriptase elongates chromosomal ends in dividing cells. hTERT activity is reduced in neurons in AD. | EGE/AE | Intravenous and intrathecal | unknown | unknown | 2019–2021 | unknown | Libella Gene Therapeutics | NCT04133649 | |
| NGF (CERE-110) | Nerve growth factor promotes the growth and development of sympathetic and parasympathetic neurons. NGF activity is reduced in neurons in AD. | EGE/AE | Nucleus basalis of Meynert | AAV2 | CAG | 2004–2015 | II | Sangamo | NCT00876863 | |
| BDNF (AAV2-BDNF) | Brain-derived neurotrophic factor promotes the development and maintenance of neuronal populations. BDNF activity is reduced in AD neurons. | EGE/AE | Intraparenchymal | AAV2 | unknown | 2021–2025 | Recruiting | Mark Tuszynski | NCT05040217 | |
| PD | AADC (AAV- hAADC-2) | Aromatic L-amino acid decarboxylase contributes to the biosynthesis of dopamine. There is a progressive loss of dopaminergic neurons expressing AADC in PD. | EGE/AE | Putamen | AAV2 | CMV | 2013–2022 | II | Voyager Ther, Neurocr. Biosci | NCT00229736 |
| GAD (AAV-GAD) | Glutamic acid decarboxylase plays a role in the biosynthesis of GABA. Progressive loss of GABAergic neurons expressing GAD reduces motor inhibition in PD. | EGE/AE | Subthalamic nucleus | AAV2 | CBA | 2003–2005 | I | Neurologix | NCT05603312 | |
| GDNF (AAV2-GDNF) | Glial cell-derived neurotrophic factor promotes the survival of neurons. There is a progressive loss of nigrostriatal dopaminergic neurons expressing GDNF in PD. | EGE/AE | Putamen | AAV2 | unknown | 2013–2022 | I | Ask Bio, NINDS | NCT04167540 | |
| NRTN (CERE-120) | Neurturin is a neurotrophic factor that promotes the survival of neuronal populations. There is a progressive loss of dopaminergic neurons expressing NRTN in PD. | EGE/AE | Putamen | AAV2 | CAG | 2005–2017 | II | Sangamo | NCT00252850 | |
| GBA1 (LY3884961) | Glucocerebrosidase plays a role in the biosynthesis of glucose and ceramide. Aberrant mutations in GBA1 in dopaminergic neurons occur in PD. | EGE/AE | Cisterna magna | AAV9 | CBA | 2020–2027 | I/II | Prevail Therapeutics | NCT04127578 | |
| FTD | GRN (PBFT02) | Granulin promotes the growth, survival, and maintenance of neuronal and microglial populations. GRN activity is reduced in neurons in FTD. | EGE/AE | Cisterna magna | AAV1, AAV9 | unknown | 2020–2027 | I/II | Passage Bio, Prevail Therapeutics | NCT04747431 |
| HD | HTT (rAAV5-miHTT) | Huntington is a cytosolic protein that regulates neuronal and glial function. There are aberrant ≥36 CAG repeats in the HTT gene in HD. | EGR using miRNA/AE | Intrastriatal | AAV5 | unknown | 2019–2026 | I/II | UniQure Biopharma | NCT04120493 |
| SMA | SMN (AVXS-101) | Survival motor neuron is a neurotrophic factor that promotes the maintenance of motor neurons. SMN activity is reduced in motor neurons in SMA. | EGE/AE | Intravenous and intrathecal | AAV9 | CBA | 2014–2035 | IV | Novartis Gene Therapy | NCT02122952 |
| Objective | Method 1 | Payload | Target | Strengths | Weaknesses | |
|---|---|---|---|---|---|---|
| Altering gene expression | Augmentation | Transgene expression | Functional gene controlled by the Pol II promoter | Heterologous gene | Low immunogenicity | Expression based on non-natural promoter |
| CRISPRa | dCas fused to a transcriptional activator (e.g., VP64), with the Pol III promoter driving sgRNA expression | DNA sequence defined by protospacer (~17–24 bases) and PAM (~2–6 bases) | Adaptable and efficient | Large size, off-target sites, immunogenicity | ||
| Suppression | ZFP | Array of zinc finger DNA binding domains | DNA promoter sequence defined by base triplets (typically 18–33 bases) | Small size, low immunogenicity | Complex selection and optimization | |
| TALE | Array of TALE DNA binding domains | DNA promoter sequence defined by base singlets | Easily sequence adaptable | Larger size and heightened immunogenicity relative to ZFPs | ||
| CRISPRi | dCas fused to transcriptional repressors (e.g., ZIM3) and sgRNA | DNA promoter sequence defined by protospacer and PAM | Adaptable, efficient | Larger size and heightened immunogenicity relative to ZFPs | ||
| RNAi | shRNA expression from the Pol II or III promoter | mRNA transcript defined by shRNA sequence | Adaptable, few off-target sites | Can compete with natural miRNA processing | ||
| Gene editing | Knockout | ZFN | ZFPs fused to an endonuclease (e.g., Fok1) | DNA gene sequence defined by base triplets | Low immunogenicity | Low efficiency, non-uniform, knockout |
| TALEN | TALE fused to an endonuclease (e.g., Fok1) | DNA gene sequence defined by base singlets | Easily sequence adaptable | Larger size and heightened immunogenicity relative to ZFPs | ||
| CRISPR-Cas via NHEJ/MMEJ | Cas nuclease or nCas nickase and sgRNA(s) | DNA gene sequence defined by protospacer and PAM | Adaptable and efficient | Large size, off-target sites, immunogenicity | ||
| Directed mutagenesis | CRISPR-Cas via HDR | Cas nickase (nCas), plus sgRNA(s) and repair template | DNA gene sequence defined by protospacer and PAM | Accurate large insertions | Large size, off-target sites, non-uniform insertion | |
| BE | Inactive Cas (dCas) fused to base editor and sgRNA | DNA gene base defined by protospacer and PAM | Few off-target sites, generates precise edits | Large size, low efficiency, only single base edits | ||
| PE | Cas nickases (nCas) fused to reverse transcriptase (e.g., M-MLV) and pegRNA and sgRNA | DNA gene sequence defined by protospacer and PAM | Few off-target sites, generates precise edits | Large size, low efficiency, only for small edits |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Vasan, L.; Kartono, B.; Clifford, K.; Attarpour, A.; Sharma, R.; Mandrozos, M.; Kim, A.; Zhao, W.; Belotserkovsky, A.; et al. Advances in Recombinant Adeno-Associated Virus Vectors for Neurodegenerative Diseases. Biomedicines 2023, 11, 2725. https://doi.org/10.3390/biomedicines11102725
Li L, Vasan L, Kartono B, Clifford K, Attarpour A, Sharma R, Mandrozos M, Kim A, Zhao W, Belotserkovsky A, et al. Advances in Recombinant Adeno-Associated Virus Vectors for Neurodegenerative Diseases. Biomedicines. 2023; 11(10):2725. https://doi.org/10.3390/biomedicines11102725
Chicago/Turabian StyleLi, Leyao, Lakshmy Vasan, Bryan Kartono, Kevan Clifford, Ahmadreza Attarpour, Raghav Sharma, Matthew Mandrozos, Ain Kim, Wenda Zhao, Ari Belotserkovsky, and et al. 2023. "Advances in Recombinant Adeno-Associated Virus Vectors for Neurodegenerative Diseases" Biomedicines 11, no. 10: 2725. https://doi.org/10.3390/biomedicines11102725
APA StyleLi, L., Vasan, L., Kartono, B., Clifford, K., Attarpour, A., Sharma, R., Mandrozos, M., Kim, A., Zhao, W., Belotserkovsky, A., Verkuyl, C., & Schmitt-Ulms, G. (2023). Advances in Recombinant Adeno-Associated Virus Vectors for Neurodegenerative Diseases. Biomedicines, 11(10), 2725. https://doi.org/10.3390/biomedicines11102725






