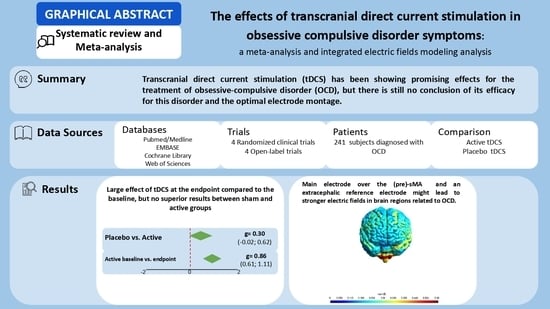
The Effects of Transcranial Direct Current Stimulation in Obsessive–Compulsive Disorder Symptoms: A Meta-Analysis and Integrated Electric Fields Modeling Analysis
Abstract
1. Background
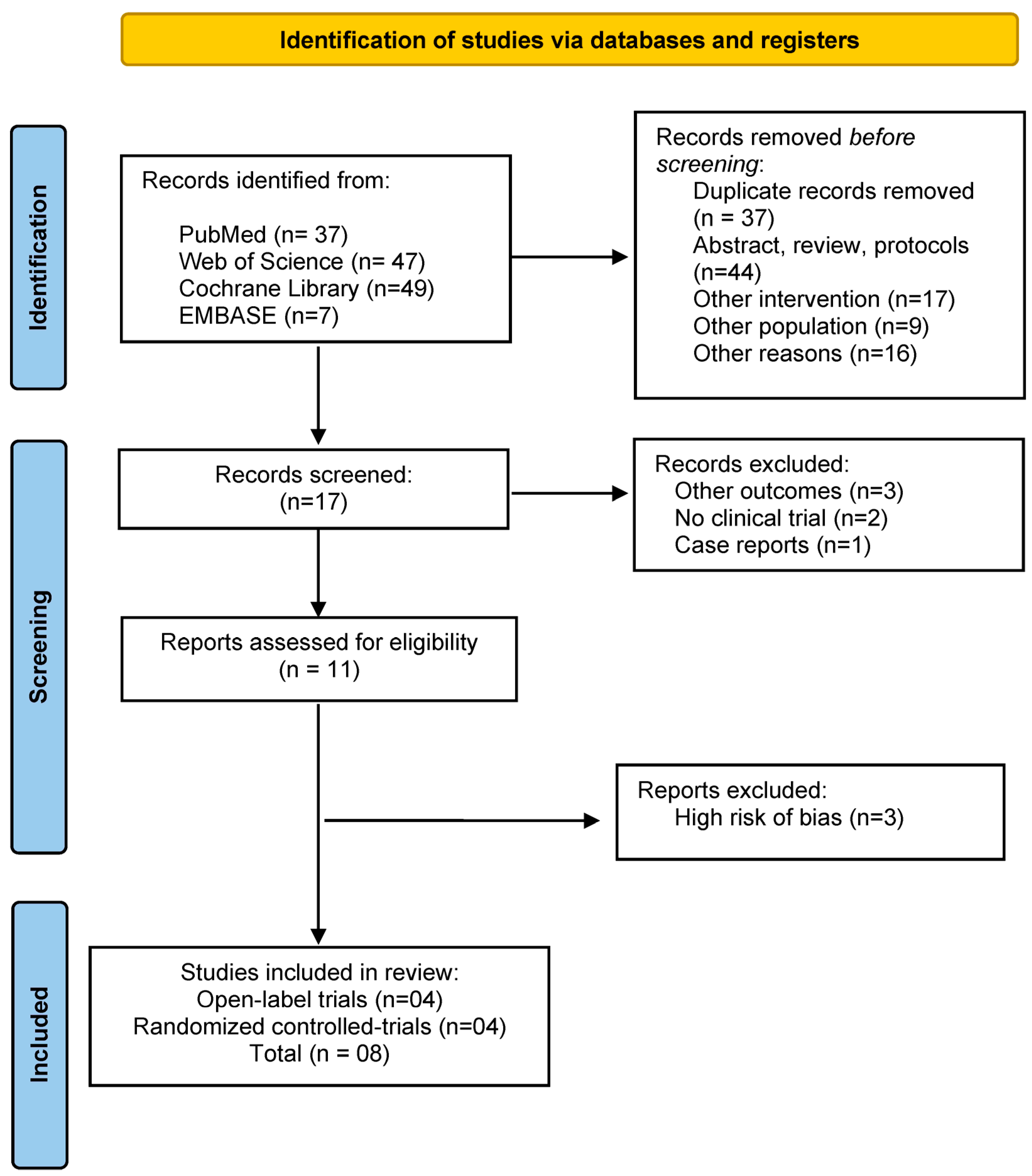
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Quality Assessment
2.3. Data Extraction
2.4. Outcomes
2.5. Electric Field Modeling
2.6. Data Analysis
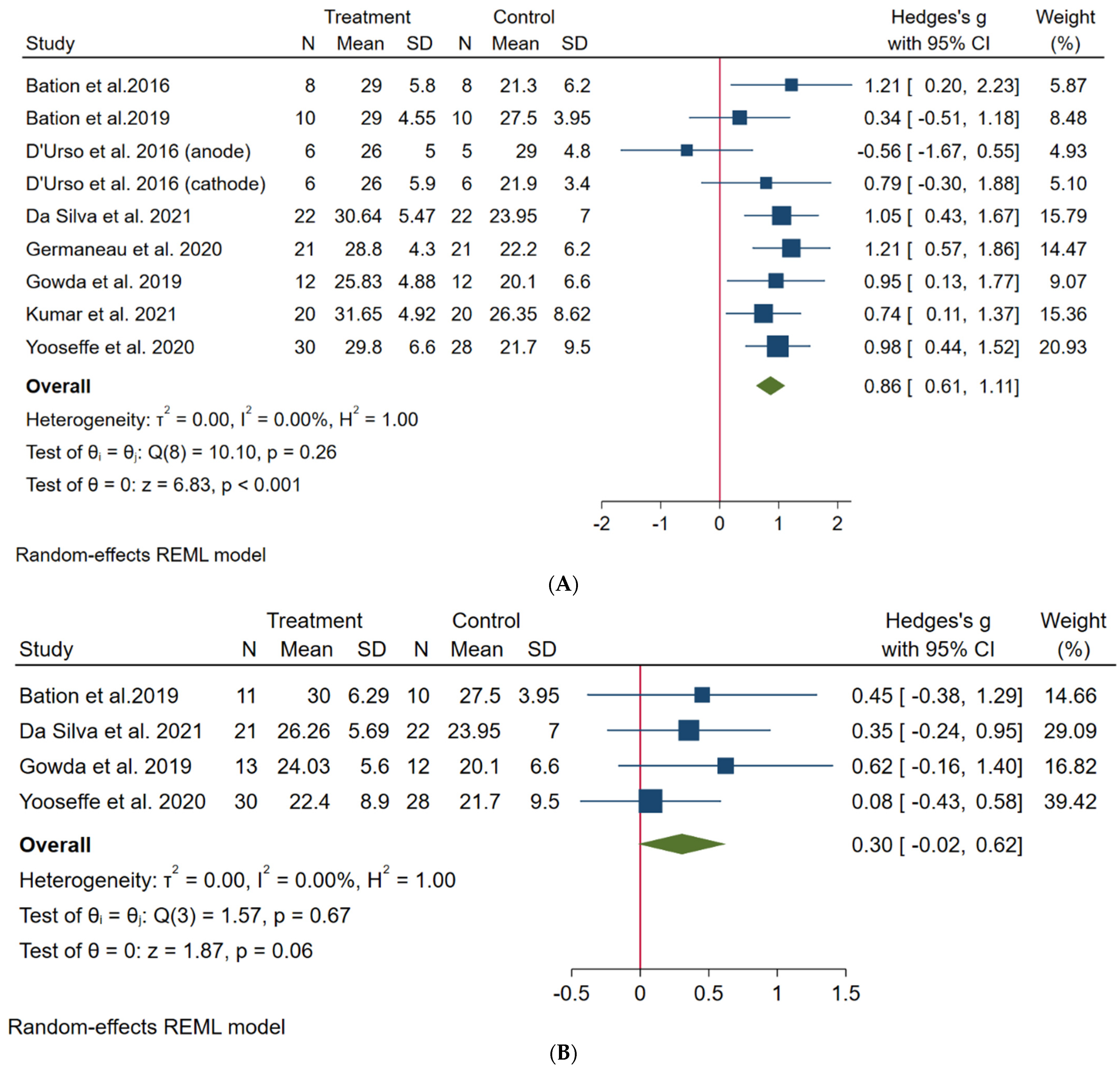
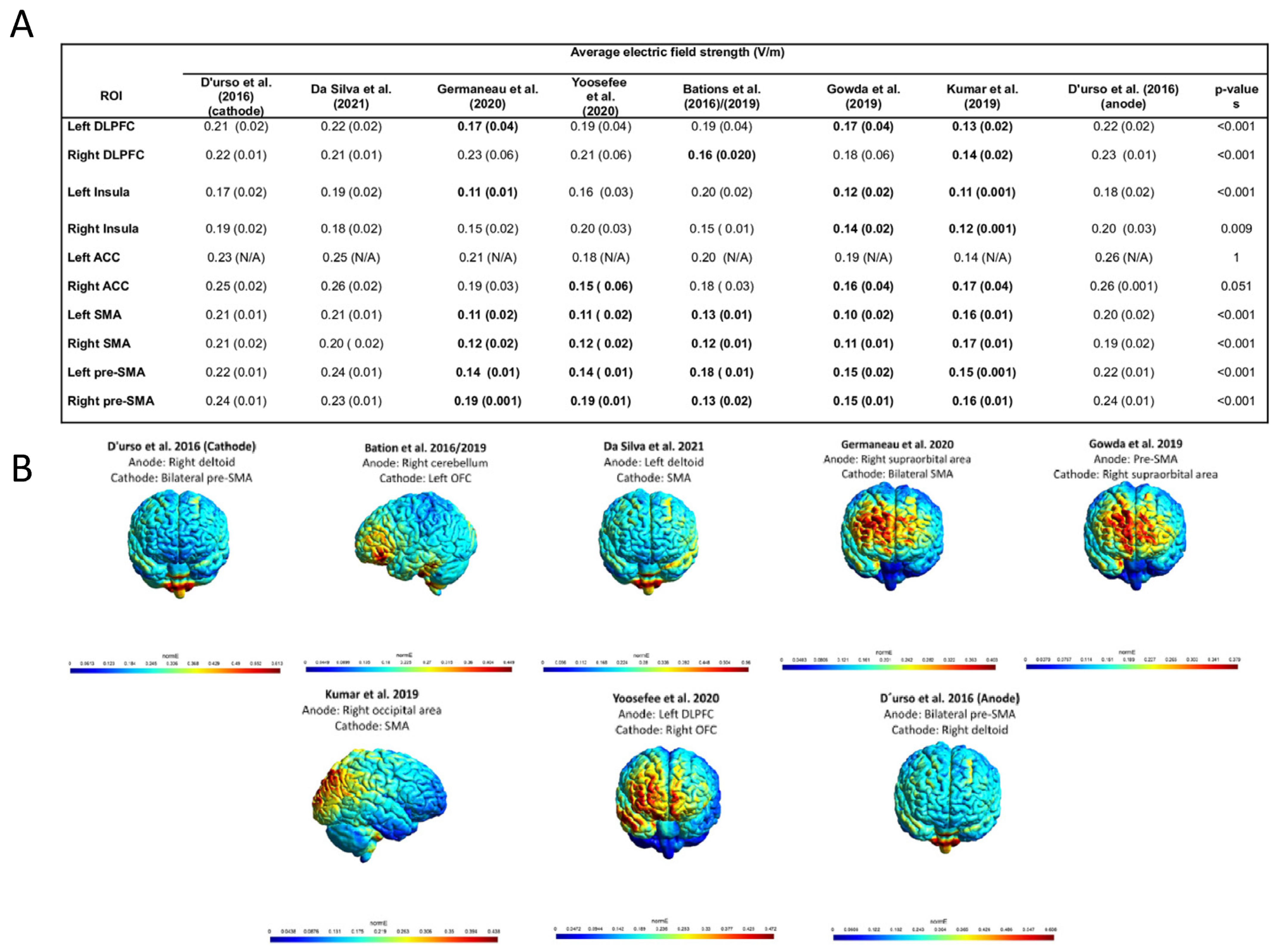
3. Results
3.1. Primary Outcome
3.2. Meta-Regression Analysis
3.3. Electric Field Modeling Analysis
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brock, H.; Hany, M. Obsessive-compulsive disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021; NBK553162. [Google Scholar]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive-Compulsive Disorder. Nat. Rev. Dis. Prim. 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan-Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the Human Ventromedial Prefrontal Cortex Support Fear Learning, Fear Extinction or Both? A Commentary on Subregional Contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Bloch, M.H.; Mathews, C.A. Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. JAMA 2017, 317, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Denys, D. Pharmacotherapy of Obsessive-Compulsive Disorder and Obsessive-Compulsive Spectrum Disorders. Psychiatr. Clin. N. Am. 2006, 29, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Denys, D.; Mantione, M.; Figee, M.; van den Munckhof, P.; Koerselman, F.; Westenberg, H.; Bosch, A.; Schuurman, R. Deep Brain Stimulation of the Nucleus Accumbens for Treatment-Refractory Obsessive-Compulsive Disorder. Arch. Gen. Psychiatry 2010, 67, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Bloch, M.H. Pharmacological Treatment of Obsessive-Compulsive Disorder. Psychiatr. Clin. N. Am. 2014, 37, 375–391. [Google Scholar] [CrossRef]
- Kammen, A.; Cavaleri, J.; Lam, J.; Frank, A.C.; Mason, X.; Choi, W.; Penn, M.; Brasfield, K.; Van Noppen, B.; Murray, S.B.; et al. Neuromodulation of OCD: A Review of Invasive and Non-Invasive Methods. Front. Neurol. 2022, 13, 909264. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories Are Not Written in Stone: Re-Writing Fear Memories by Means of Non-Invasive Brain Stimulation and Optogenetic Manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of Non-Invasive Brain Stimulation on Cognitive Functioning in Brain Disorders: A Meta-Analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Klimke, A.; Nitsche, M.A.; Maurer, K.; Voss, U. Case Report: Successful Treatment of Therapy-Resistant OCD With Application of Transcranial Alternating Current Stimulation (tACS). Brain Stimul. 2016, 9, 463–465. [Google Scholar] [CrossRef]
- Bergfeld, I.O.; Dijkstra, E.; Graat, I.; de Koning, P.; van den Boom, B.J.G.; Arbab, T.; Vulink, N.; Denys, D.; Willuhn, I.; Mocking, R.J.T. Invasive and Non-Invasive Neurostimulation for OCD. Curr. Top. Behav. Neurosci. 2021, 49, 399–436. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Shiozawa, P.; Cook, I.A.; Sato, I.A.; Kaku, C.B.; Guimarães, F.B.; Sachdev, P.; Sarkhel, S.; Cordeiro, Q. Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: An Updated Systematic Review and Meta-Analysis. J. ECT 2016, 32, 262–266. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala-Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr. Biol. 2020, 30, 3672–3679.e4. [Google Scholar] [CrossRef]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive Human Brain Stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial Direct Current Stimulation: State of the Art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Harika-Germaneau, G.; Heit, D.; Chatard, A.; Thirioux, B.; Langbour, N.; Jaafari, N. Treating Refractory Obsessive–compulsive Disorder with Transcranial Direct Current Stimulation: An Open Label Study. Brain Behav. 2020, 10, e01648. [Google Scholar] [CrossRef]
- Bation, R.; Mondino, M.; Le Camus, F.; Saoud, M.; Brunelin, J. Transcranial Direct Current Stimulation in Patients with Obsessive Compulsive Disorder: A Randomized Controlled Trial. Eur. Psychiatry 2019, 62, 38–44. [Google Scholar] [CrossRef]
- Bation, R.; Poulet, E.; Haesebaert, F.; Saoud, M.; Brunelin, J. Transcranial Direct Current Stimulation in Treatment-Resistant Obsessive-Compulsive Disorder: An Open-Label Pilot Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 153–157. [Google Scholar] [CrossRef]
- Silva, R.M.F.; Brunoni, A.R.; Goerigk, S.; Batistuzzo, M.C.; Costa, D.L.C.; Diniz, J.B.; Padberg, F.; D’Urso, G.; Miguel, E.C.; Shavitt, R.G. Efficacy and Safety of Transcranial Direct Current Stimulation as an Add-on Treatment for Obsessive-Compulsive Disorder: A Randomized, Sham-Controlled Trial. Neuropsychopharmacology 2021, 46, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Brunoni, A.R.; Mazzaferro, M.P.; Anastasia, A.; de Bartolomeis, A.; Mantovani, A. Transcranial Direct Current Stimulation for Obsessive-Compulsive Disorder: A Randomized, Controlled, Partial Crossover Trial. Depress. Anxiety 2016, 33, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Yoosefee, S.; Amanat, M.; Salehi, M.; Mousavi, S.V.; Behzadmanesh, J.; Safary, V.; Yoonesi, A.; Salehi, B. The Safety and Efficacy of Transcranial Direct Current Stimulation as Add-on Therapy to Fluoxetine in Obsessive-Compulsive Disorder: A Randomized, Double-Blind, Sham-Controlled, Clinical Trial. BMC Psychiatry 2020, 20, 570. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.M.; Narayanaswamy, J.C.; Hazari, N.; Bose, A.; Chhabra, H.; Balachander, S.; Bhaskarapillai, B.; Shivakumar, V.; Venkatasubramanian, G.; Reddy, Y.C.J. Efficacy of Pre-Supplementary Motor Area Transcranial Direct Current Stimulation for Treatment Resistant Obsessive Compulsive Disorder: A Randomized, Double Blinded, Sham Controlled Trial. Brain Stimul. 2019, 12, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, N.; Verma, R. Safety and Efficacy of Adjunctive Transcranial Direct Current Stimulation in Treatment-Resistant Obsessive-Compulsive Disorder: An Open-Label Trial. Indian J. Psychiatry 2019, 61, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Najafi, K.; Fakour, Y.; Zarrabi, H.; Heidarzadeh, A.; Khalkhali, M.; Yeganeh, T.; Farahi, H.; Rostamkhani, M.; Najafi, T.; Shabafroz, S.; et al. Efficacy of Transcranial Direct Current Stimulation in the Treatment: Resistant Patients Who Suffer from Severe Obsessive-Compulsive Disorder. Indian J. Psychol. Med. 2017, 39, 573–578. [Google Scholar] [CrossRef]
- Acevedo, N.; Bosanac, P.; Pikoos, T.; Rossell, S.; Castle, D. Therapeutic Neurostimulation in Obsessive-Compulsive and Related Disorders: A Systematic Review. Brain Sci. 2021, 11, 948. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Akbari, S.; Hassani-Abharian, P.; Tajeri, B. The Effect of Transcranial Direct Current Stimulation (tDCS) on Cerebellum in Reduction of the Symptoms of Obsessive-Compulsive Disorder. Neurocase 2022, 28, 135–139. [Google Scholar] [CrossRef]
- Shafiezadeh, S.; Eshghi, M.; Dokhaei, Z.; Mohajeri, H.; MohammadShirazi, A.; Mirsadeghi, S.; Hasani Abharian, P. Effect of Transcranial Direct Current Stimulation on Dorsolateral Prefrontal Cortex to Reduce the Symptoms of the Obsessive-Compulsive Disorder. Basic Clin. Neurosci. 2021, 12, 675–680. [Google Scholar] [CrossRef]
- Ekeu-Wei, I.T.; Blackburn, G.A.; Giovannettone, J. Accounting for the Effects of Climate Variability in Regional Flood Frequency Estimates in Western Nigeria. J. Water Resour. Prot. 2020, 12, 690–713. [Google Scholar] [CrossRef]
- Li, T.; Higgins, J.P.T.; Deeks, J.J. Collecting data. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 109–141. [Google Scholar] [CrossRef]
- Saturnino, G.B.; Madsen, K.H.; Thielscher, A. Electric Field Simulations for Transcranial Brain Stimulation Using FEM: An Efficient Implementation and Error Analysis. J. Neural Eng. 2019, 16, 066032. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Correction: Measurements and Models of Electric Fields in the In Vivo Human Brain during Transcranial Electric Stimulation. eLife 2018, 7, e35178. [Google Scholar] [CrossRef]
- Nielsen, J.D.; Madsen, K.H.; Puonti, O.; Siebner, H.R.; Bauer, C.; Madsen, C.G.; Saturnino, G.B.; Thielscher, A. Automatic Skull Segmentation from MR Images for Realistic Volume Conductor Models of the Head: Assessment of the State-of-the-Art. Neuroimage 2018, 174, 587–598. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef]
- Shephard, E.; Stern, E.R.; van den Heuvel, O.A.; Costa, D.L.C.; Batistuzzo, M.C.; Godoy, P.B.G.; Lopes, A.C.; Brunoni, A.R.; Hoexter, M.Q.; Shavitt, R.G.; et al. Toward a Neurocircuit-Based Taxonomy to Guide Treatment of Obsessive-Compulsive Disorder. Mol. Psychiatry 2021, 26, 4583–4604. [Google Scholar] [CrossRef]
- Melsen, W.G.; Bootsma, M.C.J.; Rovers, M.M.; Bonten, M.J.M. The Effects of Clinical and Statistical Heterogeneity on the Predictive Values of Results from Meta-Analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The Heterogeneity Statistic I(2) Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Senço, N.M.; Huang, Y.; D’Urso, G.; Parra, L.C.; Bikson, M.; Mantovani, A.; Shavitt, R.G.; Hoexter, M.Q.; Miguel, E.C.; Brunoni, A.R. Transcranial Direct Current Stimulation in Obsessive-Compulsive Disorder: Emerging Clinical Evidence and Considerations for Optimal Montage of Electrodes. Expert Rev. Med. Devices 2015, 12, 381–391. [Google Scholar] [CrossRef]
- Da Silva, R.d.M.F.; Batistuzzo, M.C.; Shavitt, R.G.; Miguel, E.C.; Stern, E.; Mezger, E.; Padberg, F.; D’Urso, G.; Brunoni, A.R. Transcranial Direct Current Stimulation in Obsessive-Compulsive Disorder: An Update in Electric Field Modeling and Investigations for Optimal Electrode Montage. Expert Rev. Neurother. 2019, 19, 1025–1035. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Sampaio-Junior, B.; Moffa, A.H.; Aparício, L.V.; Gordon, P.; Klein, I.; Rios, R.M.; Razza, L.B.; Loo, C.; Padberg, F.; et al. Noninvasive Brain Stimulation in Psychiatric Disorders: A Primer. Braz. J. Psychiatry 2019, 41, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef]
- Roth, Y.; Zangen, A. Reaching Deep Brain Structures: The H-coils. In Transcranial Magnetic Stimulation; Neuromethods; Springer: New York, NY, USA, 2014; pp. 57–65. ISBN 9781493908783. [Google Scholar] [CrossRef]
- Hyde, J.; Carr, H.; Kelley, N.; Seneviratne, R.; Reed, C.; Parlatini, V.; Garner, M.; Solmi, M.; Rosson, S.; Cortese, S.; et al. Efficacy of Neurostimulation across Mental Disorders: Systematic Review and Meta-Analysis of 208 Randomized Controlled Trials. Mol. Psychiatry 2022, 27, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Ekhtiari, H.; Antal, A.; Auvichayapat, P.; Baeken, C.; Bensenor, I.M.; Bikson, M.; Boggio, P.; Borroni, B.; Brighina, F.; et al. Digitalized Transcranial Electrical Stimulation: A Consensus Statement. Clinic Neurophysiol. 2022, 143, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Volpato, C.; Piccione, F.; Cavinato, M.; Duzzi, D.; Schiff, S.; Foscolo, L.; Venneri, A. Modulation of Affective Symptoms and Resting State Activity by Brain Stimulation in a Treatment-Resistant Case of Obsessive-Compulsive Disorder. Neurocase 2013, 19, 360–370. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A Systematic Review on Reporting and Assessment of Adverse Effects Associated with Transcranial Direct Current Stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Razza, L.B.; De Smet, S.; Moffa, A.; Sudbrack-Oliveira, P.; Vanderhasselt, M.-A.; Brunoni, A.R. Follow-up Effects of Transcranial Direct Current Stimulation (tDCS) for the Major Depressive Episode: A Systematic Review and Meta-Analysis. Psychiatry Res. 2021, 302, 114024. [Google Scholar] [CrossRef]
- Riley, R.D.; Lambert, P.C.; Abo-Zaid, G. Meta-Analysis of Individual Participant Data: Rationale, Conduct, and Reporting. BMJ 2010, 340, c221. [Google Scholar] [CrossRef]
- Guzick, A.G.; Schweissing, E.; Tendler, A.; Sheth, S.A.; Goodman, W.K.; Storch, E.A. Do Exposure Therapy Processes Impact the Efficacy of Deep TMS for Obsessive-Compulsive Disorder? J. Obs. Compuls. Relat. Disord. 2022, 35, 100756. [Google Scholar] [CrossRef]
| Demographic Data | Clinical Data | tDCS Strategy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Sample Size | Mean Age | % Female | Treat. Strategy | TRD | Number of Sessions | Current Intensity | Duration (min) | Electrode Position | Electrode Size (cm2) | Study Design | Risk of Bias |
| Bation et al. (2016) [21] | 8 | 44.2 (13.8) | 75 | Add-on | Yes | 10 | 2 | 20 | Anode: right cerebellum Cathode: left OFC | 35 | Open label trial | Moderate |
| Bation et al. (2019) [20] | 21 | 43 (15.9) | 57.14 | Add-on | Yes | 10 | 2 | 20 | Anode: right cerebellum Cathode: left OFC | 35 | RCT | Low |
| Da Silva et al. (2021) [22] | 44 | 37.65 (11.59) | 60.46 | Add-on | Yes | 20 | 2 | 30 | Anode: left deltoid Cathode: SMA | 25 | RCT | Low |
| D’Urso et al. (2016) [23] (cathode) | 6 | N/I | N/I | Add-on | Yes | 10 | 2 | 20 | Anode: right deltoid Cathode: bilateral pre-SMA | 35 and 25 | Partial cross-over, repeated measures | Moderate |
| D’Urso et al. (2016) [23] (anode) | 6 | N/I | N/I | Add-on | Yes | 10 | 2 | 20 | Anode: bilateral pre-SMA Cathode: right deltoid | 25 and 35 | Partial cross-over, repeated measures | Moderate |
| Germaneau et al. (2020) [19] | 21 | 42.7 (13) | 38.1 | Add-on | Yes | 10 | 2 | 30 | Anode: right supraorbital area Cathode: bilateral SMA | 35 | Open label trial | Moderate |
| Gowda et al. (2019) [25] | 25 | 28.37 (5.51) | 16 | Add-on | Yes | 10 | 2 | 20 | Anode: pre-SMA Cathode: right supraorbital area | 35 | RCT | Some Concers |
| Kumar et al. (2021) [26] | 20 | 21.6 (7.64) | 45 | Add-on | Yes | 20 | 2 | 20 | Anode: right occipital area Cathode: SMA | 25* | Open label trial | Moderate |
| Yoosefee et al. (2020) [24] | 60 | 37.25 (24.1) | 81.6 | Augmentation | N/I | 24 | 2 | 20 | Anode: left DLPFC Cathode: right OFC | 35 | RCT | Low |
| Coef (B) | 95% CI | p | ||
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Augmentation Strategy | −0.02 | −0.88 | 0.46 | 0.54 |
| Sessions per day | 0.49 | −0.18 | 1.18 | 0.15 |
| Session duration | −0.27 | −0.09 | 0.41 | 0.43 |
| Number of weeks | −0.05 | −0.16 | 0.48 | 0.27 |
| Session total | −0.03 | −0.08 | 0.01 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, B.S.; Cavendish, B.A.; da Silva, P.H.R.; Suen, P.J.C.; Marinho, K.A.P.; Valiengo, L.d.C.L.; Vanderhasselt, M.-A.; Brunoni, A.R.; Razza, L.B. The Effects of Transcranial Direct Current Stimulation in Obsessive–Compulsive Disorder Symptoms: A Meta-Analysis and Integrated Electric Fields Modeling Analysis. Biomedicines 2023, 11, 80. https://doi.org/10.3390/biomedicines11010080
Pinto BS, Cavendish BA, da Silva PHR, Suen PJC, Marinho KAP, Valiengo LdCL, Vanderhasselt M-A, Brunoni AR, Razza LB. The Effects of Transcranial Direct Current Stimulation in Obsessive–Compulsive Disorder Symptoms: A Meta-Analysis and Integrated Electric Fields Modeling Analysis. Biomedicines. 2023; 11(1):80. https://doi.org/10.3390/biomedicines11010080
Chicago/Turabian StylePinto, Bianca Silva, Beatriz Araújo Cavendish, Pedro Henrique Rodrigues da Silva, Paulo Jeng Chian Suen, Kalian Almeida Pereira Marinho, Leandro da Costa Lane Valiengo, Marie-Anne Vanderhasselt, André Russowsky Brunoni, and Laís Boralli Razza. 2023. "The Effects of Transcranial Direct Current Stimulation in Obsessive–Compulsive Disorder Symptoms: A Meta-Analysis and Integrated Electric Fields Modeling Analysis" Biomedicines 11, no. 1: 80. https://doi.org/10.3390/biomedicines11010080
APA StylePinto, B. S., Cavendish, B. A., da Silva, P. H. R., Suen, P. J. C., Marinho, K. A. P., Valiengo, L. d. C. L., Vanderhasselt, M.-A., Brunoni, A. R., & Razza, L. B. (2023). The Effects of Transcranial Direct Current Stimulation in Obsessive–Compulsive Disorder Symptoms: A Meta-Analysis and Integrated Electric Fields Modeling Analysis. Biomedicines, 11(1), 80. https://doi.org/10.3390/biomedicines11010080