Fatty Liver as Potential Biomarker of Atherosclerotic Damage in Familial Combined Hyperlipidemia
Abstract
1. Introduction
2. Materials and Methods
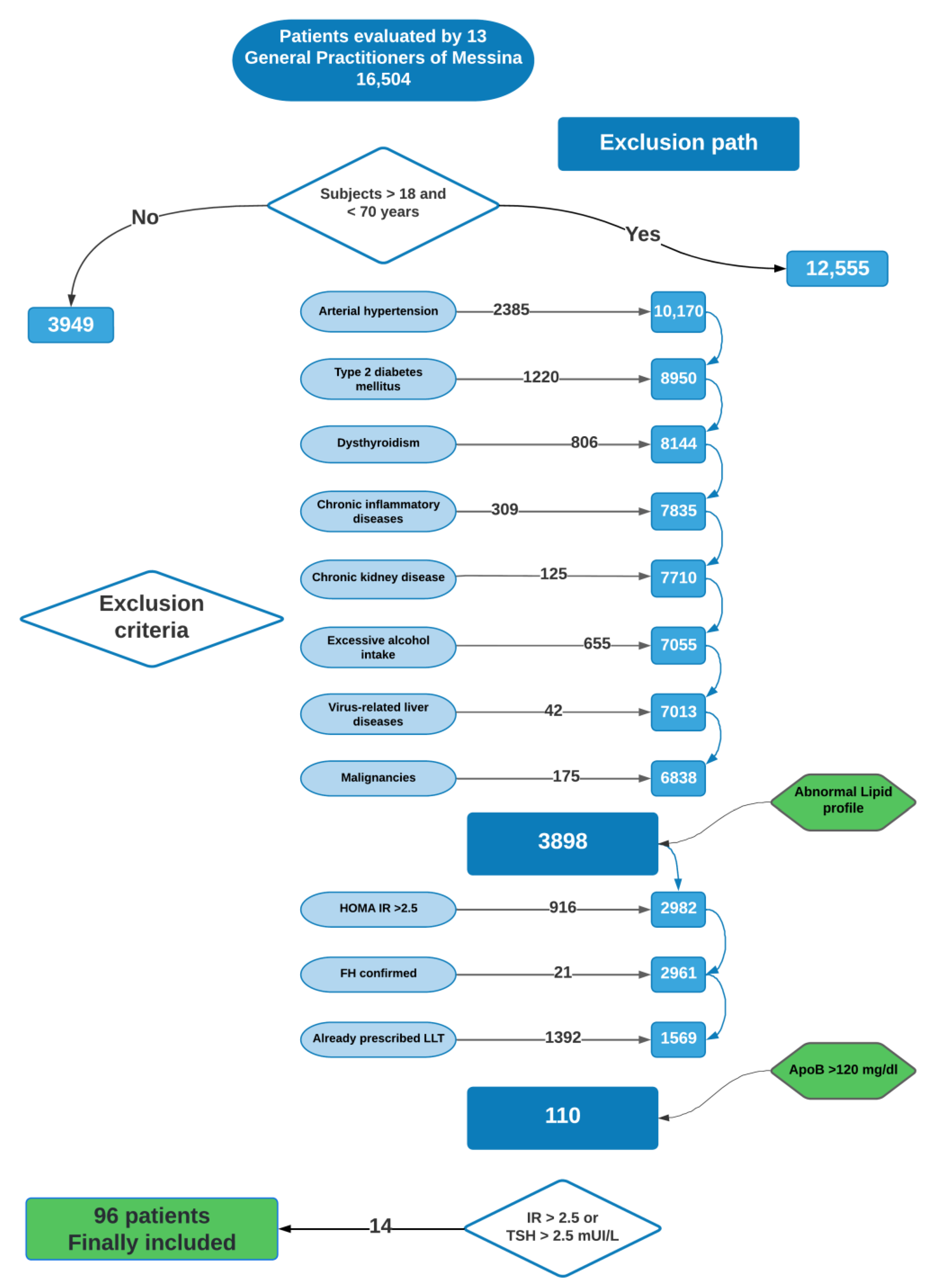
2.1. Patients Included in the Study
2.2. Clinical and Laboratory Assessment
2.3. Carotid Artery Evaluation
2.4. Non-Invasive Assessment of Liver Steatosis and Fibrosis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trinder, M.; Vikulova, D.; Pimstone, S.; Mancini, G.B.J.; Brunham, L.R. Polygenic architecture and cardiovascular risk of familial combined hyperlipidemia. Atherosclerosis 2021, 340, 35–43. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Schrott, H.G.; Hazzard, W.R.; Bierman, E.L.; Motulsky, A.G. Hyperlipidemia in Coronary Heart Disease II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J. Clin. Investig. 1973, 52, 1544–1568. [Google Scholar] [CrossRef] [PubMed]
- Brahm, A.J.; Hegele, R.A. Combined hyperlipidemia: Familial but not (usually) monogenic. Curr. Opin. Lipidol. 2016, 27, 131–140. [Google Scholar] [CrossRef]
- Vikulova, D.N.; Trinder, M.; Mancini, G.B.J.; Pimstone, S.N.; Brunham, L.R. Familial Hypercholesterolemia, Familial Combined Hyperlipidemia, and Elevated Lipoprotein(a) in Patients with Premature Coronary Artery Disease. Can. J. Cardiol. 2021, 37, 1733–1742. [Google Scholar] [CrossRef]
- Brouwers, M.; de Graaf, J.; Simons, N.; Meex, S.; Ten Doeschate, S.; van Heertum, S.; Heidemann, B.; Luijten, J.; de Boer, D.; Schaper, N.; et al. Incidence of type 2 diabetes in familial combined hyperlipidemia. BMJ Open Diabetes Res. Care 2020, 8, e001107. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Bilderbeek-Beckers, M.A.; Georgieva, A.M.; van der Kallen, C.J.; van Greevenbroek, M.M.; de Bruin, T.W. Fatty liver is an integral feature of familial combined hyperlipidaemia: Relationship with fat distribution and plasma lipids. Clin. Sci. 2007, 112, 123–130. [Google Scholar] [CrossRef][Green Version]
- Pacifico, L.; Perla, F.M.; Roggini, M.; Andreoli, G.; D’Avanzo, M.; Chiesa, C. A Systematic Review of NAFLD-Associated Extrahepatic Disorders in Youths. J. Clin. Med. 2019, 8, 868. [Google Scholar] [CrossRef]
- Garoufi, A.; Pagoni, A.; Papadaki, M.; Marmarinos, A.; Karapostolakis, G.; Michala, L.; Soldatou, A. Cardiovascular Risk Factors and Subclinical Atherosclerosis in Greek Adolescents with Polycystic Ovary Syndrome: Its Relationship with Body Mass Index. Children 2021, 9, 4. [Google Scholar] [CrossRef]
- Adiels, M.; Taskinen, M.-R.; Packard, C.; Caslake, M.J.; Soro-Paavonen, A.; Westerbacka, J.; Vehkavaara, S.; Häkkinen, A.; Olofsson, S.-O.; Yki-Järvinen, H.; et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006, 49, 755–765. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Van Der Kallen, C.J.; Schaper, N.C.; Van Greevenbroek, M.M.; Stehouwer, C.D. Five-year incidence of type 2 diabetes mellitus in patients with familial combined hyperlipidaemia. Neth. J. Med. 2010, 68, 163–167. [Google Scholar]
- Riggio, S.; Mamone, F.; Mandraffino, G.; Maimone, S.; Alibrandi, A.; Manti, L.; Saitta, C.; Tripodi, P.F.; Sardo, M.A.; Squadrito, G.; et al. Assessment of liver stiffness in subjects affected by familial combined hyperlipidaemia with hepatic steatosis. Eur. J. Clin. Investig. 2010, 40, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Imajo, K.; Ozaki, A.; Kobayashi, T.; Iwaki, M.; Honda, Y.; Kessoku, T.; Ogawa, Y.; Yoneda, M.; Kirikoshi, H.; et al. Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver. Diagnostics 2020, 10, 129. [Google Scholar] [CrossRef]
- de Bruin, T.W.; Georgieva, A.M.; Brouwers, M.C.; Heitink, M.V.; van der Kallen, C.J.; van Greevenbroek, M.M. Radiological evidence of nonalcoholic fatty liver disease in familial combined hyperlipidemia. Am. J. Med. 2004, 116, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.Y.; Kim, M.S.; Hong, H.P.; Kang, K.A.; Jun, D.W. Comparative Assessment and External Validation of Hepatic Steatosis Formulae in a Community-Based Setting. J. Clin. Med. 2020, 9, 2851. [Google Scholar] [CrossRef]
- Saokaew, S.; Kositamongkol, C.; Charatcharoenwitthaya, P.; Srivanichakorn, W.; Washirasaksiri, C.; Chaiyakunapruk, N.; Phisalprapa, P. Comparison of noninvasive scoring systems for the prediction of nonalcoholic fatty liver disease in metabolic syndrome patients. Medicine 2020, 99, e23619. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Reesink, K.D.; van Greevenbroek, M.M.; Meinders, J.M.; van der Kallen, C.J.; Schaper, N.; Hoeks, A.P.; Stehouwer, C.D. Increased arterial stiffness in familial combined hyperlipidemia. J. Hypertens. 2009, 27, 1009–1016. [Google Scholar] [CrossRef]
- Casula, M.; Olmastroni, E.; Pirillo, A.; Catapano, A.L.; Arca, M.; Averna, M.; Bertolini, S.; Calandra, S.; Tarugi, P.; Pellegatta, F.; et al. Evaluation of the performance of Dutch Lipid Clinic Network score in an Italian FH population: The LIPIGEN study. Atherosclerosis 2018, 277, 413–418. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Casadei, A.; Floreani, M.; Catalini, R.; Serra, C.; Assanti, A.P.; Conci, P. Sonographic characteristics of carotid artery plaques: Implications for follow-up planning? J. Ultrasound 2012, 15, 151–157. [Google Scholar] [CrossRef]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid Artery Stenosis: Grayscale and Doppler Ultrasound Diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Ultrasound Q. 2003, 19, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mandraffino, G.; Aragona, C.O.; Scuruchi, M.; Mamone, F.; D’Ascola, A.; Alibrandi, A.; Cinquegrani, M.; Morace, C.; Oreto, L.; Saitta, C.; et al. Biglycan expression, earlier vascular damage and pro-atherogenic profile improvement after smoke cessation in young people. Atherosclerosis 2017, 257, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of Noninvasive Markers of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Lawitz, E.J.; Alkhouri, N.; Wong, V.W.; Romero-Gomez, M.; Okanoue, T.; Trauner, M.; Kersey, K.; Li, G.; Han, L.; et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data from the STELLAR Trials. Hepatology 2019, 70, 1521–1530. [Google Scholar] [CrossRef]
- Castera, L.; Vilgrain, V.; Angulo, P. Noninvasive evaluation of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 666–675. [Google Scholar] [CrossRef]
- Tapper, E.B.; Challies, T.; Nasser, I.; Afdhal, N.H.; Lai, M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2016, 111, 677–684. [Google Scholar] [CrossRef]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Iqbal, U.; Perumpail, B.J.; Akhtar, D.; Kim, D.; Ahmed, A. The Epidemiology, Risk Profiling and Diagnostic Challenges of Nonalcoholic Fatty Liver Disease. Medicines 2019, 6, 41. [Google Scholar] [CrossRef]
- Kantartzis, K.; Rittig, K.; Cegan, A.; Machann, J.; Schick, F.; Balletshofer, B.; Fritsche, A.; Schleicher, E.; Häring, H.-U.; Stefan, N. Fatty Liver Is Independently Associated with Alterations in Circulating HDL2 and HDL3 Subfractions. Diabetes Care 2008, 31, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Speliotes, E.K.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Meigs, J.B.; Sahani, D.V.; Hirschhorn, J.N.; O’Donnell, C.J.; Fox, C.S. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham heart study. Hepatology 2010, 51, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Soresi, M.; Noto, D.; Cefalu, A.B.; Martini, S.; Vigna, G.B.; Fonda, M.; Manzato, E.; Cattin, L.; Fellin, R.; Averna, M.R.; et al. Nonalcoholic fatty liver and metabolic syndrome in Italy: Results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. 2013, 50, 241–249. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Tessari, R.; Zenari, L.; Day, C.; Arcaro, G. Prevalence of Nonalcoholic Fatty Liver Disease and Its Association with Cardiovascular Disease Among Type 2 Diabetic Patients. Diabetes Care 2007, 30, 1212–1218. [Google Scholar] [CrossRef]
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People with Type 2 Diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144. [Google Scholar] [CrossRef]
- Assy, N.; Kaita, K.; Mymin, D.; Levy, C.; Rosser, B.; Minuk, G. Fatty Infiltration of Liver in Hyperlipidemic Patients. Dig. Dis. Sci. 2000, 45, 1929–1934. [Google Scholar] [CrossRef]
- Wu, K.-T.; Kuo, P.-L.; Su, S.-B.; Chen, Y.-Y.; Yeh, M.-L.; Huang, C.-I.; Yang, J.-F.; Lin, C.-I.; Hsieh, M.-H.; Hsieh, M.-Y.; et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J. Clin. Lipidol. 2016, 10, 420–425.e1. [Google Scholar] [CrossRef] [PubMed]
- Anja Schirbel, A.R.; Roll, S.; Baumgart, D.C.; Büning, C.; Wittig, B.; Wiedenmann, B.; Dignass, A.; Sturm, A. Impact of pain on health-ralted quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 3168–3177. [Google Scholar] [CrossRef] [PubMed]
- Skoumas, I.; Masoura, C.; Aznaouridis, K.; Metaxa, V.; Tsokanis, A.; Papadimitriou, L.; Tousoulis, D.; Pitsavos, C.; Stefanadis, C. Impact of Cardiometabolic Risk Factors on Major Cardiovascular Events in Patients with Familial Combined Hyperlipidemia. Circ. J. 2013, 77, 163–168. [Google Scholar] [CrossRef]
- Castaldo, L.; Laguzzi, F.; Strawbridge, R.J.; Baldassarre, D.; Veglia, F.; Vigo, L.; Tremoli, E.; De Faire, U.; Eriksson, P.; Smit, A.J.; et al. Genetic Variants Associated with Non-Alcoholic Fatty Liver Disease Do Not Associate with Measures of Sub-Clinical Atherosclerosis: Results from the IMPROVE Study. Genes 2020, 11, 1243. [Google Scholar] [CrossRef] [PubMed]
- Jarauta, E.; Mateo-Gallego, R.; Gilabert, R.; Plana, N.; Junyent, M.; de Groot, E.; Cenarro, A.; Masana, L.; Ros, E.; Civeira, F. Carotid atherosclerosis and lipoprotein particle subclasses in familial hypercholesterolaemia and familial combined hyperlipidaemia. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 591–597. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Meffert, P.J.; Baumeister, S.E.; Lerch, M.M.; Mayerle, J.; Kratzer, W.; Völzke, H. Development, External Validation, and Comparative Assessment of a New Diagnostic Score for Hepatic Steatosis. Am. J. Gastroenterol. 2014, 109, 1404–1414. [Google Scholar] [CrossRef]
- Bril, F.; Sninsky, J.J.; Baca, A.M.; Superko, H.R.; Portillo Sanchez, P.; Biernacki, D.; Maximos, M.; Lomonaco, R.; Orsak, B.; Suman, A.; et al. Hepatic Steatosis and Insulin Resistance, But Not Steatohepatitis, Promote Atherogenic Dyslipidemia in NAFLD. J. Clin. Endocrinol. Metab. 2016, 101, 644–652. [Google Scholar] [CrossRef]
- Oni, E.T.; Agatston, A.S.; Blaha, M.J.; Fialkow, J.; Cury, R.; Sposito, A.; Erbel, R.; Blankstein, R.; Feldman, T.; Al-Mallah, M.H.; et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; Should we care? Atherosclerosis 2013, 230, 258–267. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Ronca, A.; D’Erasmo, L.; Manfredini, M.; Baratta, F.; Pastori, D.; Di Martino, M.; Ceci, F.; Angelico, F.; Del Ben, M.; et al. HDL-Mediated Cholesterol Efflux and Plasma Loading Capacities Are Altered in Subjects with Metabolically- but not Genetically Driven Non-Alcoholic Fatty Liver Disease (NAFLD). Biomedicines 2020, 8, 625. [Google Scholar] [CrossRef]
- Xin, Z.; Zhu, Y.; Wang, S.; Liu, S.; Xu, M.; Wang, T.; Lu, J.; Chen, Y.; Zhao, Z.; Wang, W.; et al. Associations of subclinical atherosclerosis with nonalcoholic fatty liver disease and fibrosis assessed by non-invasive score. Liver Int. 2020, 40, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Kawano, T.; Koeda, M.; Yoshida, Y.; Tanabe, T.; Okubo, T.; Hayama, K.; Iwashita, A.; et al. Factors influencing subclinical atherosclerosis in patients with biopsy-proven nonalcoholic fatty liver disease. PLoS ONE 2019, 14, e0224184. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; Chang, U.I.; Kang, S.-G.; Song, S.-W.; Yang, J.M. Noninvasive Serum Fibrosis Markers are Associated with Coronary Artery Calcification in Patients with Nonalcoholic Fatty Liver Disease. Gut Liver 2019, 13, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, H.; Sumida, Y.; Kataoka, S.; Kuroda, M.; Akabame, S.; Tomiyasu, K.; Tanaka, M.; Arai, M.; Taketani, H.; Seko, Y.; et al. Association of coronary artery calcification with liver fibrosis in Japanese patients with non-alcoholic fatty liver disease. Hepatol. Res. 2016, 46, 1107–1117. [Google Scholar] [CrossRef]

| Male sex, n [%] | 44 (45.8) |
| Age, years | 53 (12) |
| BMI, Kg/m2 | 25.5 (4.9) |
| Total cholesterol, mg/dL | 246.5 (26) |
| HDL-C, mg/dL | 50.5 (21) |
| Triglycerides, mg/dL | 119 (85) |
| LDL-C, mg/dL | 169 (21) |
| Fasting glucose, mg/dL | 89 (11) |
| HbA1c, % | 5.4 (0.6) |
| Fasting insulin, mIU/L | 7.1 (3.6) |
| HOMA-IR | 1.6 (0.8) |
| AST, IU/L | 20 (10) |
| ALT, IU/L | 20 (15) |
| GGT, IU/L | 24 (26) |
| PLT count × 103/mmc | 240 (85) |
| Apolipoprotein A, mg/dL | 154.5 (49) |
| Apolipoprotein B, mg/dL | 131 (26) |
| FIB-4 | 0.9 (0.7) |
| HSI | 34.9 (6.4) |
| LSM, KPa | 5.1 (2.2) |
| cIMT, mm | 1 (0.30) |
| PWV, m/s | 9.7 (4.3) |
| Steatosis according to liver US, n [%] | |
| Absent | 32 (33.3) |
| Mild | 39 (40.6) |
| Moderate | 20 (20.8) |
| Severe | 5 (5.2) |
| Steatosis according to HSI evaluation, n [%] | |
| HSI < 30 | 11 (11.5) |
| 30 < HSI < 36 | 44 (45.8) |
| HSI > 36 | 41 (42.7) |
| Liver fibrosis according to LSM by TE, n [%] | |
| LSM < 7.9 KPa | 88 (91.7) |
| 7.9 KPa > LSM < 9.9 KPa | 7 (7.3) |
| LSM > 9.9 KPa | 1 (1) |
| Liver fibrosis according to FIB4, n [%] | |
| FIB4 < 1.30 | 71 (74) |
| 1.45 < FIB4 < 2.67 | 25 (26) |
| FIB4 > 2.67 | 0 (0) |
| Atherosclerotic Plaque | Abnormal cIMT | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Fibrosis by FIB-4 | 6.667 | 2.213–20.087 | <0.001 | 1.689 | 0.553–5.161 | 0.358 |
| Fibrosis by TE | 4.778 | 0.954–23.938 | 0.057 | 3.696 | 0.443–30.865 | 0.227 |
| Final Step of Multivariable Logistic Regression Analysis | |||
|---|---|---|---|
| Variables | OR (IC 95%) | IC 95% | p |
| FIB-4 | 6.863 | 2.167–21.739 | <0.001 |
| Smoking habit | 2.122 | 1.009–4.462 | 0.047 |
| LSM by TE | 4.031 | 0.731–22.237 | 0.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandraffino, G.; Morace, C.; Franzè, M.S.; Nassisi, V.; Sinicropi, D.; Cinquegrani, M.; Saitta, C.; Scoglio, R.; Marino, S.; Belvedere, A.; et al. Fatty Liver as Potential Biomarker of Atherosclerotic Damage in Familial Combined Hyperlipidemia. Biomedicines 2022, 10, 1770. https://doi.org/10.3390/biomedicines10081770
Mandraffino G, Morace C, Franzè MS, Nassisi V, Sinicropi D, Cinquegrani M, Saitta C, Scoglio R, Marino S, Belvedere A, et al. Fatty Liver as Potential Biomarker of Atherosclerotic Damage in Familial Combined Hyperlipidemia. Biomedicines. 2022; 10(8):1770. https://doi.org/10.3390/biomedicines10081770
Chicago/Turabian StyleMandraffino, Giuseppe, Carmela Morace, Maria Stella Franzè, Veronica Nassisi, Davide Sinicropi, Maria Cinquegrani, Carlo Saitta, Riccardo Scoglio, Sebastiano Marino, Alessandra Belvedere, and et al. 2022. "Fatty Liver as Potential Biomarker of Atherosclerotic Damage in Familial Combined Hyperlipidemia" Biomedicines 10, no. 8: 1770. https://doi.org/10.3390/biomedicines10081770
APA StyleMandraffino, G., Morace, C., Franzè, M. S., Nassisi, V., Sinicropi, D., Cinquegrani, M., Saitta, C., Scoglio, R., Marino, S., Belvedere, A., Cairo, V., Lo Gullo, A., Scuruchi, M., Raimondo, G., & Squadrito, G. (2022). Fatty Liver as Potential Biomarker of Atherosclerotic Damage in Familial Combined Hyperlipidemia. Biomedicines, 10(8), 1770. https://doi.org/10.3390/biomedicines10081770









