Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Astrocyte Cell Cultures
2.2. Analysis of Astrocytes’ Response to Cytokines
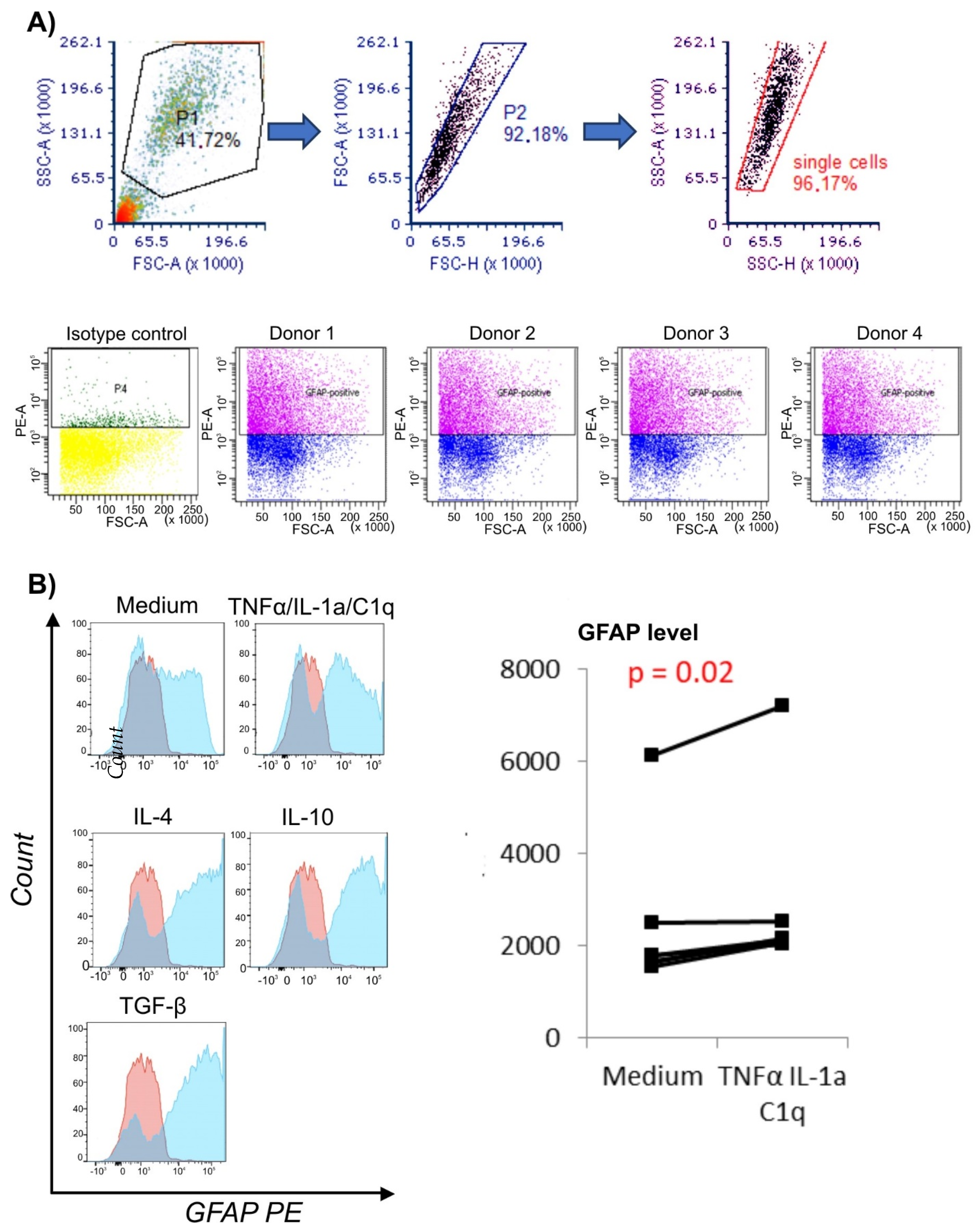
2.3. Analysis of Surface Receptor Expression and Intracellular GFAP Level with Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Proinflammatory Environment Results in Elevated GFAP Level in Astrocytes
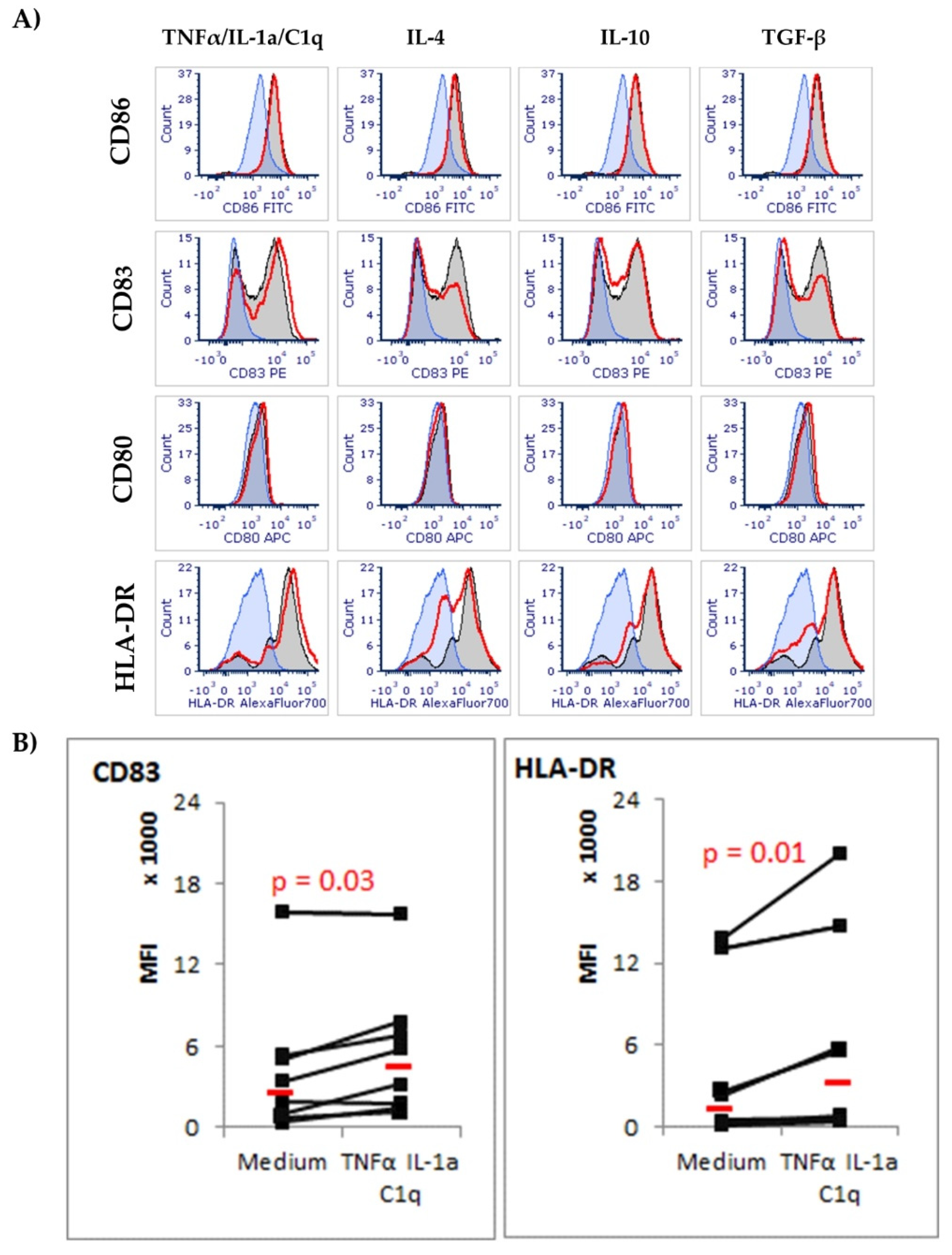
3.2. CD83 and HLA-DR Molecules Are Upregulated in Astrocytes Exposed to TNF-α/IL-1a/C1q Cytokines
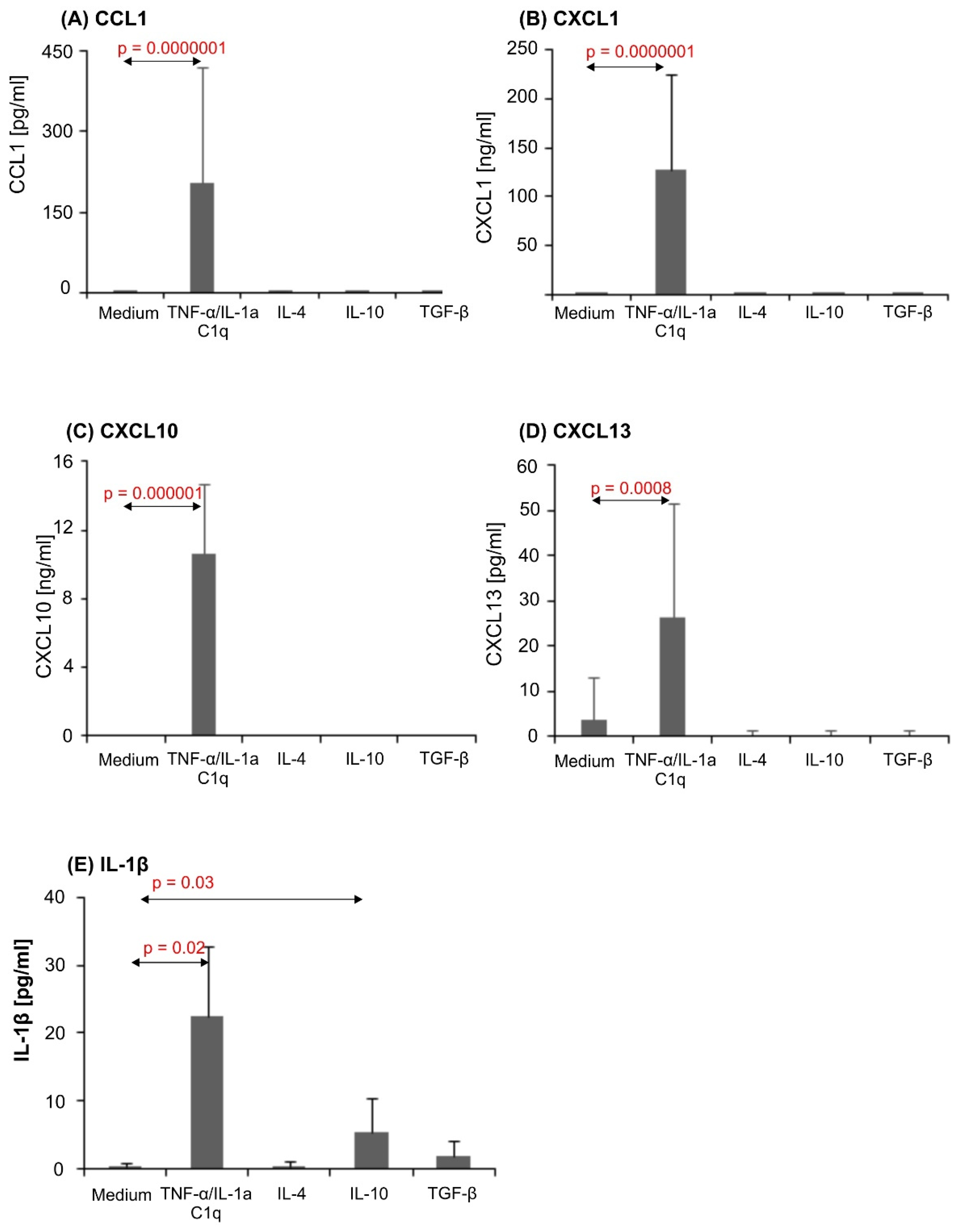
3.3. Proinflammatory Stimuli Induced Dramatic Chemokine Release in Astrocytic Cultures
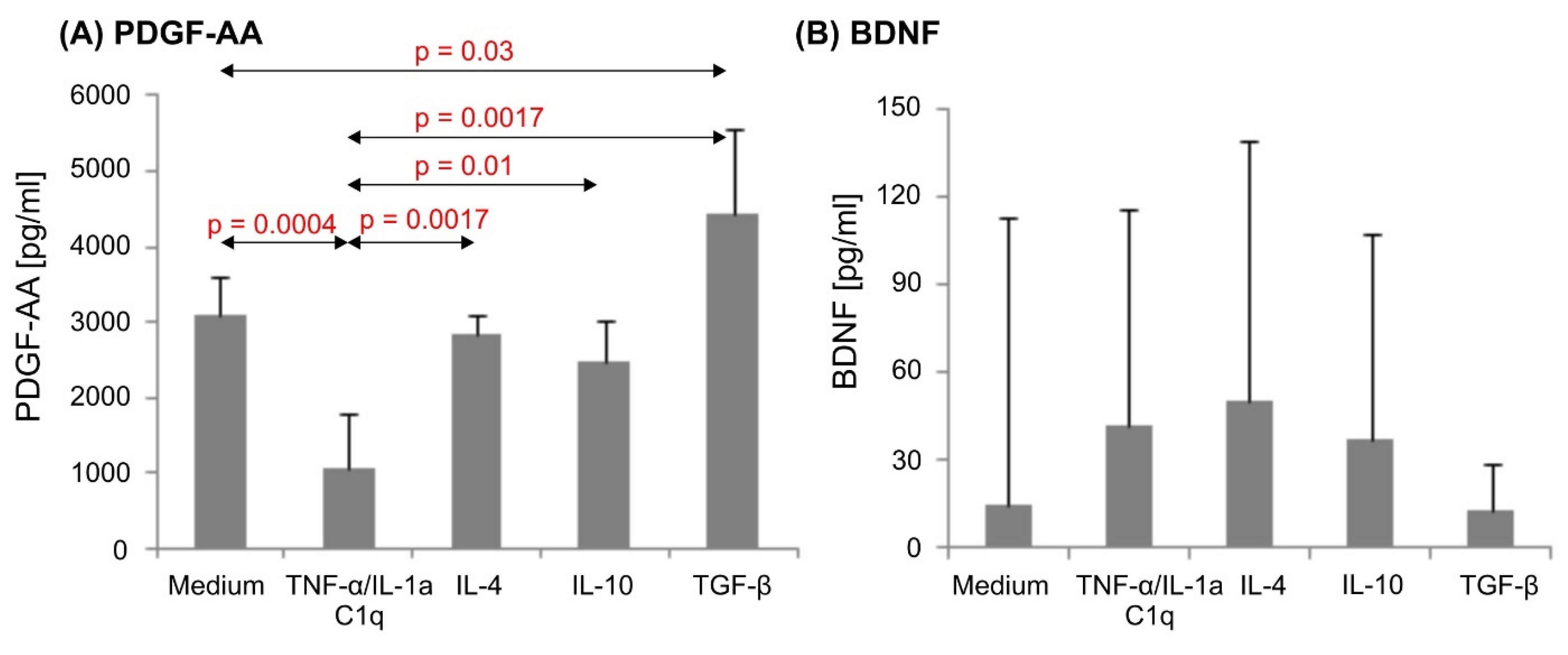
3.4. Various Cytokine Environments Differently Regulate PDGF-A Expression in Astrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eng, L.F.; Vanderhaeghen, J.J.; Bignami, A.; Gerstl, B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971, 28, 351–354. [Google Scholar] [CrossRef]
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Walz, W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000, 36, 291–300. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Quintana, F.J. Regulation of Astrocyte Functions in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Allen, N.J.; Bennett, M.L.; Foo, L.C.; Wang, G.X.; Chakraborty, C.; Smith, S.J.; Barres, B.A. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012, 486, 410–414. [Google Scholar] [CrossRef]
- Cornet, A.; Bettelli, E.; Oukka, M.; Cambouris, C.; Avellana-Adalid, V.; Kosmatopoulos, K.; Liblau, R.S. Role of astrocytes in antigen presentation and naive T-cell activation. J. Neuroimmunol. 2000, 106, 69–77. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Yang, Y.; Rosenberg, G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009, 158, 983–994. [Google Scholar] [CrossRef]
- Liberto, C.M.; Albrecht, P.J.; Herx, L.M.; Yong, V.W.; Levison, S.W. Pro-regenerative properties of cytokine-activated astrocytes. J. Neurochem. 2004, 89, 1092–1100. [Google Scholar] [CrossRef]
- Williams, A.; Piaton, G.; Lubetzki, C. Astrocytes—Friends or foes in multiple sclerosis? Glia 2007, 55, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.-H. Chemokine receptors in the central nervous system: Role in brain inflammation and neurodegenerative diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, H.; Gu, Y.-H.; Heo, J.H.; Milner, R.; del Zoppo, G.J. Use of Gel Zymography to Examine Matrix Metalloproteinase (Gelatinase) Expression in Brain Tissue or in Primary Glial Cultures. In Astrocytes: Methods and Protocols; Milner, R., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 221–233. ISBN 978-1-61779-452-0. [Google Scholar]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, S.; Montagne, L.; De Groot, C.J.A.; Van Der Valk, P. Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia 2002, 38, 24–35. [Google Scholar] [CrossRef]
- Lindholm, D.; Castrén, E.; Kiefer, R.; Zafra, F.; Thoenen, H. Transforming growth factor-beta 1 in the rat brain: Increase after injury and inhibition of astrocyte proliferation. J. Cell Biol. 1992, 117, 395–400. [Google Scholar] [CrossRef]
- Lin, Y.C.; Winokur, P.; Blake, A.; Wu, T.; Romm, E.; Bielekova, B. Daclizumab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann. Clin. Transl. Neurol. 2015, 2, 445–455. [Google Scholar] [CrossRef]
- Bailey, S.L.; Carpentier, P.A.; McMahon, E.J.; Begolka, W.S.; Miller, S.D. Innate and Adaptive Immune Responses of the Central Nervous System. Crit. Rev. Immunol. 2006, 26, 149–188. [Google Scholar] [CrossRef]
- Jensen, C.J.; Massie, A.; De Keyser, J. Immune Players in the CNS: The Astrocyte. J. Neuroimmune Pharmacol. 2013, 8, 824–839. [Google Scholar] [CrossRef]
- Ransom, B.R.; Ransom, C.B. Astrocytes: Multitalented Stars of the Central Nervous System. In Astrocytes: Methods and Protocols; Milner, R., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 3–7. ISBN 978-1-61779-452-0. [Google Scholar]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M. V Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Merlo, S.; Fagone, E.; Fruciano, M.; Sano, Y.; Kanda, T.; Sortino, M.A. Reciprocal Interplay Between Astrocytes and CD4+ Cells Affects Blood-Brain Barrier and Neuronal Function in Response to β Amyloid. Front. Mol. Neurosci. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Mills Ko, E.; Ma, J.H.; Guo, F.; Miers, L.; Lee, E.; Bannerman, P.; Burns, T.; Ko, D.; Sohn, J.; Soulika, A.M.; et al. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J. Neuroinflammation 2014, 11, 105. [Google Scholar] [CrossRef]
- Luster, A.D.; Ravetch, J. V Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med. 1987, 166, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Lloyd, A.R.; Conlon, K.; Wang, J.M.; Ortaldo, J.R.; Harada, A.; Matsushima, K.; Kelvin, D.J.; Oppenheim, J.J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med. 1993, 177, 1809–1814. [Google Scholar] [CrossRef]
- Xia, M.Q.; Bacskai, B.J.; Knowles, R.B.; Qin, S.X.; Hyman, B.T. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: In vitro ERK1/2 activation and role in Alzheimer’s disease. J. Neuroimmunol. 2000, 108, 227–235. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Tani, M.; Jensen, J.; Pierce, V.; Lucchinetti, C.; Folcik, V.A.; Qin, S.; Rottman, J.; Sellebjerg, F.; Strieter, R.M.; et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 1999, 103, 807–815. [Google Scholar] [CrossRef]
- Tani, M.; Glabinski, A.R.; Tuohy, V.K.; Stoler, M.H.; Estes, M.L.; Ransohoff, R.M. In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am. J. Pathol. 1996, 148, 889–896. [Google Scholar]
- Oh, J.-W.; Schwiebert, L.M.; Benveniste, E.N. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J. Neurovirol. 1999, 5, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Gourmala, N.; Boddeke, H.W.G.M.; Gebicke-Haerter, P.J. Lipopolysaccharide-induced expression of IP-10 mRNA in rat brain and in cultured rat astrocytes and microglia. Mol. Brain Res. 1998, 59, 256–263. [Google Scholar] [CrossRef]
- Fife, B.T.; Kennedy, K.J.; Paniagua, M.C.; Lukacs, N.W.; Kunkel, S.L.; Luster, A.D.; Karpus, W.J. CXCL10 (IFN-γ-Inducible Protein-10) Control of Encephalitogenic CD4+ T Cell Accumulation in the Central Nervous System During Experimental Autoimmune Encephalomyelitis. J. Immunol. 2001, 166, 7617–7624. [Google Scholar] [CrossRef] [PubMed]
- Glabinski, A.R.; Tani, M.; Tuohy, V.K.; Tuthill, R.J.; Ransohoff, R.M. Central Nervous System Chemokine mRNA Accumulation Follows Initial Leukocyte Entry at the Onset of Acute Murine Experimental Autoimmune Encephalomyelitis. Brain. Behav. Immun. 1995, 9, 315–330. [Google Scholar] [CrossRef]
- McTigue, D.M.; Tani, M.; Krivacic, K.; Chernosky, A.; Kelner, G.S.; Maciejewski, D.; Maki, R.; Ransohoff, R.M.; Stokes, B.T. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J. Neurosci. Res. 1998, 53, 368–376. [Google Scholar] [CrossRef]
- Bruccoleri, A.; Harry, G.J. Chemical-induced hippocampal neurodegeneration and elevations in TNFα, TNFβ, IL-1α, IP-10, and MCP-1 mRNA in osteopetrotic (op/op) mice. J. Neurosci. Res. 2000, 62, 146–155. [Google Scholar] [CrossRef]
- Wang, X.; Ellison, J.A.; Siren, A.-L.; Lysko, P.G.; Yue, T.-L.; Barone, F.C.; Shatzman, A.; Feuerstein, G.Z. Prolonged Expression of Interferon-Inducible Protein-10 in Ischemic Cortex After Permanent Occlusion of the Middle Cerebral Artery in Rat. J. Neurochem. 1998, 71, 1194–1204. [Google Scholar] [CrossRef]
- Rappert, A.; Bechmann, I.; Pivneva, T.; Mahlo, J.; Biber, K.; Nolte, C.; Kovac, A.D.; Gerard, C.; Boddeke, H.W.G.M.; Nitsch, R.; et al. CXCR3-Dependent Microglial Recruitment Is Essential for Dendrite Loss after Brain Lesion. J. Neurosci. 2004, 24, 8500–8509. [Google Scholar] [CrossRef]
- Liu, M.T.; Keirstead, H.S.; Lane, T.E. Neutralization of the Chemokine CXCL10 Reduces Inflammatory Cell Invasion and Demyelination and Improves Neurological Function in a Viral Model of Multiple Sclerosis. J. Immunol. 2001, 167, 4091–4097. [Google Scholar] [CrossRef]
- Narumi, S.; Kaburaki, T.; Yoneyama, H.; Iwamura, H.; Kobayashi, Y.; Matsushima, K. Neutralization of IFN-inducible protein 10/CXCL10 exacerbates experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2002, 32, 1784–1791. [Google Scholar] [CrossRef]
- Cepok, S.; Schreiber, H.; Hoffmann, S.; Zhou, D.; Neuhaus, O.; von Geldern, G.; Hochgesand, S.; Nessler, S.; Rothhammer, V.; Lang, M.; et al. Enhancement of Chemokine Expression by Interferon Beta Therapy in Patients with Multiple Sclerosis. Arch. Neurol. 2009, 66, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Hackstette, D.; Bauer, K.; Gudi, V.; Pul, R.; Voss, E.; Berger, K.; Kipp, M.; Baumgärtner, W.; Stangel, M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 2013, 136, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Nash, B.; Thomson, C.E.; Linington, C.; Arthur, A.T.; McClure, J.D.; McBride, M.W.; Barnett, S.C. Functional Duality of Astrocytes in Myelination. J. Neurosci. 2011, 31, 13028–13038. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, H.L.; Lautens, L.L.; Gao, J.-L.; Pease, J.; Locati, M.; Combadiere, C.; Modi, W.; Bonner, T.I.; Murphy, P.M. Identification of CCR8: A Human Monocyte and Thymus Receptor for the CC Chemokine I-309. J. Exp. Med. 1997, 186, 165–170. [Google Scholar] [CrossRef]
- Gombert, M.; Dieu-Nosjean, M.-C.; Winterberg, F.; Bünemann, E.; Kubitza, R.C.; Da Cunha, L.; Haahtela, A.; Lehtimäki, S.; Müller, A.; Rieker, J.; et al. CCL1-CCR8 Interactions: An Axis Mediating the Recruitment of T Cells and Langerhans-Type Dendritic Cells to Sites of Atopic Skin Inflammation. J. Immunol. 2005, 174, 5082–5091. [Google Scholar] [CrossRef]
- Holse, M.; Assing, K.; Poulsen, L.K. CCR3, CCR5, CCR8 and CXCR3 expression in memory T helper cells from allergic rhinitis patients, asymptomatically sensitized and healthy individuals. Clin. Mol. Allergy 2006, 4, 6. [Google Scholar] [CrossRef]
- Syrbe, U.; Siveke, J.; Hamann, A. Th1/Th2 subsets: Distinct differences in homing and chemokine receptor expression? Springer Semin. Immunopathol. 1999, 21, 263–285. [Google Scholar] [CrossRef]
- Trebst, C.; Staugaitis, S.M.; Kivisäkk, P.; Mahad, D.; Cathcart, M.K.; Tucky, B.; Wei, T.; Rani, M.R.S.; Horuk, R.; Aldape, K.D.; et al. CC Chemokine Receptor 8 in the Central Nervous System Is Associated with Phagocytic Macrophages. Am. J. Pathol. 2003, 162, 427–438. [Google Scholar] [CrossRef]
- Glabinski, A.R.; Bielecki, B.; Ransohoff, R.M. Chemokine Upregulation Follows Cytokine Expression in Chronic Relapsing Experimental Autoimmune Encephalomyelitis. Scand J. Immunol. 2003, 58, 81–88. [Google Scholar] [CrossRef]
- Akimoto, N.; Honda, K.; Uta, D.; Beppu, K.; Ushijima, Y.; Matsuzaki, Y.; Nakashima, S.; Kido, M.A.; Imoto, K.; Takano, Y.; et al. CCL-1 in the spinal cord contributes to neuropathic pain induced by nerve injury. Cell Death Dis. 2013, 4, e679. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Eskes, C.; Honegger, P.; Juillerat-Jeanneret, L.; Monnet-Tschudi, F. Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release. Glia 2002, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Loetscher, M.; Roos, R.S.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. B Cell–attracting Chemokine 1, a Human CXC Chemokine Expressed in Lymphoid Tissues, Selectively Attracts B Lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998, 187, 655–660. [Google Scholar] [CrossRef]
- Krumbholz, M.; Theil, D.; Cepok, S.; Hemmer, B.; Kivisäkk, P.; Ransohoff, R.M.; Hofbauer, M.; Farina, C.; Derfuss, T.; Hartle, C.; et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006, 129, 200–211. [Google Scholar] [CrossRef]
- Pipi, E.; Nayar, S.; Gardner, D.H.; Colafrancesco, S.; Smith, C.; Barone, F. Tertiary Lymphoid Structures: Autoimmunity Goes Local. Front. Immunol. 2018, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Columba-Cabezas, S.; Serafini, B.; Aloisi, F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2004, 148, 11–23. [Google Scholar] [CrossRef]
- Smith, J.R.; Braziel, R.M.; Paoletti, S.; Lipp, M.; Uguccioni, M.; Rosenbaum, J.T. Expression of B-cell–attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood 2003, 101, 815–821. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, A.; Cui, Y.; Liang, Z.; Li, B.; Bao, F. Borrelia burgdorferi basic membrane protein A could induce chemokine production in murine microglia cell line BV2. Microb. Pathog. 2017, 111, 174–181. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef]
- Rupprecht, T.A.; Kirschning, C.J.; Popp, B.; Kastenbauer, S.; Fingerle, V.; Pfister, H.-W.; Koedel, U. Borrelia garinii Induces CXCL13 Production in Human Monocytes through Toll-Like Receptor 2. Infect. Immun. 2007, 75, 4351–4356. [Google Scholar] [CrossRef]
- Codeluppi, S.; Svensson, C.I.; Hefferan, M.P.; Valencia, F.; Silldorff, M.D.; Oshiro, M.; Marsala, M.; Pasquale, E.B. The Rheb–mTOR Pathway Is Upregulated in Reactive Astrocytes of the Injured Spinal Cord. J. Neurosci. 2009, 29, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Krum, J.M.; Mani, N.; Rosenstein, J.M. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp. Neurol. 2008, 212, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.D.; Pringle, N.; Mosley, M.J.; Westermark, B.; Dubois-Dalcg, M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 1988, 53, 309–319. [Google Scholar] [CrossRef]
- Đặng, T.C.; Ishii, Y.; De Nguyen, V.; Yamamoto, S.; Hamashima, T.; Okuno, N.; Nguyen, Q.L.; Sang, Y.; Ohkawa, N.; Saitoh, Y.; et al. Powerful Homeostatic Control of Oligodendroglial Lineage by PDGFRα in Adult Brain. Cell Rep. 2019, 27, 1073–1089.e5. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, X. Directed migration of retinal astrocytes by PDGF signaling. Acta Ophthalmol. 2017, 95. [Google Scholar] [CrossRef]
- Silberstein, F.C.; De Simone, R.; Levi, G.; Aloisi, F. Cytokine-Regulated Expression of Platelet-Derived Growth Factor Gene and Protein in Cultured Human Astrocytes. J. Neurochem. 1996, 66, 1409–1417. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 2571. [Google Scholar] [CrossRef]
- Vana, A.C.; Flint, N.C.; Harwood, N.E.; Le, T.Q.; Fruttiger, M.; Armstrong, R.C. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J. Neuropathol. Exp. Neurol. 2007, 66, 975–988. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar]
- Lynch, G.; Rex, C.S.; Chen, L.Y.; Gall, C.M. The substrates of memory: Defects, treatments, and enhancement. Eur. J. Pharmacol. 2008, 585, 2–13. [Google Scholar] [CrossRef]
- de Pins, B.; Cifuentes-Díaz, C.; Farah, A.T.; López-Molina, L.; Montalban, E.; Sancho-Balsells, A.; López, A.; Ginés, S.; Delgado-García, J.M.; Alberch, J.; et al. Conditional BDNF Delivery from Astrocytes Rescues Memory Deficits, Spine Density, and Synaptic Properties in the 5xFAD Mouse Model of Alzheimer Disease. J. Neurosci. 2019, 39, 2441–2458. [Google Scholar] [CrossRef] [PubMed]
- Vondran, M.W.; Clinton-Luke, P.; Honeywell, J.Z.; Dreyfus, C.F. BDNF+/− mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia 2010, 58, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Wang, X.; Wang, Z.-Y.; Wang, Y.-Z.; Chen, L.-W.; Luo, Z.-J. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J. Neurosci. Res. 2013, 91, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.J.; Chou, C.-L.; Israel, D.D.; Xu, W.; Regan, J.W. Activation of EP2 prostanoid receptors in human glial cell lines stimulates the secretion of BDNF. Neurochem. Int. 2009, 54, 439–446. [Google Scholar] [CrossRef]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflügers Arch. -Eur. J. Physiol. 2017, 469, 593–610. [Google Scholar] [CrossRef]
- Alderson, R.F.; Curtis, R.; Alterman, A.L.; Lindsay, R.M.; DiStefano, P.S. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and Schwann cells in vitro. Brain Res. 2000, 871, 210–222. [Google Scholar] [CrossRef]
- Dougherty, K.D.; Dreyfus, C.F.; Black, I.B. Brain-Derived Neurotrophic Factor in Astrocytes, Oligodendrocytes, and Microglia/Macrophages after Spinal Cord Injury. Neurobiol. Dis. 2000, 7, 574–585. [Google Scholar] [CrossRef]
- Fulmer, C.G.; VonDran, M.W.; Stillman, A.A.; Huang, Y.; Hempstead, B.L.; Dreyfus, C.F. Astrocyte-Derived BDNF Supports Myelin Protein Synthesis after Cuprizone-Induced Demyelination. J. Neurosci. 2014, 34, 8186–8196. [Google Scholar] [CrossRef]
- Miyamoto, N.; Maki, T.; Shindo, A.; Liang, A.C.; Maeda, M.; Egawa, N.; Itoh, K.; Lo, E.K.; Lok, J.; Ihara, M.; et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J. Neurosci. 2015, 35, 14002–14008. [Google Scholar] [CrossRef]
- Ernsberger, U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008, 333, 353–371. [Google Scholar] [CrossRef][Green Version]
- Pozas, E.; Ibáñez, C.F. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron 2005, 45, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Bresjanac, M.; Antauer, G. Reactive astrocytes of the quinolinic acid-lesioned rat striatum express GFRalpha1 as well as GDNF in vivo. Exp. Neurol. 2000, 164, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Sadeghian, M.; Leung, C.C.M.; Jenner, P.; Rose, S. Lipopolysaccharide-induced nigral inflammation leads to increased IL-1β tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci. Lett. 2012, 510, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Tokumine, J.; Kakinohana, O.; Cizkova, D.; Smith, D.W.; Marsala, M. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J. Neurosci. Res. 2003, 74, 552–561. [Google Scholar] [CrossRef]
- Batchelor, P.E.; Liberatore, G.T.; Wong, J.Y.; Porritt, M.J.; Frerichs, F.; Donnan, G.A.; Howells, D.W. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 1999, 19, 1708–1716. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nitta, A.; Fukumitsu, H.; Nomoto, H.; Shen, L.; Furukawa, S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport 2005, 16, 99–102. [Google Scholar] [CrossRef]
- Duarte Azevedo, M.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef]
- Deng, L.-X.; Liu, N.-K.; Wen, R.; Yang, S.-N.; Wen, X.; Xu, X.-M. Laminin-coated multifilament entubulation, combined with Schwann cells and glial cell line-derived neurotrophic factor, promotes unidirectional axonal regeneration in a rat model of thoracic spinal cord hemisection. Neural Regen. Res. 2021, 16, 186–191. [Google Scholar] [CrossRef]
- Rocha, S.M.; Cristovão, A.C.; Campos, F.L.; Fonseca, C.P.; Baltazar, G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol. Dis. 2012, 47, 407–415. [Google Scholar] [CrossRef]
- Brambilla, L.; Guidotti, G.; Martorana, F.; Iyer, A.M.; Aronica, E.; Valori, C.F.; Rossi, D. Disruption of the astrocytic TNFR1-GDNF axis accelerates motor neuron degeneration and disease progression in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2016, 25, 3080–3095. [Google Scholar] [CrossRef][Green Version]
- Goss, J.R.; O’Malley, M.E.; Zou, L.; Styren, S.D.; Kochanek, P.M.; DeKosky, S.T. Astrocytes Are the Major Source of Nerve Growth Factor Upregulation Following Traumatic Brain Injury in the Rat. Exp. Neurol. 1998, 149, 301–309. [Google Scholar] [CrossRef]
- Lipnik-Stangelj, M.; Surcheva, S.; Ferjan, I.; Vlaskovska, M. Astrocytes and Chronic Pain Mechanisms—The Role of Histamine, IL-1β and NGF. Biotechnol. Biotechnol. Equip. 2013, 27, 3534–3539. [Google Scholar] [CrossRef]
- Boutros, T.; Croze, E.; Yong, V.W. Interferon-beta is a potent promoter of nerve growth factor production by astrocytes. J. Neurochem. 1997, 69, 939–946. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpakowski, P.; Ksiazek-Winiarek, D.; Turniak-Kusy, M.; Pacan, I.; Glabinski, A. Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli. Biomedicines 2022, 10, 1769. https://doi.org/10.3390/biomedicines10081769
Szpakowski P, Ksiazek-Winiarek D, Turniak-Kusy M, Pacan I, Glabinski A. Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli. Biomedicines. 2022; 10(8):1769. https://doi.org/10.3390/biomedicines10081769
Chicago/Turabian StyleSzpakowski, Piotr, Dominika Ksiazek-Winiarek, Malgorzata Turniak-Kusy, Ilona Pacan, and Andrzej Glabinski. 2022. "Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli" Biomedicines 10, no. 8: 1769. https://doi.org/10.3390/biomedicines10081769
APA StyleSzpakowski, P., Ksiazek-Winiarek, D., Turniak-Kusy, M., Pacan, I., & Glabinski, A. (2022). Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli. Biomedicines, 10(8), 1769. https://doi.org/10.3390/biomedicines10081769





