A Preliminary Study for Tunable Optical Assessment of Exhaled Breath Ammonia Based on Ultrathin Tetrakis(4-sulfophenyl)porphine Nanoassembled Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
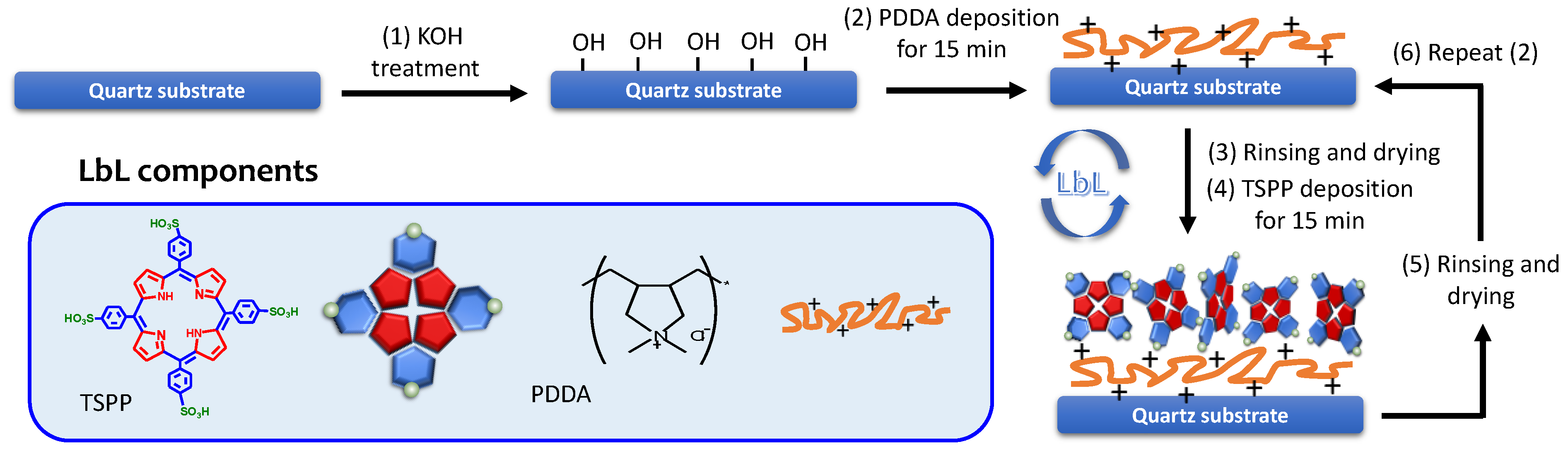
2.2. Film Preparation
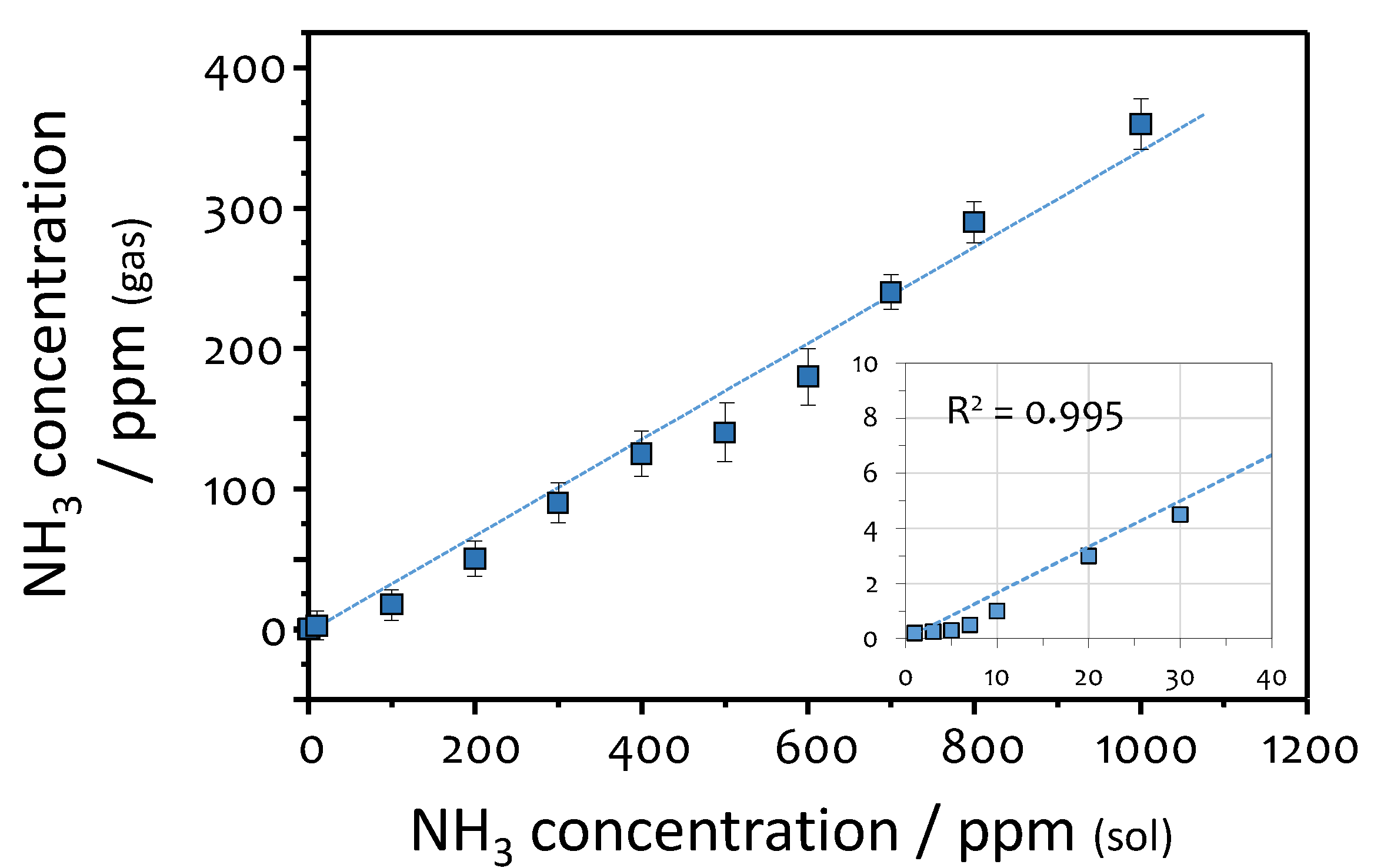
2.3. Calibration of the Sensor Response to Ammonia
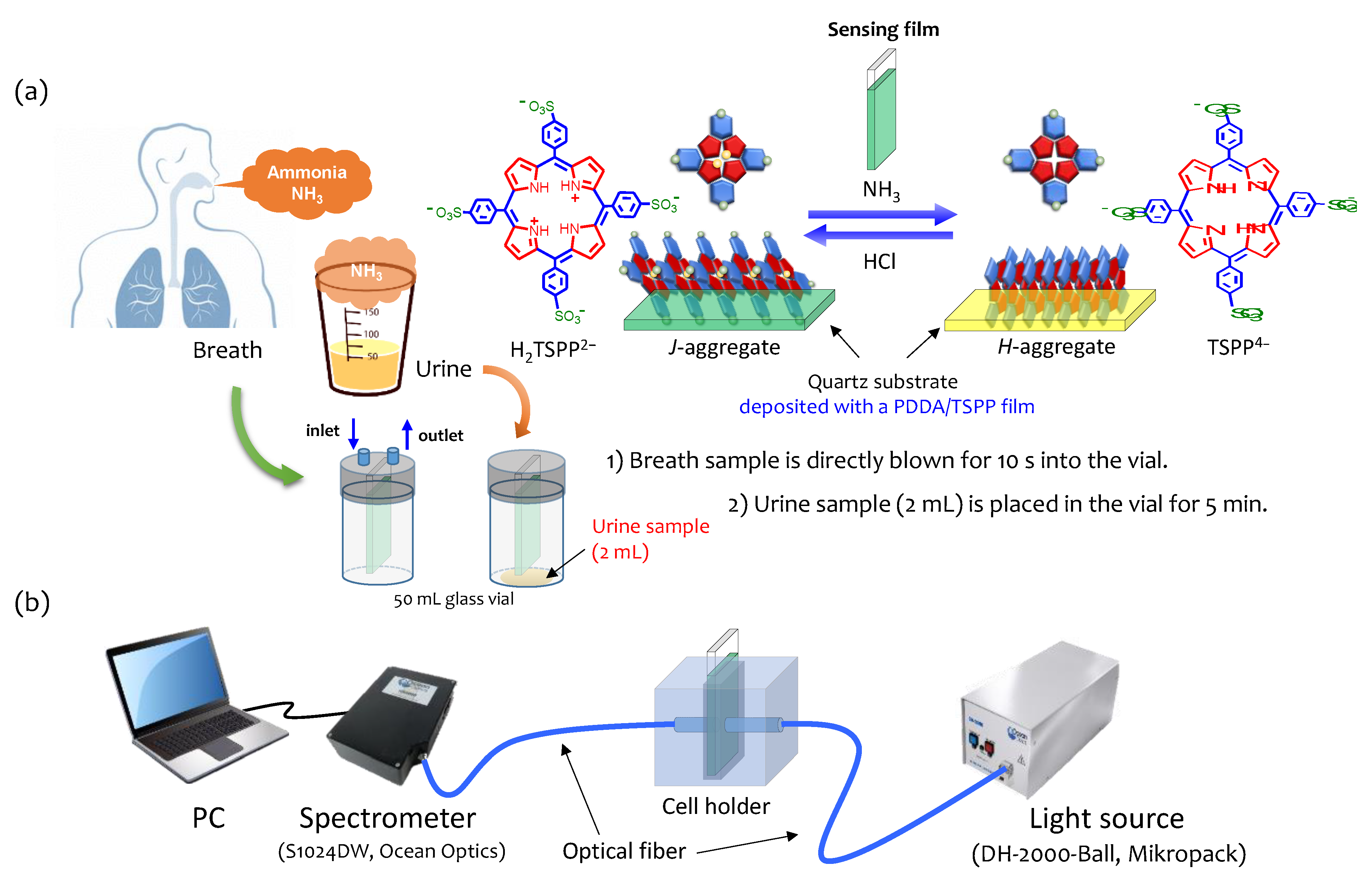
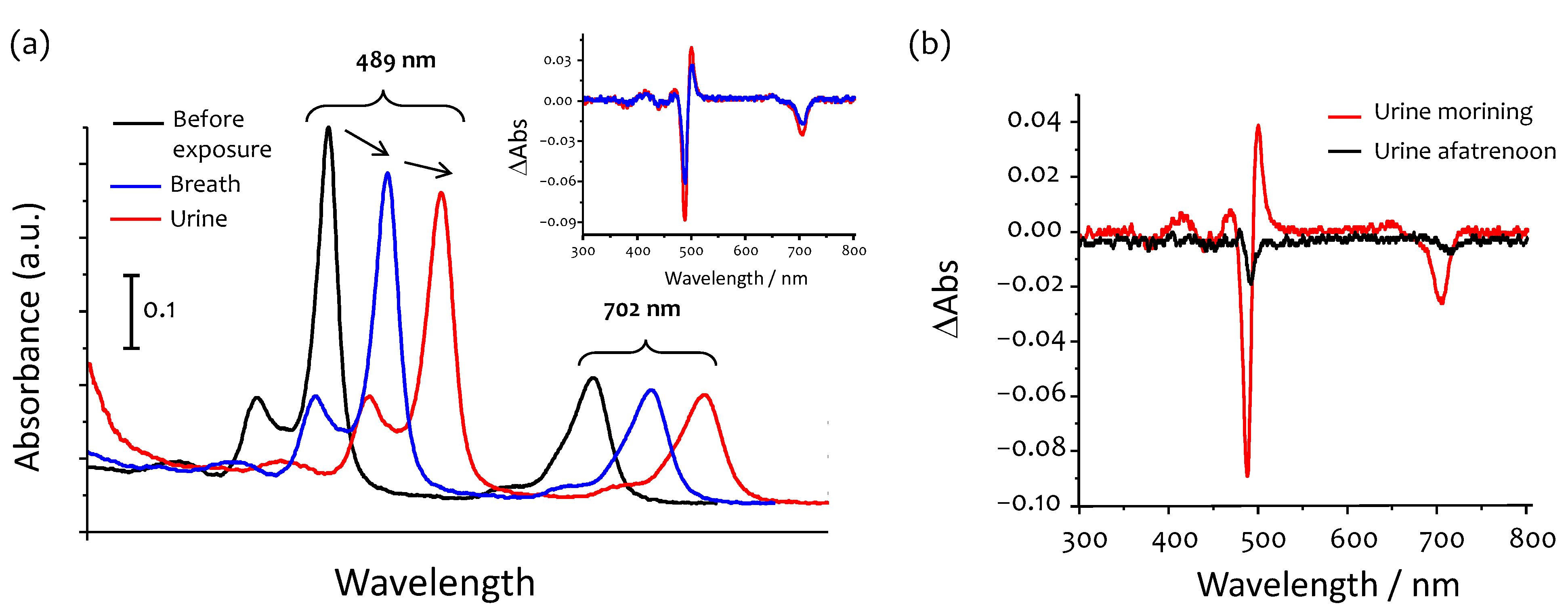
2.4. Measurements of Ammonia in Breath and Urine
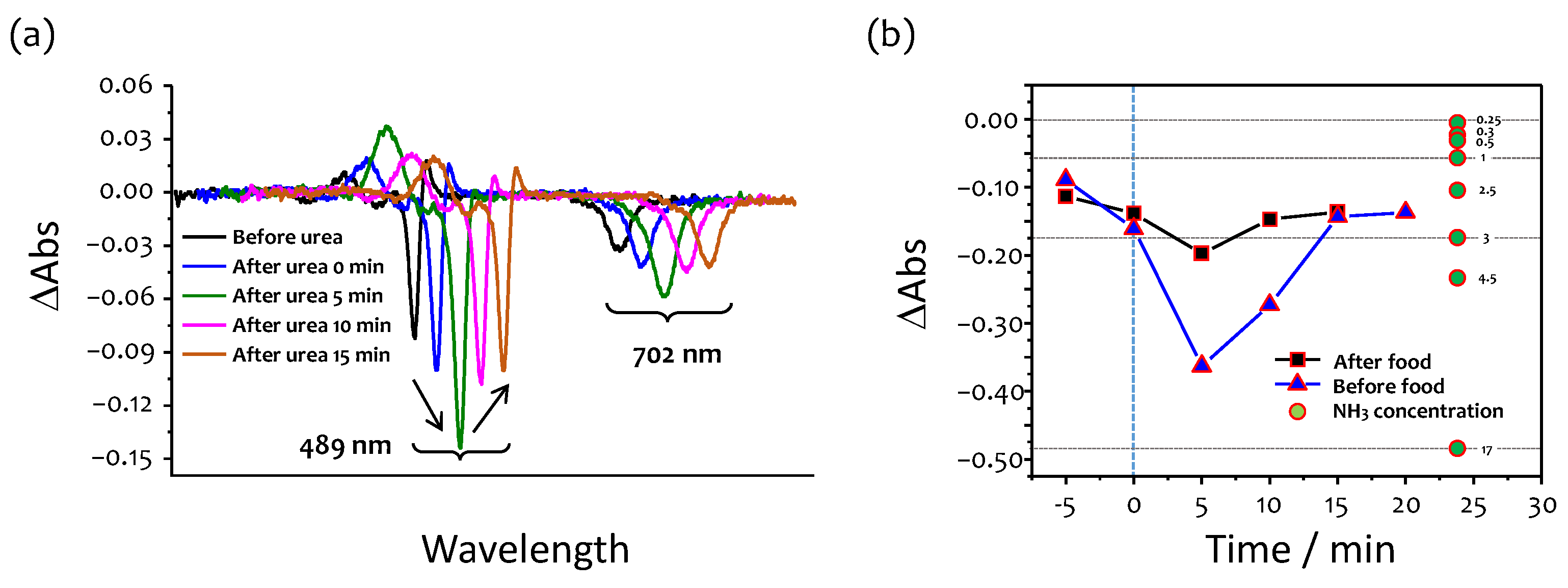
2.5. Urea Test
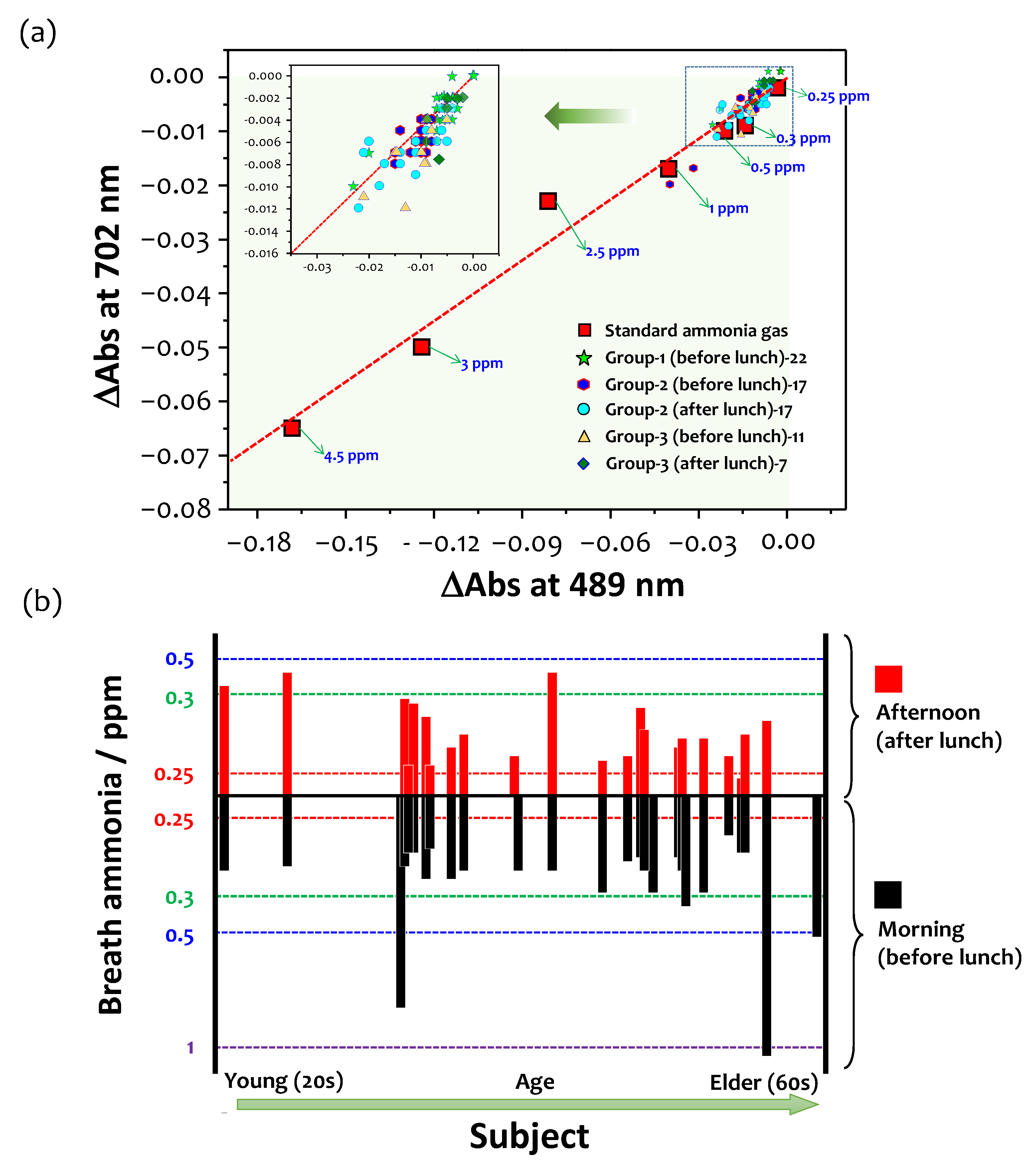
2.6. A Large-Scale Study
3. Results and Discussion
3.1. PDDA/TSPP Film Deposition and Sensitivity Improvement
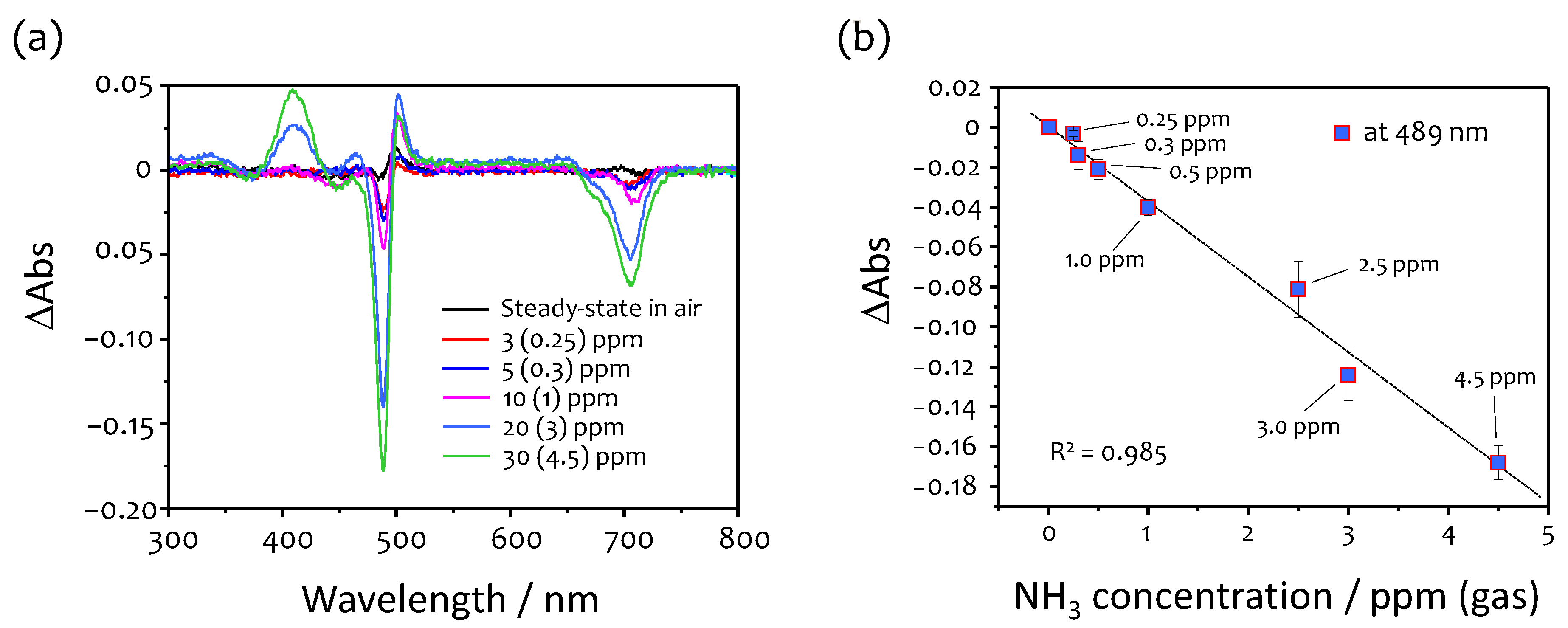
3.2. Response to Ammonia
3.3. Humidified Breath Ammonia Detection and Selectivity
3.4. Ammonia in Urine
3.5. Effects of Food Consumption
3.6. Urea Test
3.7. A Large-Scale Study and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brannelly, N.T.; Hamilton-Shield, J.P.; Killard, A.J. The measurement of ammonia in human breath and its potential in clinical diagnostics. Crit. Rev. Anal. Chem. 2016, 46, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry, SIFT-MS. Physiol. Meas. 2006, 27, 321–337. [Google Scholar] [CrossRef]
- Liu, L.; Fei, T.; Guan, X.; Zhao, H.; Zhang, T. Humidity-activated ammonia sensor with excellent selectivity for exhaled breath analysis. Sens. Actuators B Chem. 2021, 334, 129625. [Google Scholar] [CrossRef]
- Shahmoradi, A.; Hosseini, A.; Akbarinejad, A.; Alizadeh, N. Noninvasive detection of ammonia in the breath of hemodialysis patients using a highly sensitive ammonia sensor based on a polypyrrole/sulfonated graphene nanocomposite. Anal. Chem. 2021, 93, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent advances in ammonia gas sensors based on carbon nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef]
- Barsotti, R.J. Measurement of ammonia in blood. J. Pediatr. 2001, 138, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.P. Ammonia, a troublesome analyte. Clin. Biochem. 2014, 47, 753. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. What is the real utility of breath ammonia concentration measurements in medicine and physiology? J. Breath Res. 2018, 12, 027102. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.V.; Covert, D.S.; Frank, R. A Method for continuous measurement of ammonia in respiratory airways. J. Appl. Physiol. 1979, 46, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Kuwabara, Y.; Murata, H.; Kobashi, K.; Watanabe, A. Measurement of the Expiratory Ammonia Concentration and Its Clinical Significance. Metab. Brain Dis. 1997, 12, 161–169. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.; Eng, S.; Bhattacharya, R.; Rulyak, S.; Hubbard, T.; Putnam, D.; Kearney, D.J. Breath ammonia testing for diagnosis of hepatic encephalopathy. Dig. Dis. Sci. 2005, 50, 1780–1784. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Vaittinen, O.; Metsälä, M.; Lehto, M.; Forsblom, C.; Groop, P.H.; Halonen, L. Ammonia in breath and emitted from skin. J. Breath Res. 2013, 7, 017109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germanese, D.; Colantonio, S.; D’Acunto, M.; Romagnoli, V.; Salvati, A.; Brunetto, M. An E-Nose for the Monitoring of Severe Liver Impairment: A Preliminary Study. Sensors 2019, 19, 3656. [Google Scholar] [CrossRef] [Green Version]
- Brannelly, N.T.; Killard, A.J. An electrochemical sensor device for measuring blood ammonia at the point of care. Talanta 2017, 167, 296–301. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Wang, T.; Pysanenko, A.; Dryahina, K.; Španěl, P.; Smith, D. Analysis of breath, exhaled via the mouth and nose, and the air in the oral cavity. J. Breath Res. 2008, 2, 037013. [Google Scholar] [CrossRef]
- Hibbard, T.; Killard, A.J. Breath ammonia analysis: Clinical application and measurement. Crit. Rev. Anal. Chem. 2011, 41, 21–35. [Google Scholar] [CrossRef]
- Korposh, S.; Selyanchyn, R.; Yasukochi, W.; Lee, S.-W.; James, S.; Tatam, R. Optical fibre long period grating with a nanoporous coating formed from silica nanoparticles for ammonia sensing in water. Mater. Chem. Phys. 2012, 133, 784–792. [Google Scholar] [CrossRef]
- Wang, T.; Korposh, S.; James, S.; Tatam, R.; Lee, S.-W. Optical fiber long period grating sensor with a polyelectrolyte alternate thin film for gas sensing of amine odors. Sens. Actuators B Chem. 2013, 185, 117–124. [Google Scholar] [CrossRef]
- Korposh, S.; Kodaira, S.; Selyanchyn, R.; Ledezma, F.H.; James, S.W.; Lee, S.-W. Porphyrin-nanoassembled fiber-optic gas sensor fabrication: Optimization of parameters for sensitive ammonia gas detection. Opt. Laser Technol. 2018, 101, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Korposh, S.O.; Takahara, N.; Ramsden, J.J.; Lee, S.-W.; Kunitake, T. Nano-assembled thin film gas sensors. I. Ammonia detection by a porphyrin-based multilayer film. J. Biol. Phys. Chem. 2006, 6, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochalski, P.; King, J.; Haas, M.; Unterkofler, K.; Amann, A.; Mayer, G. Blood and breath profiles of volatile organic compounds in patients with end-stage renal disease. BMC Nephrol. 2014, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornienko, E.A.; Dmitrienko, M.A. Ama rapid urease test and helic ammonia breath test for H. pylori detection. In Handbook for Specialists; Saint-Petersburg State Pediatric Medicine Academy: Saint-Petersburg, Russia, 2010. [Google Scholar]
- Desimoni, E.; Brunetti, B. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta 2015, 6, 355. [Google Scholar]
- Liu, L.; Morgan, S.P.; Correia, R.; Lee, S.-W.; Korposh, S. Multi-Parameter Optical Fiber Sensing of Gaseous Ammonia and Carbon Dioxide. J. Light. Technol. 2020, 38, 2037–2045. [Google Scholar] [CrossRef]
- Ahmed, S.; Park, Y.; Okuda, H.; Ono, S.; Korposh, S.; Lee, S.-W. Fabrication of Humidity-Resistant Optical Fiber Sensor for Ammonia Sensing using Diazo Resin-Photocrosslinked Films with a Porphyrin-Polystyrene Binary Mixture. Sensors 2021, 21, 6176. [Google Scholar] [CrossRef]
- Diskin, A.M.; Spanel, P.; Smith, D. Increase of acetone and ammonia in urine headspace and breath during ovulation quantified using selected ion flow tube mass spectrometry. Physiol. Meas. 2003, 24, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Pojman, J.A.; Scott, S.K.; Wrobel, M.M.; Taylor, A.F. Base-catalyzed feedback in the urea-urease reaction. J. Phys. Chem. B 2010, 114, 14059–14063. [Google Scholar] [CrossRef]
- Pandey, S.; Nanda, K.K. Au nanocomposite based chemiresistive ammonia sensor for health monitoring. ACS Sens. 2016, 1, 55–62. [Google Scholar] [CrossRef]
- Toda, K.; Li, J.; Dasgupta, P.K. Measurement of ammonia in human breath with a liquid-film conductivity sensor. Anal. Chem. 2006, 78, 7284–7291. [Google Scholar] [CrossRef]
- Solga, S.F.; Mudalel, M.L.; Spacek, L.A.; Risby, T.H. Fast and accurate exhaled breath ammonia measurement. J. Vis. Exp. 2014, 88, 51658. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S.; Posch, H.E. Fibre-optic fluorescing sensor for ammonia. Anal. Chim. Acta 1986, 185, 321–327. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Zamarreño, C.R.; Matías, I.R.; Arregui, F.J.; Domínguez Cruz, R.F.; May-Arrioja, D.A. A fiber optic ammonia sensor using a universal pH indicator. Sensors 2014, 143, 4060–4073. [Google Scholar] [CrossRef]
- Yagi, T.; Kuboki, N.; Suzuki, Y.; Uchino, N.; Nakamura, K.; Yoshida, K. Fiber-optic ammonia sensors utilizing rectangular-cladding eccentric-core fiber. Opt. Rev. 1997, 4, 596–600. [Google Scholar] [CrossRef]
- Cao, W.; Duan, Y. Optical fiber-based evanescent ammonia sensor. Sens. Actuators B Chem. 2005, 110, 252–259. [Google Scholar] [CrossRef]
- Galbarra, D.; Arregui, F.J.; Matias, I.R.; Claus, R.O. Ammonia optical fiber sensor based on self-assembled zirconia thin films. Smart Mater. Struct. 2005, 14, 739–744. [Google Scholar] [CrossRef]
- Chu, C.-S.; Chen, Y.-F. Development of ratiometric optical fiber sensor for ammonia gas detection. In 25th Optical Fiber Sensors Conference (OFS), Proceedings of the SPIE 10323, Jeju, Korea, 23 April 2017; International Society for Optical Engineering: Washington, DC, USA, 2017; Volume 10323, p. 103231P. [Google Scholar] [CrossRef]
- Tiwari, D.; Mullaney, K.; Korposh, S.; James, S.W.; Lee, S.-W.; Tatam, R.P. An ammonia sensor based on Lossy Mode Resonances on a tapered optical fibre coated with porphyrin-incorporated titanium dioxide. Sens. Actuators B Chem. 2017, 242, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Yasukochi, W.; Korposh, S.; James, S.W.; Tatam, R.P.; Lee, S.-W. A long period grating optical fiber sensor with nano-assembled porphyrin layers for detecting ammonia gas. Sens. Actuators B Chem. 2016, 228, 573–580. [Google Scholar] [CrossRef]



| Type of Sensor | Sensitive Element | Lower Detection Limit (LDL) or Lowest Measured Concentration (LMC) | Response Time | Reference |
|---|---|---|---|---|
| Evanescent wave | Universal pH indicator | 10 ppm (LMC) | 5 min | [35] |
| Bromocresol purple/bromocresol green, dip coating sol‒gel | 9 ppm (LMC) 0.014 dB/ppm | 8 s | [36] | |
| Bromocresol purple, sol‒gel | 145 ppm (LMC) | 10 s | [37] | |
| Nanoassembled PDDA/TSPP | 6 ppm | 15 s | [20,21] | |
| Reflection | Nanoassembled ZrO2/PSS * | 1 wt% (LMC) | ‒ | [38] |
| Oxazine 170 perchlorate | 200 ppm (LMC) | ‒ | [39] | |
| Lossy mode resonance | TMPyP **-doped TiO2 nanocoating | 0.1 ppm (LMC) | 30 s | [40] |
| Grating | Nanoassembled PDDA/TSPP | 0.67 ppm (LDL) | ‒ | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korposh, S.; Lee, S.-W. A Preliminary Study for Tunable Optical Assessment of Exhaled Breath Ammonia Based on Ultrathin Tetrakis(4-sulfophenyl)porphine Nanoassembled Films. Chemosensors 2021, 9, 269. https://doi.org/10.3390/chemosensors9090269
Korposh S, Lee S-W. A Preliminary Study for Tunable Optical Assessment of Exhaled Breath Ammonia Based on Ultrathin Tetrakis(4-sulfophenyl)porphine Nanoassembled Films. Chemosensors. 2021; 9(9):269. https://doi.org/10.3390/chemosensors9090269
Chicago/Turabian StyleKorposh, Sergiy, and Seung-Woo Lee. 2021. "A Preliminary Study for Tunable Optical Assessment of Exhaled Breath Ammonia Based on Ultrathin Tetrakis(4-sulfophenyl)porphine Nanoassembled Films" Chemosensors 9, no. 9: 269. https://doi.org/10.3390/chemosensors9090269
APA StyleKorposh, S., & Lee, S.-W. (2021). A Preliminary Study for Tunable Optical Assessment of Exhaled Breath Ammonia Based on Ultrathin Tetrakis(4-sulfophenyl)porphine Nanoassembled Films. Chemosensors, 9(9), 269. https://doi.org/10.3390/chemosensors9090269







