Rational Design and Synthesis of Large Stokes Shift 2,6-Sulphur-Disubstituted BODIPYs for Cell Imaging
Abstract
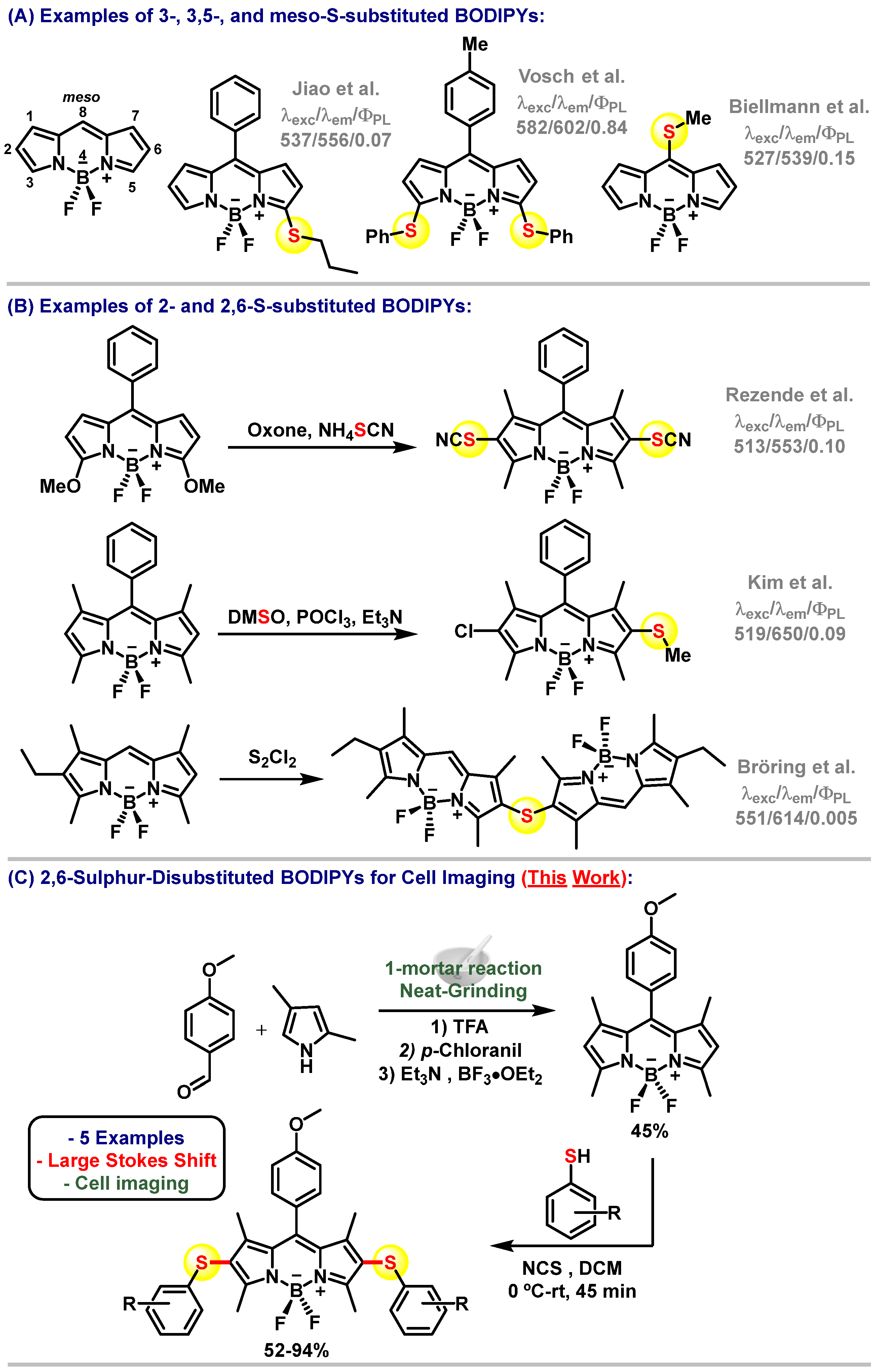
1. Introduction
2. Materials and Methods
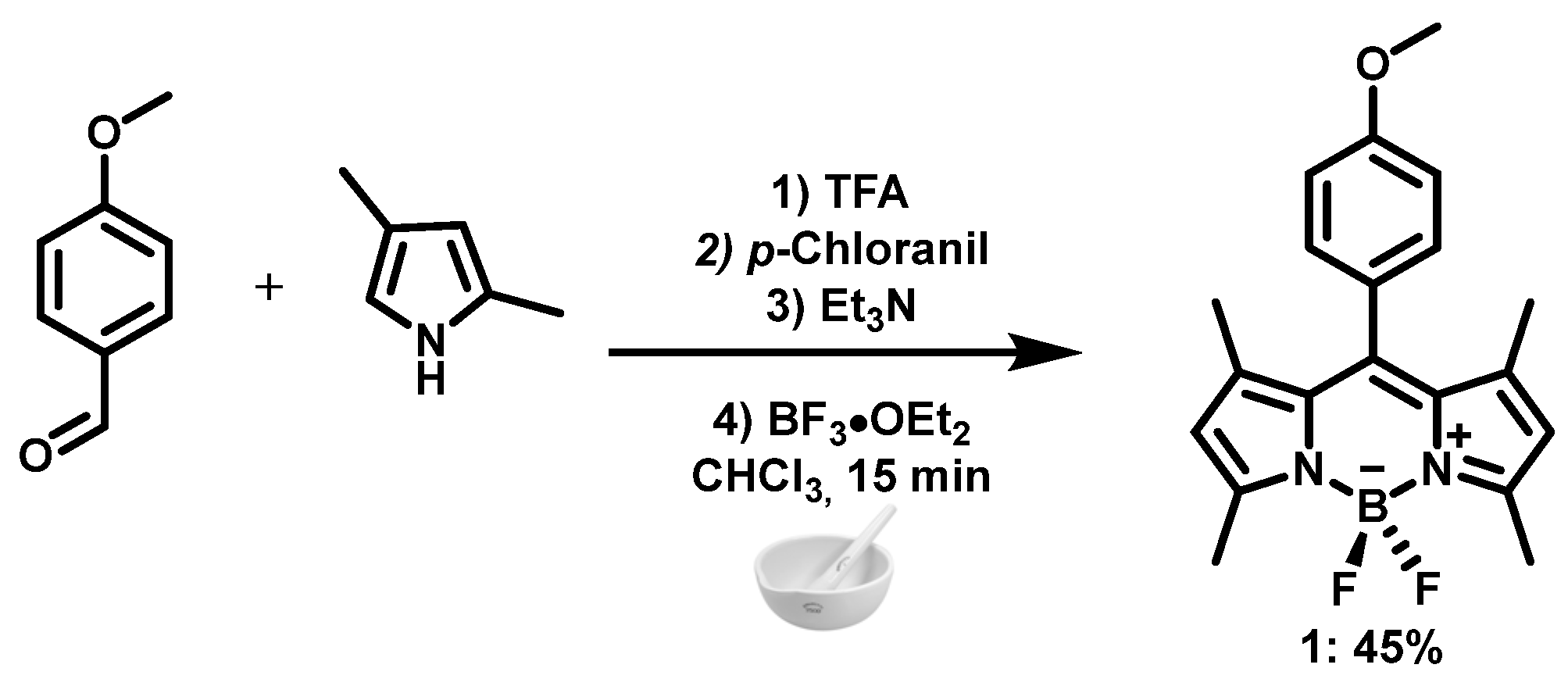
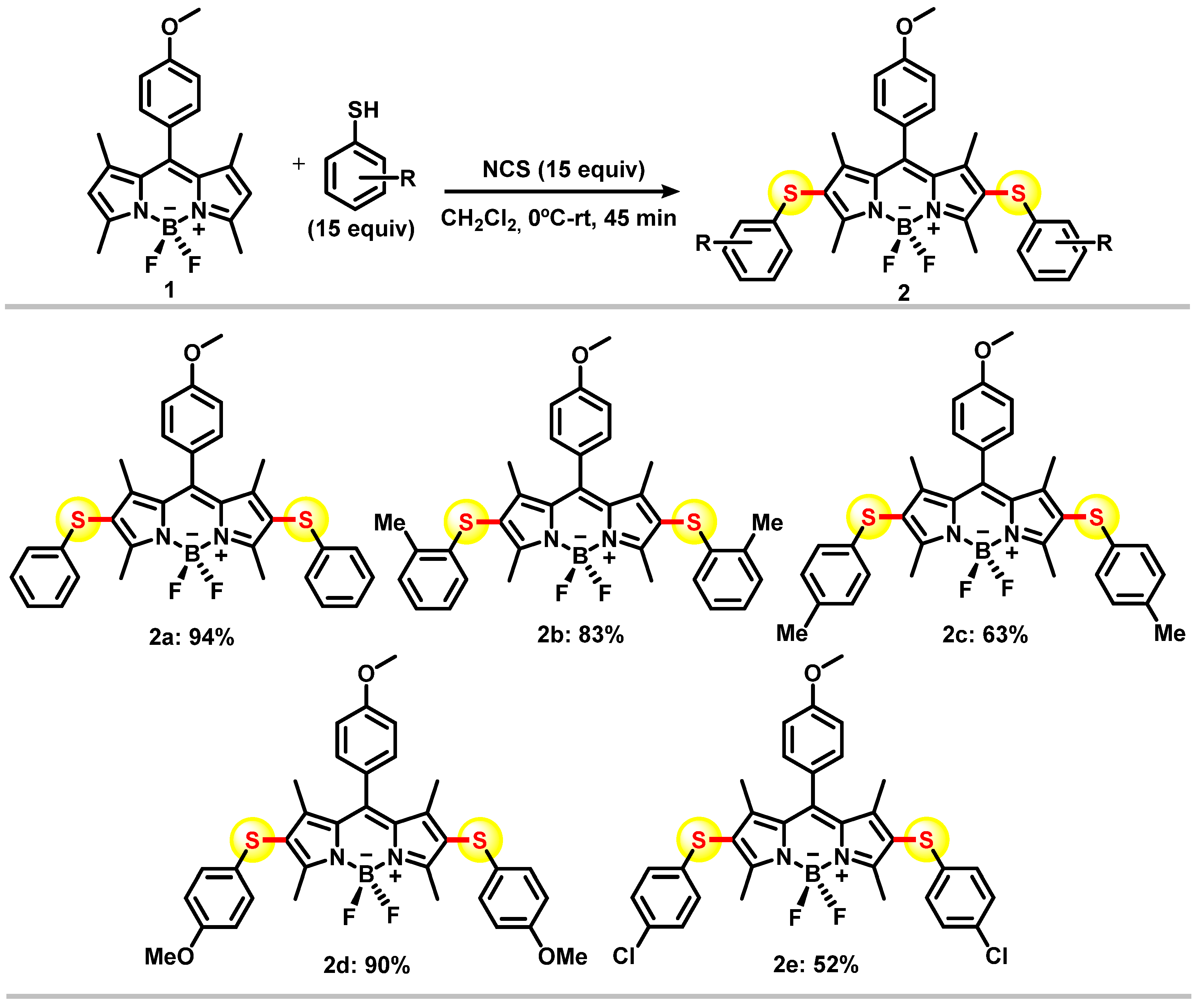
2.1. Chemistry
2.2. Photophysical Measurements
2.3. Biological Experiments
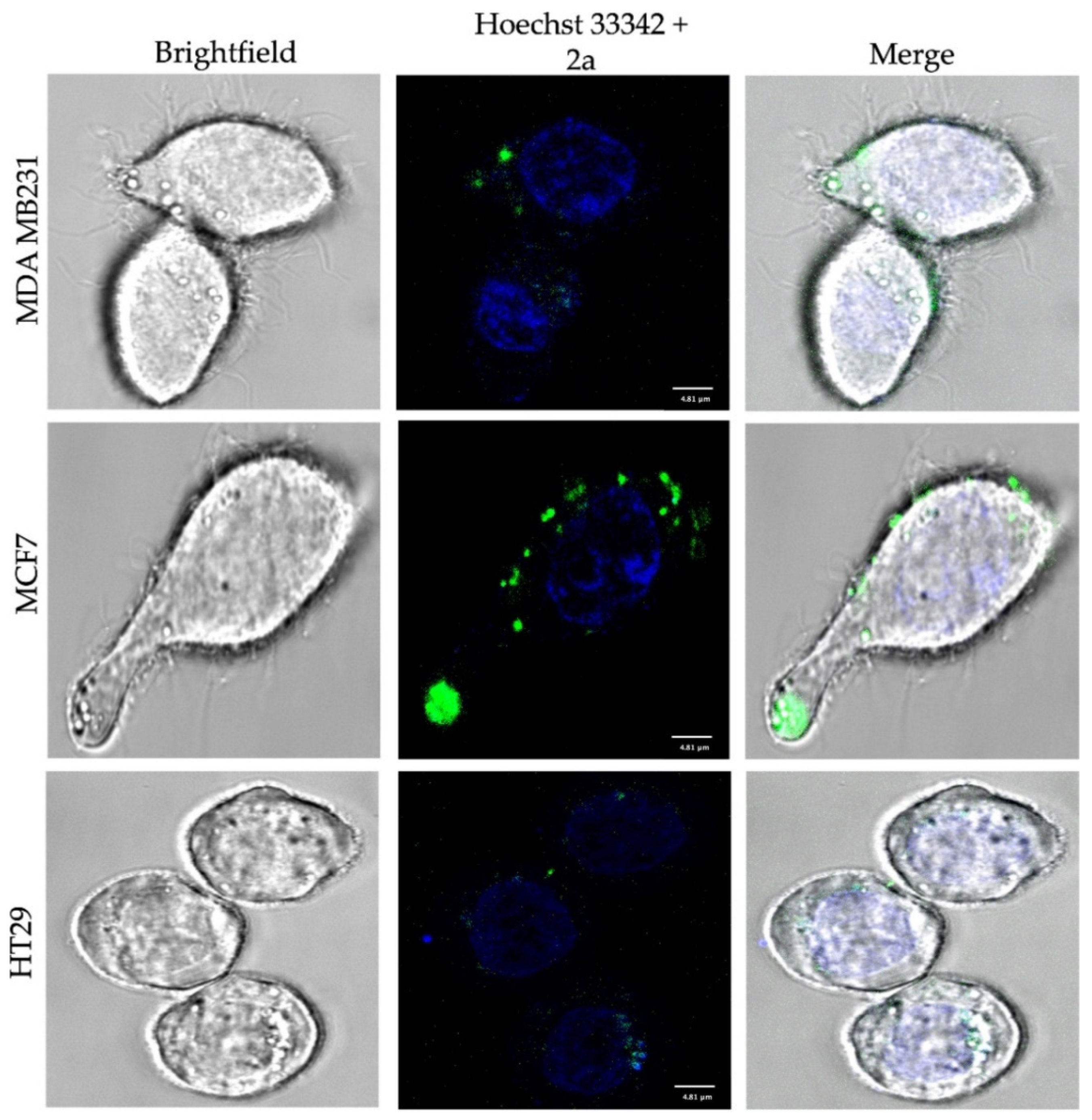
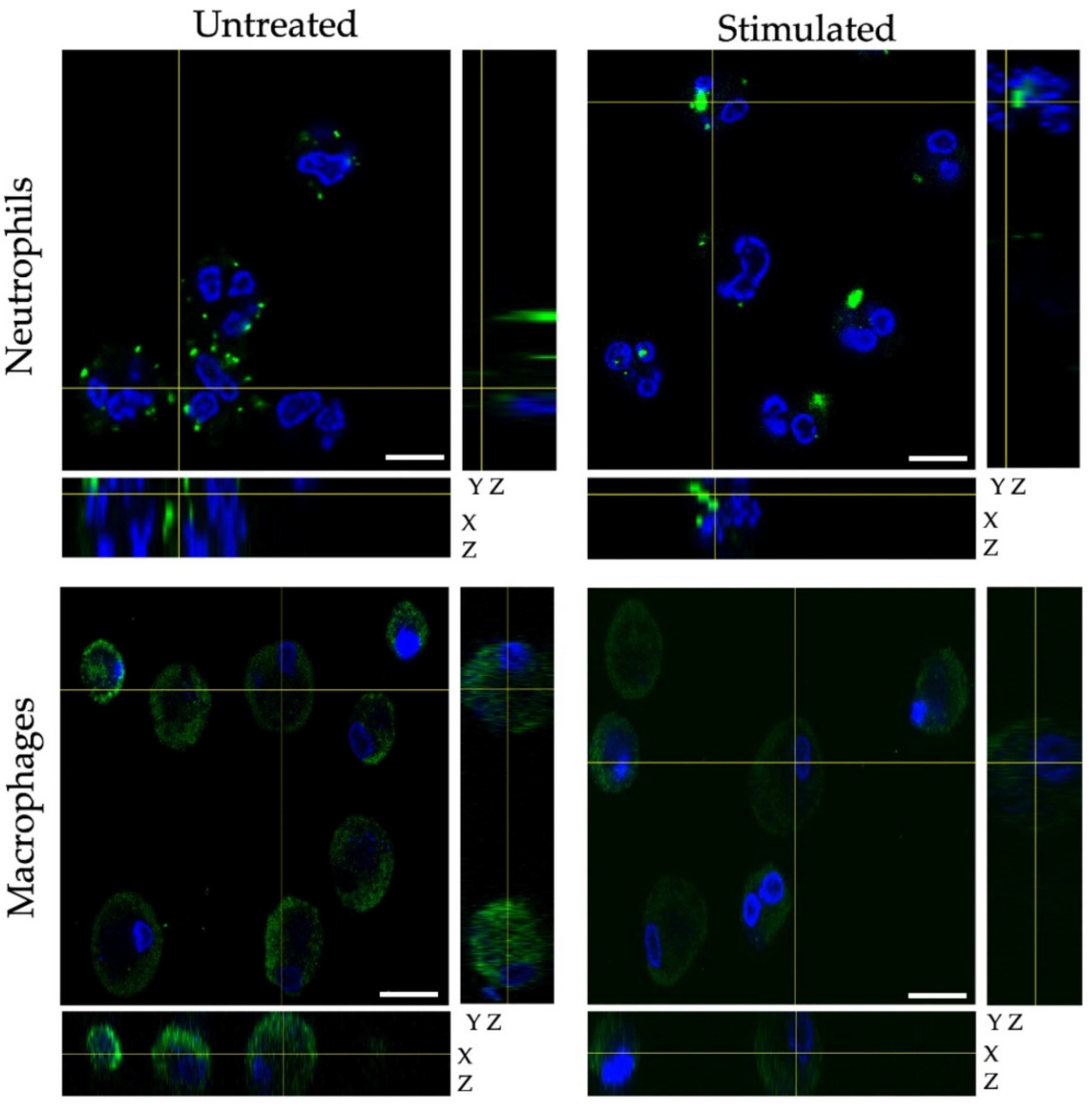
2.4. Fluorescence Confocal Microscopy
2.5. Crystallography
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Mestres, G.; Singh, V.P.; Effati, P.; Poon, J.F.; Engman, L.; Ott, M.K. Selenium- and Tellurium-Based Antioxidants for Modulating Inflammation and Effects on Osteoblastic Activity. Antioxidants 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Zugman, T.; Bandeira, P.; Dalmolin, M.; Scariot, D.; Garcia, F.; De Oliveira, A.; Nakamura, C.; Piovan, L. Complementary Performance of Organoselenides and Organotellurides as Antimicrobials Agents. J. Braz. Chem. Soc. 2021, 32, 642–675. [Google Scholar] [CrossRef]
- Galant, L.S.; Rafique, J.; Braga, A.L.; Braga, F.C.; Saba, S.; Radi, R.; da Rocha, J.B.T.; Santi, C.; Monsalve, M.; Farina, M.; et al. The Thiol-Modifier Effects of Organoselenium Compounds and Their Cytoprotective Actions in Neuronal Cells. Neurochem. Res. 2021, 46, 120–130. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Lima, D.J.B.; Valença, W.O.; Cavalcanti, B.C.; Pessoa, C.; Rafique, J.; Braga, A.L.; Jacob, C.; da Silva Junior, E.N.; Da Cruz, E.H.G. Synthesis of Selenium-Quinone Hybrid Compounds with Potential Antitumor Activity via Rh-Catalyzed C-H Bond Activation and Click Reactions. Molecules 2017, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Kharma, A.; Jacob, C.; Bozzi, Í.A.O.; Jardim, G.A.M.; Braga, A.L.; Salomão, K.; Gatto, C.C.; Silva, M.F.S.; Pessoa, C.; Stangier, M.; et al. Electrochemical Selenation/Cyclization of Quinones: A Rapid, Green and Efficient Access to Functionalized Trypanocidal and Antitumor Compounds. Eur. J. Org. Chem. 2020, 2020, 4474–4486. [Google Scholar] [CrossRef]
- Vieira, A.A.; Brandão, I.R.; Valença, W.O.; de Simone, C.A.; Cavalcanti, B.C.; Pessoa, C.; Carneiro, T.R.; Braga, A.L.; da Silva, E.N. Hybrid compounds with two redox centres: Modular synthesis of chalcogen-containing lapachones and studies on their antitumor activity. Eur. J. Med. Chem. 2015, 101, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; de Moliner, F.; Fernandez, A.; Kuru, E.; Asiimwe, N.L.; Lee, J.-S.; Hamilton, L.; Sieger, D.; Bravo, I.R.; Elliot, A.M.; et al. Photoactivatable metabolic warheads enable precise and safe ablation of target cells in vivo. Nat. Commun. 2021, 12, 2369. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Fernandez, A.; Barth, N.D.; De Moliner, F.; Horrocks, M.H.; Herrington, C.S.; Abad, J.L.; Delgado, A.; Kelly, L.; Chang, Z.; et al. SCOTfluors: Small, Conjugatable, Orthogonal, and Tunable Fluorophores for In Vivo Imaging of Cell Metabolism. Angew. Chem. Int. Ed. 2019, 58, 6911–6915. [Google Scholar] [CrossRef]
- Fernandez, A.; Thompson, E.J.; Pollard, J.W.; Kitamura, T.; Vendrell, M. A Fluorescent Activatable AND-Gate Chemokine CCL2 Enables In Vivo Detection of Metastasis-Associated Macrophages. Angew. Chem. Int. Ed. 2019, 58, 16894–16898. [Google Scholar] [CrossRef]
- Mellanby, R.J.; Scott, J.I.; Mair, I.; Fernandez, A.; Saul, L.; Arlt, J.; Moral, M.; Vendrell, M. Tricarbocyanine N-triazoles: The scaffold-of-choice for long-term near-infrared imaging of immune cells in vivo. Chem. Sci. 2018, 9, 7261–7270. [Google Scholar] [CrossRef]
- Zhao, C.; Fernandez, A.; Avlonitis, N.; Velde, G.V.; Bradley, M.; Read, N.D.; Vendrell, M. Searching for the Optimal Fluorophore to Label Antimicrobial Peptides. ACS Comb. Sci. 2016, 18, 689–696. [Google Scholar] [CrossRef]
- Mendive-Tapia, L.; Subiros-Funosas, R.; Zhao, C.; Albericio, F.; Read, N.D.; Lavilla, R.; Vendrell, M. Preparation of a Trp-BODIPY fluorogenic amino acid to label peptides for enhanced live-cell fluorescence imaging. Nat. Protoc. 2017, 12, 1588–1619. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Yeo, H.C.; Kang, N.-Y.; Kim, H.; Lin, J.; Ha, H.-H.; Vendrell, M.; Lee, J.-S.; Chandran, Y.; Lee, D.-Y.; et al. Mechanistic elements and critical factors of cellular reprogramming revealed by stepwise global gene expression analyses. Stem Cell Res. 2014, 12, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; He, H.; Wang, S.; Lei, Z.; Zhang, F. ROS/RNS and Base Dual Activatable Merocyanine-Based NIR-II Fluorescent Molecular Probe for in vivo Biosensing. Angew. Chem. Int. Ed. Engl. 2021, 60, 26337–26341. [Google Scholar] [CrossRef]
- Gao, M.; Lee, S.H.; Park, S.H.; Ciaramicoli, L.M.; Kwon, H.Y.; Cho, H.; Jeong, J.; Chang, Y.T. Neutro-phil-Selective Fluorescent Probe Development through Metabolism-Oriented Live-Cell Distinction. Angew. Chem. Int. Ed. Engl. 2021, 60, 23743–23749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Jaschke, A.; Sunbul, M. A Color-Shifting Near-Infrared Fluorescent Aptamer-Fluorophore Module for Live-Cell RNA Imaging. Angew. Chem. Int. Ed. Engl. 2021, 60, 21441–21448. [Google Scholar] [CrossRef]
- Scott, J.I.; Deng, Q.; Vendrell, M. Near-Infrared Fluorescent Probes for the Detection of Cancer-Associated Proteases. ACS Chem. Biol. 2021, 16, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Oe, M.; Miki, K.; Ohe, K. An enzyme-triggered turn-on fluorescent probe based on carboxylate-induced detachment of a fluorescence quencher. Org. Biomol. Chem. 2020, 18, 8620–8624. [Google Scholar] [CrossRef]
- Nobori, T.; Kawamura, M.; Yoshida, R.; Joichi, T.; Kamino, K.; Kishimura, A.; Baba, E.; Mori, T.; Katayama, Y. Fluorescence Signal Amplification by Using beta-Galactosidase for Flow Cytometry; Advantages of an Endogenous Activity-Free Enzyme. Anal. Chem. 2020, 92, 3069–3076. [Google Scholar] [CrossRef]
- Yao, C.; Li, Y.; Wang, Z.; Song, C.; Hu, X.; Liu, S. Cytosolic NQO1 Enzyme-Activated Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Polymeric Vesicles. ACS Nano 2020, 14, 1919–1935. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.; Gao, J.; Xu, G.; Zhu, T.; Gu, X.; Guo, Z.; Zhu, W.-H.; Zhao, C. A molecular design strategy toward enzyme-activated probes with near-infrared I and II fluorescence for targeted cancer imaging. Chem. Sci. 2019, 10, 7222–7227. [Google Scholar] [CrossRef]
- Li, X.; Bottini, M.; Zhang, L.; Zhang, S.; Chen, J.; Zhang, T.; Liu, L.; Rosato, N.; Ma, X.; Shi, X.; et al. Core–Satellite Nanomedicines for in Vivo Real-Time Monitoring of Enzyme-Activatable Drug Release by Fluorescence and Photoacoustic Dual-Modal Imaging. ACS Nano 2018, 13, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Barth, N.D.; Subiros-Funosas, R.; Mendive-Tapia, L.; Duffin, R.; Shields, M.A.; Cartwright, J.A.; Henriques, S.T.; Sot, J.; Goñi, F.M.; Lavilla, R.; et al. A fluorogenic cyclic peptide for imaging and quantification of drug-induced apoptosis. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Cheng, Z.; Kuru, E.; Sachdeva, A.; Vendrell, M. Fluorescent amino acids as versatile building blocks for chemical biology. Nat. Rev. Chem. 2020, 4, 275–290. [Google Scholar] [CrossRef]
- Yraola, F.; Ventura, R.; Vendrell, M.; Colombo, A.; Fernàndez, J.-C.; De La Figuera, N.; Fernández-Forner, D.; Royo, M.; Forns, P.; Albericio, F. A Re-evaluation of the Use of Rink, BAL, and PAL Resins and Linkers. QSAR Comb. Sci. 2004, 23, 145–152. [Google Scholar] [CrossRef]
- Zerfas, B.L.; Coleman, R.A.; Salazar-Chaparro, A.F.; Macatangay, N.J.; Trader, D.J. Fluorescent Probes with Un-natural Amino Acids to Monitor Proteasome Activity in Real-Time. ACS Chem. Biol. 2020, 15, 2588–2596. [Google Scholar] [CrossRef]
- Kuru, E.; Lambert, C.; Rittichier, J.; Till, R.; Ducret, A.; Derouaux, A.; Gray, J.; Biboy, J.; Vollmer, W.; VanNieuwenhze, M.; et al. Fluorescent D-amino-acids reveal bi-cellular cell wall modifications important for Bdellovibrio bacteriovorus predation. Nat. Microbiol. 2017, 2, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Harkiss, A.H.; Sutherland, A. Recent advances in the synthesis and application of fluorescent α-amino acids. Org. Biomol. Chem. 2016, 14, 8911–8921. [Google Scholar] [CrossRef]
- Cheruku, P.; Huang, J.-H.; Yen, H.-J.; Iyer, R.S.; Rector, K.D.; Martinez, J.S.; Wang, H.-L. Tyrosine-derived stimuli responsive, fluorescent amino acids. Chem. Sci. 2015, 6, 1150–1158. [Google Scholar] [CrossRef]
- Krueger, A.T.; Imperiali, B. Fluorescent Amino Acids: Modular Building Blocks for the Assembly of New Tools for Chemical Biology. ChemBioChem 2013, 14, 788–799. [Google Scholar] [CrossRef]
- Subiros-Funosas, R.; Ho, V.C.L.; Barth, N.D.; Mendive-Tapia, L.; Pappalardo, M.; Barril, X.; Ma, R.; Zhang, C.B.; Qian, B.Z.; Sintes, M.; et al. Fluorogenic Trp(redBODIPY) cyclopeptide targeting keratin 1 for imaging of aggressive carcinomas. Chem. Sci. 2019, 11, 1368–1374. [Google Scholar] [CrossRef]
- Fernandez, A.; Vermeren, M.; Humphries, D.; Subiros-Funosas, R.; Barth, N.; Campana, L.; MacKinnon, A.; Feng, Y.; Vendrell, M. Chemical Modulation of in Vivo Macrophage Function with Subpopulation-Specific Fluorescent Prodrug Conjugates. ACS Central Sci. 2017, 3, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Subiros-Funosas, R.; Mendive-Tapia, L.; Sot, J.; Pound, J.D.; Barth, N.; Varela, Y.; Goni, F.M.; Paterson, M.; Gregory, C.D.; Albericio, F.; et al. A Trp-BODIPY cyclic peptide for fluorescence la-belling of apoptotic bodies. Chem. Commun. 2017, 53, 945–948. [Google Scholar] [CrossRef]
- Li, W.; Gong, Q.; Guo, X.; Wu, Q.; Chang, F.; Wang, H.; Zhang, F.; Hao, E.; Jiao, L. Synthesis, Reactivity, and Properties of a Class of pi-Extended BODIPY Derivatives. J. Org. Chem. 2021, 86, 17110–17118. [Google Scholar] [CrossRef] [PubMed]
- Maleckaitė, K.; Dodonova, J.; Toliautas, S.; Žilėnaitė, R.; Jurgutis, D.; Karabanovas, V.; Tumkevičius, S.; Vyšniauskas, A. Designing a Red-Emitting Viscosity-Sensitive BODIPY Fluorophore for Intracellular Viscosity Imaging. Chem.—Eur. J. 2021, 27, 16768–16775. [Google Scholar] [CrossRef] [PubMed]
- Nodeh-Farahani, D.; Bentley, J.N.; Crilley, L.R.; Caputo, C.B.; VandenBoer, T.C. A boron dipyrromethene (BODIPY) based probe for selective passive sampling of atmospheric nitrous acid (HONO) indoors. Analyst 2021, 146, 5756–5766. [Google Scholar] [CrossRef]
- Shi, W.-J.; Feng, L.-X.; Wang, X.; Huang, Y.; Wei, Y.-F.; Ma, H.-J.; Wang, W.; Xiang, M.; Gao, L. A near-infrared-emission aza-BODIPY-based fluorescent probe for fast, selective, and “turn-on” detection of HClO/ClO−. Talanta 2021, 233, 122581. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.R.; Nemykin, V.N.; Ziegler, C.J. BOSHPY Fluorophores: BODIPY Analogues with Single Atom Controlled Aggregation. Org. Lett. 2021, 23, 5246–5250. [Google Scholar] [CrossRef]
- Deng, P.; Xiao, F.; Wang, Z.; Jin, G. A Novel BODIPY Quaternary Ammonium Salt-Based Fluorescent Probe: Synthesis, Physical Properties, and Live-Cell Imaging. Front. Chem. 2021, 9, 650006. [Google Scholar] [CrossRef]
- Pandey, V.; Raza, K.; Sonowal, M.; Gupta, I. BODIPY based red emitters: Synthesis, computational and biological studies. Bioorg. Chem. 2020, 106, 104467. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem.—Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2019, 18, 10–27. [Google Scholar] [CrossRef]
- Wang, J.; Gong, Q.; Wang, L.; Hao, E.; Jiao, L. The main strategies for tuning BODIPY fluorophores into photosensi-tizers. J. Porphyr. Phthalocyanines 2020, 24, 603–635. [Google Scholar] [CrossRef]
- Karges, J.; Kuang, S.; Maschietto, F.; Blacque, O.; Ciofini, I.; Chao, H.; Gasser, G. Rationally designed ruthenium complexes for 1- and 2-photon photodynamic therapy. Nat. Commun. 2020, 11, 3262. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances im-munogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef]
- Squeo, B.M.; Pasini, M. BODIPY platform: A tunable tool for green to NIR OLEDs. Supramol. Chem. 2020, 32, 56–70. [Google Scholar] [CrossRef]
- Kue, C.S.; Ng, S.Y.; Voon, S.H.; Kamkaew, A.; Chung, L.Y.; Kiew, L.V.; Lee, H.B. Recent strategies to improve boron dipyrromethene (BODIPY) for photodynamic cancer therapy: An updated review. Photochem. Photobiol. Sci. 2018, 17, 1691–1708. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Lv, F.; Tang, B.; Hao, E.; Liu, Q.; Wang, H.; Jiao, L. Transition-metal-free regioselective cross-coupling of BODIPYs with thiols. Chem. Commun. 2019, 55, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Goud, T.V.; Tutar, A.; Biellmann, J.-F. Synthesis of 8-heteroatom-substituted 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene dyes (BODIPY). Tetrahedron 2006, 62, 5084–5091. [Google Scholar] [CrossRef]
- Fron, E.; Coutiño-Gonzalez, E.; Pandey, L.; Sliwa, M.; Van der Auweraer, M.; De Schryver, F.C.; Thomas, J.; Dong, Z.; Leen, V.; Smet, M.; et al. Synthesis and photophysical characterization of chalcogen sub-stituted BODIPY dyes. New J. Chem. 2009, 33, 1490–1496. [Google Scholar] [CrossRef]
- Palao, E.; Slanina, T.; Klán, P. Construction of the carbon–chalcogen (S, Se, Te) bond at the 2,6-positions of BODIPY via Stille cross-coupling reaction. Chem. Commun. 2016, 52, 11951–11954. [Google Scholar] [CrossRef]
- Mulay, S.V.; Choi, M.; Jang, Y.J.; Kim, Y.; Jon, S.; Churchill, D.G. Enhanced Fluorescence Turn-on Imaging of Hy-pochlorous Acid in Living Immune and Cancer Cells. Chemistry 2016, 22, 9642–9648. [Google Scholar] [CrossRef]
- Manjare, S.T.; Kim, J.; Lee, Y.; Churchill, D.G. Facile meso-BODIPY Annulation and Selective Sensing of Hypochlorite in Water. Org. Lett. 2013, 16, 520–523. [Google Scholar] [CrossRef]
- Rezende, L.C.; Melo, S.M.; Boodts, S.; Verbelen, B.; Emery, F.S.; Dehaen, W. Thiocyanation of 3-substituted and 3,5-disubstituted BODIPYs and its application for the synthesis of new fluorescent sensors. Dye. Pigment. 2018, 154, 155–163. [Google Scholar] [CrossRef]
- Kim, T.-I.; Park, S.; Choi, Y.; Kim, Y. A BODIPY-Based Probe for the Selective Detection of Hypochlorous Acid in Living Cells. Chem.—Asian J. 2011, 6, 1358–1361. [Google Scholar] [CrossRef]
- Ahrens, J.; Böker, B.; Brandhorst, K.; Funk, M.; Bröring, M. Sulfur-Bridged BODIPY DYEmers. Chem.—Eur. J. 2013, 19, 11382–11395. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.P.; Dzyuba, S.V.; Yoon, T.P. Expeditious, mechanochemical synthesis of BODIPY dyes. Beilstein J. Org. Chem. 2013, 9, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Wurth, C.; Gonzalez, M.G.; Niessner, R.; Panne, U.; Haisch, C.; Genger, U.R. Determination of the absolute fluo-rescence quantum yield of rhodamine 6G with optical and photoacoustic methods—Providing the basis for fluorescence quantum yield standards. Talanta 2012, 90, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Dransfield, I.; Buckle, A.M.; Savill, J.S.; McDowall, A.; Haslett, C.; Hogg, N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J. Immunol. 1994, 153, 1254–1263. [Google Scholar] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Schlachter, A.; Fleury, A.; Tanner, K.; Soldera, A.; Habermeyer, B.; Guilard, R.; Harvey, P. The TDDFT Excitation Energies of the BODIPYs; The DFT and TDDFT Challenge Continues. Molecules 2021, 26, 1780. [Google Scholar] [CrossRef] [PubMed]
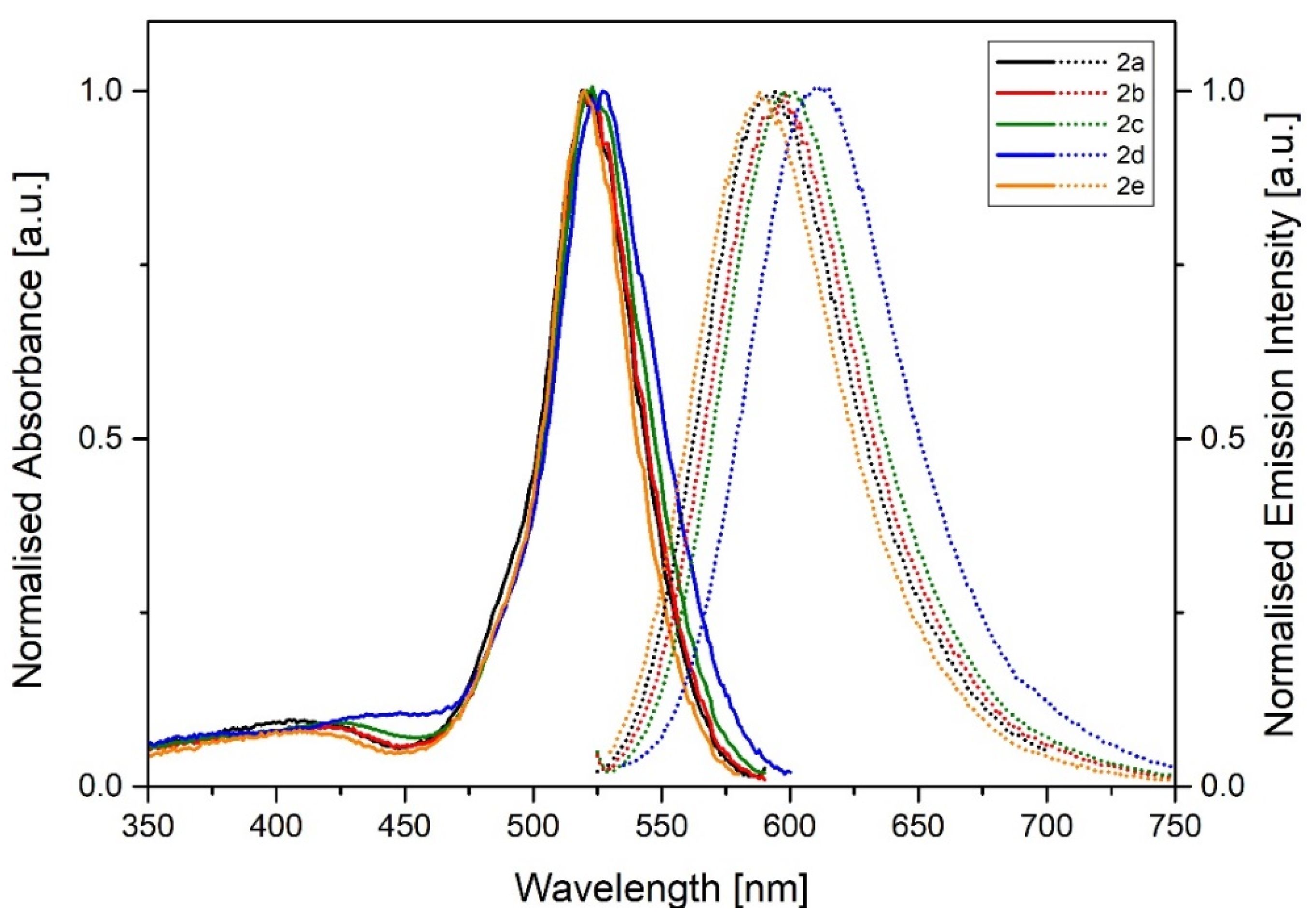
| Compound | Solvent | λabs [nm] | λem [nm] | Stokes Shift [cm−1] | ΦPL |
|---|---|---|---|---|---|
| 2a | Toluene | 521 | 595 | 2387 | 0.27 |
| THF | 520 | 593 | 2340 | 0.18 | |
| Acetone | 516 | 597 | 2629 | 0.1 | |
| MeOH | 516 | 600 | 2713 | 0.1 | |
| DMSO | 517 | 605 | 2813 | 0.07 | |
| Water (1% DMSO) | 516 | 606 | 2878 | 0.02 | |
| 2b | Toluene | 523 | 599 | 2426 | 0.24 |
| THF | 520 | 599 | 2536 | 0.13 | |
| Acetone | 519 | 600 | 2601 | 0.08 | |
| MeOH | 517 | 602 | 2731 | 0.05 | |
| DMSO | 518 | 613 | 2991 | 0.07 | |
| Water (1% DMSO) | 519 | 612 | 2928 | 0.03 | |
| 2c | Toluene | 524 | 599 | 2389 | 0.2 |
| THF | 521 | 602 | 2583 | 0.12 | |
| Acetone | 518 | 607 | 2831 | 0.05 | |
| MeOH | 519 | 607 | 2793 | 0.05 | |
| DMSO | 522 | 616 | 2923 | 0.02 | |
| Water (1% DMSO) | 520 | 614 | 2944 | < 0.01 | |
| 2d | Toluene | 519 | 618 | 3087 | 0.14 |
| THF | 517 | 619 | 3187 | 0.03 | |
| Acetone | 516 | 619 | 3225 | < 0.01 | |
| MeOH | 514 | 619 | 3300 | < 0.01 | |
| DMSO | 513 | 620 | 3364 | < 0.01 | |
| Water (1% DMSO) | 515 | 622 | 3340 | < 0.01 | |
| 2e | Toluene | 521 | 590 | 2245 | 0.26 |
| THF | 517 | 589 | 2364 | 0.2 | |
| Acetone | 512 | 592 | 2639 | 0.12 | |
| MeOH | 515 | 591 | 2497 | 0.11 | |
| DMSO | 518 | 599 | 2610 | 0.05 | |
| Water (1% DMSO) | 513 | 600 | 2826 | 0.01 | |
| BODIPY-FL | DMSO | 503 | 509 | 234 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reese, A.E.; Lochenie, C.; Geddis, A.; Machado, L.A.; de Souza, M.C.; Marques, F.F.C.; de Simone, C.A.; Gouvêa, M.M.; Pedrosa, L.F.; da Silva Júnior, E.N.; et al. Rational Design and Synthesis of Large Stokes Shift 2,6-Sulphur-Disubstituted BODIPYs for Cell Imaging. Chemosensors 2022, 10, 19. https://doi.org/10.3390/chemosensors10010019
Reese AE, Lochenie C, Geddis A, Machado LA, de Souza MC, Marques FFC, de Simone CA, Gouvêa MM, Pedrosa LF, da Silva Júnior EN, et al. Rational Design and Synthesis of Large Stokes Shift 2,6-Sulphur-Disubstituted BODIPYs for Cell Imaging. Chemosensors. 2022; 10(1):19. https://doi.org/10.3390/chemosensors10010019
Chicago/Turabian StyleReese, Abigail E., Charles Lochenie, Ailsa Geddis, Luana A. Machado, Marcos C. de Souza, Flávia F. C. Marques, Carlos A. de Simone, Marcos M. Gouvêa, Leandro F. Pedrosa, Eufrânio N. da Silva Júnior, and et al. 2022. "Rational Design and Synthesis of Large Stokes Shift 2,6-Sulphur-Disubstituted BODIPYs for Cell Imaging" Chemosensors 10, no. 1: 19. https://doi.org/10.3390/chemosensors10010019
APA StyleReese, A. E., Lochenie, C., Geddis, A., Machado, L. A., de Souza, M. C., Marques, F. F. C., de Simone, C. A., Gouvêa, M. M., Pedrosa, L. F., da Silva Júnior, E. N., & Vendrell, M. (2022). Rational Design and Synthesis of Large Stokes Shift 2,6-Sulphur-Disubstituted BODIPYs for Cell Imaging. Chemosensors, 10(1), 19. https://doi.org/10.3390/chemosensors10010019






