Atomistic Descriptions of Gas-Surface Interactions on Tin Dioxide
Abstract
:1. Introduction
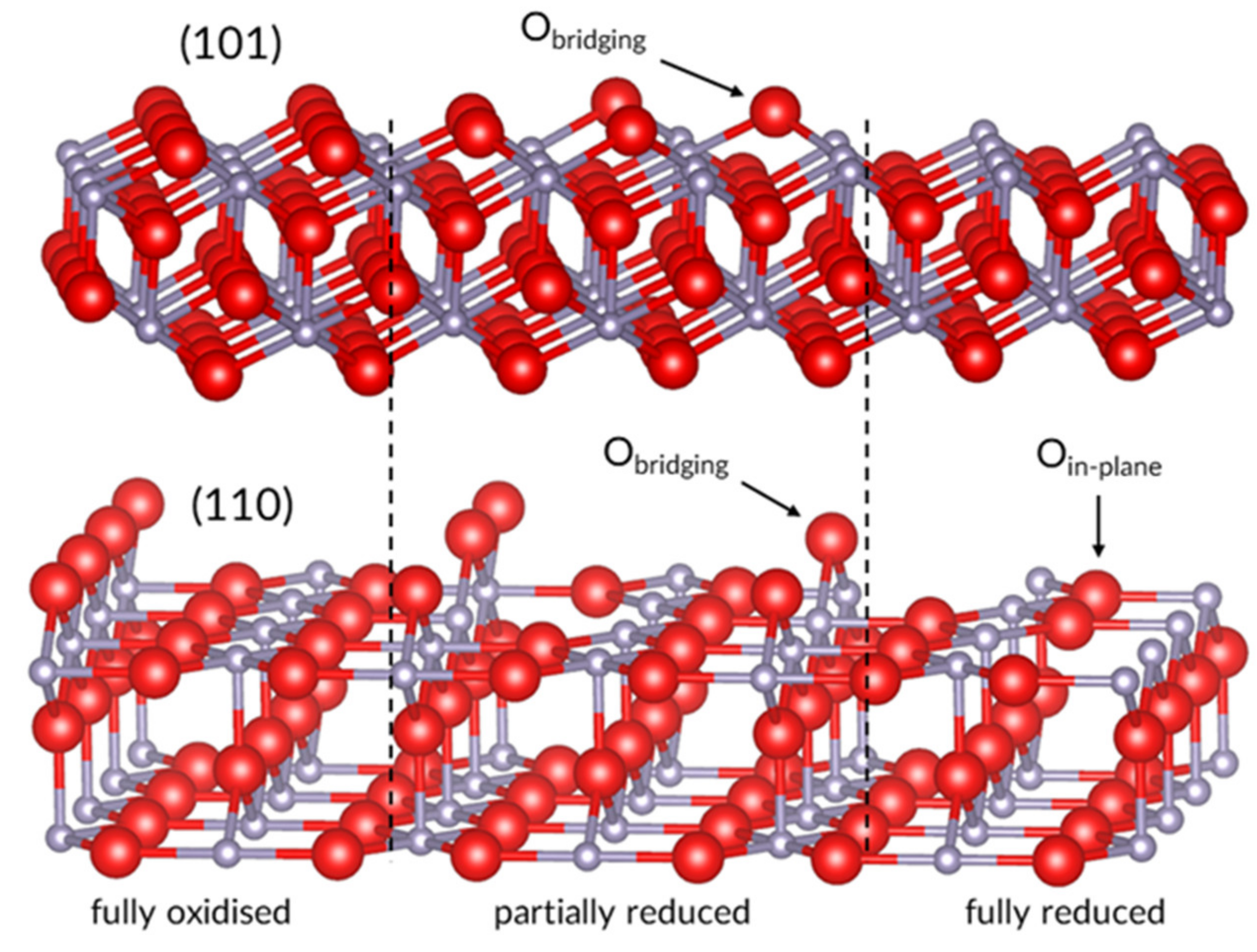
2. The Dynamic Surfaces of Tin Dioxide
3. Interactions with Oxygen
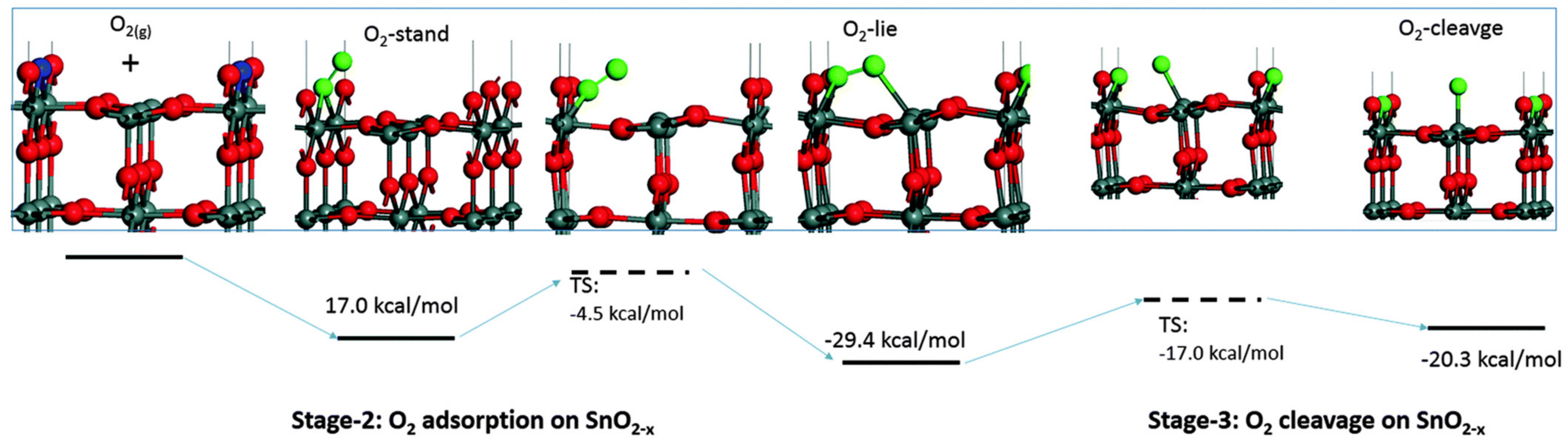
3.1. Insight from Computational Studies
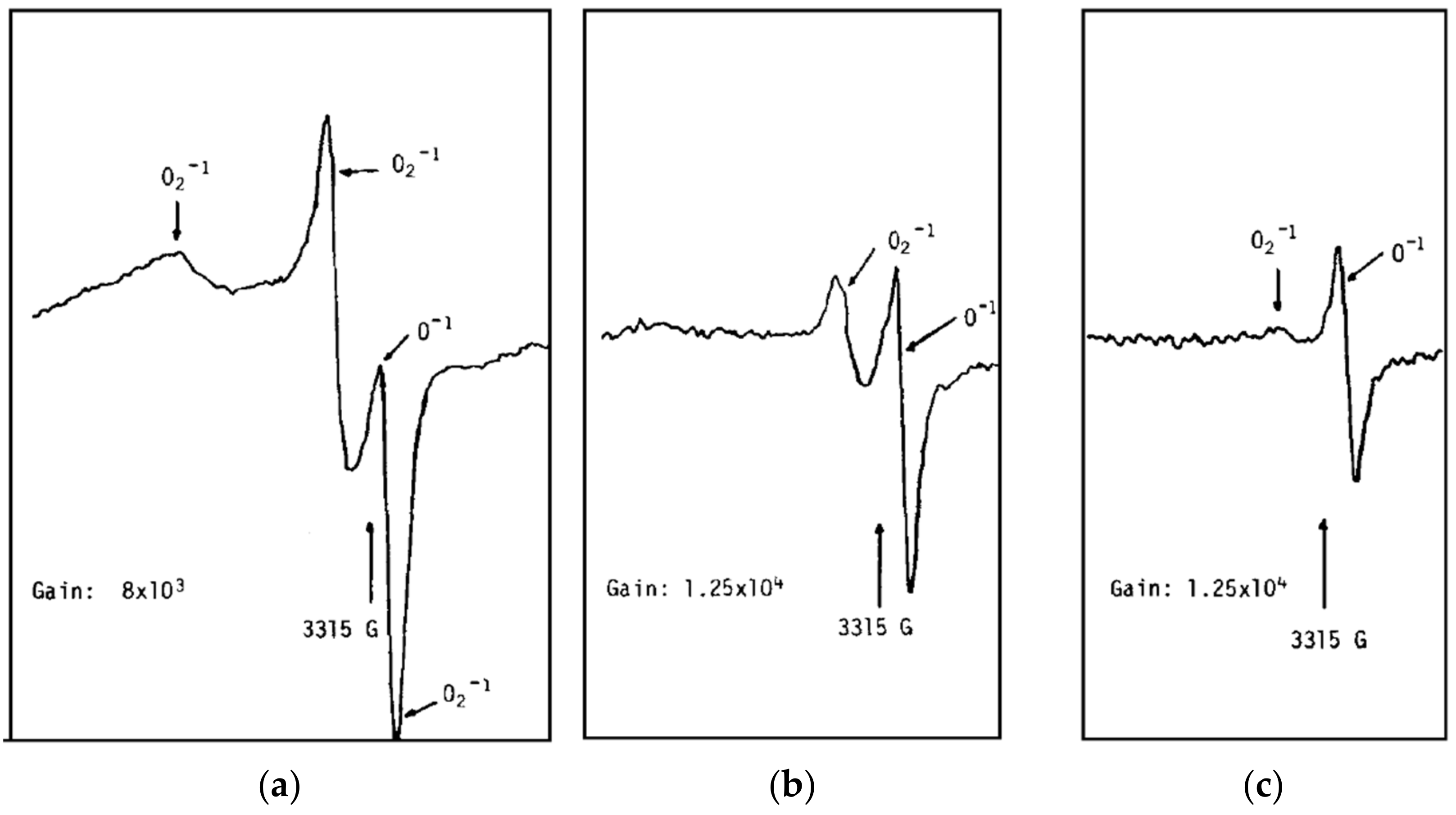
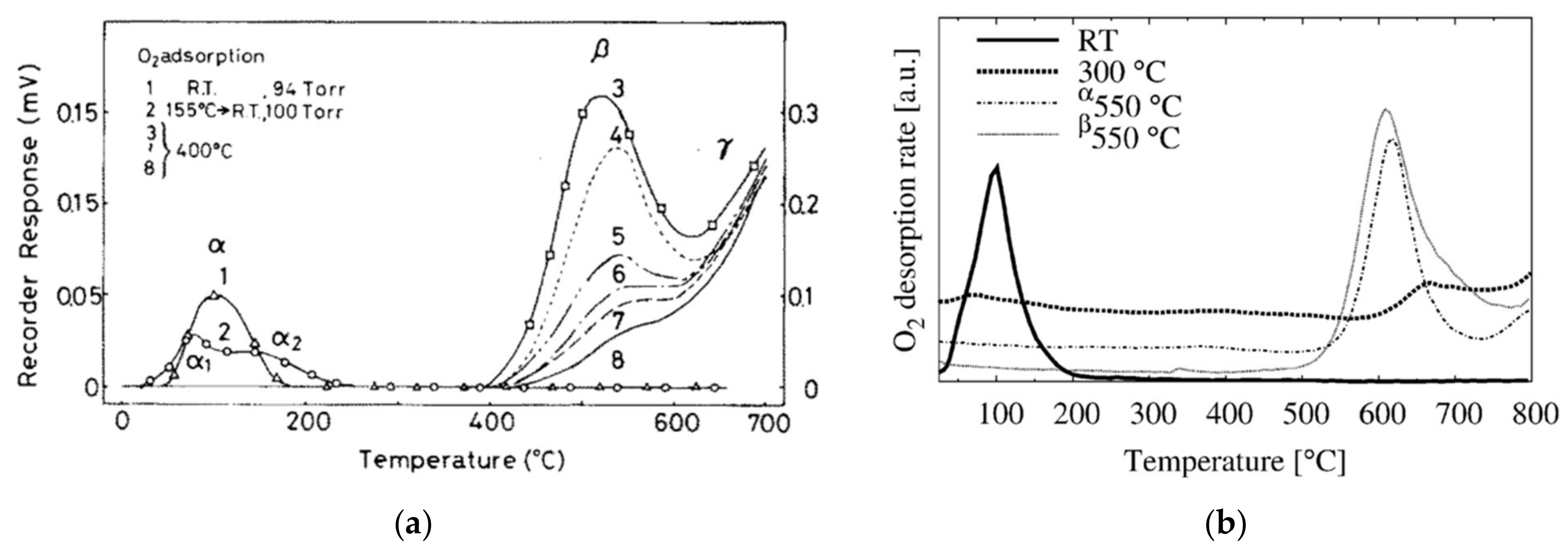
3.2. What Species Were Observed Spectroscopically?
4. Interactions with Reducing Gases
4.1. Ionosorption Based Models—Reactions with Preadsorbed Oxygen
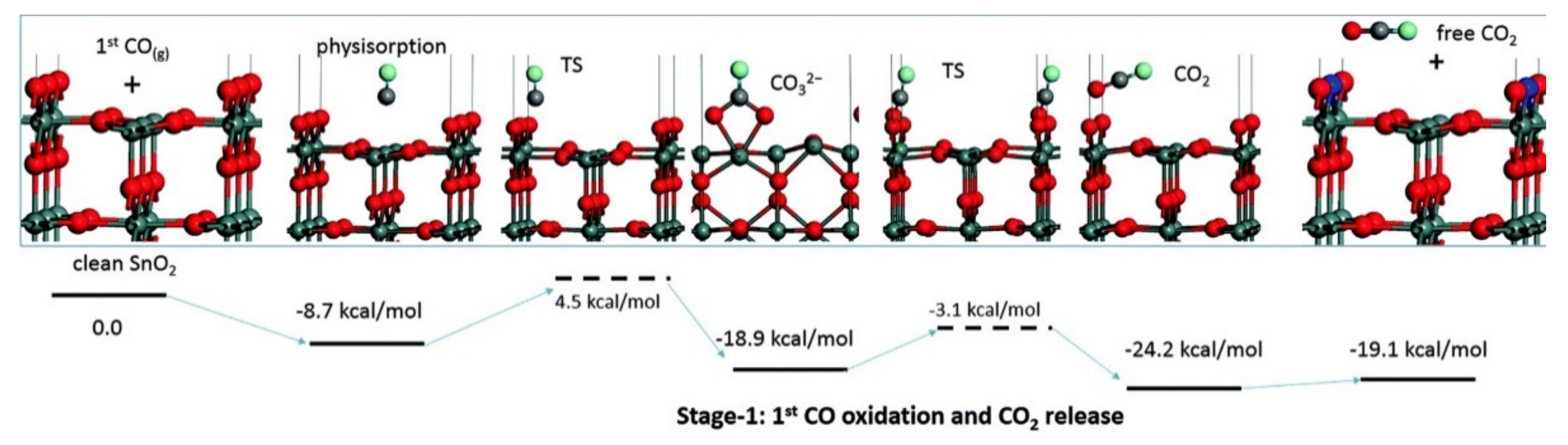
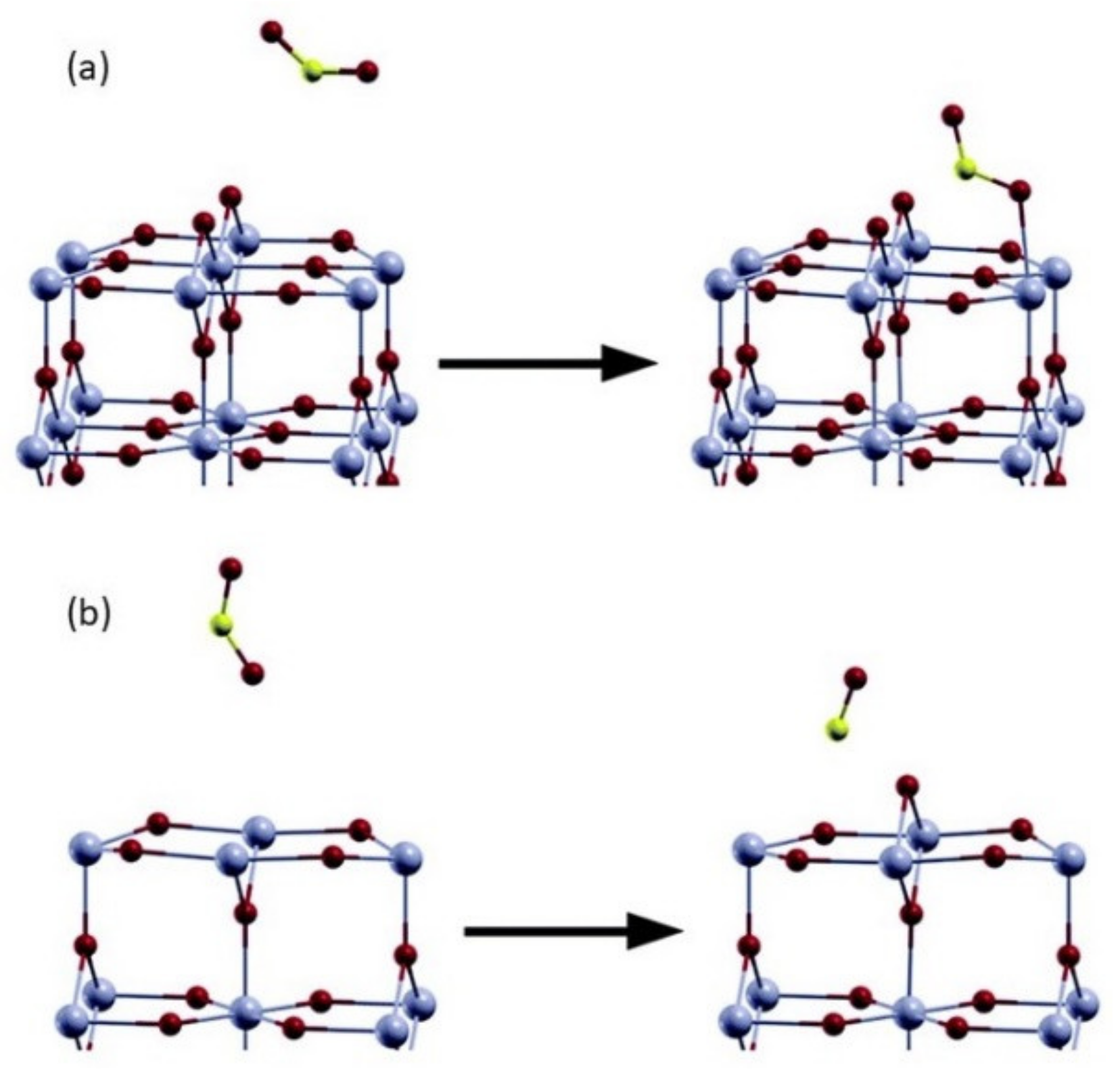
4.2. Direct Adsorption of Reducing Gases
4.3. Reduction of the Surface
5. Interactions with Oxidising Gases—NO2
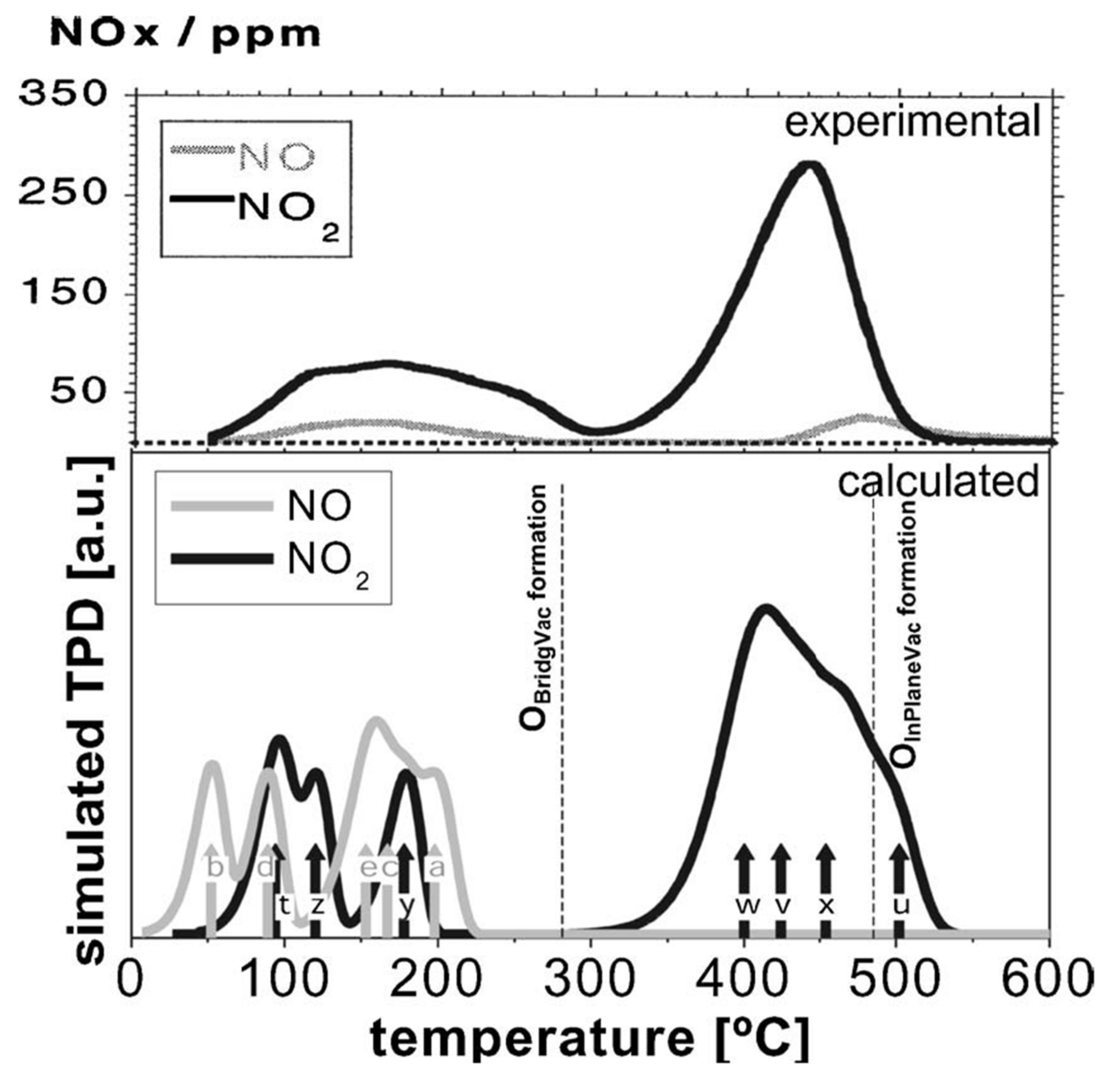
5.1. Adsorption onto Stoichiometric Surfaces
5.2. Adsorption onto Reduced Surfaces
6. Oxygen Vacancies versus Gas Sensing Mechanism
7. Conclusions
Funding
Conflicts of Interest
References
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully Inkjet Printed SnO2 Gas Sensor on Plastic Substrate. Sens. Actuators B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- Devabharathi, N.; Umarji, A.M.; Dasgupta, S. Fully Inkjet-Printed Mesoporous SnO2-Based Ultrasensitive Gas Sensors for Trace Amount NO2 Detection. ACS Appl. Mater. Interfaces 2020, 12, 57207–57217. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Lu, Z.; Wei, Z.; Xu, L.; Gui, Y. Recent Advances of SnO2-Based Sensors for Detecting Fault Characteristic Gases Extracted from Power Transformer Oil. Front. Chem. 2018, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huang, K.; Yuan, F.; Xie, C. Gas Sensing Properties and in Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study of Acetone Adsorption and Reactions on SnO2 Films. Sens. Mater. 2014, 26, 649–663. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Q.; Zhang, S.; He, Z. The Enhanced H2 Selectivity of SnO2 Gas Sensors with the Deposited SiO2 Filters on Surface of the Sensors. Sensors 2019, 19, 2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, Q.; Lv, R.; Wu, D.; Zhang, S. Enhancing Formaldehyde Selectivity of SnO2 Gas Sensors with the ZSM-5 Modified Layers. Sensors 2021, 21, 3947. [Google Scholar] [CrossRef]
- Ding, J.; McAvoy, T.J.; Cavicchi, R.E.; Semancik, S. Surface State Trapping Models for SnO2-Based Microhotplate Sensors. Sens. Actuators B Chem. 2001, 77, 597–613. [Google Scholar] [CrossRef]
- Bârsan, N.; Hübner, M.; Weimar, U. Conduction Mechanisms in SnO2 Based Polycrystalline Thick Film Gas Sensors Exposed to CO and H2 in Different Oxygen Backgrounds. Sens. Actuators B Chem. 2011, 157, 510–517. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Morrison, S.R. Mechanism of Semiconductor Gas Sensor Operation. Sens. Actuators 1987, 11, 283–287. [Google Scholar] [CrossRef]
- Heiland, G. Homogeneous Semiconducting Gas Sensors. Sens. Actuators 1981, 2, 343–361. [Google Scholar] [CrossRef]
- Geistlinger, H. Electron Theory of Thin-Film Gas Sensors. Sens. Actuators B Chem. 1993, 17, 47–60. [Google Scholar] [CrossRef]
- Göpel, W.; Schierbaum, K.D. SnO2 Sensors: Current Status and Future Prospects. Sens. Actuators B Chem. 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Hauffe, K. The Application of the Theory of Semiconductors to Problems of Heterogeneous Catalysis. Adv. Catal. 1955, 7, 213–257. [Google Scholar] [CrossRef]
- Pulkkinen, U.; Rantala, T.T.; Rantala, T.S.; Lantto, V. Kinetic Monte Carlo Simulation of Oxygen Exchange of SnO2 Surface. J. Mol. Catal. A Chem. 2001, 166, 15–21. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. Chem. Phys. Chem. 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, D.; Yang, L.; Wang, X.; Xu, G.; Yang, Z. Direct CO Oxidation by Lattice Oxygen on the SnO2(110) Surface: A DFT Study. Phys. Chem. Chem. Phys. 2014, 16, 12488–12494. [Google Scholar] [CrossRef]
- Hong, S.N.; Kye, Y.H.; Yu, C.J.; Jong, U.G.; Ri, G.C.; Choe, C.S.; Kim, K.H.; Han, J.M. Ab Initio Thermodynamic Study of the SnO2 (110) Surface in an O2 and NO Environment: A Fundamental Understanding of the Gas Sensing Mechanism for NO and NO2. Phys. Chem. Chem. Phys. 2016, 18, 31566–31578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorokhta, M.; Khalakhan, I.; Vondráček, M.; Tomeček, D.; Vorokhta, M.; Marešová, E.; Nováková, J.; Vlček, J.; Fitl, P.; Novotný, M.; et al. Investigation of Gas Sensing Mechanism of SnO2 Based Chemiresistor Using near Ambient Pressure XPS. Surf. Sci. 2018, 677, 284–290. [Google Scholar] [CrossRef]
- Bolzan, A.A.; Fong, C.; Kennedy, B.J.; Howard, C.J. Structural Studies of Rutile-Type Metal Dioxides. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 373–380. [Google Scholar] [CrossRef]
- Fröhlich, D.; Kenklies, R.; Helbig, R. Band-Gap Assignment in SnO2 by Two-Photon Spectroscopy. Phys. Rev. Lett. 1978, 41, 1750–1751. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Cristofori, D.; Scotti, R.; Morazzoni, F. New Insights into the SnO2 Sensing Mechanism Based on the Properties of Shape Controlled Tin Oxide Nanoparticles. Chem. Mater. 2013, 25, 3675–3686. [Google Scholar] [CrossRef]
- Buckeridge, J.; Catlow, C.R.A.; Farrow, M.R.; Logsdail, A.J.; Scanlon, D.O.; Keal, T.W.; Sherwood, P.; Woodley, S.M.; Sokol, A.A.; Walsh, A. Deep vs Shallow Nature of Oxygen Vacancies and Consequent N-Type Carrier Concentrations in Transparent Conducting Oxides. Phys. Rev. Mater. 2018, 2, 56–59. [Google Scholar] [CrossRef] [Green Version]
- De Frésart, E.; Darville, J.; Gilles, J.M. Influence of the Surface Reconstruction on the Work Function and Surface Conductance of (110) SnO2. Appl. Surf. Sci. 1982, 11–12, 637–651. [Google Scholar] [CrossRef]
- Kamp, B.; Merkle, R.; Lauck, R.; Maier, J. Chemical Diffusion of Oxygen in Tin Dioxide: Effects of Dopants and Oxygen Partial Pressure. J. Solid State Chem. 2005, 178, 3027–3039. [Google Scholar] [CrossRef]
- Batzill, M.; Katsiev, K.; Burst, J.M.; Diebold, U.; Chaka, A.M.; Delley, B. Gas-Phase-Dependent Properties of SnO2 (110), (100), and (101) Single-Crystal Surfaces: Structure, Composition, and Electronic Properties. Phys. Rev. B-Condens. Matter Mater. Phys. 2005, 72, 1–20. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagasawa, Y.; Shimomura, S.; Tabata, K.; Suzuki, E. A Density Functional Theory Study of the Interaction of Oxygen with a Reduced SnO2 (110) Surface. Chem. Phys. Lett. 2000, 316, 477–482. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Chen, Y.; Hu, J. Sensing Mechanism of SnO2 (110) Surface to CO: Density Functional Theory Calculations. J. Phys. Chem. C 2014, 118, 28548–28561. [Google Scholar] [CrossRef]
- Eslamian, M.; Salehi, A.; Nadimi, E. The Role of Oxygen Vacancies on SnO2 Surface in Reducing Cross-Sensitivity between Ambient Humidity and CO: A First Principles Investigation. Surf. Sci. 2021, 708, 121817. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, L.; He, C.; Ma, D.; Lu, Z. Adsorption and Oxidation of NO on Various SnO2(1 1 0) Surfaces: A Density Functional Theory Study. Sens. Actuators B Chem. 2015, 221, 717–722. [Google Scholar] [CrossRef]
- Prades, J.D.; Cirera, A.; Morante, J.R.; Pruneda, J.M.; Ordejón, P. Ab Initio Study of NOx Compounds Adsorption on SnO2 Surface. Sens. Actuators B Chem. 2007, 126, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Ducéré, J.-M.; Hemeryck, A.; Estève, A.; Rouhani, M.D.; Landa, G.; Ménini, P.; Tropis, C.; Maisonnat, A.; Fau, P.; Chaudret, B. A Computational Chemist Approach to Gas Sensors: Modeling the Response of SnO2 to CO, O2, and H2O Gases. J. Comput. Chem. 2012, 33, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Abokifa, A.A.; Haddad, K.; Fortner, J.; Lo, C.S.; Biswas, P. Sensing Mechanism of Ethanol and Acetone at Room Temperature by SnO2 Nano-Columns Synthesized by Aerosol Routes: Theoretical Calculations Compared to Experimental Results. J. Mater. Chem. A 2018, 6, 2053–2066. [Google Scholar] [CrossRef]
- Oviedo, J.; Gillan, M.J. Energetics and Structure of Stoichiometric SnO2 Surfaces Studied by First-Principles Calculations. Surf. Sci. 2000, 463, 93–101. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagasawa, Y.; Murakami, A.; Tabata, K. Stability of Oxygen Anions and Hydrogen Abstraction from Methane on Reduced SnO2 (110) Surface. Int. J. Quantum Chem. 1998, 69, 669–678. [Google Scholar] [CrossRef]
- Cox, D.F.; Fryberger, T.B.; Semancik, S. Oxygen Vacancies and Defect Electronic States on the SnO2 (110)-1 × 1 Surface. Phys. Rev. B 1988, 38, 2072–2083. [Google Scholar] [CrossRef]
- Sopiha, K.V.; Malyi, O.I.; Persson, C.; Wu, P. Chemistry of Oxygen Ionosorption on SnO2 Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 33664–33676. [Google Scholar] [CrossRef]
- Trani, F.; Causà, M.; Ninno, D.; Cantele, G.; Barone, V. Density Functional Study of Oxygen Vacancies at the SnO2 Surface and Subsurface Sites. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 77, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Liang, J.; Liu, Y.; Liu, Y.; Xu, X.; Fang, X.; Zhong, W.; Wang, X. Identifying Surface Active Sites of SnO2: Roles of Surface O2−, O22− Anions and Acidic Species Played for Toluene Deep Oxidation. Ind. Eng. Chem. Res. 2019, 58, 18569–18581. [Google Scholar] [CrossRef]
- Simion, C.E.; Schipani, F.; Papadogianni, A.; Stanoiu, A.; Budde, M.; Oprea, A.; Weimar, U.; Bierwagen, O.; Barsan, N. Conductance Model for Single-Crystalline/Compact Metal Oxide Gas-Sensing Layers in the Nondegenerate Limit: Example of Epitaxial SnO2 (101). ACS Sens. 2019, 4, 2420–2428. [Google Scholar] [CrossRef]
- Chang, S. Oxygen Chemisorption on Tin Oxide: Correlation between Electrical Conductivity and EPR Measurements. J. Vac. Sci. Technol. 1980, 17, 366–369. [Google Scholar] [CrossRef]
- Che, M.; Tench, A.J. Characterization and Reactivity of Mononuclear Oxygen Species on Oxide Surfaces. Adv. Catal. 1982, 31, 77–133. [Google Scholar] [CrossRef]
- Coronado, J.M.; Maira, A.J.; Conesa, J.C.; Yeung, K.L.; Augugliaro, V.; Soria, J. EPR Study of the Surface Characteristics of Nanostructured TiO2 under UV Irradiation. Langmuir 2001, 17, 5368–5374. [Google Scholar] [CrossRef]
- Coronado, J.M.; Soria, J. ESR Study of the Initial Stages of the Photocatalytic Oxidation of Toluene over TiO2 Powders. Catal. Today 2007, 123, 37–41. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bontempi, E.; Canevali, C.; Depero, L.E.; Mari, C.M.; Ruffo, R.; Scotti, R.; Tondello, E.; Morazzoni, F. Can Electron Paramagnetic Resonance Measurements Predict the Electrical Sensitivity of SnO2-Based Film? Appl. Magn. Reson. 2002, 22, 89–100. [Google Scholar] [CrossRef]
- Grishina, D.A.; Mironov, A.A.; Pentegov, I.S.; Marikutsa, A.V.; Konstantinova, E.A. Electron Spin Resonance Characterization of Defects in Sensor Materials Based on Nanocrystalline Tin Dioxide. Opt. Micro-Nanometrol. IV 2012, 8430, 84300A. [Google Scholar] [CrossRef]
- Che, M.; Tench, A.J. Characterization and Reactivity of Molecular Oxygen Species on Oxide Surfaces. Adv. Catal. 1983, 32, 1–148. [Google Scholar] [CrossRef]
- Volodin, A.M.; Cherkashin, A.E. ESR Spectra of O2−on SnO2. Effect of Adsorbed CO on the Conditions of Stabilization of O2−. React. Kinet. Catal. Lett. 1981, 17, 323–327. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of Tin Oxide Surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Matsuura, Y.; Takahata, K.; Ihokura, K. Mechanism of Gas Sensitivity Change with Time of SnO2 Gas Sensors. Sens. Actuators 1988, 14, 223–232. [Google Scholar] [CrossRef]
- Matsuura, Y.; Takahata, K.; Matsuura, S. Temperature Programmed Desorption Study on Mechanism of Long-Term Deterioration of SnO2 Gas Sensor. Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1990, 58, 1154–1161. [Google Scholar] [CrossRef]
- Cavicchi, R.; Tarlov, M.; Semancik, S. Preparation of Well-ordered, Oxygen-rich SnO2 (110) Surfaces via Oxygen Plasma Treatment. J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 2347–2352. [Google Scholar] [CrossRef]
- Saukko, S.; Lassi, U.; Lantto, V.; Kroneld, M.; Novikov, S.; Kuivalainen, P.; Rantala, T.T.; Mizsei, J. Experimental Studies of O2-SnO2 Surface Interaction Using Powder, Thick Films and Monocrystalline Thin Films. Thin Solid Film. 2005, 490, 48–53. [Google Scholar] [CrossRef]
- Capone, S.; Siciliano, P.; Quaranta, F.; Rella, R.; Epifani, M.; Vasanelli, L. Moisture Influence and Geometry Effect of Au and Pt Electrodes on CO Sensing Response of SnO2 Microsensors Based on Sol-Gel Thin Film. Sens. Actuators B Chem. 2001, 77, 503–511. [Google Scholar] [CrossRef]
- Hahn, S.H.; Bârsan, N.; Weimar, U.; Ejakov, S.G.; Visser, J.H.; Soltis, R.E. CO Sensing with SnO2thick Film Sensors: Role of Oxygen and Water Vapour. Thin Solid Film. 2003, 436, 17–24. [Google Scholar] [CrossRef]
- Umar, A.; Ammar, H.Y.; Kumar, R.; Almas, T.; Ibrahim, A.A.; AlAssiri, M.S.; Abaker, M.; Baskoutas, S. Efficient H2 Gas Sensor Based on 2D SnO2 Disks: Experimental and Theoretical Studies. Int. J. Hydrog. Energy 2020, 45, 26388–26401. [Google Scholar] [CrossRef]
- Suematsu, K.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Ambient Oxygen Partial Pressure on the Hydrogen Response of SnO2 Semiconductor Gas Sensors. J. Electrochem. Soc. 2019, 166, B618–B622. [Google Scholar] [CrossRef]
- Grossmann, K.; Pavelko, R.G.; Barsan, N.; Weimar, U. Interplay of H2, Water Vapor and Oxygen at the Surface of SnO2 Based Gas Sensors—An Operando Investigation Utilizing Deuterated Gases. Sens. Actuators B Chem. 2012, 166–167, 787–793. [Google Scholar] [CrossRef]
- Hübner, M.; Pavelko, R.G.; Barsan, N.; Weimar, U. Influence of Oxygen Backgrounds on Hydrogen Sensing with SnO2 Nanomaterials. Sens. Actuators B Chem. 2011, 154, 264–269. [Google Scholar] [CrossRef]
- Choi, P.G.; Izu, N.; Shirahata, N.; Masuda, Y. SnO2 Nanosheets for Selective Alkene Gas Sensing. ACS Appl. Nano Mater. 2019, 2, 1820–1827. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a Novel Methane Gas Sensor Based on SnO2 Nanorods-Nanoporous Graphene Hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Shi, C.; Li, L.; Qin, H.; Hu, J. Sensing Mechanism of SnO2 (1 1 0) Surface to H2: Density Functional Theory Calculations. Sens. Actuators B Chem. 2015, 220, 279–287. [Google Scholar] [CrossRef]
- Barsan, N.; Rebholz, J.; Weimar, U. Conduction Mechanism Switch for SnO2 Based Sensors during Operation in Application Relevant Conditions; Implications for Modeling of Sensing. Sens. Actuators B Chem. 2015, 207, 455–459. [Google Scholar] [CrossRef]
- Santarossa, G.; Hahn, K.; Baiker, A. Free Energy and Electronic Properties of Water Adsorption on the SnO2 (110) Surface. Langmuir 2013, 29, 5487–5499. [Google Scholar] [CrossRef]
- Leblanc, E.; Perier-Camby, L.; Thomas, G.; Gibert, R.; Primet, M.; Gelin, P. NOx Adsorption onto Dehydroxylated or Hydroxylated Tin Dioxide Surface. Application to SnO2-Based Sensors. Sens. Actuators B Chem. 2000, 62, 67–72. [Google Scholar] [CrossRef]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient Humidity Influence on CO Detection with SnO2 Gas Sensing Materials. A Combined DRIFTS/DFT Investigation. J. Phys. Chem. C 2017, 121, 25064–25073. [Google Scholar] [CrossRef] [Green Version]
- Thoren, W.; Kohl, D.; Heiland, G. Kinetic Studies of the Decomposition of CH3COOH and CH3COOD on SnO2 Single Crystals. Surf. Sci. 1985, 162, 402–410. [Google Scholar] [CrossRef]
- Tamaki, J.; Nagaishi, M.; Teraoka, Y.; Miura, N.; Yamazoe, N.; Moriya, K.; Nakamura, Y. Adsorption Behavior of CO and Interfering Gases on SnO2. Surf. Sci. 1989, 221, 183–196. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Liu, Z.; Li, L.; Shi, C.; Qin, H.; Hu, J. CO2-Sensing Properties and Mechanism of Nano-SnO2 Thick-Film Sensor. Sens. Actuators B Chem. 2016, 227, 73–84. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, J.Y.; Park, H.C.; Kim, K.H. CO2-Sensing Characteristics of SnO2 Thick Film by Coating Lanthanum Oxide. Sens. Actuators B Chem. 2000, 62, 61–66. [Google Scholar] [CrossRef]
- Hsu, K.C.; Fang, T.H.; Hsiao, Y.J.; Chan, C.A. Highly Response CO2 Gas Sensor Based on Au-La2O3 Doped SnO2 Nanofibers. Mater. Lett. 2020, 261, 127144. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Pala, R.G.S.; Shapovalov, V.; Metiu, H. CO Oxidation by Rutile TiO2 (110) Doped with V, W, Cr, Mo, and Mn. J. Phys. Chem. C 2008, 112, 12398–12408. [Google Scholar] [CrossRef]
- Ramesh, K.; Chen, L.; Chen, F.; Liu, Y.; Wang, Z.; Han, Y.F. Re-Investigating the CO Oxidation Mechanism over Unsupported MnO, Mn2O3 and MnO2 Catalysts. Catal. Today 2008, 131, 477–482. [Google Scholar] [CrossRef]
- Zakaryan, H.A.; Aroutiounian, V.M. Ab Initio Investigation of CO Gas Sensing Mechanism on SnO2 Surfaces. Allsensor 2017, 27, 50–55. [Google Scholar]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the Active Oxygen Species in SnO2 Based Gas Sensing Materials: An Operando IR Spectrsocopy Study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Degler, D.; Barz, N.; Dettinger, U.; Peisert, H.; Chassé, T.; Weimar, U.; Barsan, N. Extending the Toolbox for Gas Sensor Research: Operando UV/Vis Diffuse Reflectance Spectroscopy on SnO2-Based Gas Sensors. Sens. Actuators B Chem. 2016, 224, 256–259. [Google Scholar] [CrossRef]
- Ruhland, B.; Becker, T.; Müller, G. Gas-Kinetic Interactions of Nitrous Oxides with SnO2surfaces. Sens. Actuators B Chem. 1998, 50, 85–94. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Pagnier, T. In Situ Raman Spectroscopy Study of NO2 Adsorption onto Nanocrystalline Tin (IV) Oxide. J. Raman Spectrosc. 2006, 37, 1272–1277. [Google Scholar] [CrossRef]
- Maeng, S.; Kim, S.W.; Lee, D.H.; Moon, S.E.; Kim, K.C.; Maiti, A. SnO2 Nanoslab as NO2 Sensor: Identification of the NO2 Sensing Mechanism on a SnO2 Surface. ACS Appl. Mater. Interfaces 2014, 6, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Rodriguez, J.A.; Law, M.; Kung, P.; McKinney, J.R.; Yang, P. SnO2 Nanoribbons as NO2 Sensors: Insights from First Principles Calculations. Nano Lett. 2003, 3, 1025–1028. [Google Scholar] [CrossRef]
- Epifani, M.; Prades, J.D.; Comini, E.; Pellicer, E.; Avella, M.; Faglia, G.; Cirera, A.; Scotti, R.; Morazzoni, F.; Morante, J.R. The Role of Surface Oxygen Vacancies in the NO2 Sensing Properties of SnO2 Nanocrystals. J. Phys. Chem. C 2008, 112, 19540–19546. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, C.; Yuan, G.; Gao, S. SnO2nanocrystals with Abundant Oxygen Vacancies: Preparation and Room Temperature NO2sensing. J. Alloys Compd. 2016, 681, 43–49. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, W.; Zhao, X.; Jiang, X.; Lin, S.; Zhen, Z.; Chen, W.; Xie, D.; Zhu, H. High-Response Room-Temperature NO2 Sensor and Ultrafast Humidity Sensor Based on SnO2 with Rich Oxygen Vacancy. ACS Appl. Mater. Interfaces 2019, 11, 13441–13449. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.D.; Cirera, A.; Morante, J.R. First-Principles Study of NOx and SO2 Adsorption onto SnO2 (110). J. Electrochem. Soc. 2007, 154, H675. [Google Scholar] [CrossRef]
- Watkins, G.D. Negative-U Properties for Defects in Solids. In Advances in Solid State Physics; Springer: Berlin/Heidelberg, Germany, 1984; Volume 24, pp. 163–189. [Google Scholar] [CrossRef]
- Lany, S.; Zakutayev, A.; Mason, T.O.; Wager, J.F.; Poeppelmeier, K.R.; Perkins, J.D.; Berry, J.J.; Ginley, D.S.; Zunger, A. Surface Origin of High Conductivities in Undoped In2O3 Thin Films. Phys. Rev. Lett. 2012, 108, 2–6. [Google Scholar] [CrossRef] [Green Version]
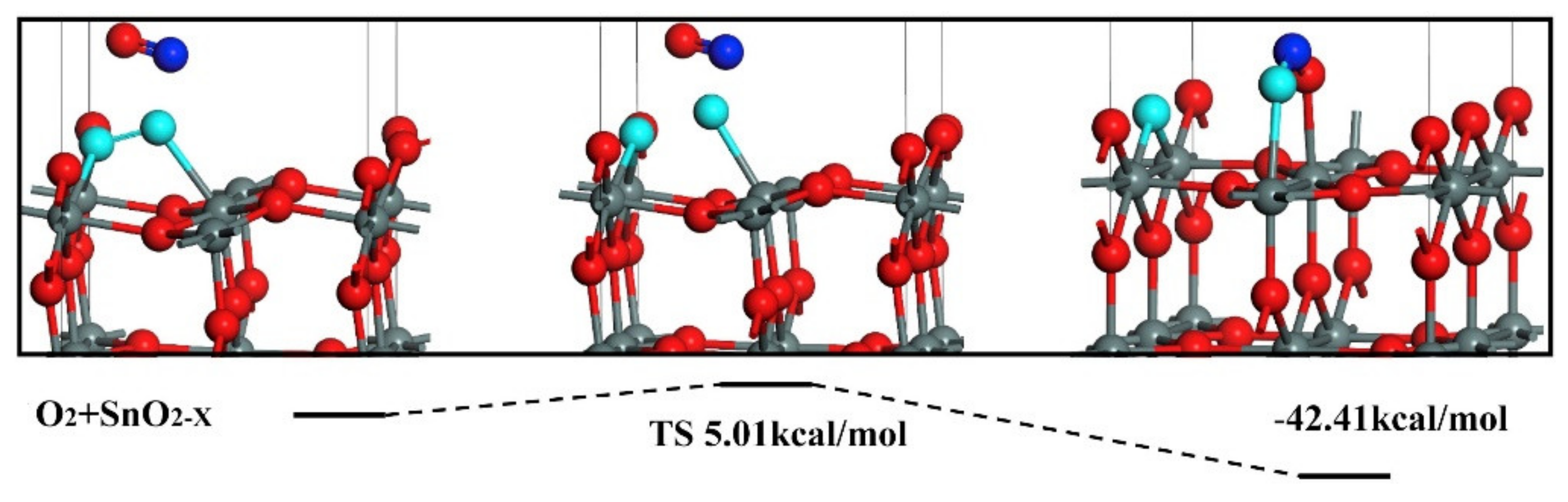
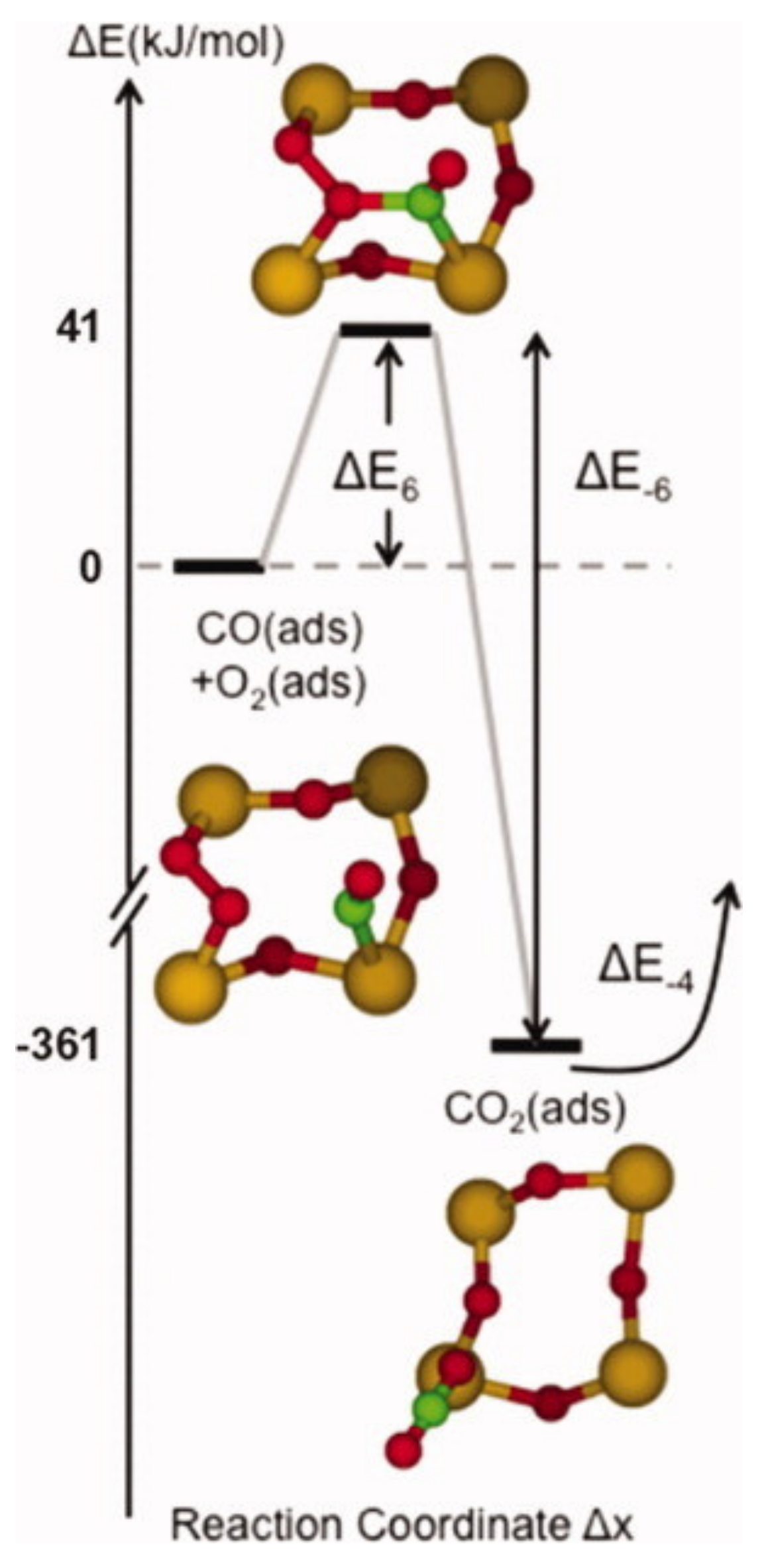
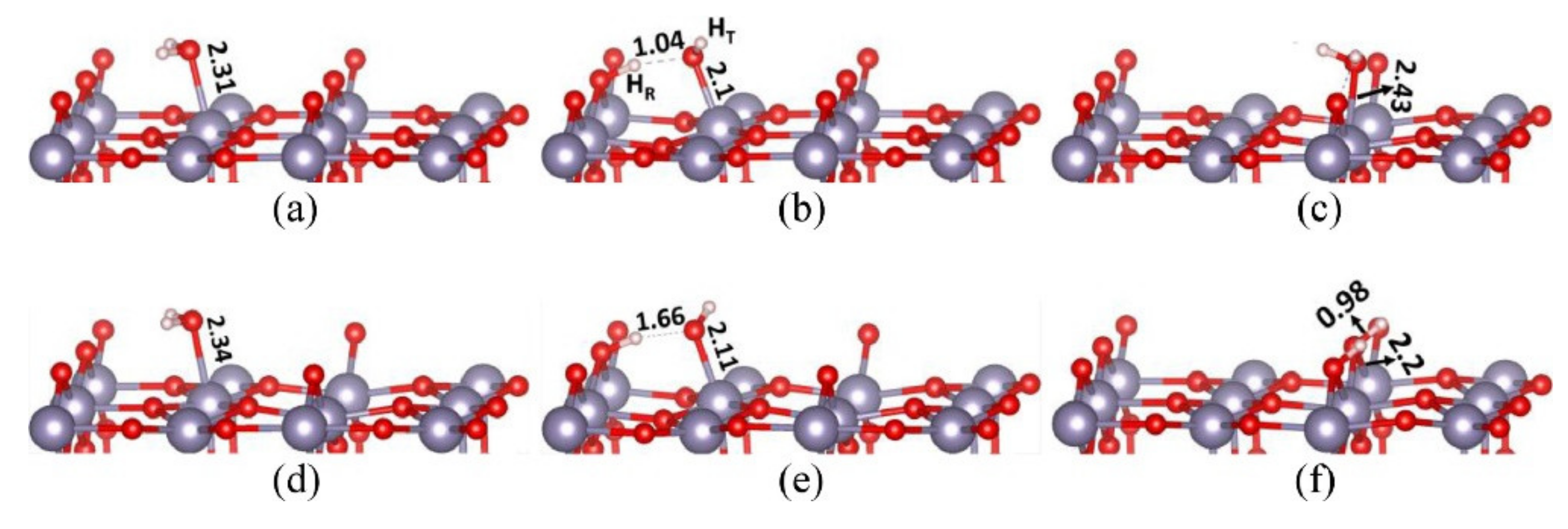

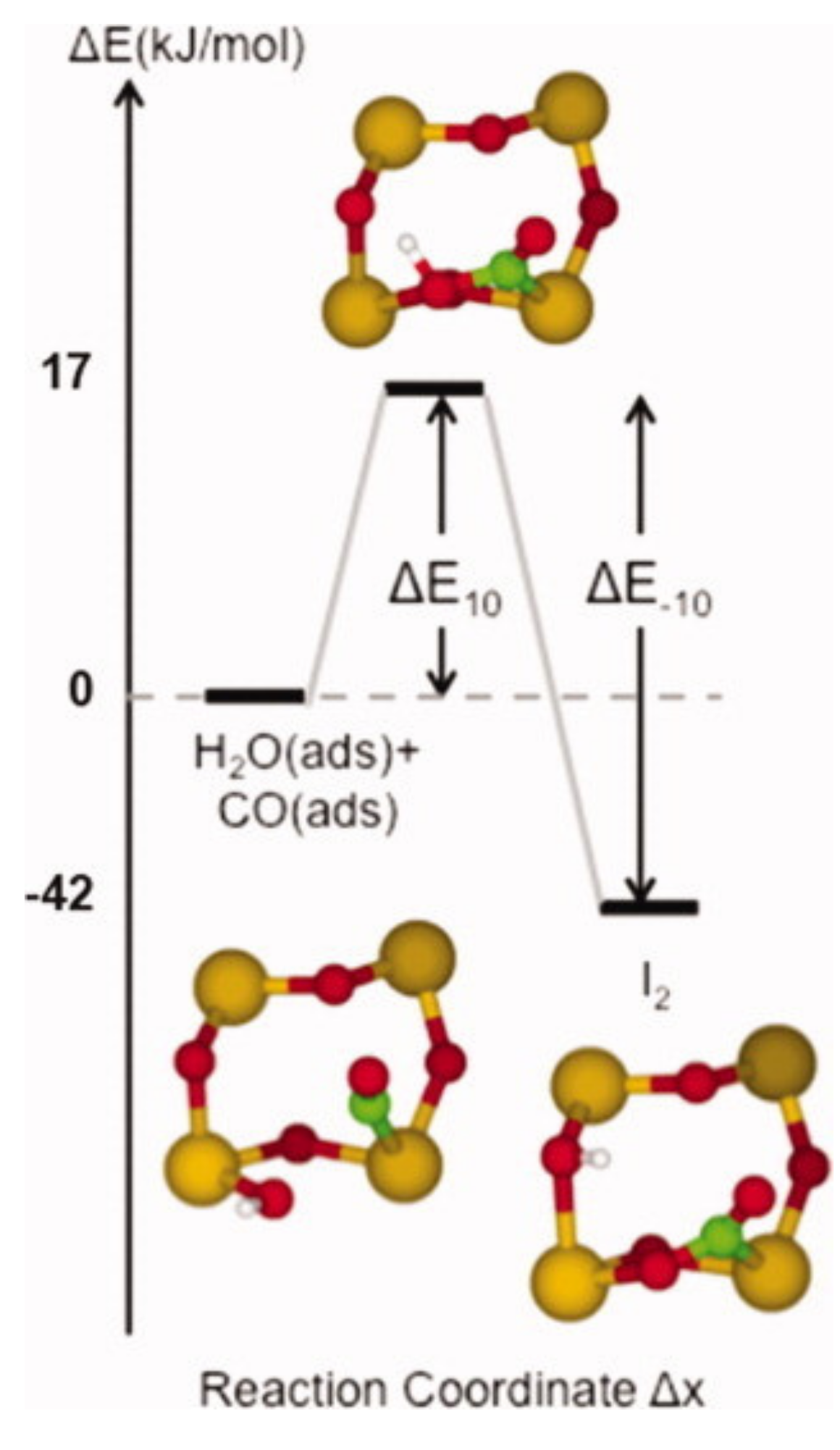

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharski, S.; Blackman, C. Atomistic Descriptions of Gas-Surface Interactions on Tin Dioxide. Chemosensors 2021, 9, 270. https://doi.org/10.3390/chemosensors9090270
Kucharski S, Blackman C. Atomistic Descriptions of Gas-Surface Interactions on Tin Dioxide. Chemosensors. 2021; 9(9):270. https://doi.org/10.3390/chemosensors9090270
Chicago/Turabian StyleKucharski, Stefan, and Chris Blackman. 2021. "Atomistic Descriptions of Gas-Surface Interactions on Tin Dioxide" Chemosensors 9, no. 9: 270. https://doi.org/10.3390/chemosensors9090270
APA StyleKucharski, S., & Blackman, C. (2021). Atomistic Descriptions of Gas-Surface Interactions on Tin Dioxide. Chemosensors, 9(9), 270. https://doi.org/10.3390/chemosensors9090270







