A Comprehensive Review on Raman Spectroscopy Applications
Abstract
:1. Introduction
2. Raman Spectroscopy: Principles and Instrumentation
2.1. Theoretical Principles: A Quantum Mechanical Approach
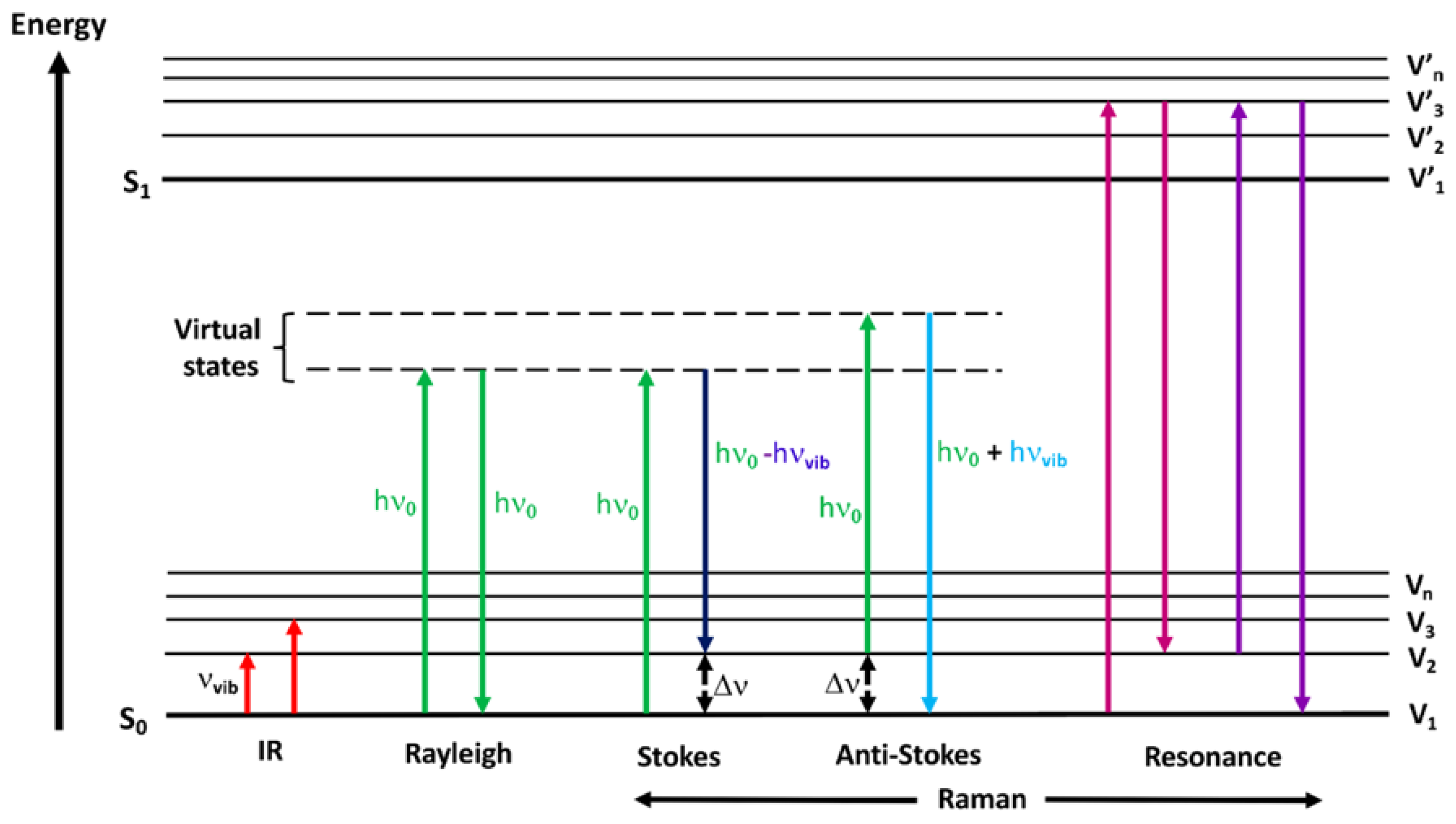
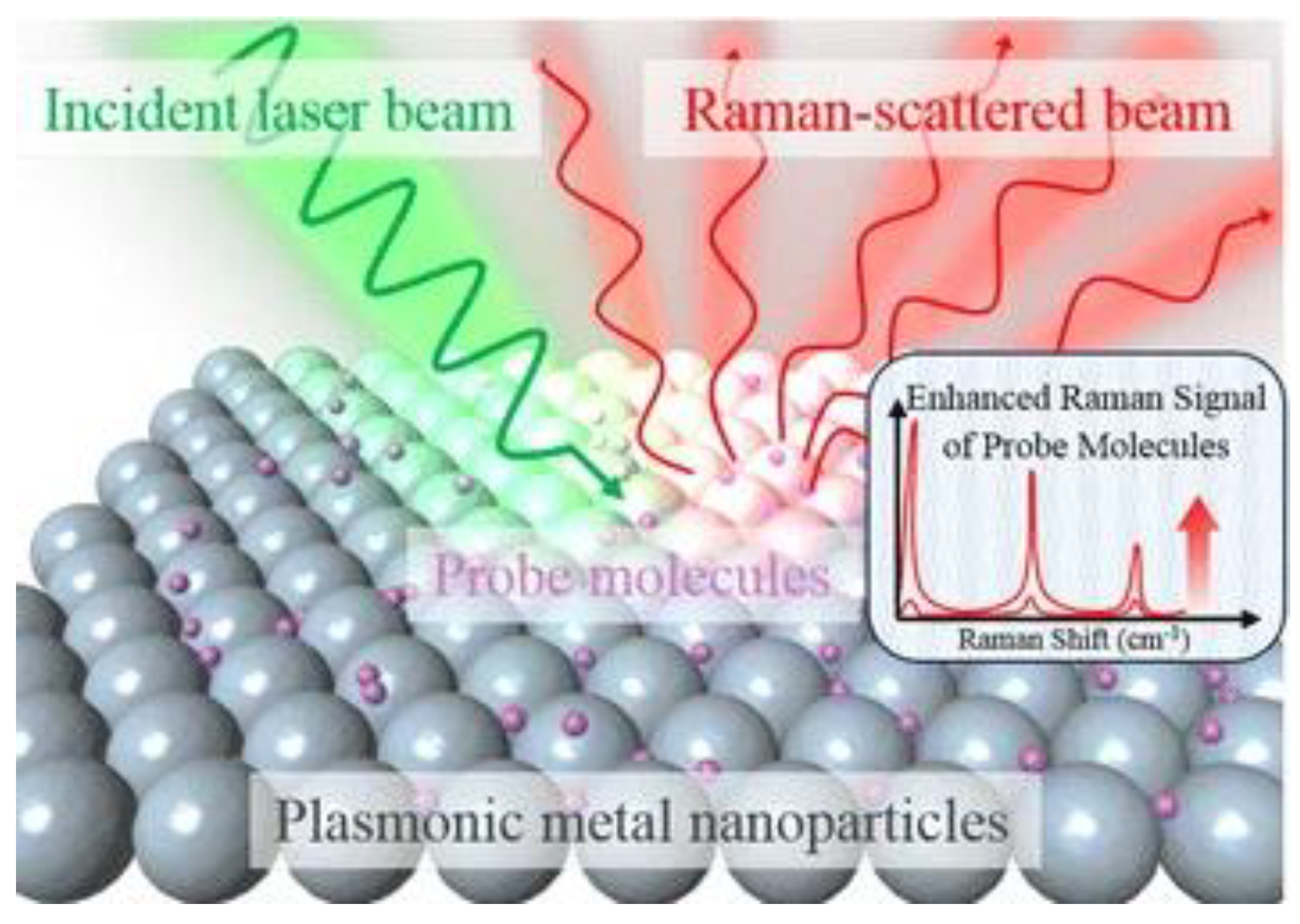
2.2. Theoretical Background of Surface Enhanced Raman Spectroscopy Techniques
2.3. Instrumentation: Raman and microRaman
3. Applications of Raman Spectroscopy
3.1. Carbon Based Materials
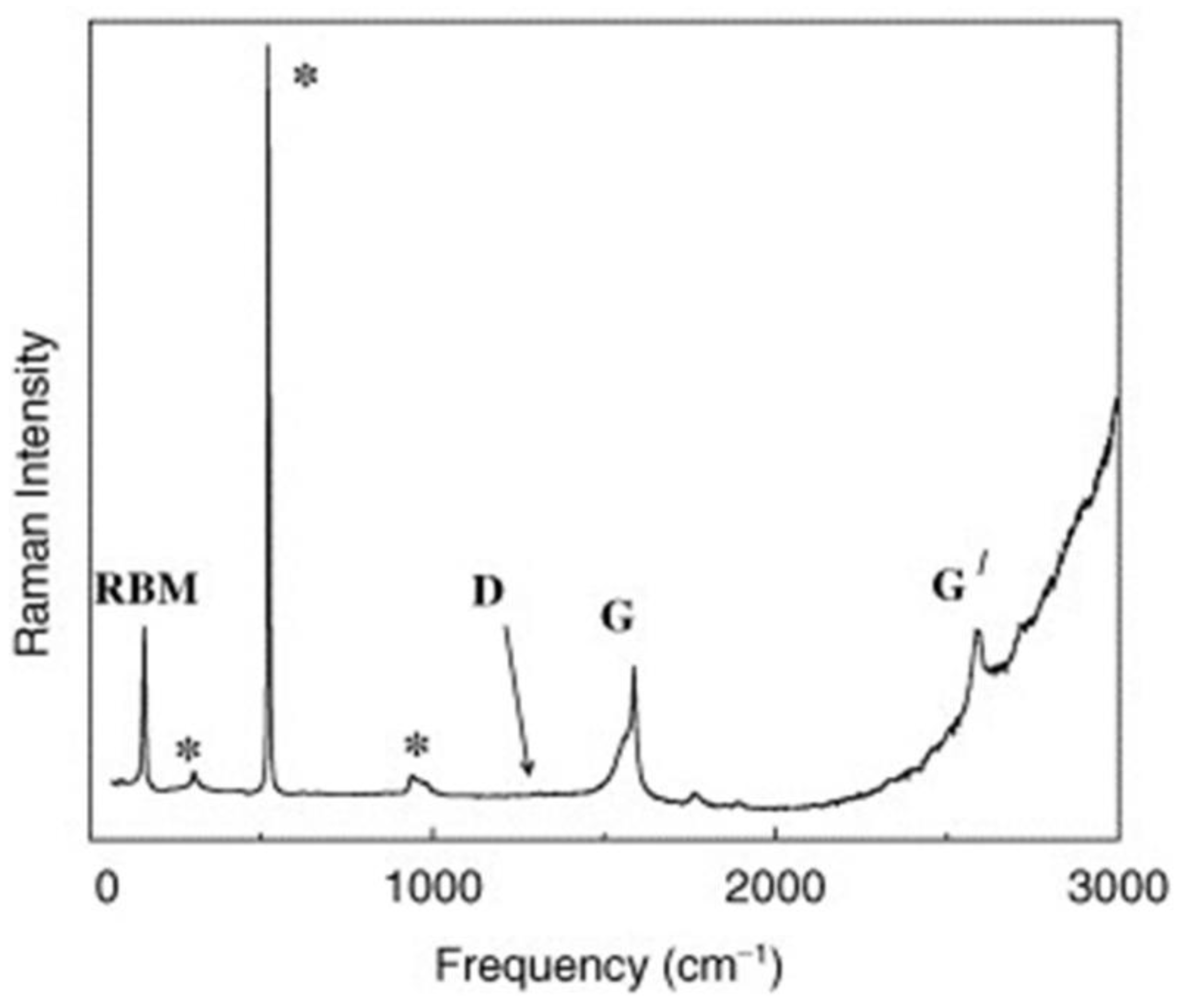
3.1.1. Carbon Nanotubes
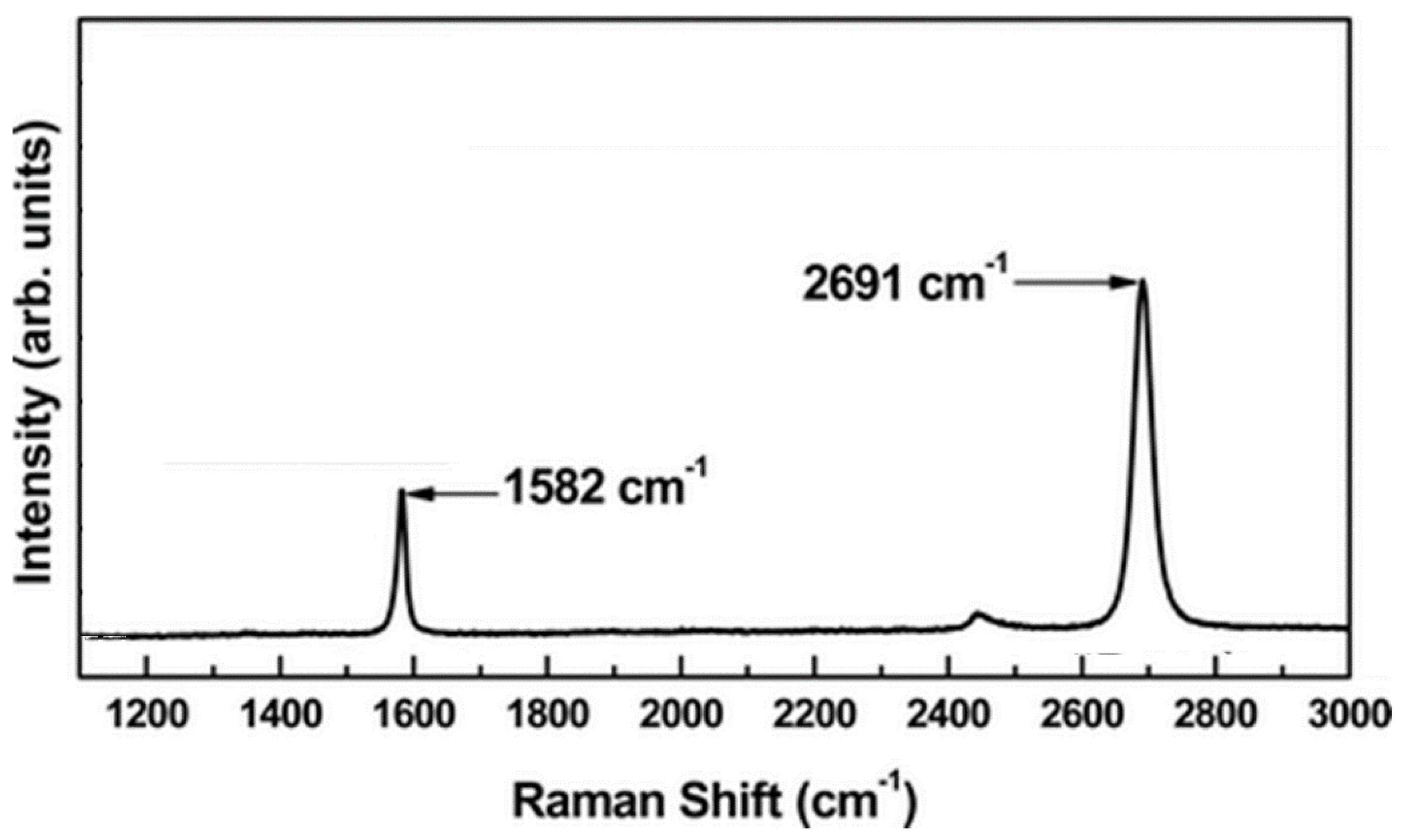
3.1.2. Graphene and Graphene-Like Materials
3.1.3. Amorphous and Disordered Carbon
3.2. Inorganic Materials
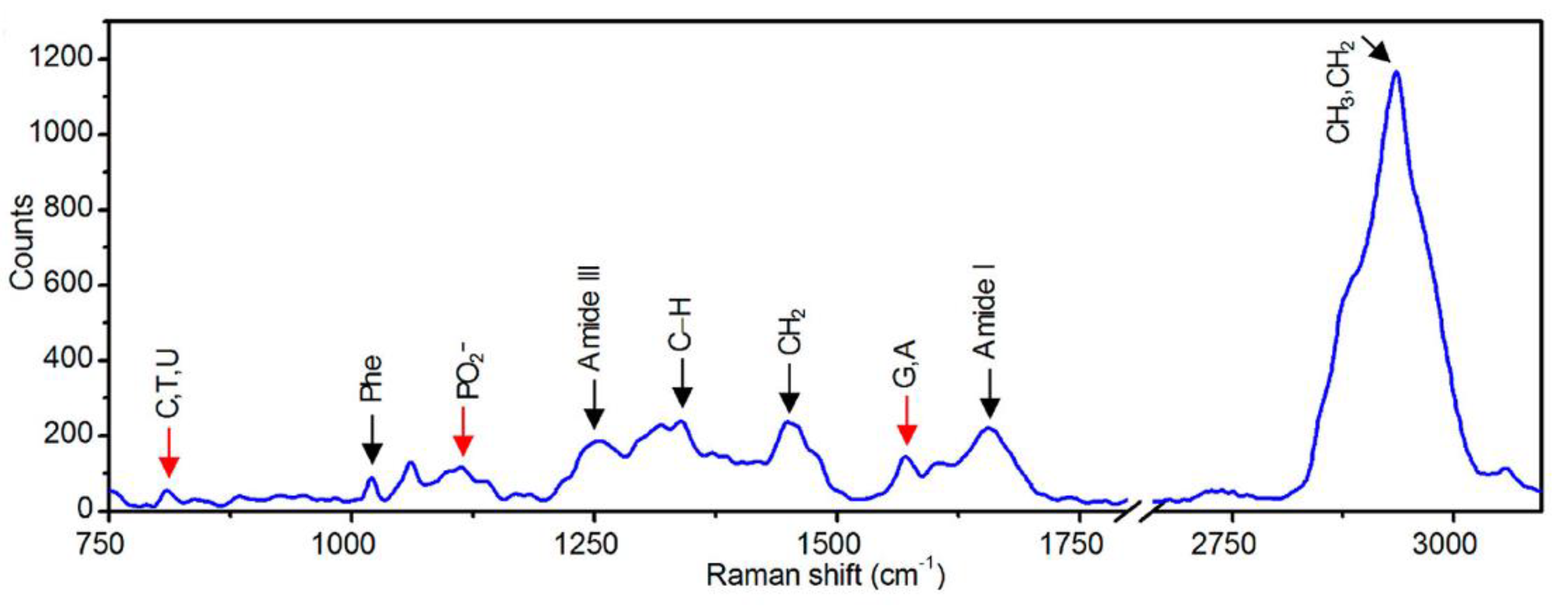
3.2.1. Biological Applications
3.2.2. Mineralogy
3.3. Advanced Applications
3.3.1. Cultural Heritage
3.3.2. Quality Control and Analytical Processes
4. Considerations about the Limitations and Advantages of Raman Spectroscopy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scotter, C.N.G. Non-destructive spectroscopic techniques for the measurement of food quality. Trends Food Sci. Technol. 1997, 8, 285–292. [Google Scholar] [CrossRef]
- Bicchieri, M.; Sodo, A.; Piantanida, G.; Coluzza, C. Analysis of degraded papers by non-destructive spectroscopic techniques. J. Raman Spectrosc. 2006, 37, 1186–1192. [Google Scholar] [CrossRef]
- Pelletier, M.J. Analytical Applications of Raman Spectroscopy; Blackwell Science: Oxford, UK, 1999. [Google Scholar]
- Raman, C.V. A new radiation. Indian J. Phys. 1928, 2, 387–398. [Google Scholar] [CrossRef]
- Landsberg, G.; Mandelstam, L. Eine neue Erscheinung bei der Lichtzerstreuung in Krystallen. Die Nat. 1928, 16, 557–558. [Google Scholar]
- Platonenko, V.T.; Khokhlov, R.V. On the mechanism of operation of a raman laser. J. Exptl. Theor. Phys. 1966, 19, 555–559. [Google Scholar]
- Hendra, P.J.; Stratton, P.M. Laser-raman spectroscopy. Chem. Rev. 1969, 69, 325–344. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Ying, Y. Applications of Raman Spectroscopy in Agricultural Products and Food Analysis: A Review. Appl. Spectrosc. Rev. 2011, 46, 539–560. [Google Scholar] [CrossRef]
- Parimaladevi, R.; Sathe, V.; Mahalingam, U. Graphene boosted silver nanoparticles as surface enhanced Raman spectroscopic sensors and photocatalysts for removal of standard and industrial dye contaminants. Sens. Actuators B Chem. 2019, 281, 679–688. [Google Scholar] [CrossRef]
- Auer, B.M.; Skinner, J.L. IR and Raman spectra of liquid water: Theory and interpretation. J. Chem. Phys. 2008, 128, 224511. [Google Scholar] [CrossRef]
- Picard, A.; Daniel, I.; Montagnac, G.; Oger, P. In situ monitoring by quantitative Raman spectroscopy of alcoholic fermentation by Saccharomyces cerevisiae under high pressure. Extremophiles 2007, 11, 445–452. [Google Scholar] [CrossRef]
- Rohleder, D.R.; Kocherscheidt, G.; Gerbe, K.; Köhler, W.; Möcks, J.; Petrich, W.H. Comparison of mid-infrared and Raman spectroscopy in the quantitative analysis of serum. J. Biomed. Opt. 2005, 10, 31108–31118. [Google Scholar] [CrossRef] [Green Version]
- Jung, N.; Crowther, A.C.; Kim, N.; Kim, P.; Brus, L. Raman Enhancement on Graphene: Adsorbed and Intercalated Molecular Species. ACS Nano 2010, 4, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Jorabchi, H.; Wilson, R.H.; Belton, P.S.; Edwards-Webb, J.D.; Coxon, D.T. Quantitative analysis of oils and fats by Fourier transform Raman spectroscopy. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 1449–1458. [Google Scholar] [CrossRef]
- Frausto-Reyes, C.; Medina-Gutiérrez, C.; Sato-Berrú, R.; Sahagún, L.R. Qualitative study of ethanol content in tequilas by Raman spectroscopy and principal component analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2657–2662. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Pergamon Press: New York, NY, USA, 2013. [Google Scholar]
- Kudelski, A. Analytical applications of Raman spectroscopy. Talanta 2008, 76, 1–8. [Google Scholar] [CrossRef]
- Ferraro, J.R. Introductory to Raman Spectroscopy; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Geraldes, C.F.G.C. Introduction to Infrared and Raman-Based Biomedical Molecular Imaging and Comparison with Other Modalities. Molecules 2020, 25, 5547. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef]
- Naik, G.V.; Kim, J.; Boltasseva, A. Oxides and nitrides as alternative plasmonic materials in the optical range. Opt. Mater. Express 2011, 1, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Jeon, T.Y.; Kim, D.J.; Park, S.-G.; Kim, S.-H.; Kim, D.-H. Nanostructured plasmonic substrates for use as SERS sensors. Nano Converg. 2016, 3, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kooij, E.S.; Ahmed, W.; Zandvliet, H.J.; Poelsema, B. Localized plasmons in noble metal nanospheroids. J. Phys. Chem. C 2011, 115, 10321–10332. [Google Scholar] [CrossRef]
- Tsuneda, T.; Iwasa, T.; Taketsugu, T. Roles of silver nanoclusters in surface-enhanced Raman spectroscopy. J. Chem. Phys. 2019, 151, 094102. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.; Blandón, J.; Riascos, H. Localized Plasmon resonance in metal nanoparticles using Mie theory. J. Phys. Conf. Ser. 2017, 850, 012017. [Google Scholar] [CrossRef]
- Horvath, H. Gustav Mie and the scattering and absorption of light by particles: Historic developments and basics. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 787–799. [Google Scholar] [CrossRef]
- Drude, P. Zur elektronentheorie der metalle. Ann. Der Phys. 1900, 306, 566–613. [Google Scholar] [CrossRef] [Green Version]
- Coronado, E.A.; Schatz, G.C. Surface plasmon broadening for arbitrary shape nanoparticles: A geometrical probability approach. J. Chem. Phys. 2003, 119, 3926–3934. [Google Scholar] [CrossRef]
- Kreibig, U. Electronic properties of small silver particles: The optical constants and their temperature dependence. J. Phys. F Met. Phys. 1974, 4, 999. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman scattering and biophysics. J. Phys. Condens. Matter 2002, 14, R597. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Puebla, R.A. Effects of the Excitation Wavelength on the SERS Spectrum. J. Phys. Chem. Lett. 2012, 3, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Kerker, M.; Siiman, O.; Bumm, L.; Wang, D.-S. Surface enhanced Raman scattering (SERS) of citrate ion adsorbed on colloidal silver. Appl. Opt. 1980, 19, 3253–3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Kerker, M. Enhanced Raman scattering by molecules adsorbed at the surface of colloidal spheroids. Phys. Rev. B 1981, 24, 1777–1790. [Google Scholar] [CrossRef]
- Zeman, E.J.; Schatz, G.C. An accurate electromagnetic theory study of surface enhancement factors for silver, gold, copper, lithium, sodium, aluminum, gallium, indium, zinc, and cadmium. J. Phys. Chem. 1987, 91, 634–643. [Google Scholar] [CrossRef]
- Xu, H.; Aizpurua, J.; Käll, M.; Apell, P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering. Phys. Rev. E 2000, 62, 4318–4324. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Ohtaka, K. Surface enhanced Raman scattering by metal spheres. I. Cluster effect. J. Phys. Soc. Jpn. 1983, 52, 3853–3864. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, X.L.; Shen, J.C.; Xia, Y.; Shen, P.N.; Chu, P.K. Silver fractal networks for surface-enhanced Raman scattering substrates. Appl. Surf. Sci. 2008, 254, 5399–5402. [Google Scholar] [CrossRef]
- Weitz, D.; Oliveria, M. Fractal structures formed by kinetic aggregation of aqueous gold colloids. Phys. Rev. Lett. 1984, 52, 1433–1436. [Google Scholar] [CrossRef]
- Shalaev, V.M. Electromagnetic properties of small-particle composites. Phys. Rep. 1996, 272, 61–137. [Google Scholar] [CrossRef]
- Sarychev, A.K.; Shalaev, V.M. Electromagnetic field fluctuations and optical nonlinearities in metal-dielectric composites. Phys. Rep. 2000, 335, 275–371. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kartha, V.B.; Manoharan, R.; Deinum, G.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Detection and identification of a single DNA base molecule using surface-enhanced Raman scattering (SERS). Phys. Rev. E 1998, 57, R6281. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Aldeanueva-Potel, P.; Mateo-Mateo, C.; Rodríguez-Lorenzo, L.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M. Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J. R. Soc. Interface 2010, 7, S435–S450. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rico, E.; Alvarez-Puebla, R.A.; Guerrini, L. Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: From fundamental studies to real-life applications. Chem. Soc. Rev. 2018, 47, 4909–4923. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, W.; Zhong, H.; Yang, F.; Fu, W.; Ling, Y.; Zhang, Z. Robust quantitative SERS analysis with Relative Raman scattering intensities. Talanta 2021, 221, 121465. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, M.; DiLella, D.; Maynard, K. Surface Raman spectroscopy of a number of cyclic aromatic molecules adsorbed on silver: Selection rules and molecular reorientation. Langmuir 1988, 4, 67–76. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons: Cichester, UK, 2006. [Google Scholar]
- Stöckle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]
- Fiederling, K.; Abasifard, M.; Richter, M.; Deckert, V.; Gräfe, S.; Kupfer, S. The chemical effect goes resonant–a full quantum mechanical approach on TERS. Nanoscale 2020, 12, 6346–6359. [Google Scholar] [CrossRef]
- Bailo, E.; Deckert, V. Tip-Enhanced Raman Spectroscopy of Single RNA Strands: Towards a Novel Direct-Sequencing Method. Angew. Chem. Int. Ed. 2008, 47, 1658–1661. [Google Scholar] [CrossRef]
- Rasmussen, A.; Deckert, V. Surface- and tip-enhanced Raman scattering of DNA components. J. Raman Spectrosc. 2006, 37, 311–317. [Google Scholar] [CrossRef]
- He, Z.; Qiu, W.; Kizer, M.E.; Wang, J.; Chen, W.; Sokolov, A.V.; Wang, X.; Hu, J.; Scully, M.O. Resolving the Sequence of RNA Strands by Tip-Enhanced Raman Spectroscopy. ACS Photonics 2020, 8, 424–430. [Google Scholar] [CrossRef]
- Yeo, B.-S.; Mädler, S.; Schmid, T.; Zhang, W.; Zenobi, R. Tip-Enhanced Raman Spectroscopy Can See More: The Case of Cytochrome c. J. Phys. Chem. C 2008, 112, 4867–4873. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Hoehse, M.; Gornushkin, I.; Merk, S.; Panne, U. Assessment of suitability of diode pumped solid state lasers for laser induced breakdown and Raman spectroscopy. J. Anal. At. Spectrom. 2011, 26, 414–424. [Google Scholar] [CrossRef]
- Ariese, F.; Meuzelaar, H.; Kerssens, M.M.; Buijs, J.B.; Gooijer, C. Picosecond Raman spectroscopy with a fast intensified CCD camera for depth analysis of diffusely scattering media. Analyst 2009, 134, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Shepherd, I. Fluorescence suppression in time-resolved Raman spectra. J. Phys. E Sci. Instrum. 1977, 10, 617–620. [Google Scholar] [CrossRef]
- Yoo, W.S.; Kim, J.H.; Han, S.M. Multiwavelength Raman characterization of silicon stress near through-silicon vias and its inline monitoring applications. J. Micro/Nanolithography MEMS MOEMS 2014, 13, 011205. [Google Scholar] [CrossRef]
- Sekar, S.K.V.; Mosca, S.; Farina, A.; Martelli, F.; Taroni, P.; Valentini, G.; Cubeddu, R.; Pifferi, A. Frequency offset Raman spectroscopy (FORS) for depth probing of diffusive media. Opt. Express 2017, 25, 4585–4597. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, B.; Dombrowski, K. Experimental challenges of stress measurements with resonant micro-Raman spectroscopy. J. Raman Spectrosc. 1999, 30, 893–897. [Google Scholar] [CrossRef]
- Wermelinger, T.; Charpentier, C.; Yüksek, M.D.; Spolenak, R. Measuring stresses in thin metal films by means of Raman microscopy using silicon as a strain gage material. J. Raman Spectrosc. 2009, 40, 1849–1857. [Google Scholar] [CrossRef]
- Zhao, Q.; Wagner, H.D. Raman spectroscopy of carbon–nanotube–based composites. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2407–2424. [Google Scholar] [CrossRef]
- Hashida, T.; Jeong, Y.G.; Hua, Y.; Hsu, S.L.; Paul, C.W. Spectroscopic study on morphology evolution in polymer blends. Macromolecules 2005, 38, 2876–2882. [Google Scholar] [CrossRef]
- Van Overbeke, E.; Devaux, J.; Legras, R.; Carter, J.; McGrail, P.; Carlier, V. Phase separation in epoxy-copolyethersulphone blends: Morphologies and local characterisation by micro-Raman spectroscopy. Polymer 2003, 44, 4899–4908. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.; Hartschuh, A.; Novotny, L. Chirality changes in carbon nanotubes studied with near-field Raman spectroscopy. Nano Lett. 2007, 7, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Fan, X.; Ni, Z.; Shen, Z.; Feng, Y.; Zou, B. Orientation-dependent Raman spectroscopy of single wurtzite CdS nanowires. J. Phys. Chem. C 2008, 112, 1865–1870. [Google Scholar] [CrossRef]
- Livneh, T.; Zhang, J.; Cheng, G.; Moskovits, M. Polarized Raman scattering from single GaN nanowires. Phys. Rev. B 2006, 74, 035320. [Google Scholar] [CrossRef]
- Aqel, A.; Abou El-Nour, K.M.; Ammar, R.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Dresselhaus, G.; Saito, R. Raman spectroscopy on one isolated carbon nanotube. Phys. B Condens. Matter 2002, 323, 15–20. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Jorio, A.; Souza Filho, A.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Hofmann, M. The big picture of Raman scattering in carbon nanotubes. Vib. Spectrosc. 2007, 45, 71–81. [Google Scholar] [CrossRef]
- Cooper, C.; Young, R.; Halsall, M. Investigation into the deformation of carbon nanotubes and their composites through the use of Raman spectroscopy. Compos. Part A Appl. Sci. Manuf. 2001, 32, 401–411. [Google Scholar] [CrossRef]
- Kang, D.; Hakamatsuka, M.; Kojima, K.; Tachibana, M. Influence of heating and laser irradiation on the Raman D band in single-wall carbon nanotubes. Diam. Relat. Mater. 2010, 19, 578–580. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Zhao, X.; Ando, Y.; Qin, L.-C.; Kataura, H.; Maniwa, Y.; Saito, R. Radial breathing modes of multiwalled carbon nanotubes. Chem. Phys. Lett. 2002, 361, 169–174. [Google Scholar] [CrossRef]
- Gohil, S.; Ghosh, S. Surface enhanced Raman scattering from multiwalled carbon nanotubes at low temperatures. Appl. Phys. Lett. 2010, 96, 143108. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef] [Green Version]
- Maciel, I.O.; Anderson, N.; Pimenta, M.A.; Hartschuh, A.; Qian, H.; Terrones, M.; Terrones, H.; Campos-Delgado, J.; Rao, A.M.; Novotny, L. Electron and phonon renormalization near charged defects in carbon nanotubes. Nat. Mater. 2008, 7, 878–883. [Google Scholar] [CrossRef]
- Rao, A.; Jorio, A.; Pimenta, M.; Dantas, M.; Saito, R.; Dresselhaus, G.; Dresselhaus, M. Polarized Raman study of aligned multiwalled carbon nanotubes. Phys. Rev. Lett. 2000, 84, 1820–1823. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, F.; Koinkar, P.; Avasthi, D.; Pivin, J.; More, M. Effect of intense laser and energetic ion irradiation on Raman modes of Multiwalled Carbon Nanotubes. Thin Solid Film 2009, 517, 4322–4324. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Yano, T.; Khoklang, K.; Krukowski, P.; Akai-Kasaya, M.; Saito, A.; Kuwahara, Y. Nanoscale analysis of multiwalled carbon nanotube by tip-enhanced Raman spectroscopy. Carbon 2016, 99, 642–648. [Google Scholar] [CrossRef]
- Asiya, S.; Kyzas, G.Z.; Pal, K.; de Souza, F.G. Graphene functionalized hybrid nanomaterials for industrial-scale applications: A systematic review. J. Mol. Struct. 2021, 130518. [Google Scholar]
- Nanda, S.S.; Kim, M.J.; Yeom, K.S.; An, S.S.A.; Ju, H.; Yi, D.K. Raman spectrum of graphene with its versatile future perspectives. TrAC Trends Anal. Chem. 2016, 80, 125–131. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Calizo, I.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Temperature Dependence of the Raman Spectra of Graphene and Graphene Multilayers. Nano Lett. 2007, 7, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Zhou, W.; Shi, W.; Dang, W.; Li, X.; Liang, B. The split-up of G band and 2D band in temperature-dependent Raman spectra of suspended graphene. Opt. Laser Technol. 2021, 139, 106960. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Tanimura, M.; Tachibana, M. Temperature dependence of the Raman spectra of multilayer graphene nanoribbons fabricated by unzipping method. Diam. Relat. Mater. 2020, 109, 108047. [Google Scholar] [CrossRef]
- Al-Hazmi, F.; Beall, G.W.; Al-Ghamdi, A.; Alshahrie, A.; Shokr, F.; Mahmoud, W.E. Raman and ellipsometry spectroscopic analysis of graphene films grown directly on Si substrate via CVD technique for estimating the graphene atomic planes number. J. Mol. Struct. 2016, 1118, 275–278. [Google Scholar] [CrossRef]
- Koo, E.; Ju, S.-Y. Role of residual polymer on chemical vapor grown graphene by Raman spectroscopy. Carbon 2015, 86, 318–324. [Google Scholar] [CrossRef]
- Woo, Y.S.; Lee, D.W.; Kim, U.J. General Raman-based method for evaluating the carrier mobilities of chemical vapor deposited graphene. Carbon 2018, 132, 263–270. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Gokturk, P.A.; Kakenov, N.; Kocabas, C.; Suzer, S. Raman and X-ray photoelectron spectroscopic studies of graphene devices for identification of doping. Appl. Surf. Sci. 2017, 425, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Ferralis, N.; Maboudian, R.; Carraro, C. Evidence of Structural Strain in Epitaxial Graphene Layers on 6H-SiC(0001). Phys. Rev. Lett. 2008, 101, 156801. [Google Scholar] [CrossRef] [Green Version]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Robertson, J.; O’Reilly, E.P. Electronic and atomic structure of amorphous carbon. Phys. Rev. B 1987, 35, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef] [Green Version]
- Mariotto, G.; Vinegoni, C.; Jacobsohn, L.G.; Freire, F.L. Raman spectroscopy and scanning electron microscopy investigation of annealed amorphous carbon–germanium films deposited by d.c. magnetron sputtering. Diam. Relat. Mater. 1999, 8, 668–672. [Google Scholar] [CrossRef]
- Cui, W.G.; Lai, Q.B.; Zhang, L.; Wang, F.M. Quantitative measurements of sp3 content in DLC films with Raman spectroscopy. Surf. Coat. Technol. 2010, 205, 1995–1999. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Lin, Y.; Wang, F. A ternary phase diagram for amorphous carbon. Carbon 2015, 94, 202–213. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Kleinsorge, B.; Adamopoulos, G.; Robertson, J.; Milne, W.I.; Stolojan, V.; Brown, L.M.; LiBassi, A.; Tanner, B.K. Determination of bonding in amorphous carbons by electron energy loss spectroscopy, Raman scattering and X-ray reflectivity. J. Non-Cryst. Solids 2000, 266–269, 765–768. [Google Scholar] [CrossRef]
- Gilkes, K.W.R.; Sands, H.S.; Batchelder, D.N.; Milne, W.I.; Robertson, J. Direct observation of sp3 bonding in tetrahedral amorphous carbon UV Raman spectroscopy. J. Non-Cryst. Solids 1998, 227-230, 612–616. [Google Scholar] [CrossRef]
- Pardanaud, C.; Martin, C.; Roubin, P.; Giacometti, G.; Hopf, C.; Schwarz-Selinger, T.; Jacob, W. Raman spectroscopy investigation of the H content of heated hard amorphous carbon layers. Diam. Relat. Mater. 2013, 34, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.-X.; Yao, K.-L.; Liu, Z.-L. Substrate bias effect on structure of tetrahedral amorphous carbon films by Raman spectroscopy. Diam. Relat. Mater. 2007, 16, 1746–1751. [Google Scholar] [CrossRef]
- Liu, F.-X.; Yao, K.-L.; Liu, Z.-L. Substrate tilting effect on structure of tetrahedral amorphous carbon films by Raman spectroscopy. Surf. Coat. Technol. 2007, 201, 7235–7240. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
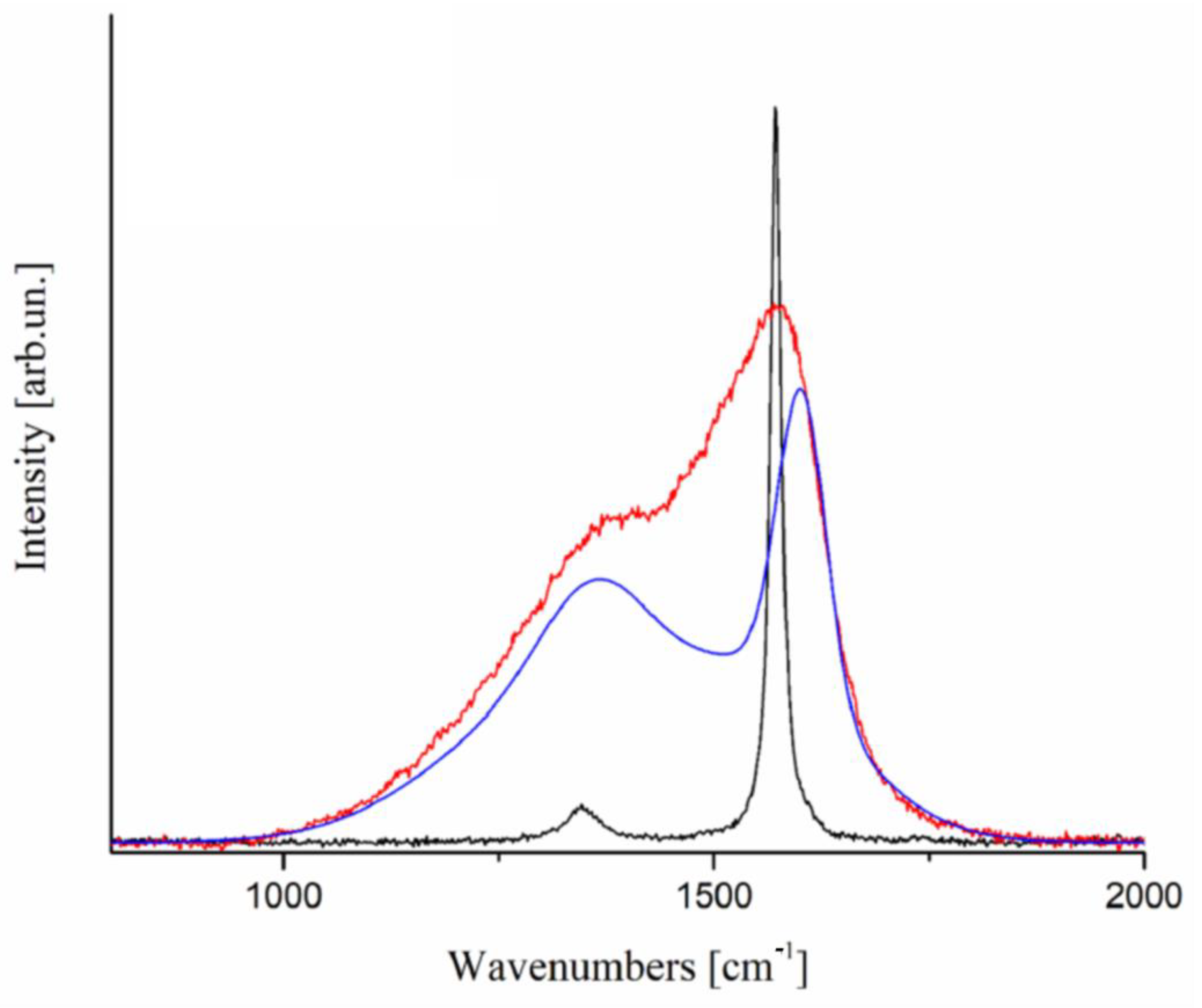
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the Novel Mixed Gaussian-Lorentzian Lineshape in the Analysis of the Raman Signal of Biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef]
- Giorcelli, M.; Bartoli, M.; Sanginario, A.; Padovano, E.; Rosso, C.; Rovere, M.; Tagliaferro, A. High-Temperature Annealed Biochar as a Conductive Filler for the Production of Piezoresistive Materials for Energy Conversion Application. ACS Appl. Electron. Mater. 2021, 3, 838–844. [Google Scholar] [CrossRef]
- Savi, P.; Yasir, M.; Bartoli, M.; Giorcelli, M.; Longo, M. Electrical and Microwave Characterization of Thermal Annealed Sewage Sludge Derived Biochar Composites. Appl. Sci. 2020, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Noori, A.; Bartoli, M.; Frache, A.; Piatti, E.; Giorcelli, M.; Tagliaferro, A. Development of Pressure-Responsive PolyPropylene and Biochar-Based Materials. Micromachines 2020, 11, 339. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, M.; Nasir, M.A.; Passaglia, E.; Spiniello, R.; Jagdale, P.; Rosso, C.; Giorcelli, M.; Rovere, M.; Tagliaferro, A. Influence of pyrolytic thermal history on olive pruning biochar and related epoxy composites mechanical properties. J. Compos. Mater. 2020, 54, 1863–1873. [Google Scholar] [CrossRef]
- Giorcelli, M.; Bartoli, M. Development of Coffee Biochar Filler for the Production of Electrical Conductive Reinforced Plastic. Polymers 2019, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, M.; Giorcelli, M.; Rovere, M.; Jagdale, P.; Tagliaferro, A.; Chae, M.; Bressler, D.C. Shape tunability of carbonized cellulose nanocrystals. SN Appl. Sci. 2019, 1, 1661–1676. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, M.; Giorcelli, M.; Rosso, C.; Rovere, M.; Jagdale, P.; Tagliaferro, A. Influence of Commercial Biochar Fillers on Brittleness/Ductility of Epoxy Resin Composites. Appl. Sci. 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Papaefthymiou, G.C. Nanoparticle magnetism. Nano Today 2009, 4, 438–447. [Google Scholar] [CrossRef]
- Iskandar, F. Nanoparticle processing for optical applications—A review. Adv. Powder Technol. 2009, 20, 283–292. [Google Scholar] [CrossRef]
- Bhaumik, A.; Shearin, A.; Delong, R.; Wanekaya, A.; Ghosh, K. Probing the Interaction at the Nano–Bio Interface Using Raman Spectroscopy: ZnO Nanoparticles and Adenosine Triphosphate Biomolecules. J. Phys. Chem. C 2014, 118, 18631–18639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agressott, E.V.; de Moura, T.A.; Marinho, N.L.; Vasconcelos, T.d.L.; Cunha, F.A.; Fechine, P.; de Souza Filho, A.G.; Paschoal, A.R. Tip-Enhanced Raman spectroscopy investigations of core-shell Ag-proteins nanoparticles synthesized by Rhodotorula mucilaginosa and Rhodotorula glutinis fungi. Vib. Spectrosc. 2020, 110, 103104. [Google Scholar] [CrossRef]
- Alula, M.T.; Lemmens, P.; Bo, L.; Wulferding, D.; Yang, J.; Spende, H. Preparation of silver nanoparticles coated ZnO/Fe3O4 composites using chemical reduction method for sensitive detection of uric acid via surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2019, 1073, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Caprara, D.; Ripanti, F.; Capocefalo, A.; Sarra, A.; Brasili, F.; Petrillo, C.; Fasolato, C.; Postorino, P. DNA-functionalized gold nanoparticle assemblies for Surface Enhanced Raman Scattering. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124399. [Google Scholar] [CrossRef] [Green Version]
- El-Said, W.A.; Alshitari, W.; Choi, J.-w. Controlled fabrication of gold nanobipyramids/polypyrrole for shell-isolated nanoparticle-enhanced Raman spectroscopy to detect γ-aminobutyric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117890. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Peñalver, E.; Villaseñor, M.J.; Contento, A.M.; Ríos, Á. Erythrosine B–coated gold nanoparticles as an analytical sensing tool for the proper determination of both compounds based on surface-enhanced Raman spectroscopy. Microchem. J. 2020, 157, 104937. [Google Scholar] [CrossRef]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. RSC Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef] [Green Version]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, L.; Pazos-Perez, N.; Garcia-Rico, E.; Alvarez-Puebla, R. Cancer characterization and diagnosis with SERS-encoded particles. Cancer Nanotechnol. 2017, 8, 1–24. [Google Scholar] [CrossRef]
- D’Acunto, M.; Gaeta, R.; Capanna, R.; Franchi, A. contribution of Raman Spectroscopy to Diagnosis and Grading of chondrogenic tumors. Sci. Rep. 2020, 10, 2155. [Google Scholar] [CrossRef]
- Sbroscia, M.; Di Gioacchino, M.; Ascenzi, P.; Crucitti, P.; di Masi, A.; Giovannoni, I.; Longo, F.; Mariotti, D.; Naciu, A.M.; Palermo, A. Thyroid cancer diagnosis by Raman spectroscopy. Sci. Rep. 2020, 10, 13342. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.-Y.; Ning, Y.-J.; Jiang, Z.-F.; Zhu, Y.-Y.; Guo, J.; Fan, X.-Y.; Zhang, Y.-B. The efficacy of Raman spectroscopy in lung cancer diagnosis: The first diagnostic meta-analysis. Lasers Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Giamougiannis, P.; Morais, C.L.; Grabowska, R.; Ashton, K.M.; Wood, N.J.; Martin-Hirsch, P.L.; Martin, F.L. A comparative analysis of different biofluids towards ovarian cancer diagnosis using Raman microspectroscopy. Anal. Bioanal. Chem. 2021, 413, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.-W.; Ma, D.-Y.; Fu, J.-J.; Lu, Y.-F.; Zhao, Y.; Xu, X.-Y.; Yin, J.-H. Fluorescence imaging and Raman spectroscopy applied for the accurate diagnosis of breast cancer with deep learning algorithms. Biomed. Opt. Express 2020, 11, 3673–3683. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Y.; Huang, L.; He, W.; Wang, S.; Wang, C.; Zhou, G.; Chen, Y.; Zhou, X.; Huang, Y. Label-free diagnosis for colorectal cancer through coffee ring-assisted surface-enhanced Raman spectroscopy on blood serum. J. Biophotonics 2020, 13, e201960176. [Google Scholar] [CrossRef]
- Correia, N.A.; Batista, L.T.; Nascimento, R.J.; Cangussú, M.C.; Crugeira, P.J.; Soares, L.G.; Silveira, L., Jr.; Pinheiro, A.L. Detection of prostate cancer by Raman spectroscopy: A multivariate study on patients with normal and altered PSA values. J. Photochem. Photobiol. B Biol. 2020, 204, 111801. [Google Scholar] [CrossRef]
- Téllez-Plancarte, A.; Haro-Poniatowski, E.; Picquart, M.; Morales-Méndez, J.G.; Lara-Cruz, C.; Jiménez-Salazar, J.E.; Damián-Matsumura, P.; Escobar-Alarcón, L.; Batina, N. Development of a nanostructured platform for identifying HER2-heterogeneity of breast cancer cells by surface-enhanced raman scattering. Nanomaterials 2018, 8, 549. [Google Scholar] [CrossRef] [Green Version]
- Ryzhikova, E.; Ralbovsky, N.M.; Sikirzhytski, V.; Kazakov, O.; Halamkova, L.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Raman spectroscopy and machine learning for biomedical applications: Alzheimer’s disease diagnosis based on the analysis of cerebrospinal fluid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119188. [Google Scholar] [CrossRef]
- Gurian, E.; Giraudi, P.; Rosso, N.; Tiribelli, C.; Bonazza, D.; Zanconati, F.; Giuricin, M.; Palmisano, S.; de Manzini, N.; Sergo, V. Differentiation between stages of non-alcoholic fatty liver diseases using surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2020, 1110, 190–198. [Google Scholar] [CrossRef]
- Kaewseekhao, B.; Nuntawong, N.; Eiamchai, P.; Roytrakul, S.; Reechaipichitkul, W.; Faksri, K. Diagnosis of active tuberculosis and latent tuberculosis infection based on Raman spectroscopy and surface-enhanced Raman spectroscopy. Tuberculosis 2020, 121, 101916. [Google Scholar] [CrossRef]
- Chen, C.; Yang, L.; Zhao, J.; Yuan, Y.; Chen, C.; Tang, J.; Yang, H.; Yan, Z.; Wang, H.; Lv, X. Urine Raman spectroscopy for rapid and inexpensive diagnosis of chronic renal failure (CRF) using multiple classification algorithms. Optik 2020, 203, 164043. [Google Scholar] [CrossRef]
- Kashif, M.; Majeed, M.I.; Hanif, M.A.; ur Rehman, A. Surface Enhanced Raman Spectroscopy of the serum samples for the diagnosis of Hepatitis C and prediction of the viral loads. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118729. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, S.; Khan, M.B.; Amin, A.; Bilal, M.; Nawaz, H.; Hassan, M. Optical diagnosis of hepatitis B virus infection in blood plasma using Raman spectroscopy and chemometric techniques. J. Raman Spectrosc. 2020, 51, 1067–1077. [Google Scholar] [CrossRef]
- Rickard, J.J.; Di-Pietro, V.; Smith, D.J.; Davies, D.J.; Belli, A.; Oppenheimer, P.G. Rapid optofluidic detection of biomarkers for traumatic brain injury via surface-enhanced Raman spectroscopy. Nat. Biomed. Eng. 2020, 4, 610–623. [Google Scholar] [CrossRef]
- Su, Y.; Xu, S.; Zhang, J.; Chen, X.; Jiang, L.-P.; Zheng, T.; Zhu, J.-J. Plasmon near-field coupling of bimetallic nanostars and a hierarchical bimetallic SERS “hot field”: Toward ultrasensitive simultaneous detection of multiple cardiorenal syndrome biomarkers. Anal. Chem. 2018, 91, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Low, J.S.Y.; Thevarajah, T.M.; Chang, S.W.; Goh, B.T.; Khor, S.M. Biosensing based on surface-enhanced Raman spectroscopy as an emerging/next-generation point-of-care approach for acute myocardial infarction diagnosis. Crit. Rev. Biotechnol. 2020, 40, 1191–1209. [Google Scholar] [CrossRef]
- Carlomagno, C.; Banfi, P.; Gualerzi, A.; Picciolini, S.; Volpato, E.; Meloni, M.; Lax, A.; Colombo, E.; Ticozzi, N.; Verde, F. Human salivary Raman fingerprint as biomarker for the diagnosis of Amyotrophic Lateral Sclerosis. Sci. Rep. 2020, 10, 10175. [Google Scholar] [CrossRef] [PubMed]
- Acri, G.; Venuti, V.; Costa, S.; Testagrossa, B.; Pellegrino, S.; Crupi, V.; Majolino, D. Raman Spectroscopy as Noninvasive Method of Diagnosis of Pediatric Onset Inflammatory Bowel Disease. Appl. Sci. 2020, 10, 6974. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Biji, P.; Panthalingal, M.K.; Krishna, C.M.; Rajkumar, S.; Joshi, D.S.; Sundaram, N. Development of integrated microfluidic platform coupled with Surface-enhanced Raman Spectroscopy for diagnosis of COVID-19. Med. Hypotheses 2021, 146, 110356. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Li, L.; Lu, S.; Yin, Y.; Su, Y.; Zeng, Y.; Luo, M.; Ma, M.; Zhou, H.; Orlandini, L. An efficient primary screening of COVID-19 by serum Raman spectroscopy. J. Raman Spectrosc. 2021, 52, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Elsharif, A.M. Rapid Detection of COVID 19 via Surface Enhanced Raman Spectroscopy. Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 715–720. [Google Scholar] [CrossRef]
- Kneipp, K.; Haka, A.S.; Kneipp, H.; Badizadegan, K.; Yoshizawa, N.; Boone, C.; Shafer-Peltier, K.E.; Motz, J.T.; Dasari, R.R.; Feld, M.S. Surface-enhanced raman spectroscopy in single living cells using gold nanoparticles. Appl. Spectrosc. 2002, 56, 150–154. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Bai, Y.; Wang, W.; Liang, J.; Cheng, J.-X. Fingerprinting a Living Cell by Raman Integrated Mid-Infrared Photothermal Microscopy. Anal. Chem. 2019, 91, 10750–10756. [Google Scholar] [CrossRef]
- Wattanavichean, N.; Nishida, I.; Ando, M.; Kawamukai, M.; Yamamoto, T.; Hamaguchi, H.o. Organelle specific simultaneous Raman/green fluorescence protein microspectroscopy for living cell physicochemical studies. J. Biophotonics 2020, 13, e201960163. [Google Scholar] [CrossRef]
- Xie, C.; Goodman, C.; Dinno, M.A.; Li, Y.-Q. Real-time Raman spectroscopy of optically trapped living cells and organelles. Opt. Express 2004, 12, 6208–6214. [Google Scholar] [CrossRef]
- Puppels, G.; De Mul, F.; Otto, C.; Greve, J.; Robert-Nicoud, M.; Arndt-Jovin, D.; Jovin, T. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature 1990, 347, 301–303. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kajimoto, S.; Nakabayashi, T. Experimental evaluation of the density of water in a cell by Raman microscopy. J. Phys. Chem. Lett. 2017, 8, 5241–5245. [Google Scholar] [CrossRef]
- Gurjar, R.S.; Backman, V.; Perelman, L.T.; Georgakoudi, I.; Badizadegan, K.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Imaging human epithelial properties with polarized light- scattering spectroscopy. Nat. Med. 2001, 7, 1245–1248. [Google Scholar] [CrossRef]
- Backman, V.; Gurjar, R.; Badizadegan, K.; Itzkan, I.; Dasari, R.R.; Perelman, L.T.; Feld, M.S. Polarized light scattering spectroscopy for quantitative measurement of epithelial cellular structures in situ. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.G.; Valdez, T.A.; Georgakoudi, I.; Backman, V.; Fuentes, C.; Kabani, S.; Laver, N.; Wang, Z.; Boone, C.W.; Dasari, R.R.; et al. Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer 2003, 97, 1681–1692. [Google Scholar] [CrossRef]
- Nasdala, L.; Schmidt, C. Applications of Raman spectroscopy in mineralogy and geochemistry. Elem. Int. Mag. Mineral. Geochem. Petrol. 2020, 16, 99–104. [Google Scholar] [CrossRef]
- Schmidt, C.; Steele-MacInnis, M.; Watenphul, A.; Wilke, M. Calibration of zircon as a Raman spectroscopic pressure sensor to high temperatures and application to water-silicate melt systems. Am. Mineral. 2013, 98, 643–650. [Google Scholar] [CrossRef]
- Beyssac, O. New trends in Raman spectroscopy: From high-resolution geochemistry to planetary exploration. Elem. Int. Mag. Mineral. Geochem. Petrol. 2020, 16, 117–122. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, Z.; Wu, P.; Wang, J.; Cao, H.; Chen, J. Mineralogy of Lunar Meteorite Northwest Africa 10480 by Raman Spectroscopic Studies. In Proceedings of the 52nd Lunar and Planetary Science Conference, Virtual Conference, 15–19 March 2021; p. 2003. [Google Scholar]
- Cao, H.; Chen, J.; Fu, X.; Ling, Z. Raman and infrared spectroscopic perspectives of lunar meteorite Northwest Africa 4884. J. Raman Spectrosc. 2020, 51, 1652–1666. [Google Scholar] [CrossRef]
- Polavaram, K.C.; Garg, N. High-fidelity and high-resolution phase mapping of granites via confocal Raman imaging. Sci. Rep. 2021, 11, 8022. [Google Scholar] [CrossRef]
- Batista, A.J.; Vianna, P.G.; Ribeiro, H.B.; de Matos, C.J.S.; Gomes, A.S.L. QR code micro-certified gemstones: Femtosecond writing and Raman characterization in Diamond, Ruby and Sapphire. Sci. Rep. 2019, 9, 8927. [Google Scholar] [CrossRef] [Green Version]
- Culka, A.; Jehlička, J. Identification of gemstones using portable sequentially shifted excitation Raman spectrometer and RRUFF online database: A proof of concept study. Eur. Phys. J. Plus 2019, 134, 130. [Google Scholar] [CrossRef]
- Committee, A.M. Raman spectroscopy in cultural heritage: Background paper. Anal. Methods 2015, 7, 4844–4847. [Google Scholar]
- Casadio, F.; Daher, C.; Bellot-Gurlet, L. Raman spectroscopy of cultural heritage materials: Overview of applications and new frontiers in instrumentation, sampling modalities, and data processing. In Analytical Chemistry for Cultural Heritage; Springer: Cham, Switerland, 2016; pp. 161–211. [Google Scholar]
- Prieto, A.; Avella, M.; Martínez, O.; Jiménez, J.; Alonso, J.; Sánchez, I.; Martín, R.; Barrera, M. Raman Spectroscopy Analysis of a Playing Card from the 18th Century. Spectrosc. Lett. 2012, 45, 114–117. [Google Scholar] [CrossRef]
- Alves, A.P.P.; de Oliveira, L.P.; Castro, A.A.; Neumann, R.; de Oliveira, L.F.; Edwards, H.G.; Sant’Ana, A.C. The structure of different cellulosic fibres characterized by Raman spectroscopy. Vib. Spectrosc. 2016, 86, 324–330. [Google Scholar] [CrossRef]
- Edwards, H.; Ellis, E.; Farwell, D.; Janaway, R. Preliminary study of the application of Fourier transform Raman spectroscopy to the analysis of degraded archaeological linen textiles. J. Raman Spectrosc. 1996, 27, 663–669. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Wehling, B.; Moens, L.; Dekeyzer, B.; Cardon, B.; von Bohlen, A.; Klockenkämper, R. Pigment investigation of a late-medieval manuscript with total reflection X-ray fluorescence and micro-Raman spectroscopy. Analyst 1999, 124, 169–172. [Google Scholar] [CrossRef]
- Bicchieri, M.; Nardone, M.; Sodo, A. Application of micro-Raman spectroscopy to the study of an illuminated medieval manuscript. J. Cult. Herit. 2000, 1, S277–S279. [Google Scholar] [CrossRef]
- Gniadecka, M.; Edwards, H.; Hart Hansen, J.; Nielsen, O.F.; Christensen, D.; Guillen, S.; Wulf, H. Near-infrared Fourier transform Raman spectroscopy of the mummified skin of the Alpine iceman, Qilakitsoq Greenland mummies and Chiribaya mummies from Peru. J. Raman Spectrosc. 1999, 30, 147–153. [Google Scholar] [CrossRef]
- Petersen, S.; Nielsen, O.F.; Christensen, D.H.; Edwards, H.G.; Farwell, D.W.; David, R.; Lambert, P.; Gniadecka, M.; Wulf, H.C. Near-infrared Fourier transform Raman spectroscopy of skin samples from the ‘Tomb of the Two Brothers’, Khnum-Nakht and Nekht-Ankh, XIIth dynasty Egyptian mummies (ca 2000 BC). J. Raman Spectrosc. 2003, 34, 375–379. [Google Scholar] [CrossRef]
- Barone, G.; Mazzoleni, P.; Raneri, S.; Jehlička, J.; Vandenabeele, P.; Lottici, P.P.; Lamagna, G.; Manenti, A.M.; Bersani, D. Raman investigation of precious jewelry collections preserved in Paolo Orsi regional museum (Siracusa, Sicily) using portable equipment. Appl. Spectrosc. 2016, 70, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Lu, T.-A.; Milande, V. Non-invasive on-site Raman study of blue-decorated early soft-paste porcelain: The use of arsenic-rich (European) cobalt ores–Comparison with huafalang Chinese porcelains. Ceram. Int. 2018, 44, 9018–9026. [Google Scholar] [CrossRef]
- Antunes, V.; Candeias, A.; Oliveira, M.J.; Longelin, S.; Serrão, V.; Seruya, A.I.; Coroado, J.; Dias, L.; Mirão, J.; Carvalho, M.L. Characterization of gypsum and anhydrite ground layers in 15th and 16th centuries Portuguese paintings by Raman Spectroscopy and other techniques. J. Raman Spectrosc. 2014, 45, 1026–1033. [Google Scholar] [CrossRef]
- Appolonia, L.; Vaudan, D.; Chatel, V.; Aceto, M.; Mirti, P. Combined use of FORS, XRF and Raman spectroscopy in the study of mural paintings in the Aosta Valley (Italy). Anal. Bioanal. Chem. 2009, 395, 2005–2013. [Google Scholar] [CrossRef]
- Martens, W.; Frost, R.L.; Kloprogge, J.T.; Williams, P.A. Raman spectroscopic study of the basic copper sulphates—Implications for copper corrosion and ‘bronze disease’. J. Raman Spectrosc. 2003, 34, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Martens, W.; Kloprogge, J.T.; Williams, P.A. Raman spectroscopy of the basic copper chloride minerals atacamite and paratacamite: Implications for the study of copper, brass and bronze objects of archaeological significance. J. Raman Spectrosc. 2002, 33, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.A.; Patience, D.B.; Rawlings, J.B. Industrial crystallization process control. IEEE Control Syst. Mag. 2006, 26, 70–80. [Google Scholar]
- Roozeboom, F.; Robson, H.E.; Chan, S.S. Laser Raman study on the crystallization of zeolites A, X and Y. Zeolites 1983, 3, 321–328. [Google Scholar] [CrossRef]
- Strachan, C.J.; Rades, T.; Gordon, K.C.; Rantanen, J. Raman spectroscopy for quantitative analysis of pharmaceutical solids. J. Pharm. Pharmacol. 2007, 59, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wikström, H.; Byrn, S.R.; Taylor, L.S. Estimation of the transition temperature for an enantiotropic polymorphic system from the transformation kinetics monitored using Raman spectroscopy. J. Pharm. Biomed. Anal. 2007, 45, 546–551. [Google Scholar] [CrossRef]
- Wang, C.; Vickers, T.J.; Mann, C.K. Direct assay and shelf-life monitoring of aspirin tablets using Raman spectroscopy. J. Pharm. Biomed. Anal. 1997, 16, 87–94. [Google Scholar] [CrossRef]
- Ali, H.; Ullah, R.; Khan, S.; Bilal, M. Raman spectroscopy and hierarchical cluster analysis for the ingredients characterization in different formulations of paracetamol and counterfeit paracetamol. Vib. Spectrosc. 2019, 102, 112–115. [Google Scholar] [CrossRef]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-enhanced Raman spectroscopy applied to food safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Paxton, W.F.; Kleinman, S.L.; Basuray, A.N.; Stoddart, J.F.; Van Duyne, R.P. Surface-enhanced Raman spectroelectrochemistry of TTF-modified self-assembled monolayers. J. Phys. Chem. Lett. 2011, 2, 1145–1149. [Google Scholar] [CrossRef]
- Ando, J.; Fujita, K.; Smith, N.I.; Kawata, S. Dynamic SERS imaging of cellular transport pathways with endocytosed gold nanoparticles. Nano Lett. 2011, 11, 5344–5348. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wagner, M.; Szkola, A.; Horn, H.; Niessner, R.; Haisch, C. Label-free in situ SERS imaging of biofilms. J. Phys. Chem. B 2010, 114, 10184–10194. [Google Scholar] [CrossRef] [PubMed]
- Piorek, B.D.; Lee, S.J.; Santiago, J.G.; Moskovits, M.; Banerjee, S.; Meinhart, C.D. Free-surface microfluidic control of surface-enhanced Raman spectroscopy for the optimized detection of airborne molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 18898–18901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, D.A.; Biggs, K.B.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy of half-mustard agent. Analyst 2006, 131, 568–572. [Google Scholar] [CrossRef]
- Sylvia, J.M.; Janni, J.A.; Klein, J.; Spencer, K.M. Surface-enhanced Raman detection of 2,4-dinitrotoluene impurity vapor as a marker to locate landmines. Anal. Chem. 2000, 72, 5834–5840. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, J.P.; Gregas, M.K.; Lauly, B.; Carter, J.C.; Angel, S.M.; Vo-Dinh, T. Trace molecular detection via surface-enhanced Raman scattering and surface-enhanced resonance Raman scattering at a distance of 15 m. Appl. Spectrosc. 2010, 64, 485–492. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.; Liz-Marzan, L. Environmental applications of plasmon assisted Raman scattering. Energy Environ. Sci. 2010, 3, 1011–1017. [Google Scholar] [CrossRef]
- Stoddart, P.; White, D. Optical fibre SERS sensors. Anal. Bioanal. Chem. 2009, 394, 1761–1774. [Google Scholar] [CrossRef]
- Sengupta, A.; Mujacic, M.; Davis, E.J. Detection of bacteria by surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2006, 386, 1379–1386. [Google Scholar] [CrossRef]
- Golightly, R.S.; Doering, W.E.; Natan, M.J. Surface-enhanced Raman spectroscopy and homeland security: A perfect match? ACS Nano 2009, 3, 2859–2869. [Google Scholar] [CrossRef]
- Wang, Y.; Serrano, S.; Santiago-Avilés, J.J. Raman characterization of carbon nanofibers prepared using electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.-I.; Li, C.-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.-N.; Duber, S. Raman microspectroscopy characterization of carbon blacks: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Dippel, B.; Heintzenberg, J. Soot characterization in atmospheric particles from different sources by NIR FT Raman spectroscopy. J. Aerosol Sci. 1999, 30, S907–S908. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- He, S.; Zhang, W.; Liu, L.; Huang, Y.; He, J.; Xie, W.; Wu, P.; Du, C. Baseline correction for Raman spectra using an improved asymmetric least squares method. Anal. Methods 2014, 6, 4402–4407. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Liu, S.; Yan, L.; Liu, T.; Zhang, T. Joint baseline-correction and denoising for Raman spectra. Appl. Spectrosc. 2015, 69, 1013–1022. [Google Scholar] [CrossRef]
- He, S.; Fang, S.; Liu, X.; Zhang, W.; Xie, W.; Zhang, H.; Wei, D.; Fu, W.; Pei, D. Investigation of a genetic algorithm based cubic spline smoothing for baseline correction of Raman spectra. Chemom. Intell. Lab. Syst. 2016, 152, 1–9. [Google Scholar] [CrossRef]
- Guo, S.; Bocklitz, T.; Popp, J. Optimization of Raman-spectrum baseline correction in biological application. Analyst 2016, 141, 2396–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Beer, T.; Vergote, G.; Baeyens, W.; Remon, J.P.; Vervaet, C.; Verpoort, F. Development and validation of a direct, non-destructive quantitative method for medroxyprogesterone acetate in a pharmaceutical suspension using FT-Raman spectroscopy. Eur. J. Pharm. Sci. 2004, 23, 355–362. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Wang, A.; Han, J.; Guo, L.; Yu, J.; Zeng, P. Database of standard Raman spectra of minerals and related inorganic crystals. Appl. Spectrosc. 1994, 48, 959–968. [Google Scholar] [CrossRef]
- Castro, K.; Pérez-Alonso, M.; Rodríguez-Laso, M.; Fernández, L.A.; Madariaga, J. On-line FT-Raman and dispersive Raman spectra database of artists’ materials (e-VISART database). Anal. Bioanal. Chem. 2005, 382, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Moens, L.; Edwards, H.G.; Dams, R. Raman spectroscopic database of azo pigments and application to modern art studies. J. Raman Spectrosc. 2000, 31, 509–517. [Google Scholar] [CrossRef]
| Materials | Features | Informations Provided | Limitations |
|---|---|---|---|
| CNTs |
|
|
|
| Graphene and graphene like materials |
|
|
|
| Amorphous carbon |
|
|
|
| Inorganic species |
|
|
|
| Organic molecules |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. https://doi.org/10.3390/chemosensors9090262
Orlando A, Franceschini F, Muscas C, Pidkova S, Bartoli M, Rovere M, Tagliaferro A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors. 2021; 9(9):262. https://doi.org/10.3390/chemosensors9090262
Chicago/Turabian StyleOrlando, Andrea, Filippo Franceschini, Cristian Muscas, Solomiya Pidkova, Mattia Bartoli, Massimo Rovere, and Alberto Tagliaferro. 2021. "A Comprehensive Review on Raman Spectroscopy Applications" Chemosensors 9, no. 9: 262. https://doi.org/10.3390/chemosensors9090262
APA StyleOrlando, A., Franceschini, F., Muscas, C., Pidkova, S., Bartoli, M., Rovere, M., & Tagliaferro, A. (2021). A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors, 9(9), 262. https://doi.org/10.3390/chemosensors9090262








