Alveolus Lung-on-a-Chip Platform: A Proposal
Abstract
:1. Introduction
1.1. COPD
1.2. Asthma
1.3. Acute Lower Respiratory Tract Infections
1.4. Tuberculosis
1.5. Lung Cancer
2. Lung Cell Culture
3. Modelling Lung Microenvironment
3.1. Organ Decellularization/Recellularization
3.2. Organoids
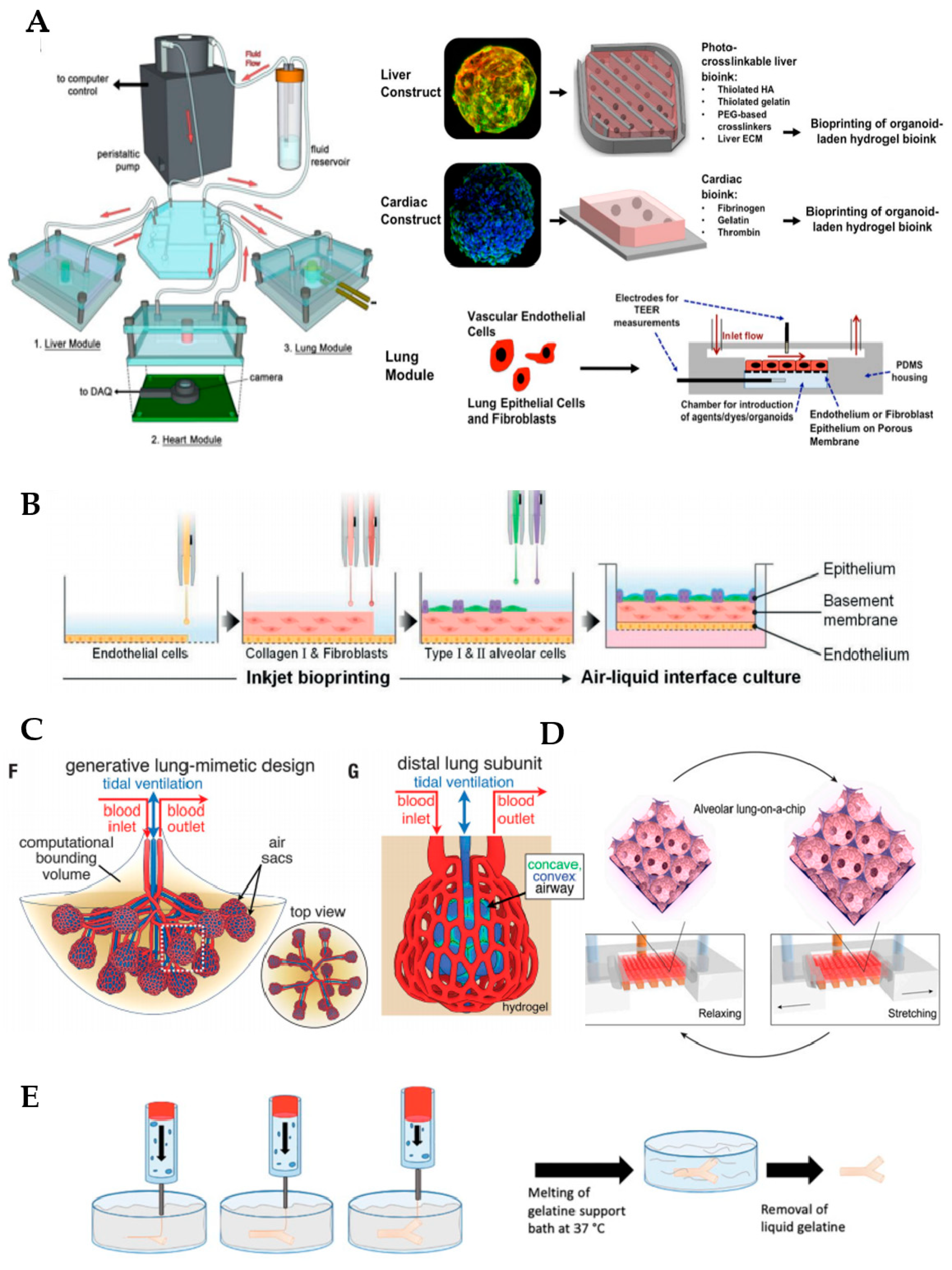
3.3. 3D Bioprinting
4. Microfluidic Lung Systems
5. Future Applications
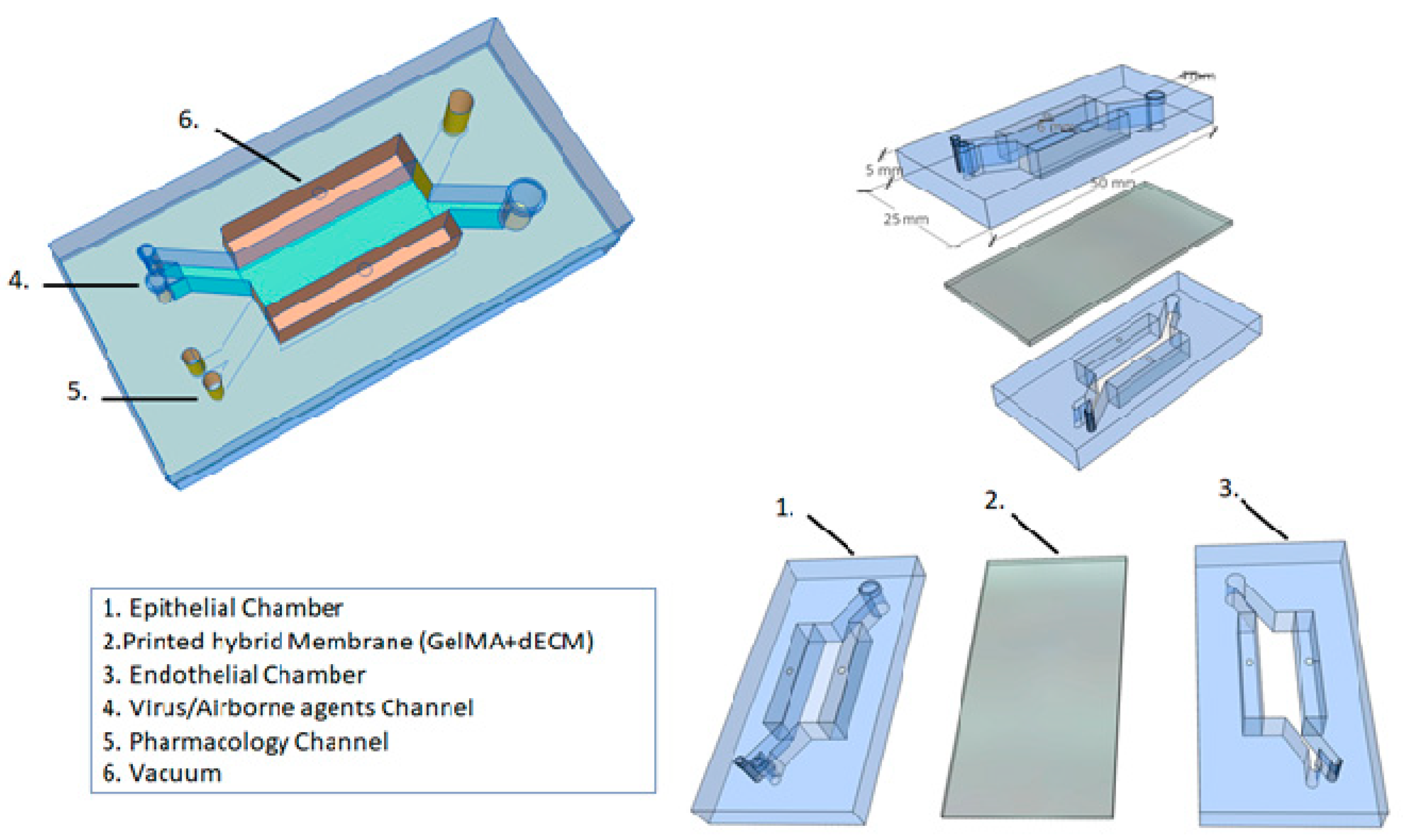
5.1. Printed Hybrid Membrane
5.2. Alveolar Lung-on-a-Chip Culture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gibson, G.J.; Loddenkemper, R.; Lundbäck, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Sheffield, UK, 2017. [Google Scholar]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 30 June 2021).
- World Health Organization (WHO). Chronic Respiratory Diseases: Asthma. Available online: https://www.who.int/news-room/q-a-detail/chronic-respiratory-diseases-asthma (accessed on 30 June 2021).
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Cereta, A.D.; Oliveira, V.R.; Costa, I.P.; Guimarães, L.L.; Afonso, J.P.; Fonseca, A.L.; Sousa, A.R.; Silva, G.A.; Mello, D.A.; Oliveira, L.V.; et al. Early Life Microbial Exposure and Immunity Training Effects on Asthma Development and Progression. Front. Med. 2021, 8, 662262. [Google Scholar] [CrossRef]
- Montefusco-Pereira, C.V.; Carvalho-Wodarz, C.S.; Seeger, J.; Kloft, C.; Michelet, R.; Lehr, C. Decoding (patho-)physiology of the lung by advanced in vitro models for developing novel anti-infectives therapies. Drug Discov. Today 2020, 26, 148–163. [Google Scholar] [CrossRef]
- Patella, V.; Florio, G.; Magliacane, D.; Giuliano, A.; Crivellaro, M.A.; Di Bartolomeo, D.; Genovese, A.; Palmieri, M.; Postiglione, A.; Ridolo, E.; et al. Air Pollution and Climate Change Task Force of the Italian Society of Allergology, Asthma and Clinical Immunology (SIAAIC). Urban air pollution and climate change: “The Decalogue: Allergy Safe Tree” for allergic and respiratory diseases care. Clin. Mol. Allergy 2018, 16, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 30 June 2021).
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 30 June 2021).
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 447–468. [Google Scholar] [CrossRef]
- Cavalheri, V.; Jenkins, S.; Cecins, N.; Gain, K.; Phillips, M.; Sanders, L.H.; Hill, K. Impairments after curative intent treatment for non-small cell lung cancer: A comparison with age and gender-matched healthy controls. Respir. Med. 2015, 109, 1332–1339. [Google Scholar] [CrossRef] [Green Version]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Aliwa-Hahnle, K.; Jing, Z.; Gibbs, J.S. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 30 June 2021).
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs.3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Asmani, M.; Velumani, S.; Li, Y.; Wawrzyniak, N.; Hsia, I.; Chen, Z.; Hinz, B.; Zhao, R. Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat. Commun. 2018, 9, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluhmki, T.; Bitzer, S.; Gindele, J.A.; Schruf, E.; Kiechle, T.; Webster, M.; Schymeinsky, J.; Ries, R.; Gantner, F.; Bischoff, D.; et al. Development of a miniaturized 96-Transwell air-liquid interface human small airway epithelial model. Sci. Rep. 2020, 10, 13022. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell 2020, 27, 890–904.e8. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.R.; Abdullatif, M.B.; Burnett, E.C.; Kempsell, K.E.; Conforti, F.; Tolley, H.; Collins, J.E.; Davies, D.E. Long Term Culture of the A549 Cancer Cell Line Promotes Multilamellar Body Formation and Differentiation towards an Alveolar Type II Pneumocyte Phenotype. PLoS ONE 2016, 11, e0164438. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Del Mar Cendra, M.; Torrents, E. Differential adaptability between reference strains and clinical isolates of Pseudomonas aeruginosa into the lung epithelium intracellular lifestyle. Virulence 2020, 11, 862–876. [Google Scholar] [CrossRef]
- Selo, M.A.; Delmas, A.S.; Springer, L.; Zoufal, V.; Sake, J.A.; Clerkin, C.G.; Huwer, H.; Schneider-Daum, N.; Lehr, C.M.; Nickel, S.; et al. Tobacco Smoke and Inhaled Drugs Alter Expression and Activity of Multidrug Resistance-Associated Protein-1 (MRP1) in Human Distal Lung Epithelial Cells in vitro. Front. Bioeng. Biotechnol. 2020, 8, 1030. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Xu, M.; Zhang, H.; Chen, Y.; Chung, K.F.; Adcock, I.M.; Li, F. Roles of TRPA1 and TRPV1 in cigarette smoke-induced airway epithelial cell injury model. Free Radic. Biol. Med. 2019, 134, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Tajima, H.; Tajiki-Nishino, R.; Watanabe, Y.; Kurata, K.; Fukuyama, T. Activation of aryl hydrocarbon receptor by benzo[a]pyrene increases interleukin 33 expression and eosinophil infiltration in a mouse model of allergic airway inflammation. J. Appl. Toxicol. 2020, 40, 1545–1553. [Google Scholar] [CrossRef]
- Pooladanda, V.; Thatikonda, S.; Bale, S.; Pattnaik, B.; Sigalapalli, D.K.; Bathini, N.B.; Singh, S.B.; Godugu, C. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation. Cell Death Dis. 2019, 10, 81. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.L.; Yang, J.Y.; Huang, J.; Kang, G.; Hu, H.B.; Xie, S. Downregulation of lysyl oxidase-like 4 LOXL4 by miR-135a-5p promotes lung cancer progression in vitro and in vivo. J. Cell Physiol. 2019, 234, 18679–18687. [Google Scholar] [CrossRef]
- Nichane, M.; Javed, A.; Sivakamasundari, V.; Ganesan, M.; Ang, L.T.; Kraus, P.; Lufkin, T.; Loh, K.M.; Lim, B. Isolation and 3D expansion of multipotent Sox9(+) mouse lung progenitors. Nat. Methods 2017, 14, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Morley, M.; Hawkins, F.; McCauley, K.B.; Jean, J.C.; Heins, H.; Na, C.L.; Weaver, T.E.; Vedaie, M.; Hurley, K.; et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 2017, 21, 472–488.e10. [Google Scholar] [CrossRef]
- Hawkins, F.; Kotton, D.N. Embryonic and induced pluripotent stem cells for lung regeneration. Ann. Am. Thorac. Soc. 2015, 1, S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Chow, C.W.; Young, E.W. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Jiang, L.; Qin, J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol. Res. 2018, 7, 1048–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.B.; Luks, A.M. West’s Respiratory Physiology: The Essentials, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Hsia, C.C.; Ravikumar, P. Role of Mechanical Stress in Lung Repair and Regeneration; Stem Cells in the Lung, Stem Cell Biology and Regenerative Medicine; Bertoncello, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma. 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.M.; Orlando, G.; Mirmalek-Sani, S.H.; Siddiqui, M.; Atala, A.; Soker, S. Whole organ decellularization–a tool for bioscaffold fabrication and organ bioengineering. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, Minnesota, 3–6 September 2009; pp. 6526–6529. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2020, 16, 927–933. [Google Scholar] [CrossRef]
- Wagner, D.E.; Bonenfant, N.R.; Sokocevic, D.; DeSarno, M.J.; Borg, Z.D.; Parsons, C.S.; Brooks, E.M.; Platz, J.J.; Khalpey, Z.I.; Hoganson, D.M.; et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 2014, 35, 2664–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, E.; Kasper, J.Y.; Unger, R.E.; Farré, R.; Kirkpatrick, C.J. Development of a bronchial wall model: Triple culture on a decellularized porcine trachea. Tissue Eng. Part C Methods 2015, 21, 909–921. [Google Scholar] [CrossRef]
- Nonaka, P.N.; Uriarte, J.J.; Campillo, N.; Melo, E.; Navajas, D.; Farré, R.; Oliveira, L.V. Mechanical properties of mouse lungs along organ decellularization by sodium dodecyl sulfate. Respir. Physiol. Neurobiol. 2014, 200, 1–5. [Google Scholar] [CrossRef]
- Jensen, T.; Roszell, B.; Zang, F.; Girard, E.; Matson, A.; Thrall, R.; Jaworski, D.M.; Hatton, C.; Weiss, D.J.; Finck, C. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng. Part C Methods 2012, 18, 632–646. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, S.E.; Ren, X.; Okamoto, T.; Guyette, J.P.; Mou, H.; Rajagopal, J.; Mathisen, D.J.; Vacanti, J.P.; Ott, H.C. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann. Thorac. Surg. 2014, 98, 1721–1729. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Moser, P.T.; Gilpin, S.E.; Okamoto, T.; Wu, T.; Tapias, L.F.; Mercier, F.E.; Linjie, X.; Ghawi, R.; Scadden, D.T.; et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat. Biotechnol. 2015, 33, 1097–1102. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Chung, M.; Fiortet, B.; Gao, X.; Katsura, H.; Hogan, B.L. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Tas, S.; Bölükbas, D.A.; Alsafadi, H.N.; Da Silva, I.A.; De Santis, M.M.; Rehnberg, E.; Tamargo, I.; Mohlin, S.; Lindstedt, S.; Wagner, D.E. Decellularized extracellular matrix hydrogels for human airway organoid culture. ERJ Open Res. 2021, 7, 101. [Google Scholar] [CrossRef]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.W.; Seol, Y.J.; Zhang, Y.S.; Shin, S.R.; Zhao, L.; Aleman, J.; et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.W. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018, 11, 015002. [Google Scholar] [CrossRef] [PubMed]
- Rehmani, S.S.; Al-Ayoubi, A.M.; Ayub, A.; Barsky, M.; Lewis, E.; Flores, R.; Lebovics, R.; Bhora, F.Y. Three-dimensional-printed bioengineered tracheal grafts: Preclinical results and potential for human use. Ann. Thorac. Surg. 2017, 104, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Park, J.A.; Kim, W.; Kim, S.; Lee, H.R.; Kim, W.J.; Yoo, J.Y.; Jung, S. All-inject-printed 3D alveolar barrier model with physiologically relevant microarchitecture. Adv. Sci. 2021, 8, 2004990. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef] [Green Version]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv. Mater. 2021, 33, 2005476. [Google Scholar] [CrossRef]
- Petrou, C.L.; D’ovidio, T.J.; Bölükbas, D.A.; Tas, S.; Brown, R.D.; Allawzi, A.; Lindstedt, S.; Nozik-Grayck, E.; Stenmark, K.R.; Wagner, D.E.; et al. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J. Mater. Chem. B 2020, 8, 6814–6826. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, T.; Liao, J.; Maharjan, S.; Xie, X.; Pérez, M.; Anaya, I.; Wang, S.; Mayer, A.T.; Kang, Z.; et al. Reversed-engineered human alveolar lung-on-a-chip model. Proc. Natl. Acad. Sci. USA 2021, 118, e2016146118. [Google Scholar] [CrossRef]
- Bessler, N.; Ogiermann, D.; Buchholz, M.B.; Santel, A.; Heidenreich, J.; Ahmmed, R.; Zaehres, H.; Brand-Saberi, B. Nydus One Syringe Extruder (NOSE): A Prusa i3 3D printer conversion for bioprinting applications utilizing the FRESH-method. HardwareX 2019, 6, e00069. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015, 15, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Jorba, I.; Beltrán, G.; Falcones, B.; Suki, B.; Farré, B.; García-Aznar, J.M.; Navajas, D. Nonlinear elasticity of the lung extracellular microenvironment is regulated by macroscale tissue strain. Acta Biomater. 2019, 92, 265–276. [Google Scholar] [CrossRef]
- Zamprogno, P.; Thoma, G.; Cencen, V.; Ferrari, D.; Putz, B.; Michler, J.; Fantner, G.E.; Guenat, O.T. Mechanical Properties of Soft Biological Membranes for Organ-on-a-Chip Assessed by Bulge Test and AFM. ACS Biomater. Sci. Eng. 2021, 7, 2990–2997. [Google Scholar] [CrossRef]
- Sellgren, K.L.; Butala, E.J.; Gilmour, B.P.; Randell, S.H.; Grego, S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip 2014, 14, 3349–3358. [Google Scholar] [CrossRef]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Nesmith, A.P.; Agarwal, A.; McCain, M.L.; Parker, K.K. Human airway musculature on a chip: An in vitro model of allergic asthmatic bronchoconstriction and bronchodilation. Lab Chip 2014, 14, 3925–3936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tas, S.; Renhberg, E.; Bölükbas, D.; Beech, J.; Kazado, L.N.; Sveningsson, I.; Arvidsson, M.; Sandberg, A.; Dahlgren, K.A.; Edthofer, A.; et al. 3D printed lung-on-a-chip device with a stretchable nanofibrous membrane for modeling ventilator induced lung injury. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tavana, H.; Kuo, C.H.; Lee, Q.Y.; Mosadegh, B.; Huh, D.; Christensen, P.J.; Grotberg, J.B.; Takayama, S. Dynamics of liquid plugs of buffer and surfactant solutions in a microengineered pulmonary airway model. Langmuir 2010, 26, 3744–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, P.N.; Falcones, B.; Farré, A.; Artigas, A.; Almendros, I.; Navajas, D. Biophysical preconditioning mesenchymal stem cells improves treatment of ventilator-induced lung injury. Arch. Bronconeumol. 2020, 56, 176–189. [Google Scholar] [CrossRef]
- Grist, S.M.; Chrostowski, L.; Cheung, K.C. Optical oxygen sensors for applications in microfluidic cell culture. Sensors 2010, 10, 9286–9316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campillo, N.; Jorba, I.; Schaedel, L.; Casals, B.; Gozal, D.; Farré, R.; Almendros, I.; Navajas, D. A novel chip for cyclic stretch and intermittent hypoxia cell exposures mimicking obstructive sleep apnea. Front. Physiol. 2016, 7, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campillo, N.; Torres, M.; Vilaseca, A.; Nonaka, P.N.; Gozal, D.; Roca-Ferrer, J.; Picado, C.; Montserrat, J.M.; Farré, R.; Navajas, D.; et al. Role of cyclooxygenase-2 on intermittent hypoxia-induced lung tumor malignancy in a mouse model of sleep apnea. Sci. Rep. 2017, 7, 44693. [Google Scholar] [CrossRef]
- Campillo, N.; Falcones, B.; Otero, J.; Colina, R.; Gozal, D.; Navajas, D.; Farré, R.; Almendros, I. Differential oxygenation in tumor microenvironment modulates macrophage and cancer cell crosstalk: Novel experimental setting and proof of concept. Front. Oncol. 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Benam, K.H.; Novak, R.; Nawroth, J.; Hirano-Kobayashi, M.; Ferrante, T.C.; Choe, Y.; Prantil-Baun, R.; Weaver, J.C.; Bahinski, A.; Parker, K.K.; et al. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016, 3, 456–466.e4. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip 2018, 18, 486–495. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campillo, N.; Oliveira, V.R.; da Palma, R.K. Alveolus Lung-on-a-Chip Platform: A Proposal. Chemosensors 2021, 9, 248. https://doi.org/10.3390/chemosensors9090248
Campillo N, Oliveira VR, da Palma RK. Alveolus Lung-on-a-Chip Platform: A Proposal. Chemosensors. 2021; 9(9):248. https://doi.org/10.3390/chemosensors9090248
Chicago/Turabian StyleCampillo, Noelia, Vinicius Rosa Oliveira, and Renata Kelly da Palma. 2021. "Alveolus Lung-on-a-Chip Platform: A Proposal" Chemosensors 9, no. 9: 248. https://doi.org/10.3390/chemosensors9090248
APA StyleCampillo, N., Oliveira, V. R., & da Palma, R. K. (2021). Alveolus Lung-on-a-Chip Platform: A Proposal. Chemosensors, 9(9), 248. https://doi.org/10.3390/chemosensors9090248






