Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection
Abstract
:1. Introduction
2. Materials and Methods
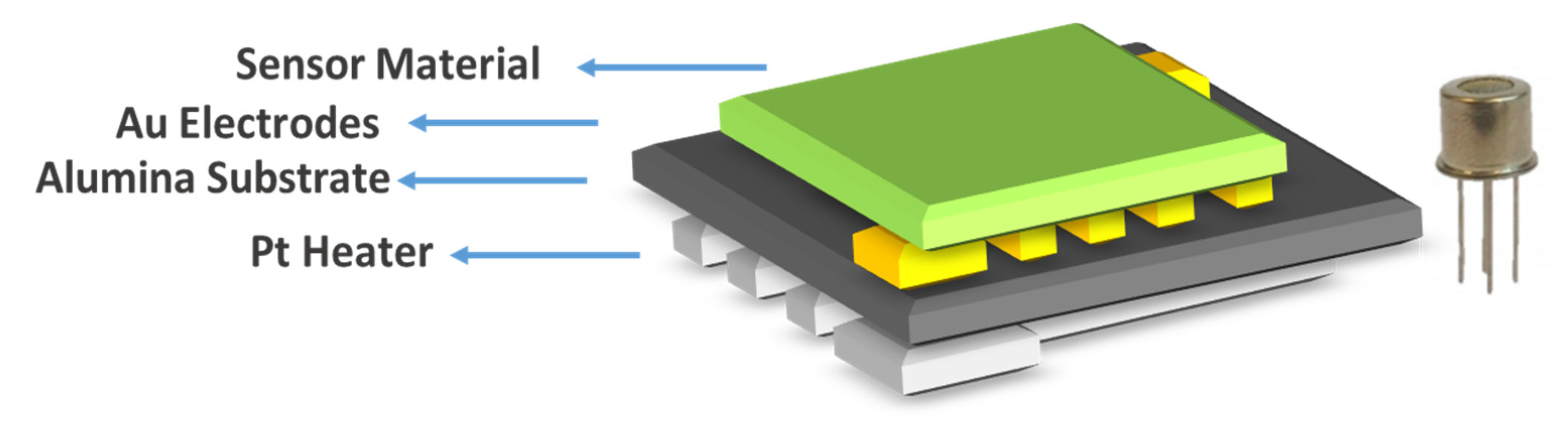
2.1. Sensor Fabrication
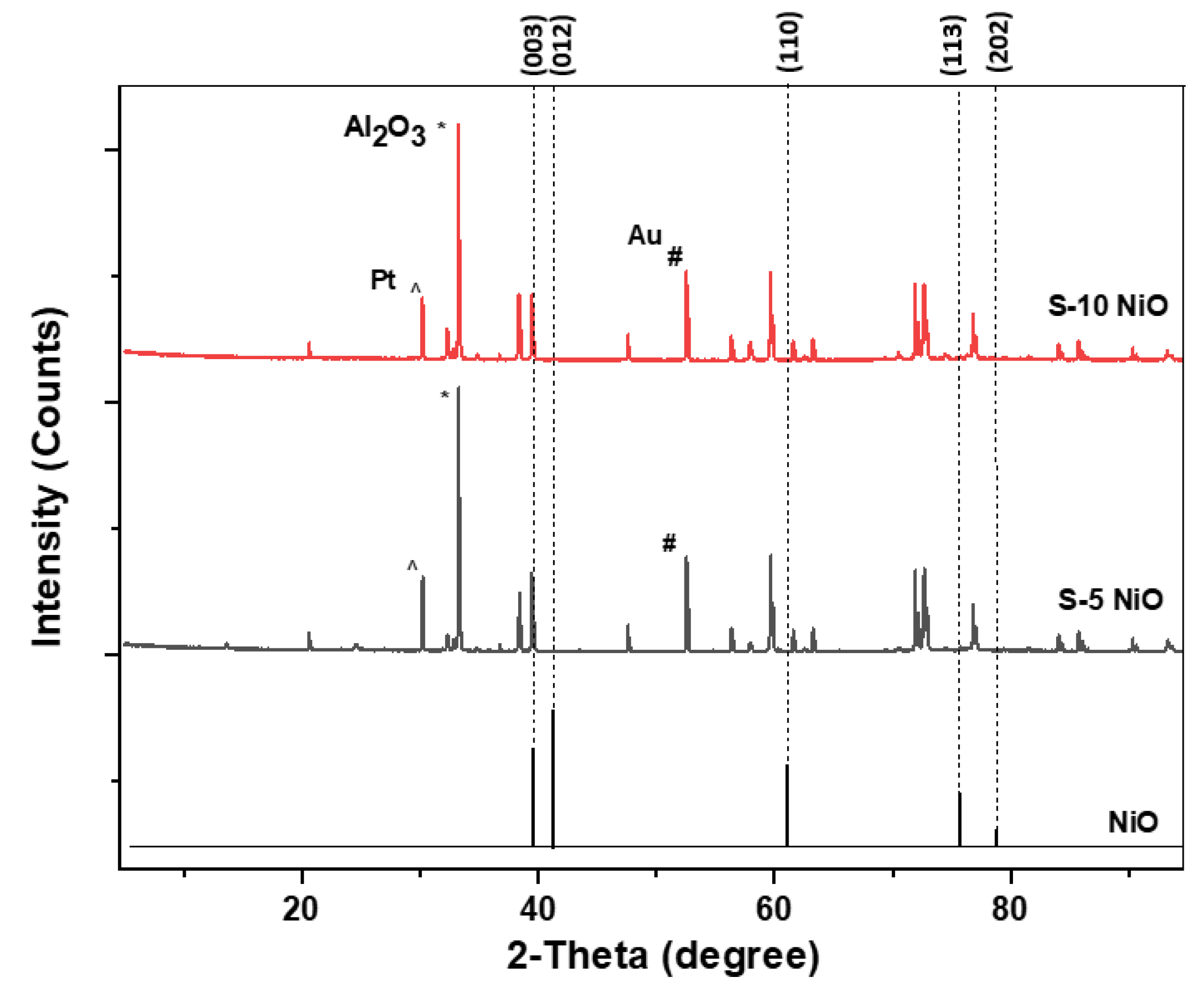
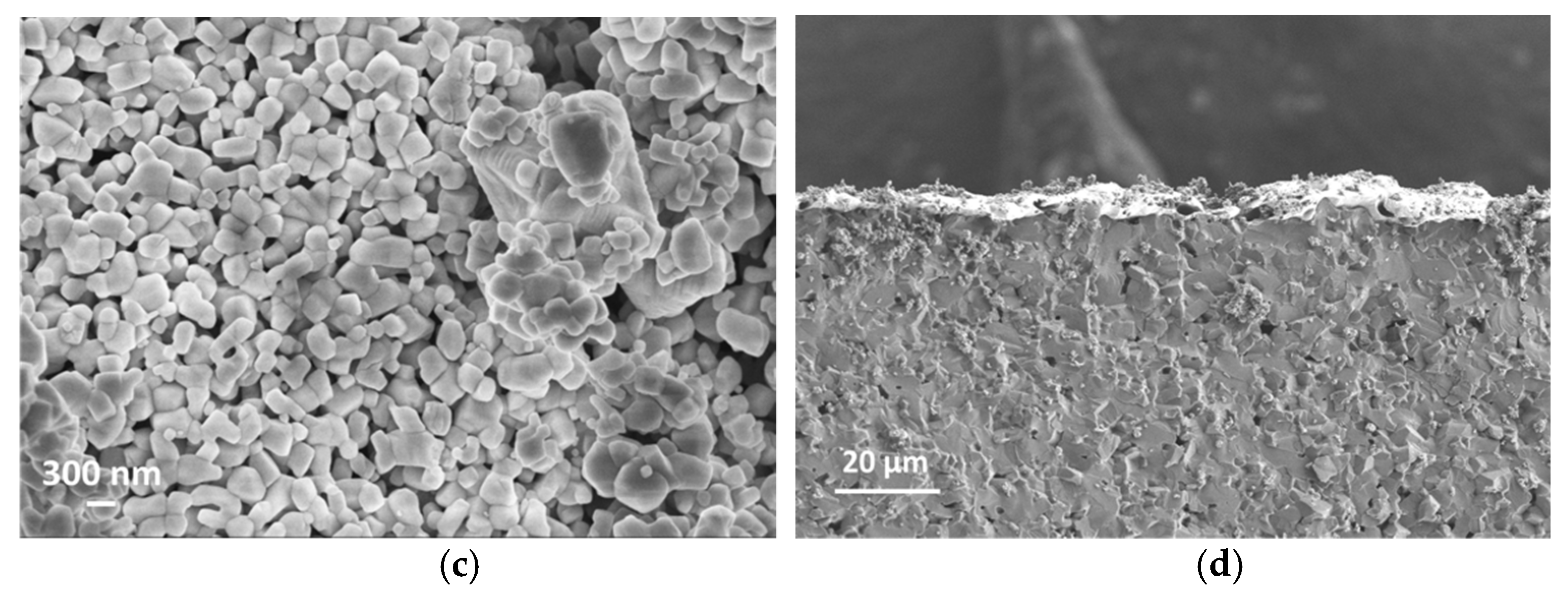
2.2. Material Characterisation
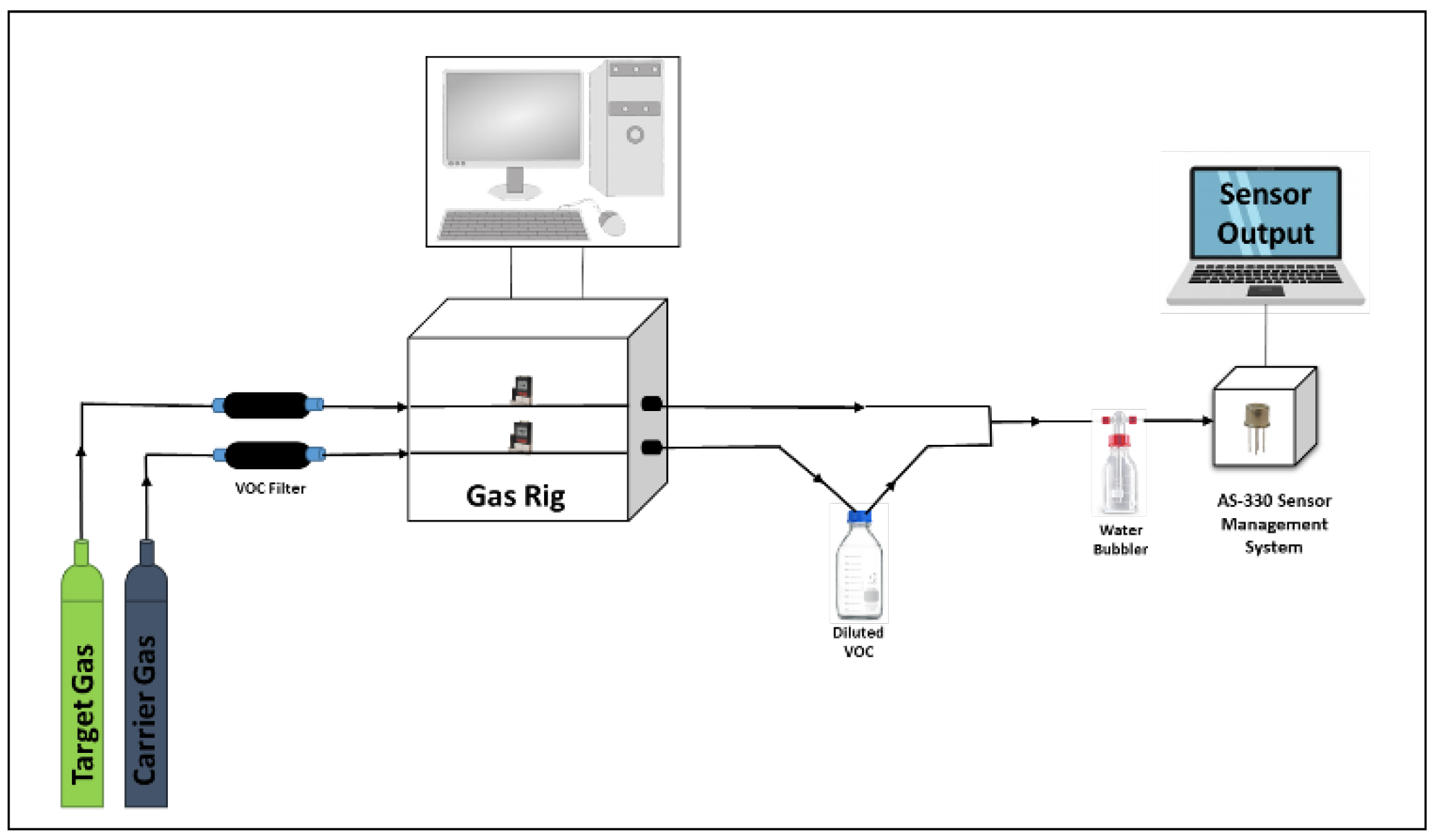
2.3. Gas Testing
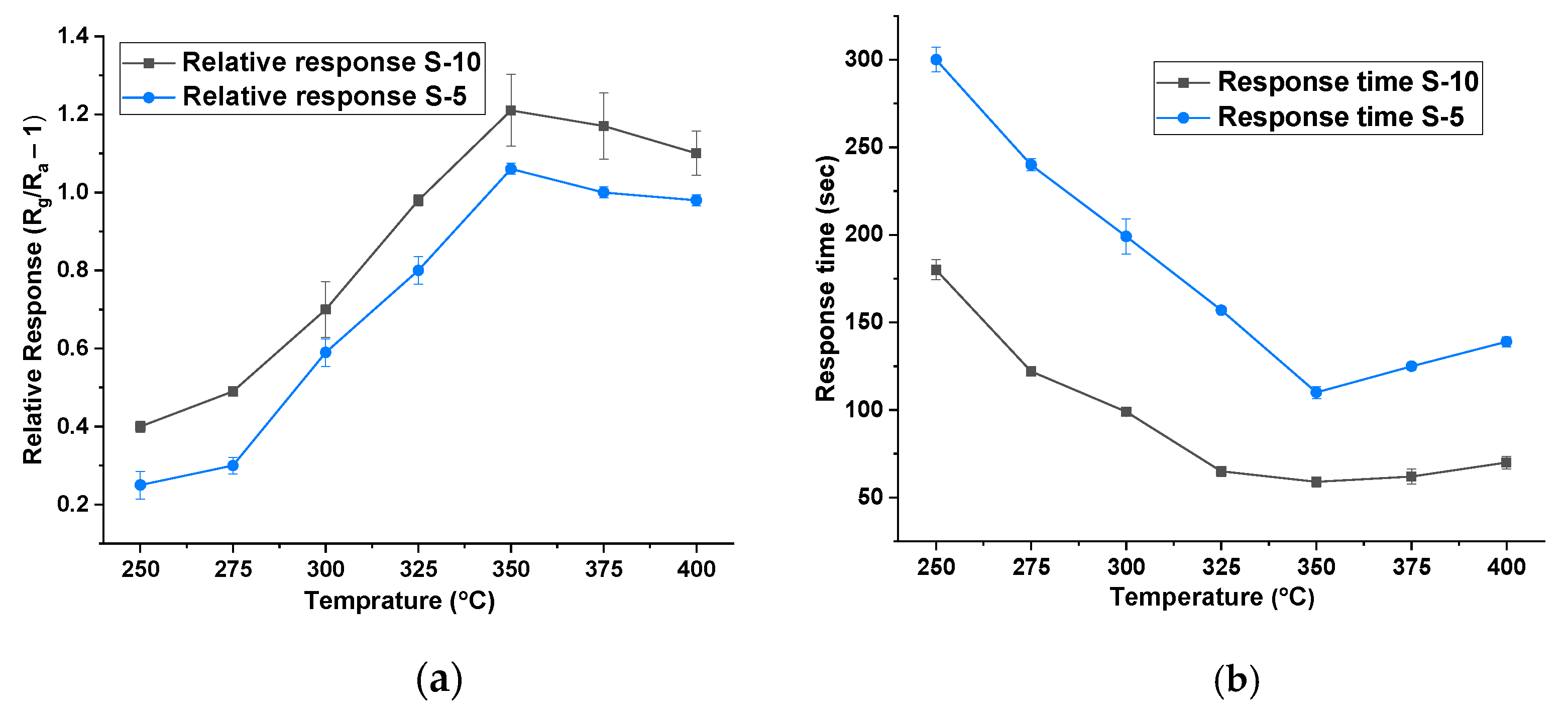
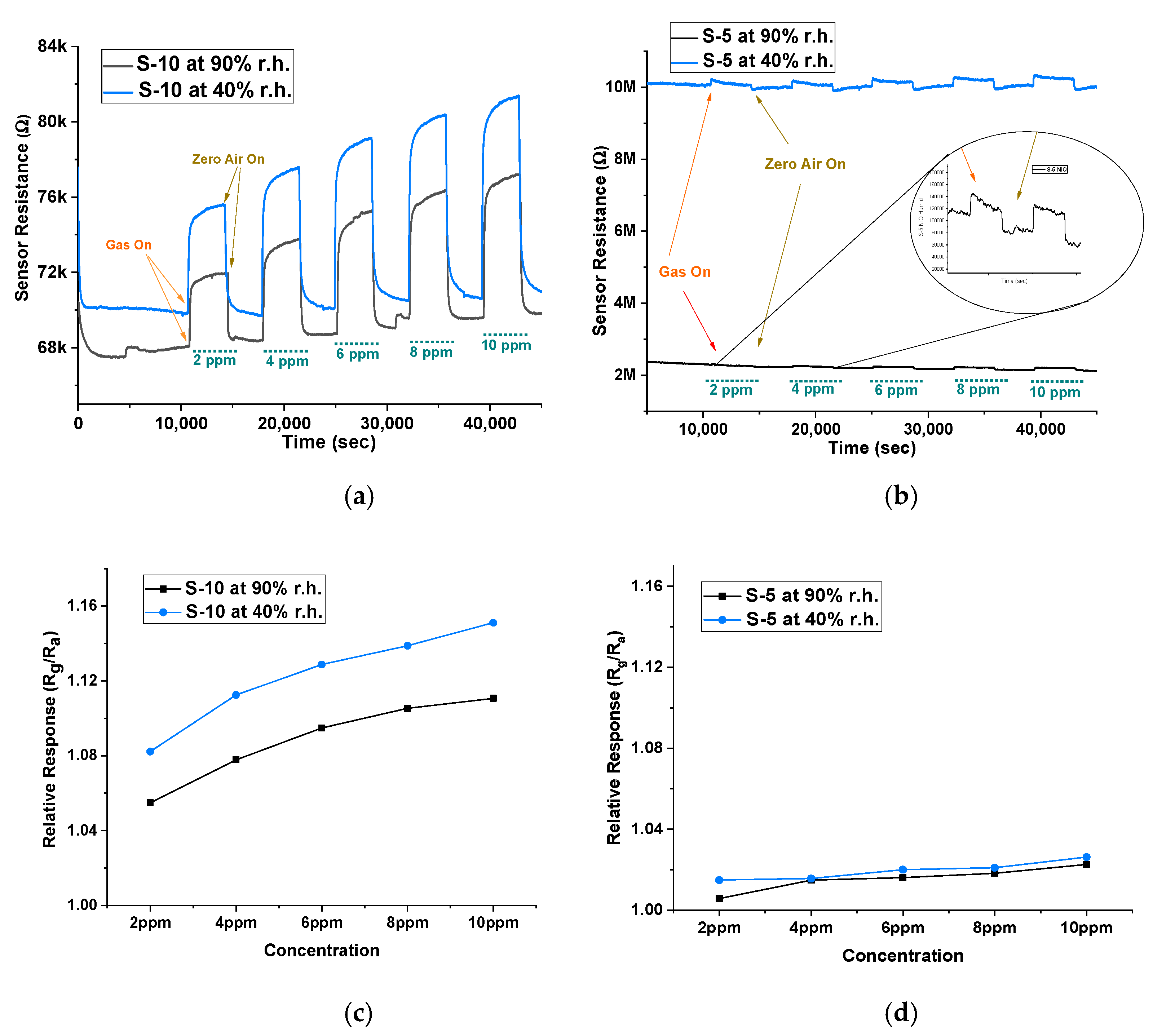
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajabi, H.; Mosleh, P.; Mandal, P.; Lea-Langton, A.; Sedighi, M. Emissions of volatile organic compounds from crude oil processing–Global emission inventory and environmental release. Sci. Total Environ. 2020, 727, 138654. [Google Scholar] [CrossRef] [Green Version]
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer Nature: Cham, Switzerland, 2014; Volume 1, ISBN 978-1-4614-7387-9. [Google Scholar]
- Kumar, P.; Deep, A.; Kim, K.H.; Brown, R.J.C. Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar] [CrossRef]
- Balouria, V.; Kumar, A.; Samanta, S.; Singh, A.; Debnath, A.K.; Mahajan, A.; Bedi, R.K.; Aswal, D.K.; Gupta, S.K. Nano-crystalline Fe2O3 thin films for ppm level detection of H2S. Sens. Actuators B Chem. 2013, 181, 471–478. [Google Scholar] [CrossRef]
- Mabrook, M.; Hawkins, P. A rapidly-responding sensor for benzene, methanol and ethanol vapours based on films of titanium dioxide dispersed in a polymer operating at room temperature. Sens. Actuators B Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Choi, U.S.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Sensing properties of SnO2-Co3O4 composites to CO and H2. Sens. Actuators B Chem. 2004, 98, 166–173. [Google Scholar] [CrossRef]
- Becker, T.; Ahlers, S.; Bosch-v.Braunmühl, C.; Müller, G.; Kiesewetter, O. Gas sensing properties of thin- and thick-film tin-oxide materials. Sens. Actuators B Chem. 2001, 77, 55–61. [Google Scholar] [CrossRef]
- Bhowmick, T.; Ambardekar, V.; Ghosh, A.; Dewan, M.; Pratim Bandyopadhyay, P.; Nag, S.; Basu Majumder, S. Multilayered and Chemiresistive Thin and Thick Film Gas Sensors for Air Quality Monitoring. Multilayer Thin Film. Versatile Appl. Mater. Eng. 2020, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Arshak, K.; Gaidan, I. Development of a novel gas sensor based on oxide thick films. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2005, 118, 44–49. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdullah, M.J.; Abdul Aziz, A.; Ahmad, H.; Low, L.Y. ZnO thin films for VOC sensing applications. Vacuum 2010, 85, 101–106. [Google Scholar] [CrossRef]
- Kanda, K.; Maekawa, T. Development of a WO3 thick-film-based sensor for the detection of VOC. Sens. Actuators B Chem. 2005, 108, 97–101. [Google Scholar] [CrossRef]
- Zhu, B.L.; Xie, C.S.; Wu, J.; Zeng, D.W.; Wang, A.H.; Zhao, X.Z. Influence of Sb, in and Bi dopants on the response of ZnO thick films to VOCs. Mater. Chem. Phys. 2006, 96, 459–465. [Google Scholar] [CrossRef]
- Iizuka, K.; Kambara, M.; Yoshida, T. Growth of tin oxide thick films by plasma spray physical vapor deposition. Sens. Actuators B Chem. 2011, 155, 551–556. [Google Scholar] [CrossRef]
- Zhu, B.L.; Xie, C.S.; Wang, A.H.; Zeng, D.W.; Song, W.L.; Zhao, X.Z. The gas-sensing properties of thick film based on tetrapod-shaped ZnO nanopowders. Mater. Lett. 2005, 59, 1004–1007. [Google Scholar] [CrossRef]
- James, F.; Fiorido, T.; Bendahan, M.; Aguir, K. Comparison between MOX sensors for low VOCs concentrations with interfering gases. In Proceedings of the All Sensors, Nice, France, 19–23 March 2017; pp. 39–40. [Google Scholar]
- Cho, B.; Lee, K.; Pyo, S.; Kim, J. Fabrication and characterization of VOC sensor array based on SnO2 and ZnO nanoparticles functionalized by metalloporphyrins. Micro Nano Syst. Lett. 2018, 6, 10. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, H.; Cai, H.; Zhang, J.; Yu, J.; Tang, Z. Hydrothermally synthesized CuO based volatile organic compound gas sensor. RSC Adv. 2014, 4, 57975–57982. [Google Scholar] [CrossRef]
- Sari, W.; Smith, P.; Leigh, S.; Covington, J. Oxygen Sensors Based on Screen Printed Platinum and Palladium Doped Indium Oxides. Proceedings 2017, 1, 401. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Zappa, D.; Ferroni, M.; Poli, N.; Campanini, M.; Negrea, R.; Comini, E. Branch-like NiO/ZnO heterostructures for VOC sensing. Sens. Actuators B Chem. 2018, 262, 477–485. [Google Scholar] [CrossRef]
- Vincent, T.A.; Xing, Y.; Cole, M.; Gardner, J.W. Investigation of the response of high-bandwidth MOX sensors to gas plumes for application on a mobile robot in hazardous environments. Sens. Actuators B Chem. 2019, 279, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Dirksen, J.A.; Duval, K.; Ring, T.A. NiO thin-film formaldehyde gas sensor. Sens. Actuators B Chem. 2001, 80, 106–115. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Nagashima, K.; Meng, G.; Dai, T.; Tong, B.; Deng, Z.; Wang, S.; Zhu, N.; Yanagida, T.; et al. Discrimination of VOCs molecules via extracting concealed features from a temperature-modulated p-type NiO sensor. Sens. Actuators B Chem. 2019, 293, 342–349. [Google Scholar] [CrossRef]
- Ayyala, S.K.; Tsang, J.H.; Blackman, C.; Covington, J.A. Comparative study of spin-coated and vapour deposited nickel oxides for detecting VOCs. Proc. IEEE Sens. 2020, 4–7. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodic, D.; Spasojevic, V.; Kusigerski, V.; Tellgren, R.; Rundlof, H. Magnetic ordering in polycrystalline NixZn1-xO Solid Solutions. Phys. Status Solidi Basic Res. 2000, 218, 527–536. [Google Scholar] [CrossRef]
- Nguyen, H.; El-Safty, S.A. Meso- and macroporous Co3O4 nanorods for effective VOC gas sensors. J. Phys. Chem. C 2011, 115, 8466–8474. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahbarpour, S.; Hosseini-Golgoo, S.M.; Jamaati, H. Reducing the destructive effect of ambient humidity variations on gas detection capability of a temperature modulated gas sensor by calcium chloride. Sens. Actuators B Chem. 2020, 331, 129091. [Google Scholar] [CrossRef]
- Faia, P.M.; Furtado, C.S. Effect of composition on electrical response to humidity of TiO2:ZnO sensors investigated by impedance spectroscopy. Sens. Actuators B Chem. 2013, 181, 720–729. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stǎnoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Saruhan, B.; Lontio Fomekong, R.; Nahirniak, S. Review: Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]

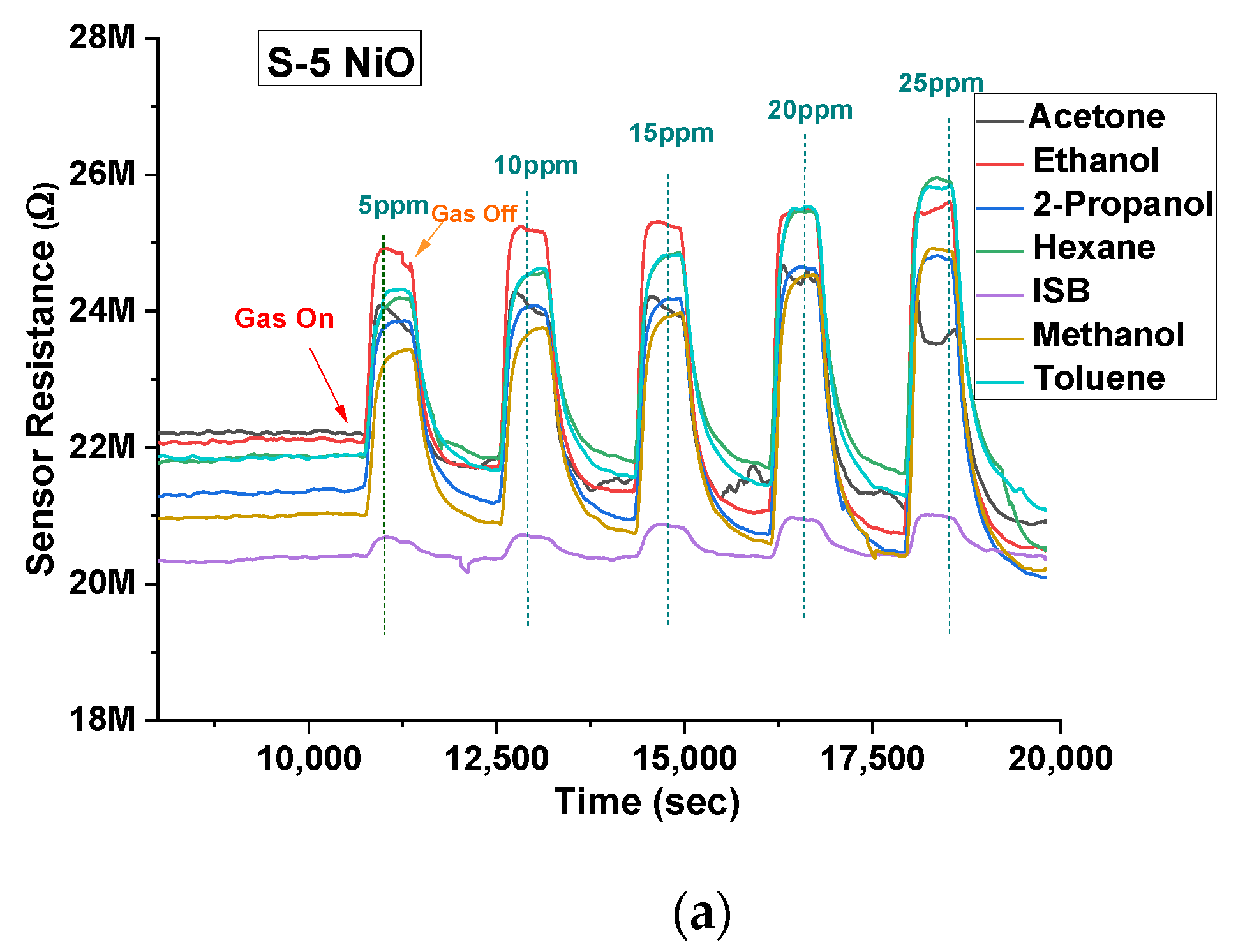
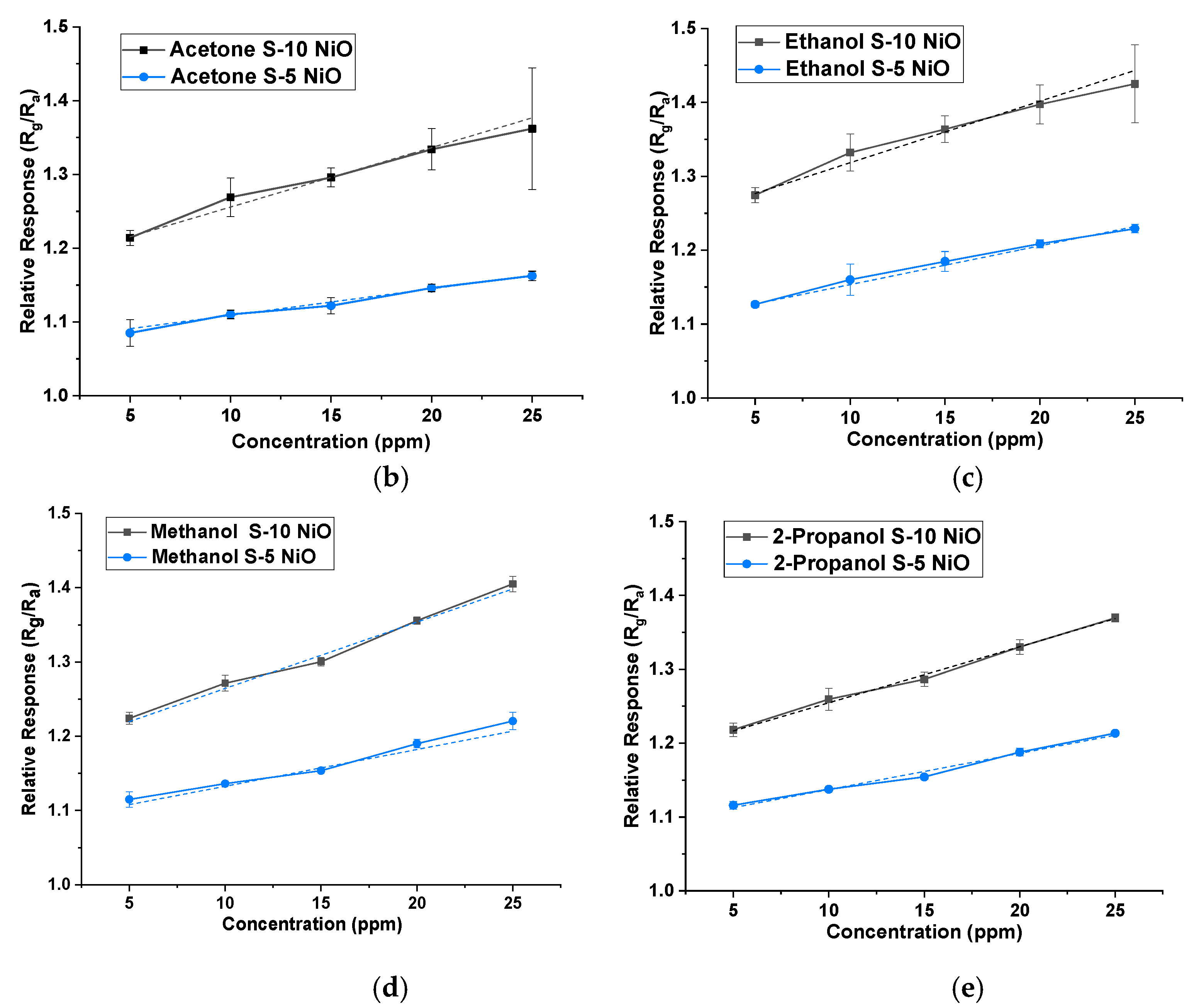
| VOC Gas | S-10 NiO (Rg/Ra) | S-5 NiO (Rg/Ra) |
|---|---|---|
| Acetone | 1.22 | 1.09 |
| Ethanol | 1.27 | 1.13 |
| 2-Propanol | 1.21 | 1.12 |
| Toluene | 1.15 | 1.11 |
| Hexane | 1.10 | 1.10 |
| Methanol | 1.22 | 1.12 |
| Isobutylene | 1.03 | 1.02 |
| VOC Gas | Target VOCs | ppm Range | Method | Thickness | Operating Temperature | Response | Reference |
|---|---|---|---|---|---|---|---|
| ZnO | Acetone isopropanol Ethanol | <1000 ppm | RF sputtering | - | 400 °C | - | [10] |
| WO3 thick film | Aromatic hydrocarbons | 0.1–500 ppm | Sputtering | 35 µm | 400 °C | - | [11] |
| Doped ZnO thick film | Benzene, Toluene, Xylene | 100 ppm | HILH | 420 °C | 2.9 | [12] | |
| SnO2 thick film | Formaldehyde | >10 ppm | Sputtering | 10 µm | 266 °C | - | [13] |
| ZnO thick film | Acetone, Alcohol, Xylene, Toluene, Benzene | 100 ppm | Vapour-phase oxidation | - | 420 °C | 0.02–0.04 | [14] |
| Doped NiO | Formaldehyde, Methanol, Formalin | 25–100 ppm | Wet chemical method | 500 nm | 600 °C | - | [21] |
| NiO thick films | Acetone, Ethanol, Toluene, Hexane, Methanol, Isobutylene, 2-propanol | <25 ppm | Spin coating | 5 µm and 10µm | 350 °C | 1.1–1.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyala, S.K.; Covington, J.A. Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection. Chemosensors 2021, 9, 247. https://doi.org/10.3390/chemosensors9090247
Ayyala SK, Covington JA. Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection. Chemosensors. 2021; 9(9):247. https://doi.org/10.3390/chemosensors9090247
Chicago/Turabian StyleAyyala, Sai Kiran, and James A. Covington. 2021. "Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection" Chemosensors 9, no. 9: 247. https://doi.org/10.3390/chemosensors9090247
APA StyleAyyala, S. K., & Covington, J. A. (2021). Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection. Chemosensors, 9(9), 247. https://doi.org/10.3390/chemosensors9090247







