Single Nanowire Gas Sensor Able to Distinguish Fish and Meat and Evaluate Their Degree of Freshness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SnO2 Nanowires
2.2. Nanowires Characterization
2.3. Sensor Fabrication
2.4. Gas Sensor Measurements
2.5. Meat and Fish Spoilage Measurement
2.6. Machine Learning
3. Results and Discussion
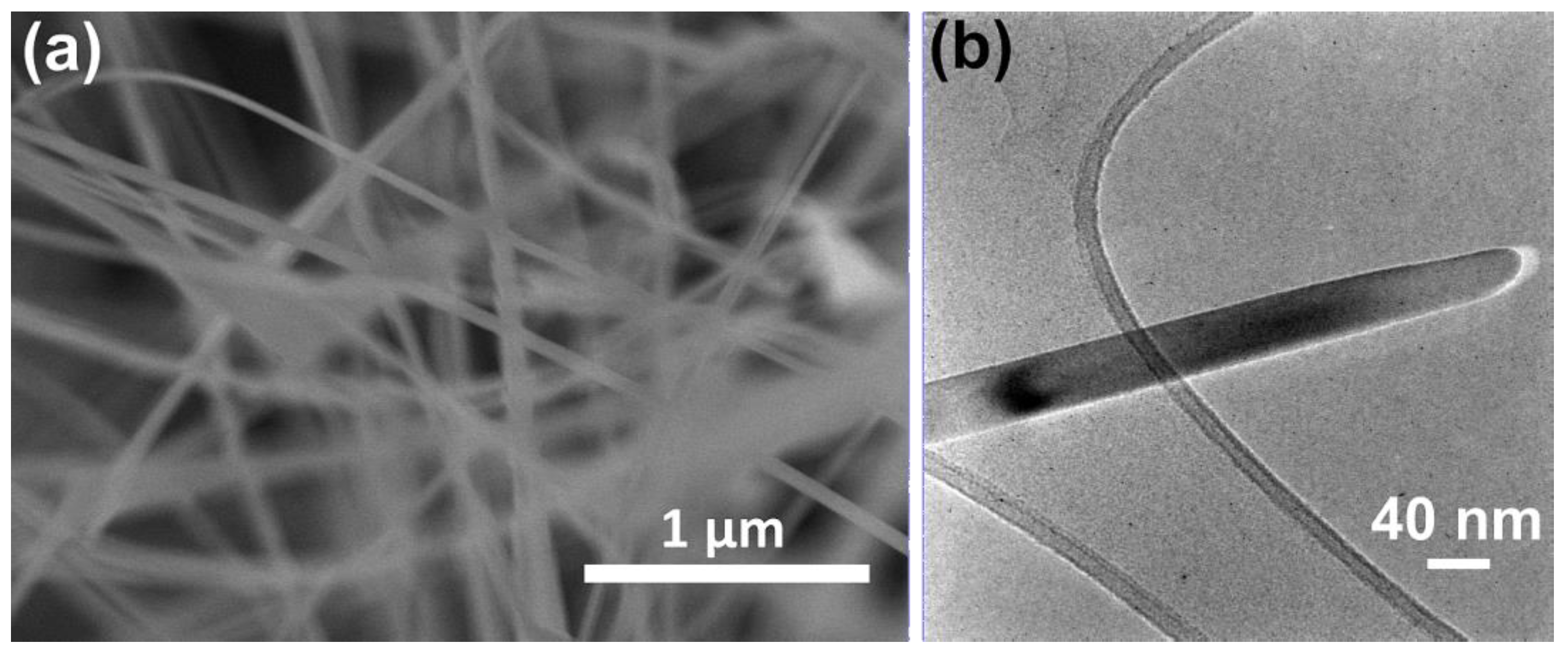
3.1. Nanowires Characterization
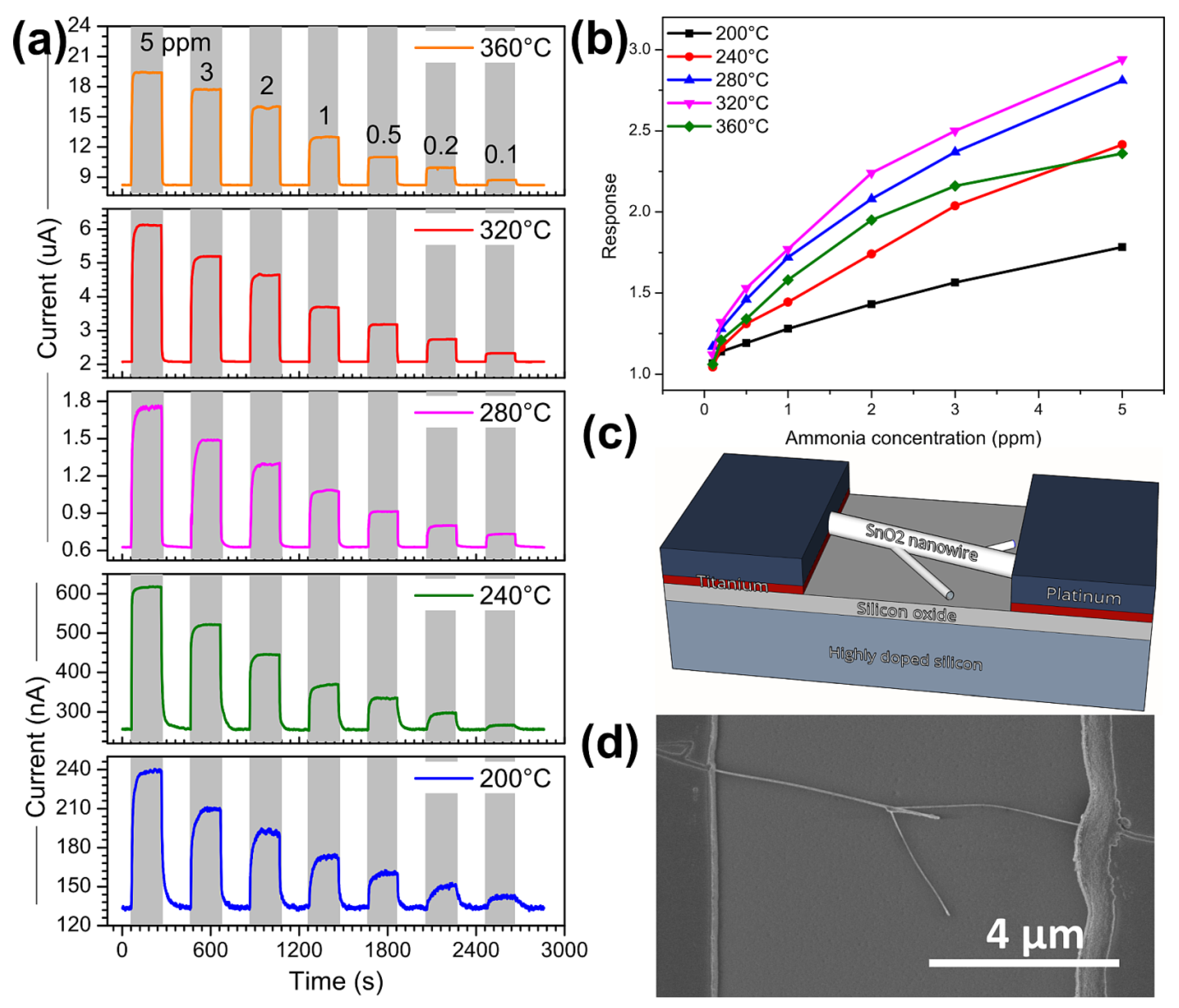
3.2. Ammonia Sensing Performance
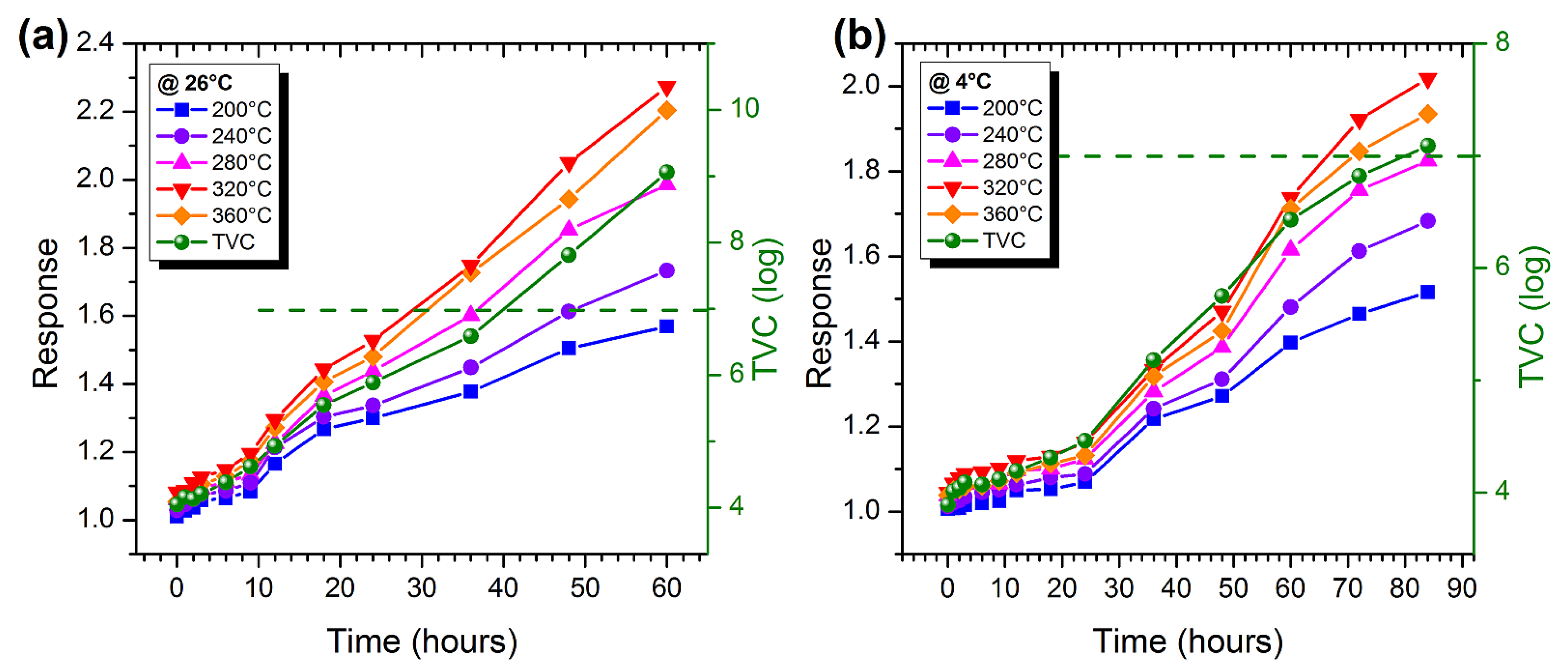
3.3. Measurements on Marble Trout and Pork Samples
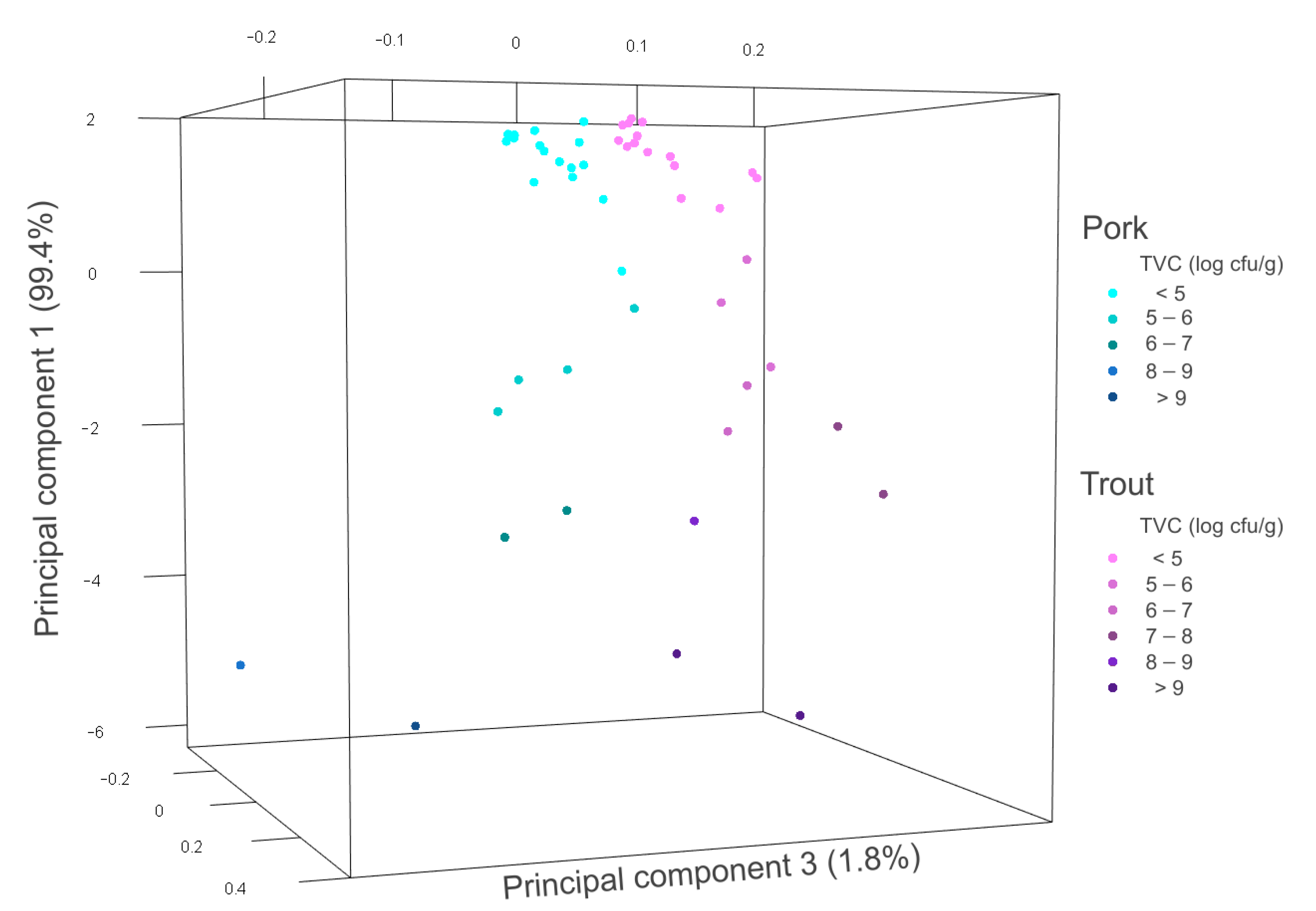
3.4. Distinction between Meat and Fish
3.5. Estimation of the Degree of Freshness of Food
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef]
- Sundström, K. Cost of Illness for Five Major Foodborne Illnesses and Sequelae in Sweden. Appl. Health Econ. Health Policy 2018, 16, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Boyer, D.; Ramaswami, A. Comparing urban food system characteristics and actions in US and Indian cities from a multi-environmental impact perspective: Toward a streamlined approach. J. Ind. Ecol. 2020, 24, 841–854. [Google Scholar] [CrossRef]
- Comi, G. (Ed.) Spoilage of Meat and Fish. In The Microbiological Quality of Food; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deisingh, A.K.; Stone, D.C.; Thompson, M. Applications of electronic noses and tongues in food analysis. Int. J. Food Sci. Technol. 2004, 39, 587–604. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Chuang, M.-Y.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J.; Yeh, P.-H.; Chen, J.-N. One-Minute Fish Freshness Evaluation by Testing the Volatile Amine Gas with an Ultrasensitive Porous-Electrode-Capped Organic Gas Sensor System. ACS Sens. 2017, 3, 531–539. [Google Scholar] [CrossRef]
- Perez de Vargas-Sansalvador, I.M.; Erenas, M.M.; Martinez-Olmos, A.; Mirza-Montoro, F.; Diamond, D.; Capitan-Vallvey, L.F. Smartphone based meat freshness detection. Talanta 2020, 216, 120985. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Li, F.; Chen, Y.; Tang, L.; Xie, J.; Yu, Q. Fast quantification of total volatile basic nitrogen (TVB-N) content in beef and pork by near-infrared spectroscopy: Comparison of SVR and PLS model. Meat Sci. 2021, 180, 108559. [Google Scholar] [CrossRef] [PubMed]
- Olafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilseng, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Thai, N.X.; Van Duy, N.; Hung, C.M.; Nguyen, H.; Tonezzer, M.; Van Hieu, N.; Hoa, N.D. Prototype edge-grown nanowire sensor array for the real-time monitoring and classification of multiple gases. J. Sci. Adv. Mater. Devices 2021, 5, 409–416. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Nagashima, K.; Takahashi, T.; Hosomi, T.; Yanagida, T. Metal-oxide nanowire molecular sensors and their promises. Chemosensors 2021, 9, 41. [Google Scholar] [CrossRef]
- Tonezzer, M.; Thai, N.X.; Gasperi, F.; Van Duy, N.; Biasioli, F. Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient. Nanomaterials 2021, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Duy, N.V.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Redwing, J.M.; Miao, X.; Li, X. Vapor-Liquid-Solid Growth of Semiconductor Nanowires. In Handbook of Crystal Growth, 2nd ed.; Thomas, F.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 399–439. [Google Scholar]
- Lin, Y.-F.; Jian, W.-B. The impact of nanocontact on nanowire based nanoelectronics. Nano Lett. 2008, 8, 3146–3150. [Google Scholar] [CrossRef]
- Todeschini, M.; Bastos da Silva Fanta, A.; Jensen, F.; Birkedal Wagner, J.; Han, A. Influence of Ti and Cr Adhesion Layers on Ultrathin Au Films. ACS Appl. Mater. Interfaces 2017, 9, 37374–37385. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Takahashi, T.; Kanai, M.; Zhang, G.; He, Y.; Nagashima, K.; Yanagida, T. Long-Term Stability of Oxide Nanowire Sensors via Heavily Doped Oxide Contact. ACS Sens. 2017, 8, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- López, A.; Baguer, B.; Goñi, P.; Rubio, E.; Gómez, J.; Mosteo, R.; Ormad, M.P. Assessment of the methodologies used in microbiological control of sewage sludge. Waste Manag. 2019, 96, 168–174. [Google Scholar] [CrossRef]
- Tonezzer, M.; Izidoro, S.C.; Moraes, J.P.A.; Dang, L.T.T. Improved Gas Selectivity Based on Carbon Modified SnO2 Nanowires. Front. Mater. Sci. 2019, 6, 277. [Google Scholar] [CrossRef]
- Bekhit, A.E.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Tsuda, N.; Nasu, K.; Fujimori, A.; Siratori, K. Electronic Conduction in Oxides, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Selectivity of catalytically modified tin dioxide to CO and NH3 gas mixtures. Chemosensors 2015, 3, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Wagh, M.S.; Jain, G.H.; Patil, D.R.; Patil, S.A.; Patil, L.A. Modified zinc oxide thick film resistors as NH3 gas sensor. Sens. Actuators B Chem. 2005, 115, 128–133. [Google Scholar] [CrossRef]
- Wu, H.; Ma, Z.; Lin, Z.; Song, H.; Yan, S.; Shi, Y. High-Sensitive Ammonia Sensors Based on Tin Monoxide Nanoshells. Nanomaterials 2019, 9, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsoumanis, K. Predictive Modeling of the Shelf Life of Fish under Nonisothermal Conditions. Appl. Environ. Microbiol. 2001, 67, 1821–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciortino, J.A.; Ravikumar, R. Chapter 5: Fish Quality Assurance. In Fishery Harbour Manual on the Prevention of Pollution, Bay of Bengal Programme; Bay of Bengal Programme: Madras, India, 1999. [Google Scholar]
- Tonezzer, M. Detection of Mackerel Fish Spoilage with a Gas Sensor Based on One Single SnO2 Nanowire. Chemosensors 2021, 9, 2. [Google Scholar] [CrossRef]


| True | |||
|---|---|---|---|
| Estimated | Sample | Pork | Trout |
| Pork | 20 | 1 | |
| Trout | 1 | 20 | |
| Pork | Estimated TVC (log cfu/g) | ||||||
|---|---|---|---|---|---|---|---|
| <5 | 5–6 | 6–7 | 7–8 | 8–9 | >9 | ||
| True TVC (log cfu/g) | <5 | 9 | |||||
| 5–6 | 4 | ||||||
| 6–7 | 2 | ||||||
| 7–8 | 2 | ||||||
| 8–9 | 1 | 2 | |||||
| > 9 | 1 | ||||||
| Marble Trout | Estimated TVC (log cfu/g) | ||||||
|---|---|---|---|---|---|---|---|
| <5 | 5–6 | 6–7 | 7–8 | 8–9 | > 9 | ||
| True TVC (log cfu/g) | <5 | 9 | |||||
| 5–6 | 3 | ||||||
| 6–7 | 2 | ||||||
| 7–8 | 1 | 2 | 1 | ||||
| 8–9 | 1 | ||||||
| >9 | 2 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonezzer, M. Single Nanowire Gas Sensor Able to Distinguish Fish and Meat and Evaluate Their Degree of Freshness. Chemosensors 2021, 9, 249. https://doi.org/10.3390/chemosensors9090249
Tonezzer M. Single Nanowire Gas Sensor Able to Distinguish Fish and Meat and Evaluate Their Degree of Freshness. Chemosensors. 2021; 9(9):249. https://doi.org/10.3390/chemosensors9090249
Chicago/Turabian StyleTonezzer, Matteo. 2021. "Single Nanowire Gas Sensor Able to Distinguish Fish and Meat and Evaluate Their Degree of Freshness" Chemosensors 9, no. 9: 249. https://doi.org/10.3390/chemosensors9090249
APA StyleTonezzer, M. (2021). Single Nanowire Gas Sensor Able to Distinguish Fish and Meat and Evaluate Their Degree of Freshness. Chemosensors, 9(9), 249. https://doi.org/10.3390/chemosensors9090249






