Gold Nanoparticles/Carbon Nanotubes and Gold Nanoporous as Novel Electrochemical Platforms for L-Ascorbic Acid Detection: Comparative Performance and Application
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
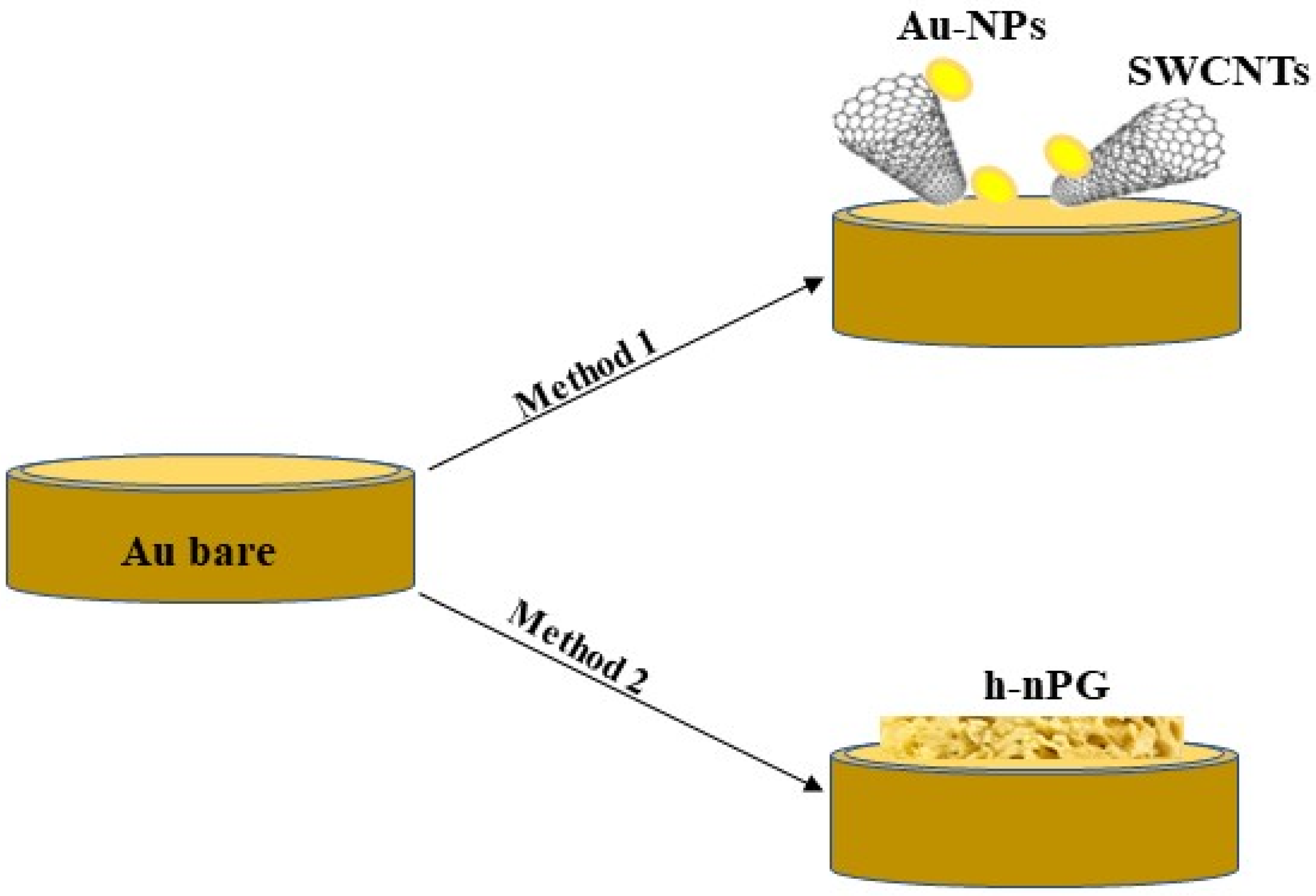
2.2. Preparation of Gold Modified Electrodes
2.3. SEM Experiments
2.4. Raman Spectroscopy
3. Results and Discussion
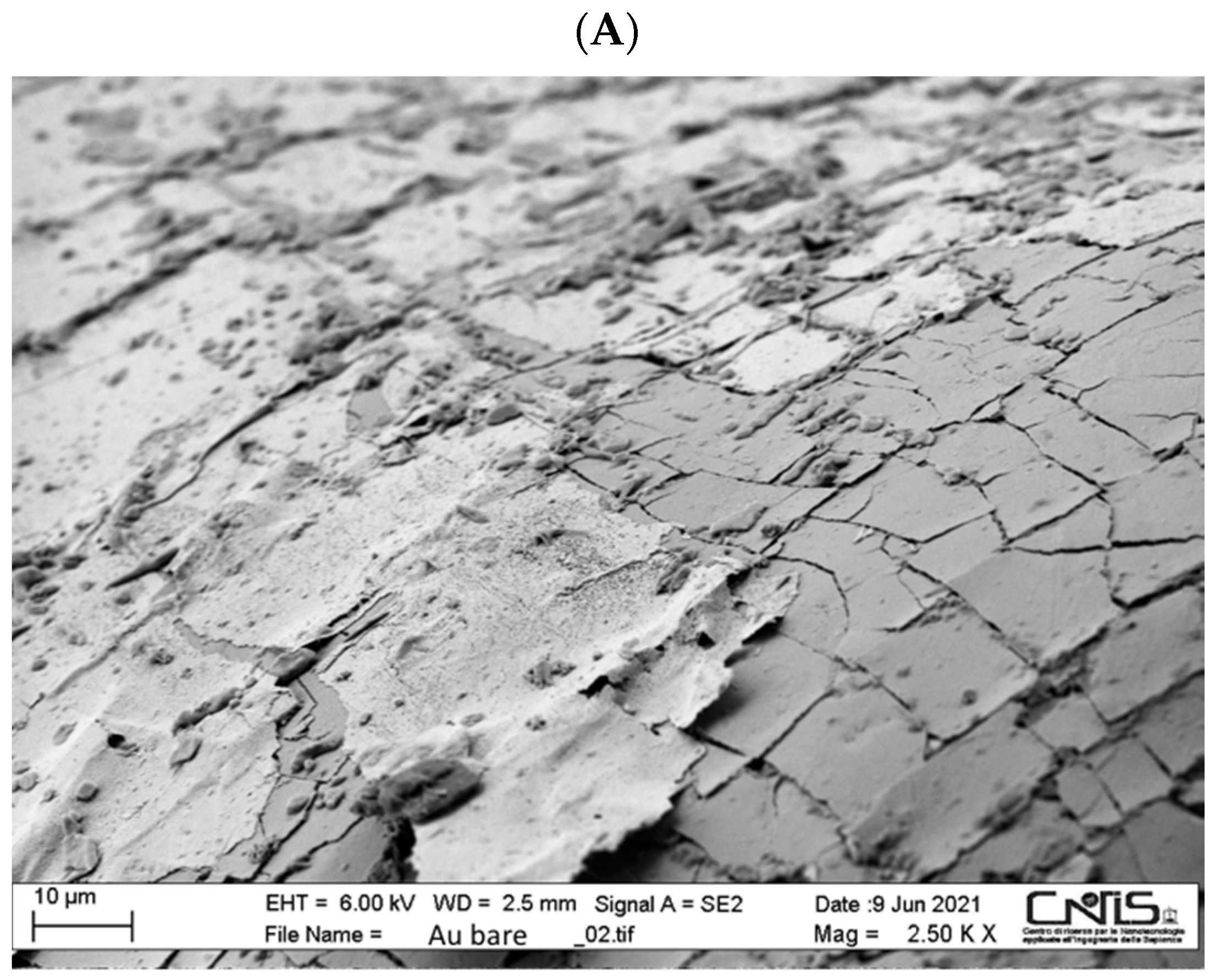
3.1. SEM Characterization
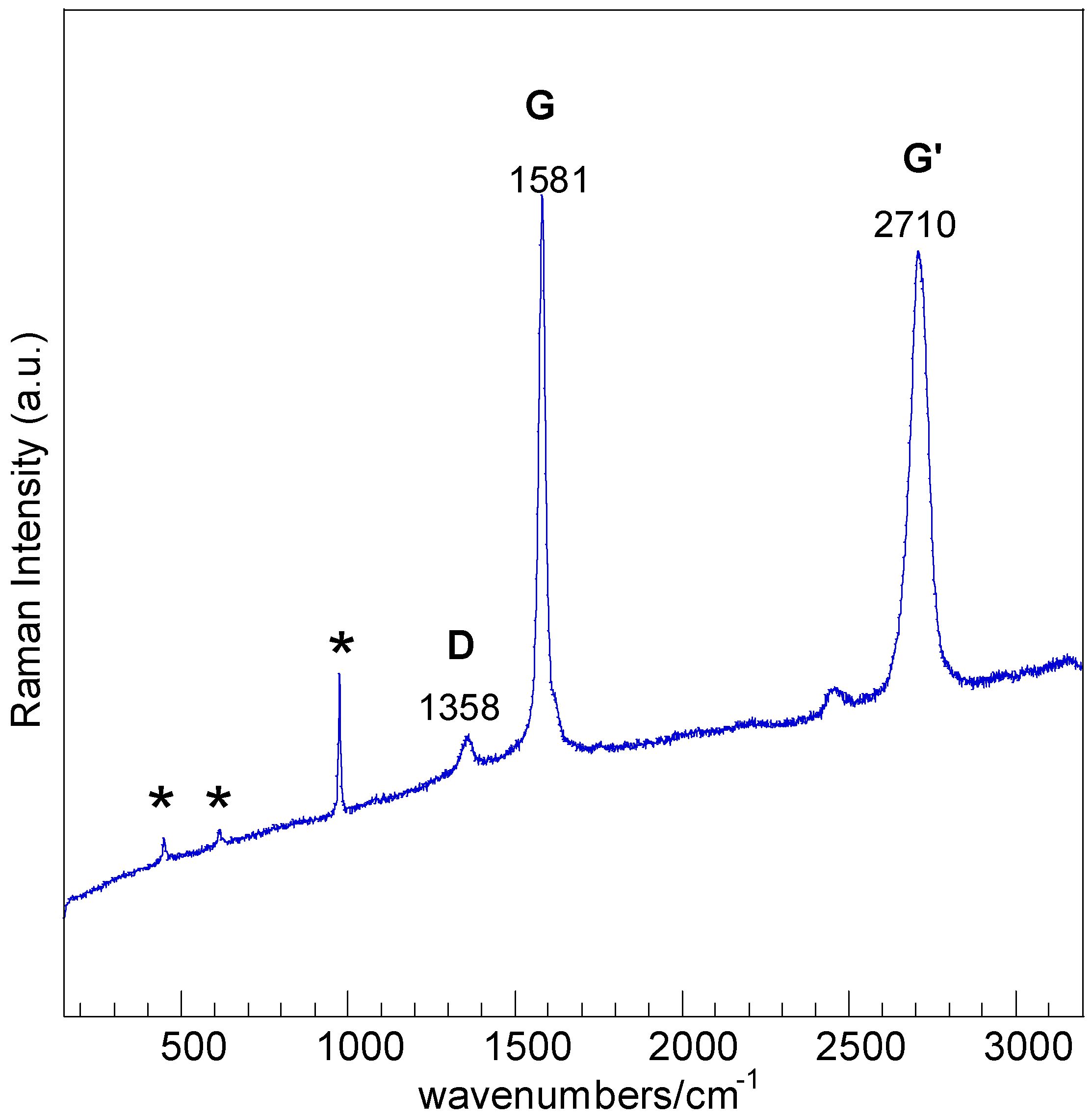
3.2. Raman Characterization
3.3. Electrochemical Characterization
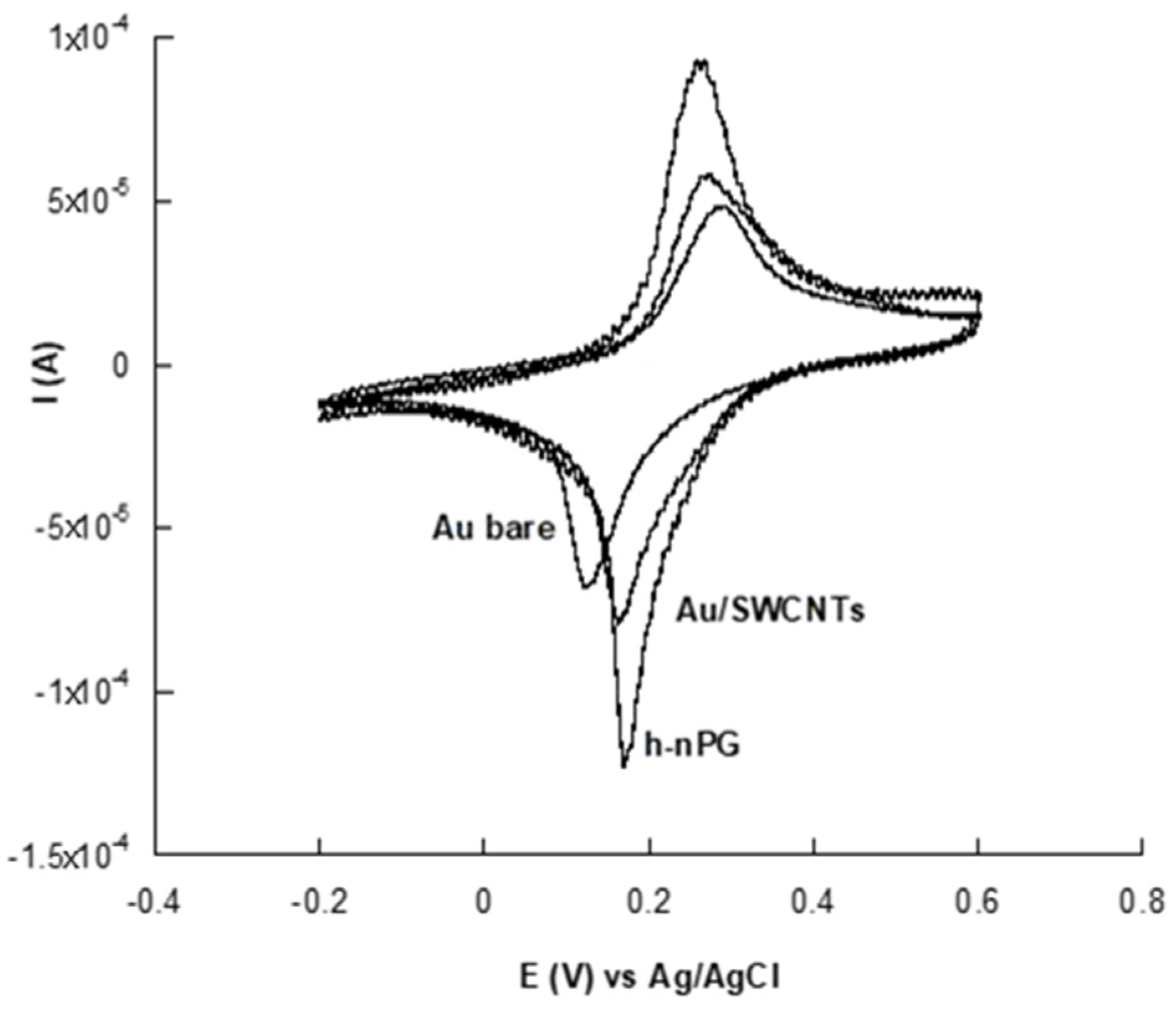
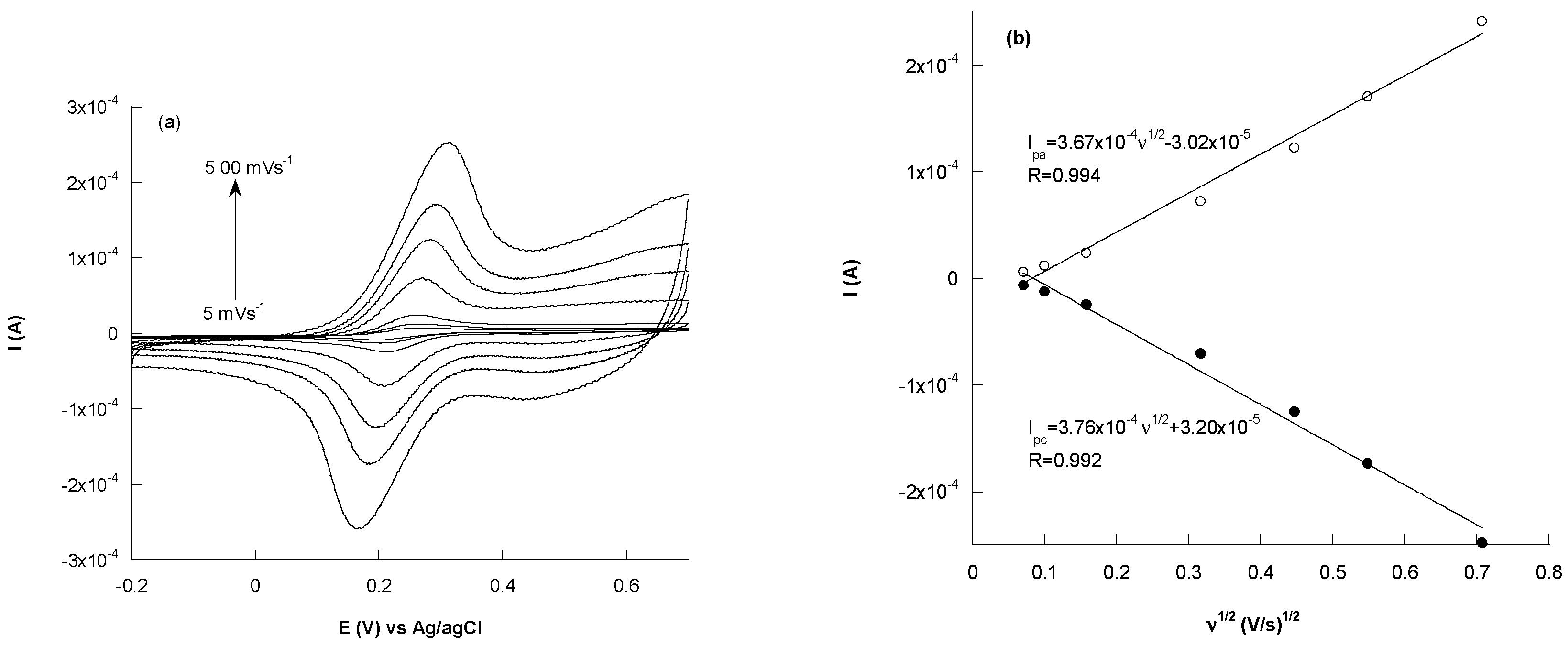
3.3.1. Cyclic Voltammetry Characterization
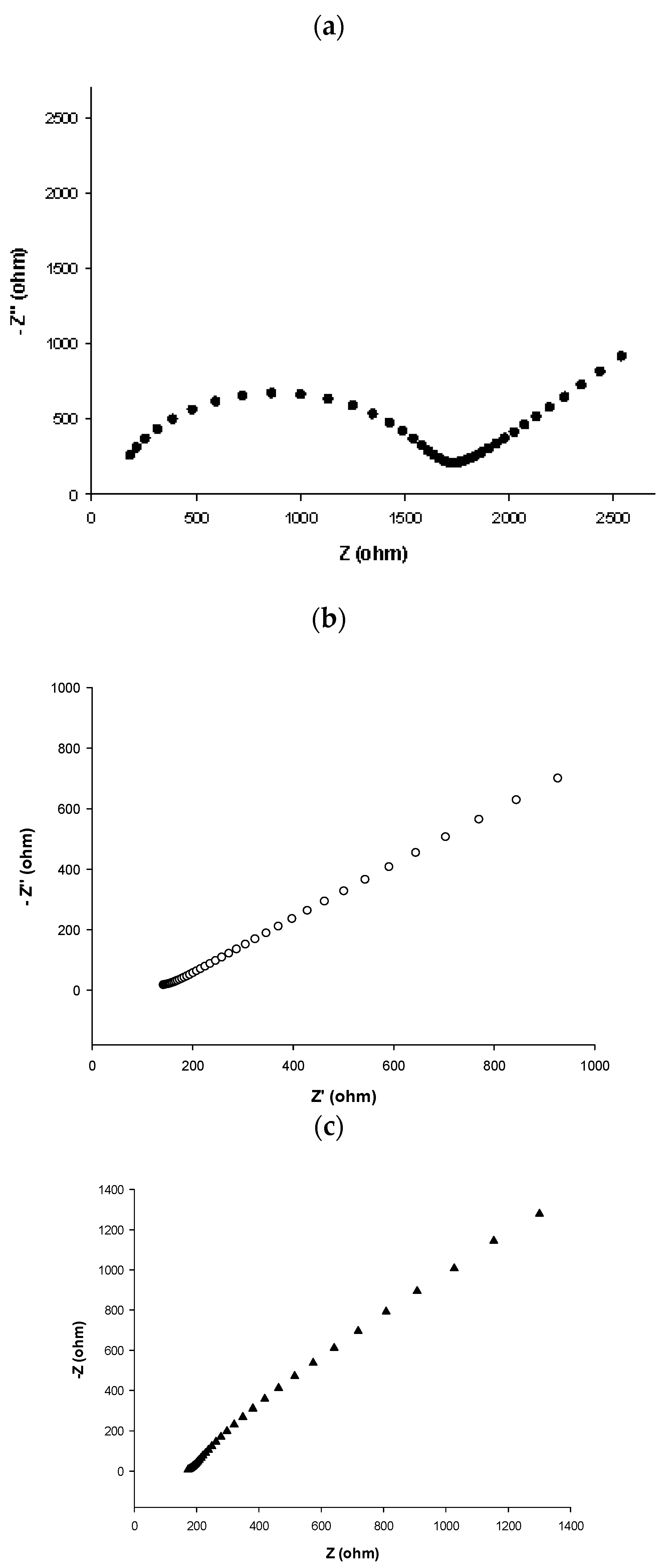
3.3.2. Electrochemical Impedance Spectroscopy Characterization
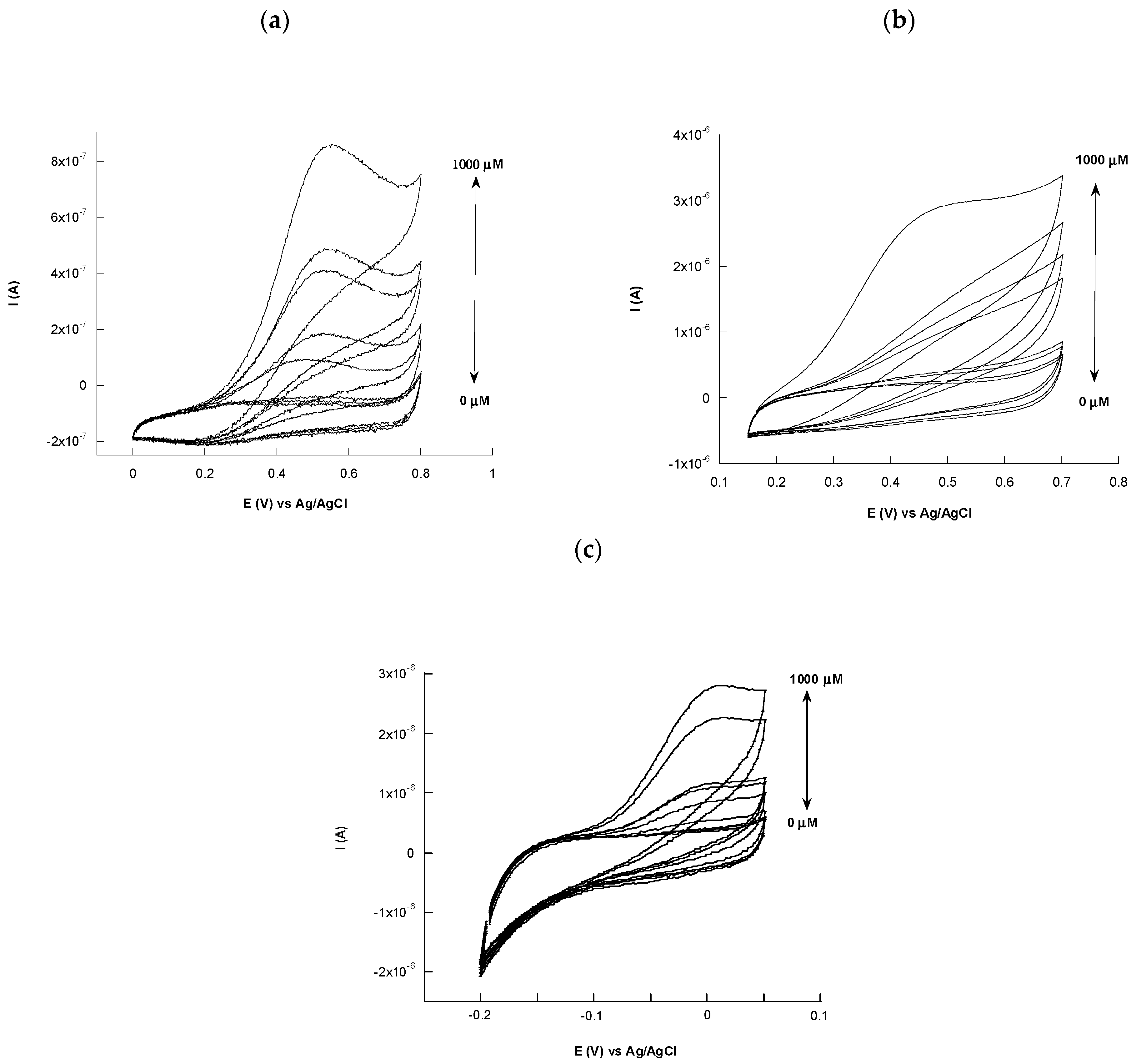
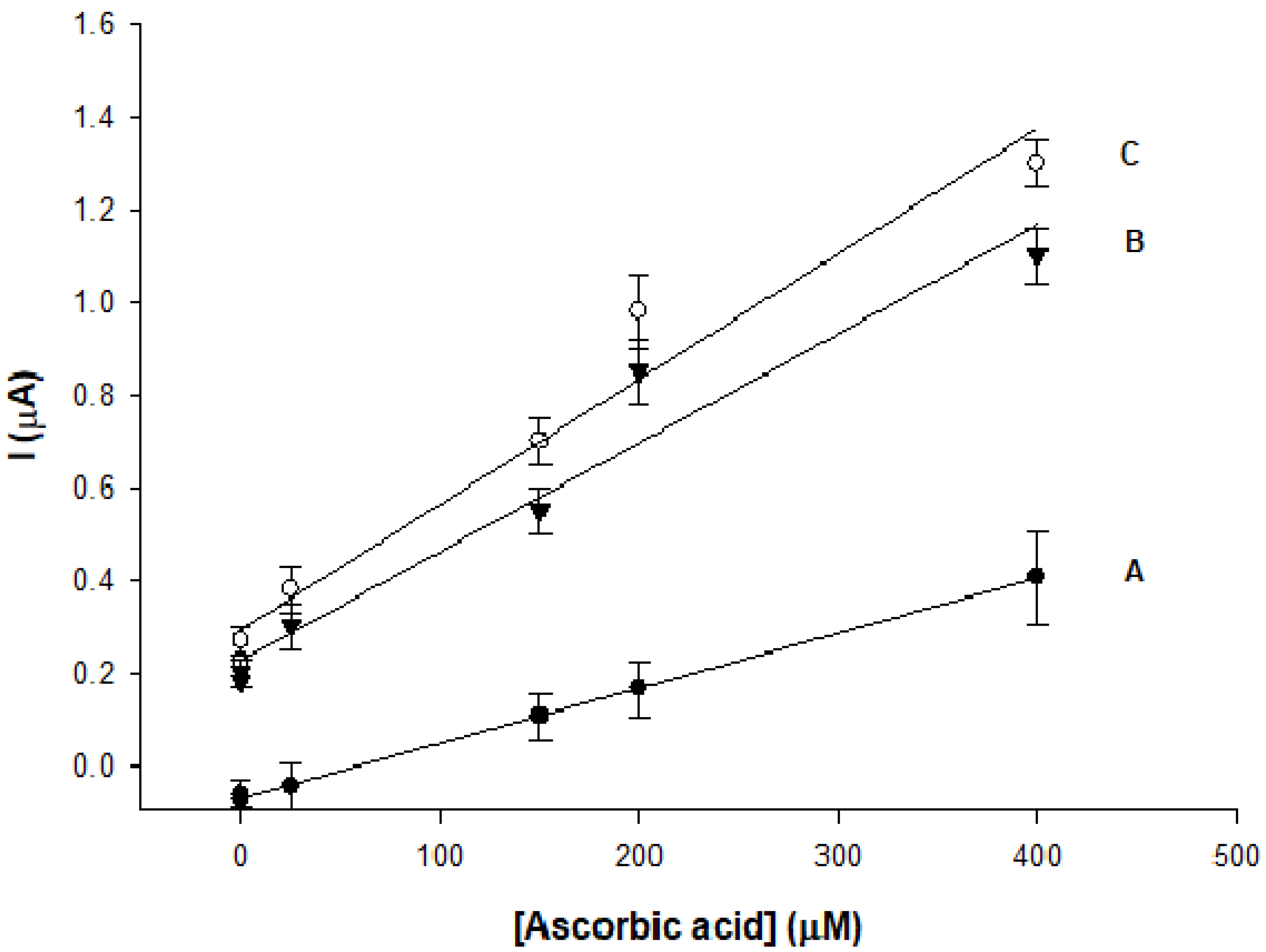
3.4. L-Ascorbic Acid Sensor
Ip (μA) = 0.002 CAA (M) + 0.22, R2 = 0.99
Ip (μA) = 0.003 CAA (M) + 0.29, R2 = 0.98
3.5. Reproducibility and Stability
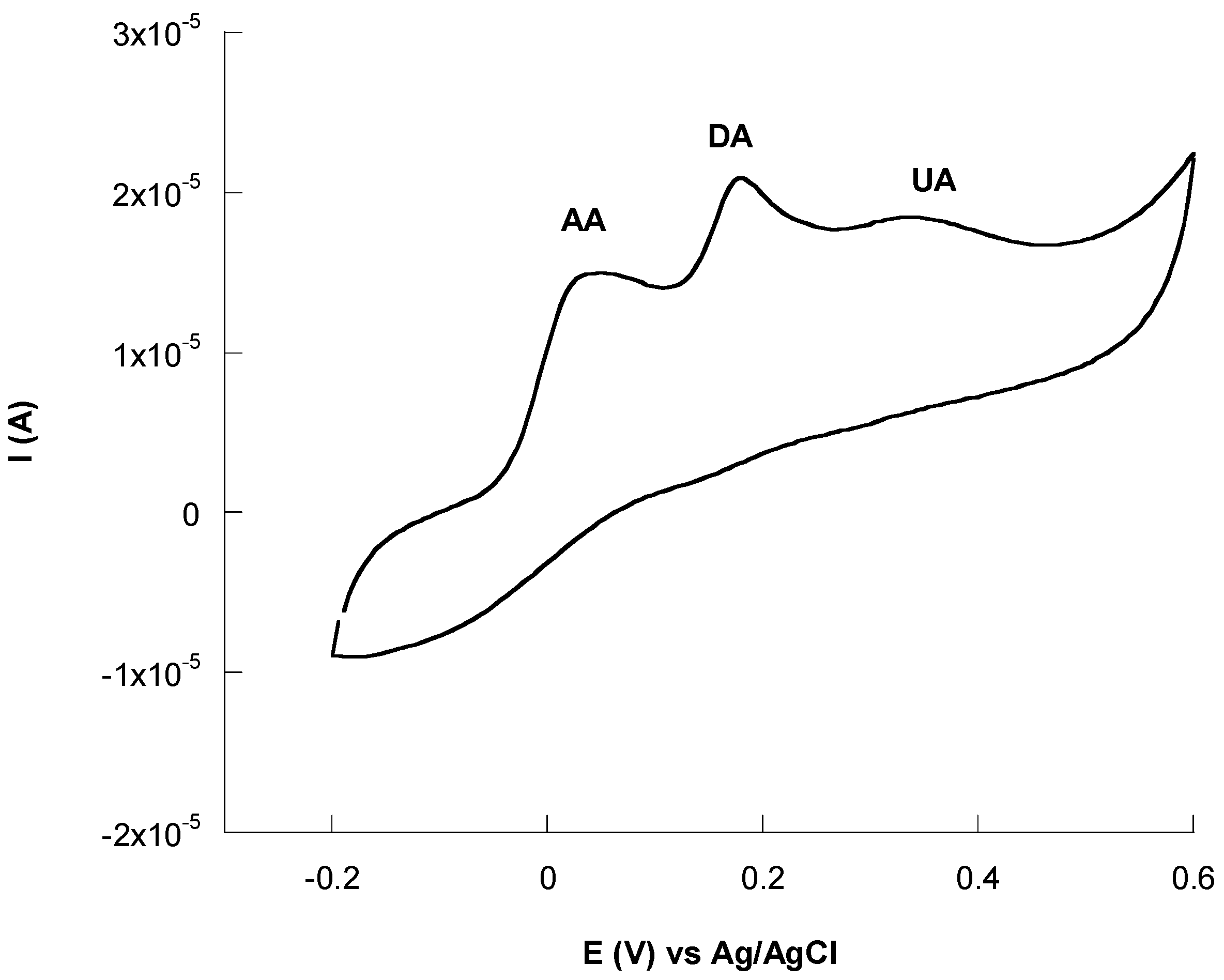
3.6. Interference Studies
3.7. Real Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Iqbal, K.; Khan, A.; Khattak, M. Biological significance of ascorbic acid (vitamin C) in human health—A review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar]
- Davies, M.B.; Partridge, D.A.; Austin, J.A. Vitamin C: Its Chemistry and Biochemistry; The Royal Society of Chemistry: Cambridge, UK, 1991. [Google Scholar]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.H.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.L.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Hemilä, H. Vitamin C supplementation and the common cold—Was Linus Pauling right or wrong? Int. J. Vitam. Nutr. Res. 1997, 67, 329–335. [Google Scholar] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Massey, L.K.; Liebman, M.; Kynast-Gales, S.A. Ascorbate increases human oxaluria and kidney stone risk. J. Nutr. 2005, 135, 1673–1677. [Google Scholar] [CrossRef] [Green Version]
- Pincemail, J.; Vanbelle, S.; Degrune, F.; Cheramy-Bien, J.P.; Charlier, C.; Chapelle, J.P.; Giet, D.; Collette, G.; Albert, A.; Defraigne, J.O. Lifestyle behaviours and plasma vitamin C and β-carotene levels from the ELAN population (Liège, Belgium). J. Nutr. Metab. 2011, 2011, 494370. [Google Scholar] [CrossRef] [Green Version]
- Biancatelli, R.M.L.C.; Berrill, M.; Marik, P.E. The antiviral properties of vitamin C. Expert Rev. Anti-Infect. Ther. 2020, 18, 99–101. [Google Scholar] [CrossRef]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondaneli, M.; Cena, H.; D’Antona, G. The long history of vitamin C: From prevention of the common cold to potential aid in the treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef]
- Carr, A.C.; Roew, S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; Backer, D.D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Int. Care 2021, 11, 5. [Google Scholar] [CrossRef]
- Law, W.S.; Kubáň, P.; Zhao, J.H.; Li, S.F.Y.; Hauser, P.C. Determination of vitamin C and preservatives in beverages by conventional capillary electrophoresis and microchip electrophoresis with capacitively coupled contactless conductivity detection. Electrophoresis 2005, 26, 4648–4658. [Google Scholar] [CrossRef]
- Wu, T.; Guan, Y.; Ye, J. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem. 2007, 100, 1573–1579. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, A.; Verma, K.K. Determination of ascorbic acid in soft drinks, preserved fruit juices and pharmaceuticals by flow injection spectrophotometry: Matrix absorbance correction by treatment with sodium hydroxide. Talanta 1995, 42, 779–787. [Google Scholar] [CrossRef]
- Tabata, M.; Morita, H. Spectrophotometric determination of a nanomolar amount of ascorbic acid using its catalytic effect on copper (II) porphyrin formation. Talanta 1997, 44, 151–157. [Google Scholar] [CrossRef]
- Alwarthan, A.A. Determination of ascorbic acid by flow injection with chemiluminescence detection. Analyst 1993, 118, 639–642. [Google Scholar] [CrossRef]
- Agater, I.B.; Jewsbury, R.A. Direct chemiluminescence determination of ascorbic acid using flow injection analysis. Anal. Chim. Acta 1997, 356, 289–294. [Google Scholar] [CrossRef]
- Chen, H.; Li, R.; Lin, L.; Guo, G.; Lin, J.-M. Determination of l-ascorbic acid in human serum by chemiluminescence based on hydrogen peroxide–sodium hydrogen carbonate–CdSe/CdS quantum dots system. Talanta 2010, 81, 1688–1696. [Google Scholar] [CrossRef]
- Verdini, R.A.; Lagier, C.M. Voltammetric iodometric titration of ascorbic acid with dead-stop end-point detection in fresh vegetables and fruit samples. J. Agric. Food Chem. 2000, 48, 2812–2817. [Google Scholar] [CrossRef]
- Suntornsuk, L.; Gritsanapun, W.; Nilkamhank, S.; Paochom, A. Quantitation of vitamin C content in herbal juice using direct titration. J. Pharm. Biomed. Anal. 2002, 28, 849–855. [Google Scholar] [CrossRef]
- Pachla, L.A.; Kissinger, P.T. Determination of ascorbic acid in foodstuffs, pharmaceuticals, and body fluids by liquid chromatography with electrochemical detection. Anal. Chem. 1976, 48, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Speek, A.; Schrijver, J.; Schreurs, W. Fluorometric determination of total vitamin C in whole blood by high-performance liquid chromatography with pre-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1984, 305, 53–60. [Google Scholar] [CrossRef]
- Iwata, T.; Yamaguchi, M.; Hara, S.; Nakamura, M.; Ohkura, Y. Determination of total ascorbic acid in human serum by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1985, 344, 351–355. [Google Scholar] [CrossRef]
- Perez-Ruiz, T.; Martinez-Lozano, C.; Tomas, V.; Fenol, J. Fluorimetric determination of total ascorbic acid by a stopped-flow mixing technique. Analyst 2001, 126, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Kumar, S.A.; Lo, P.H.; Chen, S.M. Electrochemical selective determination of ascorbic acid at redox active polymer modified electrode derived from direct blue. Biosens. Bioelectron. 2008, 24, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Dhara, K.; Debièrosad, R.M. Review on nanomaterials-enabled electrochemical sensors for ascorbic acid detection. Anal. Biochem. 2019, 586, 113415. [Google Scholar] [CrossRef] [PubMed]
- Tomita, I.N.; Manzoli, A.; Fertonani, F.L.; Yamanaka, H. Amperometric biosensor for ascorbic acid. Eclét. Quím. 2005, 30, 37–43. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Dinçkaya, E. A new enzyme electrode based on ascorbate oxidase immobilized in gelatin for specific determination of L-ascorbic acid. Talanta 1999, 50, 87–93. [Google Scholar] [CrossRef]
- Silva, T.A.; Khan, M.R.K.; Fatibello-Filho, O.; Cillinson, M.M. Simultaneous electrochemical sensing of ascorbic acid and uric acid under biofouling conditions using nanoporous gold electrodes. J. Electroanal. Chem. 2019, 846, 113160. [Google Scholar] [CrossRef]
- Barfidokht, A.; Gooding, J.J. Approaches toward allowing electroanalytical devices to be used in biological fluids. Electroanalysis 2014, 26, 1182–1196. [Google Scholar] [CrossRef]
- Wisniewski, N.; Moussy, F.; Reichter, M.W. Characterization of implantable biosensor membrane biofouling. Fresenius J. Anal. Chem. 2000, 366, 611–621. [Google Scholar] [CrossRef]
- Sha, Y.F.; Qian, L.; Ma, Y.; Bai, H.X.; Yang, X.R. Multilayer films of carbon nanotubes and redox polymer on screen-printed carbon electrodes for electrocatalysis of ascorbic acid. Talanta 2006, 70, 556–560. [Google Scholar] [CrossRef]
- Thangamuthu, R.; Kumar, S.M.; Pillai, K.C. Direct amperometric determination of l-ascorbic acid (vitamin C) at octacyanomolybdate-doped-poly(4-vinylpyridine) modified electrode in fruit juice and pharmaceuticals. Sens. Actuators B Chem. 2007, 120, 745–753. [Google Scholar] [CrossRef]
- Xi, L.; Ren, D.; Luo, J.; Zhu, Y. Electrochemical analysis of ascorbic acid using copper nanoparticles/polyaniline modified glassy carbon electrode. J. Electroanal. Chem. 2010, 650, 127–134. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Yang, M. Electrochemical sensing of L-ascorbic acid by using a glassy carbon electrode modified with a molybdophosphate film. Microchim. Acta 2019, 186, 445. [Google Scholar] [CrossRef]
- Taei, M.; Jamshidi, M.S. A voltammetric sensor for simultaneous determination of ascorbic acid, noradrenaline, acetaminophen and tryptophan. Microchem. J. 2017, 130, 108–115. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Danet, A.F.; Kalinowski, S. Ascorbic acid determination in commercial fruit juice samples by cyclic voltammetry. J. Anal. Methods Chem. 2008, 2008, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; Ma, Y.; Guo, Y.; Shao, S. Electrocatalytic oxidation and simultaneous determination of uric acid and ascorbic acid on the gold nanoparticles-modified glassy carbon electrode. Electrochim. Acta 2008, 53, 6610–6615. [Google Scholar] [CrossRef]
- Tig, G.A. Development of electrochemical sensor for detection of ascorbic acid, dopamine, uric acid and L-tryptophan based on Ag nanoparticles and poly(L-argininne)-graphene oxide composite. J. Electroanal. Chem. 2017, 807, 19–28. [Google Scholar] [CrossRef]
- Argoubi, W.; Rabti, A.; Aoun, S.B.; Raouafi, N. Sensitive detection of ascorbic acid using screen-printed electrodes modified by electroactive melanin b-like nanoparticles. RSC Adv. 2019, 9, 37384–37390. [Google Scholar] [CrossRef] [Green Version]
- Arroquia, A.; Acosta, I.; Armada, M.P.G. Self-assembled gold decorated polydopamine nanosphere as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. Mater. Sci. Eng. C 2020, 109, 110602. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, A.A.; Lam, M.; Freeman, C.Y.; Uppalapati, B.; Collinson, M.M. Potentiometric measurements in biofouling solutions: Comparison of nanoporous gold to planar gold. J. Electroanal. Soc. 2016, 163, H3083–H3087. [Google Scholar] [CrossRef] [Green Version]
- Jena, B.K.; Ray, C.R. Morphology dependent electrocatalytic activity of Au nanoparticles. Electrochem. Commun. 2008, 10, 951–954. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.C.; Chung, T.D. Electrochemical analysis based on nanoporous structures. Analyst 2012, 137, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Daggumati, P.; Matharu, Z.; Wang, L.; Seker, E. Biofouling resilient nanoporous gold electrodes for DNA sensing. Anal. Chem. 2015, 87, 8618–8622. [Google Scholar] [CrossRef] [Green Version]
- Collinson, M. Nanoporous gold electrodes and their applications in Analytical Chemistry. Int. Sch. Res. Not. Anal. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Wittstock, A.; Biener, J.; Baumer, M. Introduction to Nanoporous Gold; Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Highly sensitive membraneless fructose biosensor based on fructose dehydrogenase immobilized onto aryl thiol modified highly porous gold electrode: Characterization and application in food samples. Anal. Chem. 2018, 90, 12131–12136. [Google Scholar] [CrossRef]
- Junior, G.J.S.; Selva, J.S.G.; Sukeri, A.; Goncalves, J.M.; Regiart, M.; Bertotti, M. Fabrication of dendritic nanoporous gold via a two-step amperometric approach: Application for electrochemical detection of methyl parathion in river water samples. Talanta 2021, 226, 122130. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Furtado, V.L.; Goncalves, J.M.; Bannitz-Fernandes, R.; Netto, L.E.-S.; Araki, K.; Bertotti, M. Amperometric microsensor based on nanoporous gold for ascorbic acid detection in highly acidic biological extracts. Anal. Chim. Acta 2020, 2095, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sanzó, G.; Taurino, L.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; De Micheli, G.; Carrara, S. Bubble electrodeposition of gold porous nanocorals for the enzymatic and non-enzymatic detection of glucose. Bioelectrochemistry 2016, 112, 125–131. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Compton, R.G. Direct electrodeposition of gold nanoparticles onto indium tin oxide film coated glass: Application to the detection of arsenic (III). Anal. Sci. 2006, 22, 567–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Tasca, F.; Antiochia, R. Minimally invasive glucose monitoring using a highly porous gold microneedles-based biosensor: Characterization and application in artificial interstitial fluid. Catalysts 2019, 9, 580. [Google Scholar] [CrossRef] [Green Version]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102–021127. [Google Scholar] [CrossRef]
- Ben, K.; Kauffmann, H.; Aroui, H.; Fontana, M. Raman study of cation effect on sulfate vibration modes in solid state and in aqueous solutions. J. Raman Spectrosc. 2013, 44, 1603–1608. [Google Scholar]
- Pina, S.; Candia-Onfray, C.; Hassan, N.; Jara-Ulloa, P.; Contreras, D.; Salazar, R. Glassy carbon electrode modified with C/Au nanostructured materials for simultaneous determination of hydroquinone and cathecol in water matrices. Chemosensors 2021, 9, 88. [Google Scholar] [CrossRef]
- Oldham, K.B. Analytical expressions for the reversible Randles-Sevcik function. J. Electroanal. Chem. Interfacial Electrochem. 1979, 105, 373–375. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. A calibration-base method for the evaluation of the detection limit of an electrochemical biosensor. Electroanalysis 2007, 19, 1227–1230. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkener, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; p. 381. [Google Scholar]
- Randviir, E.P. A cross examination of electron transfer rate constants for carbon screen-printed electrodes using Electrochemical Impedance Spectroscopy and cyclic voltammetry. Electrochim. Acta 2018, 186, 179–186. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Liu, L.; Yagnik, G.B.; Zhou, F. reaction rates and mechanism of the ascorbic acid oxidation by molecular oxygen facilitated by Cu(II)-containing amyloid-β complexes and aggregates. J. Phys. Chem. B 2010, 114, 48–96. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Gonçalves, J.M.; Selva, J.S.G.; Araki, K.; Bertotti, M. Correlating selective electrocatalysis of dopamine and ascorbic acid electrooxidation at nanoporous gold surfaces with structural-defects. J. Electrochem. Soc. 2019, 166, H704. [Google Scholar] [CrossRef]
- De, S.K.; Mondal, S.; Sen, P.; Pal, U.; Pathak, B.; Rawat, K.S.; Bardhan, M.; Bhattacharya, M.; Satpati, B.; De, A.; et al. Crystal-defect-induced facet-dependent electrocatalytic activity of 3D gold nanoflowers for the selective nanomolar detection of ascorbic acid. Nanoscale 2018, 10, 13792. [Google Scholar] [CrossRef] [Green Version]
- Alden, J.A.; Compton, R.G. Automated simulation of electrode processes: Quantitative mechanistic analysis via working surface interpolation. J. Phys. Chem. B 1997, 101, 9741–9750. [Google Scholar] [CrossRef]
- Zhang, G.; He, P.; Feng, W.; Ding, S.; Chen, J.; Li, L.; He, H.; Zhang, S.; Dong, F. Carbon nanohors/poly(glycine) modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2016, 760, 24–31. [Google Scholar] [CrossRef]
- Bagheri, H.; Pajooheshpour, N.; Khoshsafar, H. A novel electrochemical platform for sensitive and simultaneous determination of dopamine, uric acid and ascorbic acid based on Fe3O4 SnO2 Gr ternary nanocomposite. Microchem. J. 2017, 131, 120–129. [Google Scholar] [CrossRef]
- El-Said, W.A.; Lee, J.H.; Oh, B.K.; Choi, J.W. 3-D nanoporous gold thin film for the simultaneous electrochemical determination of dopamine and ascorbic acid. Electrochem. Commun. 2010, 12, 1756–1759. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Zhou, G.-P.; Ji, G.-L.; Zhang, Y.; Huang, X.-R.; Ding, Y. A novel nanoporous gold modified electrode for the selective determination of dopamine in the presence of ascorbic acid. Colloids Surf. B Biointerfaces 2009, 69, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Rong, Y.; Fan, L.; Zhang, C.; Dong, W.; Li, J.; Niu, J.; Yang, C.; Shuang, S.; Dong, C.; et al. Simultaneous electrochemical sensing of serotonin, dopamine and ascorbic acid by using a nanocomposite prepared from reduced graphene oxide, Fe3O4 and hydroxypropyl-ß -cyclodextrin. Microchim. Acta 2019, 186, 751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, Y.; Yuan, R.; Chen, S.; Han, J.; Yuan, D. Facile synthesis of graphene hybrid tube-like structure for simultaneous detection of ascorbic acid, dopamine, uric acid and tryptophan. Anal. Chim. Acta 2012, 756, 7–12. [Google Scholar] [CrossRef]
- Jalalvand, A.R. Four-dimensional voltammetry: An efficient strategy for simultaneous determination of ascorbic acid and uric acid in the presence of dopamine as uncalibrated interfere. Sens. Biosens. Res. 2020, 28, 100330. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Song, Y.; Li, Y.; Li, S. Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using a gold electrode modified with carboxilated graphene and silver nanocube functionalized polydopamine nanospheres. Microchim. Acta 2018, 185, 382. [Google Scholar] [CrossRef]
- Edris, N.M.M.A.; Abdullah, J.; Kamaruzaman, S.; Sulaiman, Y. Ultrasensitive reduced graphene oxide-poly(procion)/gold nanoparticles modified glassy carbon electrode for selective and simultaneous determination of ascorbic acid, dopamine, and uric acid. J. Electrochem. Soc. 2019, 166, B664. [Google Scholar] [CrossRef]
- Huang, H.; Yue, Y.; Chen, Z.; Chen, Y.; Wu, S.; Liao, J.; Liu, S.; Wen, H. Electrochemical sensor based on a nanocomposite prepared from TmPO4 and graphene oxide for simultaneous voltammetric detection of ascorbic acid, dopamine and uric acid. Microchim. Acta 2019, 186, 189. [Google Scholar] [CrossRef]



| Sensor | Eox/mV | Ered/mV | ∆Ep/mV | Ae (mm2) | k0 (cm s−1) | ϱ |
|---|---|---|---|---|---|---|
| Au bare | 283 | 126 | 157 | 2.86 | 0.85 × 10−3 | 0.92 |
| Au/SWCNTs | 270 | 164 | 106 | 39.90 | 2.95 × 10−3 | 13.31 |
| h-nPG | 263 | 176 | 87 | 59.20 | 3.98 × 10−3 | 19.10 |
| Sensor SPE | Rs/Ω | Rct/Ω | W/ohm s1/2 | CPE/ohm s N | N | Cdl/F | k0 (cm s−1) * | i0 (A) * |
|---|---|---|---|---|---|---|---|---|
| Au bare | 97 | 1540 | 981 × 10−6 | 1 × 10−6 | 0.9 | - | 1.21 × 10−3 | 16.7 × 10−6 |
| Au/SWCNTs | 76 | 92 | 896 × 10−6 | 2.74 × 10−4 | 0.3 | - | 1.4 × 10−3 | 279 × 10−6 |
| h-nPG | 181 | 1 × 10−6 | 708 × 10−6 | - | - | 90 × 10−6 | - | - |
| Modified Electrode | Linear Range (µM) | LOD (µM) | Sensitivity (µA µM−1 cm−2) | Stability | Ref. |
|---|---|---|---|---|---|
| h-nPG/AuE (dealloying) | 320–3400 | 63.0 | - | - | [32] |
| h-nPG/AuE (electrodeposition) | 10–1100 | 2 | - | 88% after 5 weeks | [28] |
| h-nPG/AuE (electrodeposition) | 5–400 | 1.8 | 2.5 × 10−2 | 98% after 1 month | this work |
| PVP-GR/GCE | 4–1000 | 0.8 | - | 83.6% after 15 days | [75] |
| rGO/Fe3O4/HP-ß-CD/GCE | 10–350 | 3.3 | - | ~100% after 1 month | [76] |
| P(Arg)-GO/AgNPs/GCE | 4–2400 | 0.9 | - | - | [77] |
| Au-PDNs/SPCE | 10–240 | 0.2 × 10−3 | 2.2 × 10−2 | ~99% after 2 months | [78] |
| AgNC@PDA-NS/AuE | 50–4000 | 6.4 | 8.4 × 10−2 | - | [79] |
| rGO-poly(PR)/AuNPs/GCE | 0.4–110 | 0.054 | 4.9 | 87.5% over 1 month | [80] |
| GO/TmPO4/GCE | 0.06–100 | 0.4 | 0.239 | 92.33% after 2 weeks | [81] |
| poly-TB/GCE | 1–630 | 0.1 | 0.46 | 97.9% after 3 weeks | [39] |
| Interfering Compound | [AA] (M) | Recovery (%) |
|---|---|---|
| Glucose | 480 | 95 |
| L-cysteine | 490 | 98 |
| L-lysine | 495 | 99 |
| Urea | 475 | 95 |
| citric acid | 485 | 97 |
| Urine Sample | Added (M) | Detected (M) | Spectrophotometric Method (M) | Recovery (%) |
|---|---|---|---|---|
| 1 | 10.0 | 10.56 | 10.34 | 105.6 ± 0.72 |
| 2 | 20.0 | 21.16 | 20.86 | 105.8 ± 1.22 |
| 3 | 50.0 | 49.20 | 50.88 | 98.40 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortolini, C.; Tasca, F.; Venneri, M.A.; Marchese, C.; Antiochia, R. Gold Nanoparticles/Carbon Nanotubes and Gold Nanoporous as Novel Electrochemical Platforms for L-Ascorbic Acid Detection: Comparative Performance and Application. Chemosensors 2021, 9, 229. https://doi.org/10.3390/chemosensors9080229
Tortolini C, Tasca F, Venneri MA, Marchese C, Antiochia R. Gold Nanoparticles/Carbon Nanotubes and Gold Nanoporous as Novel Electrochemical Platforms for L-Ascorbic Acid Detection: Comparative Performance and Application. Chemosensors. 2021; 9(8):229. https://doi.org/10.3390/chemosensors9080229
Chicago/Turabian StyleTortolini, Cristina, Federico Tasca, Mary Anna Venneri, Cinzia Marchese, and Riccarda Antiochia. 2021. "Gold Nanoparticles/Carbon Nanotubes and Gold Nanoporous as Novel Electrochemical Platforms for L-Ascorbic Acid Detection: Comparative Performance and Application" Chemosensors 9, no. 8: 229. https://doi.org/10.3390/chemosensors9080229
APA StyleTortolini, C., Tasca, F., Venneri, M. A., Marchese, C., & Antiochia, R. (2021). Gold Nanoparticles/Carbon Nanotubes and Gold Nanoporous as Novel Electrochemical Platforms for L-Ascorbic Acid Detection: Comparative Performance and Application. Chemosensors, 9(8), 229. https://doi.org/10.3390/chemosensors9080229










