Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept
Abstract
:1. Introduction
2. Perovskites Compounds
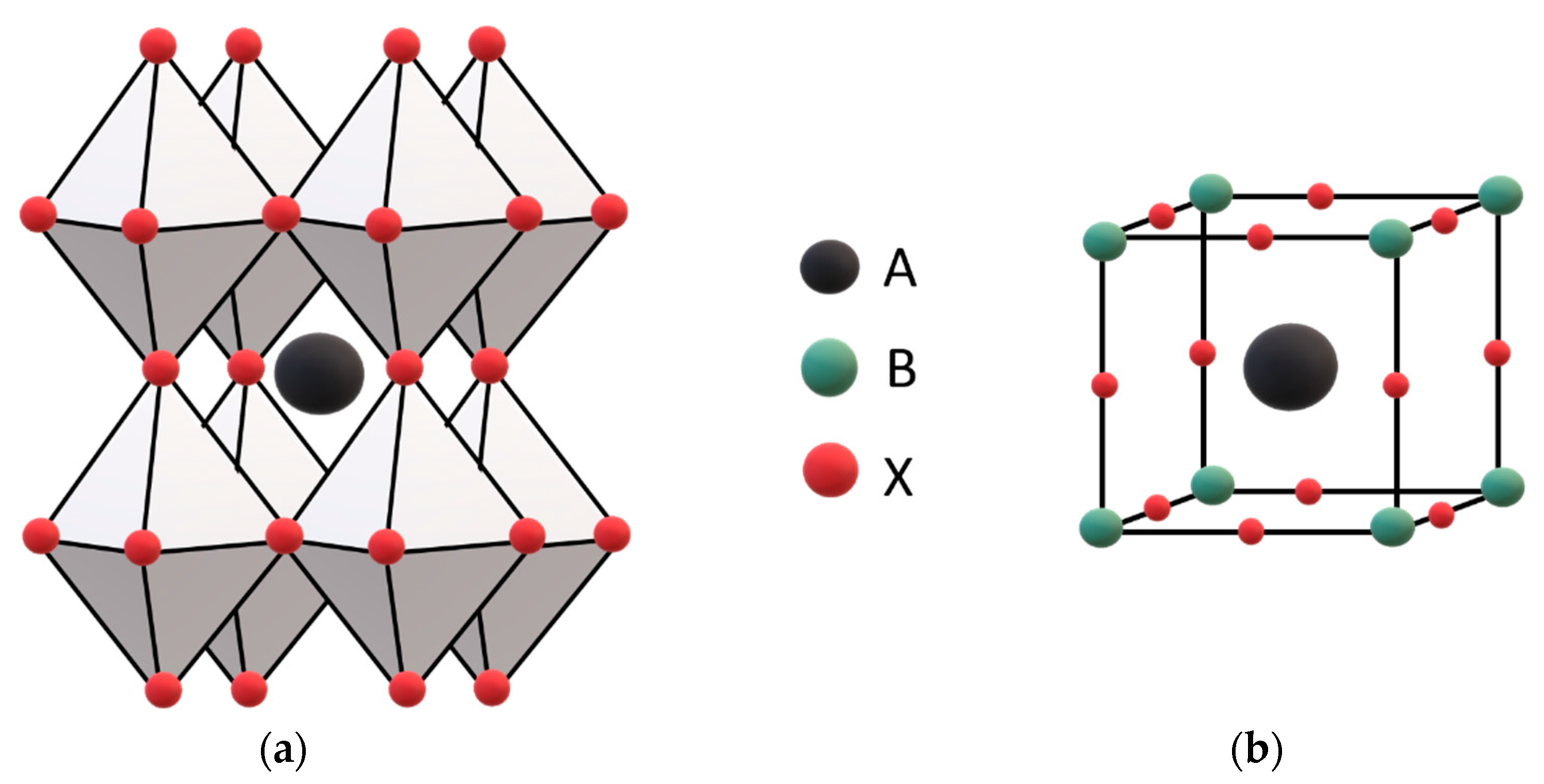
2.1. Perovskite Structure
2.2. Perovskite Oxides
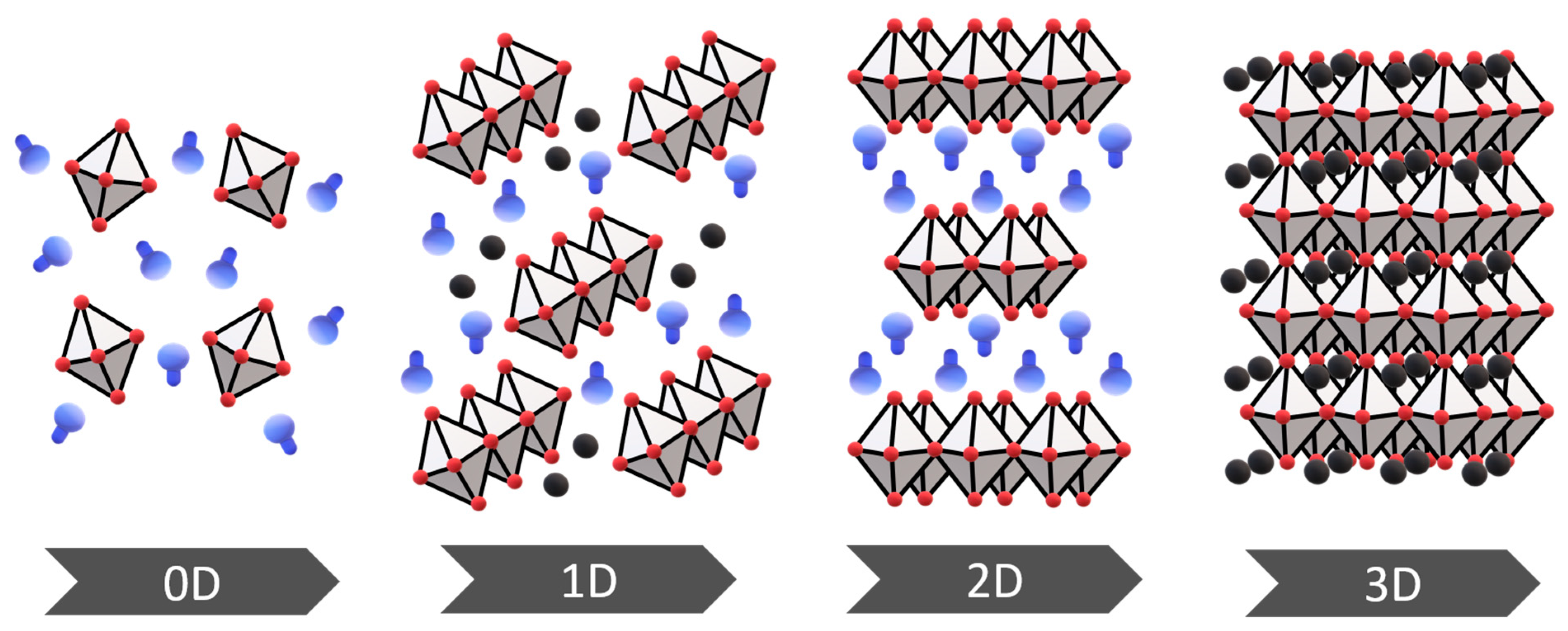
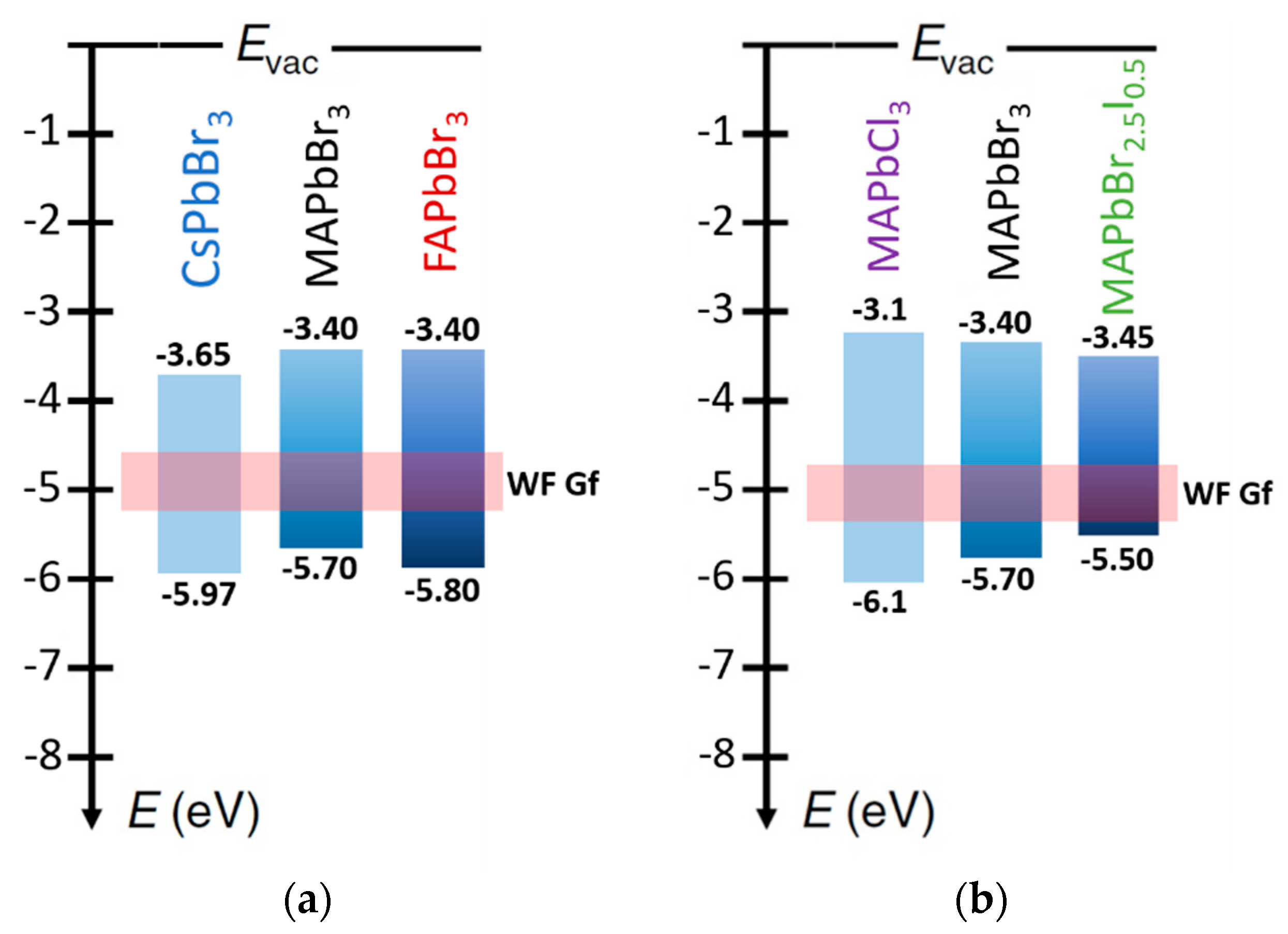
2.3. Halide Perovskites
3. Perovskite@Graphene Nanohybrids
3.1. Graphene Functionalization
3.2. Development and Characterization of Perovskite@Graphene Nanohybrids
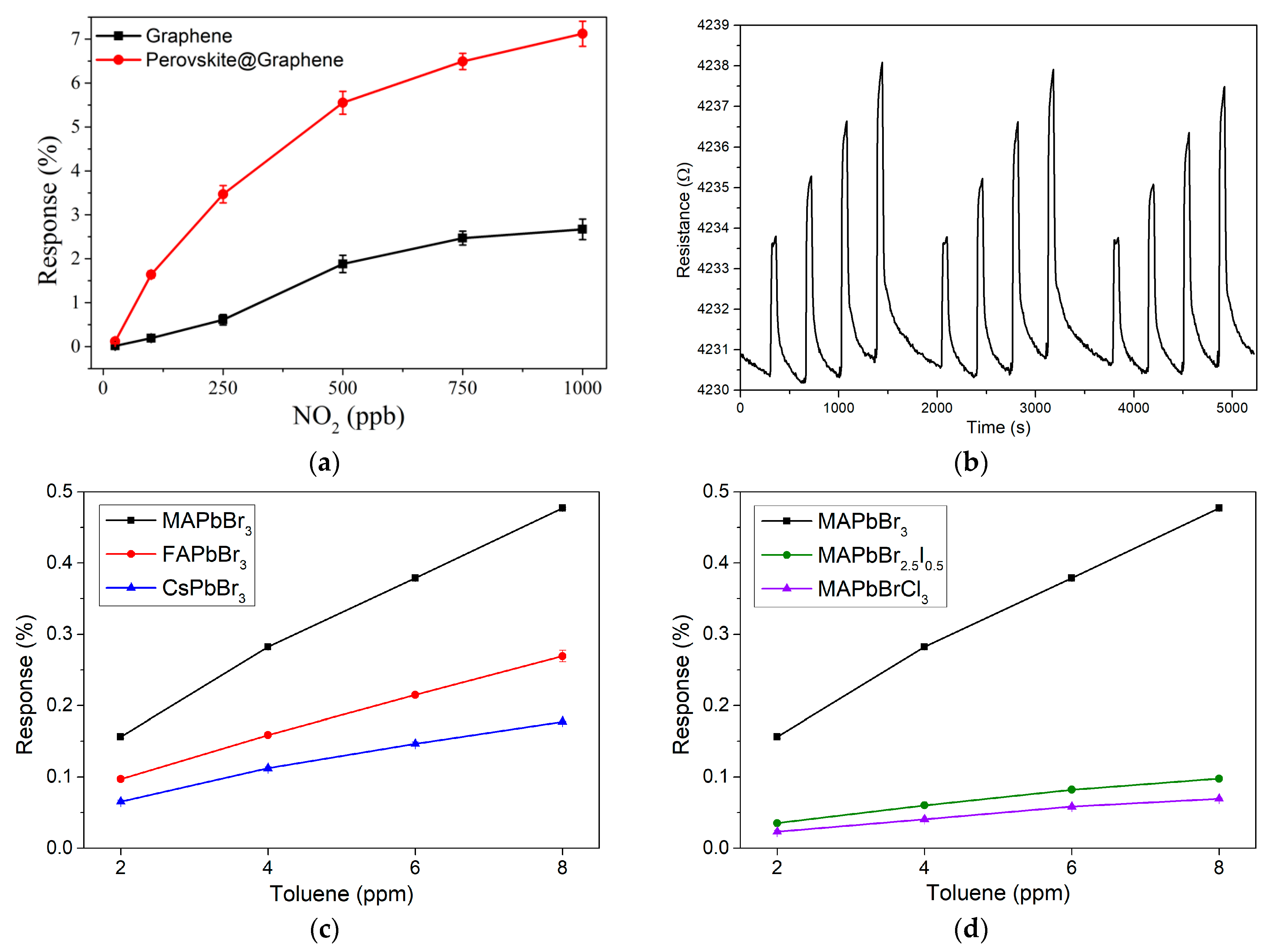
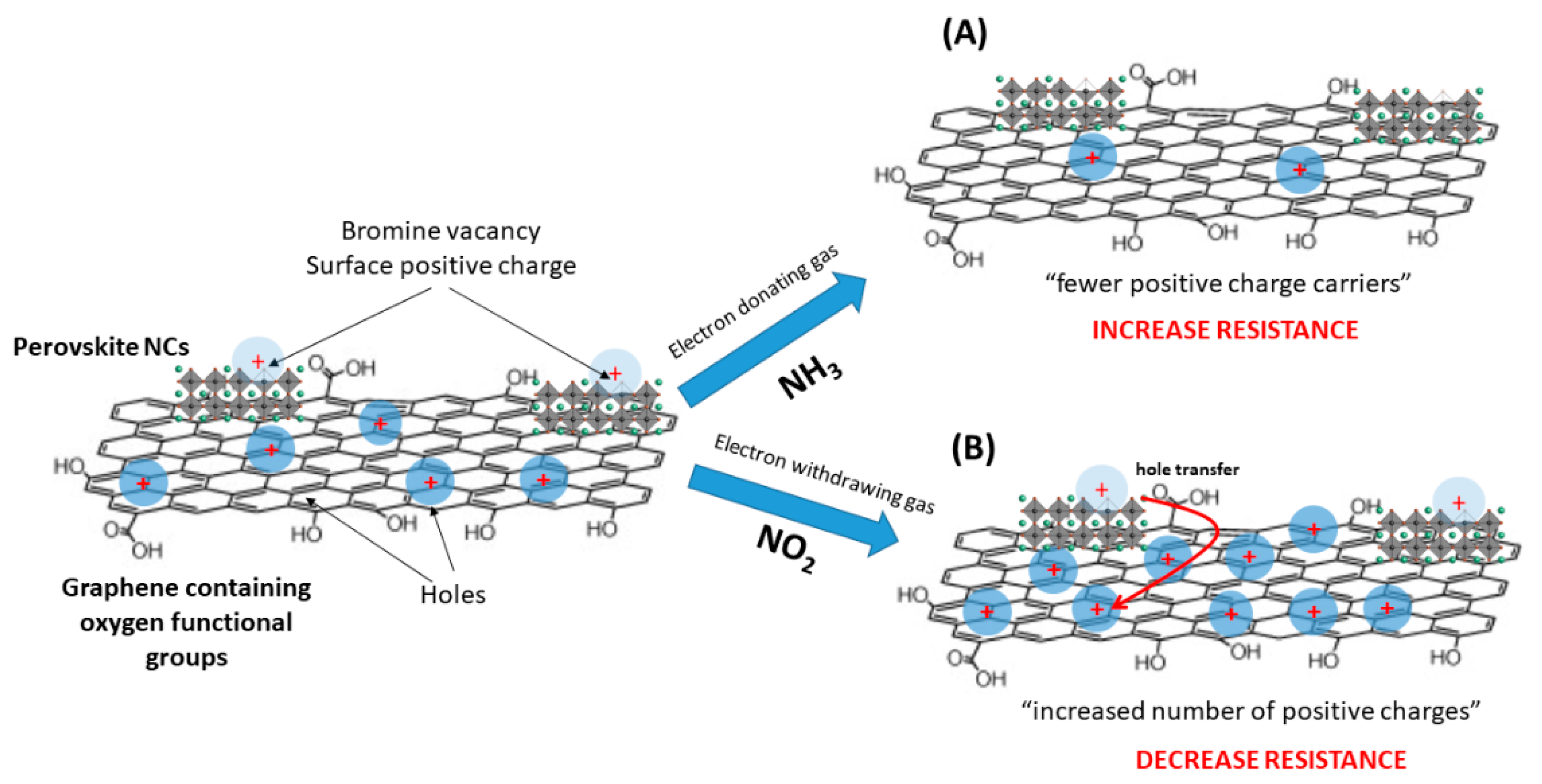
3.3. Gas Detection
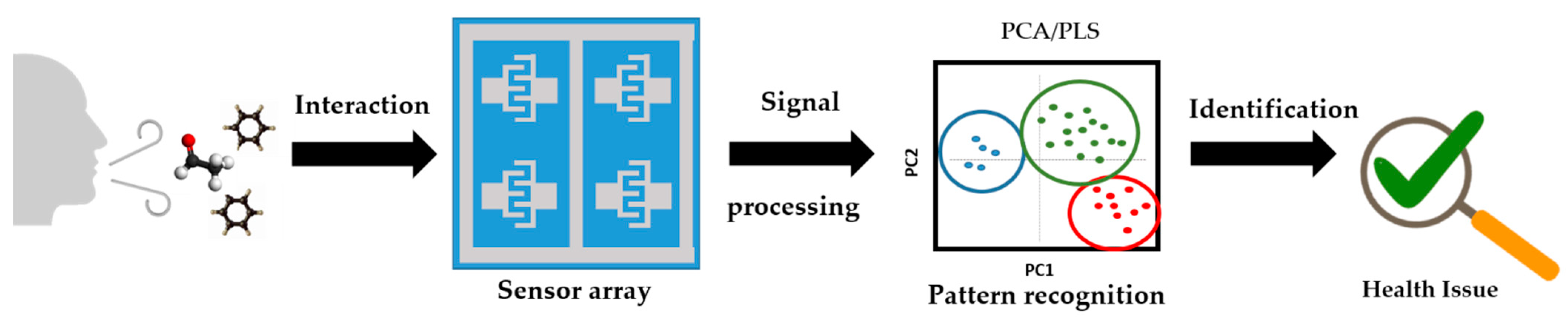
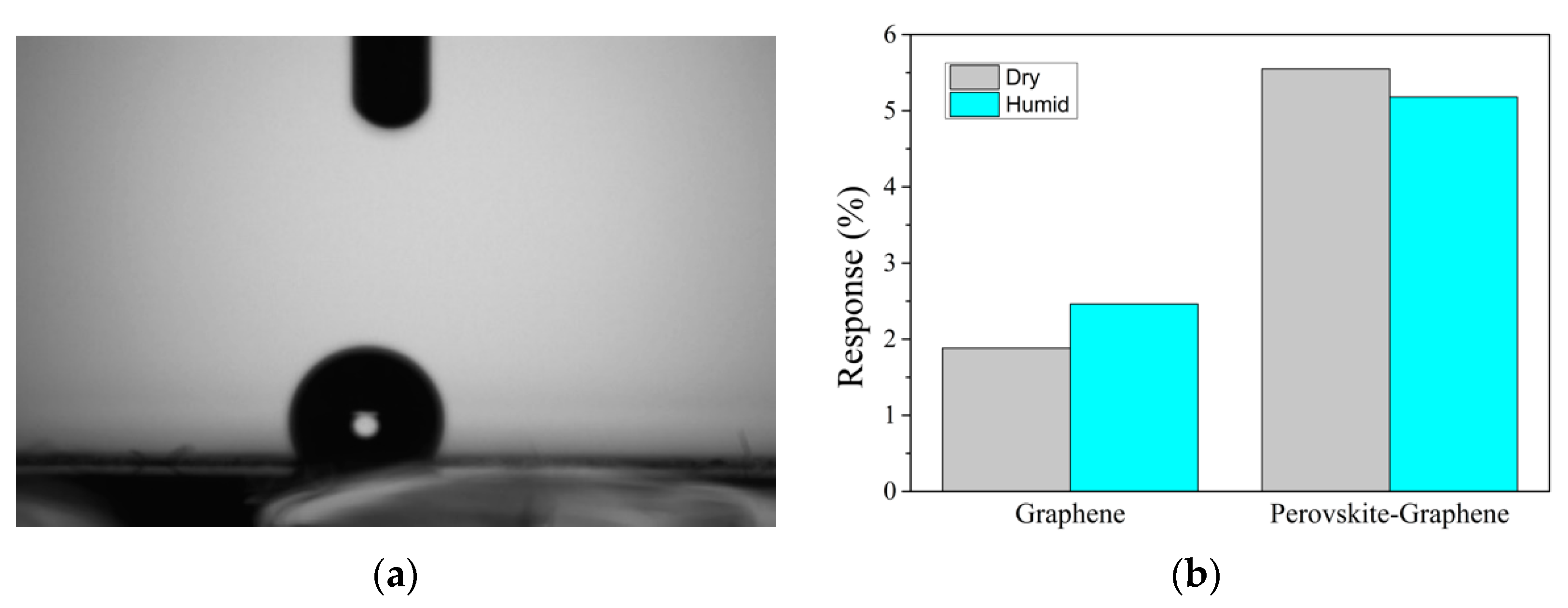
3.4. Potential Use for Breath Analysis
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Cikach, F.S.; Dweik, R.A. Cardiovascular Biomarkers in Exhaled Breath. Prog. Cardiovasc. Dis. 2012, 55, 34–43. [Google Scholar] [CrossRef] [Green Version]
- du Plessis, A.; le Roux, S.G.; Guelpa, A. Comparison of Medical and Industrial X-Ray Computed Tomography for Non-Destructive Testing. Case Stud. Nondestruct. Test. Eval. 2016, 6, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Mashir, A.; Dweik, R.A. Exhaled Breath Analysis: The New Interface between Medicine and Engineering. Adv. Powder Technol. 2009, 20, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Radogna, A.V.; Siciliano, P.A.; Sabina, S.; Sabato, E.; Capone, S. A Low-Cost Breath Analyzer Module in Domiciliary Non-Invasive Mechanical Ventilation for Remote Copd Patient Monitoring. Sensors 2020, 20, 653. [Google Scholar] [CrossRef] [Green Version]
- Grob, N.M.; Aytekin, M.; Dweik, R.A. Biomarkers in Exhaled Breath Condensate: A Review of Collection, Processing and Analysis. J. Breath Res. 2008, 2, 037004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled Breath Analysis for Lung Cancer. J. Thorac. Dis. 2013, 5, S540. [Google Scholar]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Beccaria, M.; Bobak, C.; Maitshotlo, B.; Mellors, T.R.; Purcaro, G.; Franchina, F.A.; Rees, C.A.; Nasir, M.; Black, A.; Hill, J.E. Exhaled Human Breath Analysis in Active Pulmonary Tuberculosis Diagnostics by Comprehensive Gas Chromatography-Mass Spectrometry and Chemometric Techniques. J. Breath Res. 2019, 13, 16005. [Google Scholar] [CrossRef]
- Ribes, A.; Carrera, G.; Gallego, E.; Roca, X.; Berenguer, M.J.; Guardino, X. Development and Validation of a Method for Air-Quality and Nuisance Odors Monitoring of Volatile Organic Compounds Using Multi-Sorbent Adsorption and Gas Chromatography/Mass Spectrometry Thermal Desorption System. J. Chromatogr. A 2007, 1140, 44–55. [Google Scholar] [CrossRef]
- Sola Martínez, R.A.; Pastor Hernández, J.M.; Lozano Terol, G.; Gallego-Jara, J.; García-Marcos, L.; Cánovas Díaz, M.; de Diego Puente, T. Data Preprocessing Workflow for Exhaled Breath Analysis by GC/MS Using Open Sources. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of Hazardous Volatile Organic Compounds (VOCs) by Metal Oxide Nanostructures-Based Gas Sensors: A Review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Meng, F.L.; Guo, Z.; Huang, X.J. Graphene-Based Hybrids for Chemiresistive Gas Sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Jiang, C.; Guo, H.; Qu, F.; Shimakawa, Y.; Yang, M. Integrated Sensing Array of the Perovskite-Type LnFeO3 (Ln=La, Pr, Nd, Sm) to Discriminate Detection of Volatile Sulfur Compounds. J. Hazard. Mater. 2021, 413, 125380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Du, T.; Zhu, Z.; Zhang, J.; Liu, Q. A Gas Sensor Array for the Simultaneous Detection of Multiple VOCs. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Di Natale, C.; Martinelli, E.; Pennazza, G.; Orsini, A.; Santonico, M. Data Analysis for Chemical Sensor Arrays. In Advances in Sensing with Security Applications; Springer: Dordrecht, The Netherlands, 2006; Volume 2, pp. 147–169. [Google Scholar]
- Scott, S.M.; James, D.; Ali, Z. Data Analysis for Electronic Nose Systems. Microchim. Acta 2006, 156, 183–207. [Google Scholar] [CrossRef]
- Buckley, D.J.; Black, N.C.G.; Castanon, E.G.; Melios, C.; Hardman, M.; Kazakova, O. Frontiers of Graphene and 2D Material-Based Gas Sensors for Environmental Monitoring. 2D Mater. 2020, 7, 032002. [Google Scholar] [CrossRef]
- Travan, C.; Bergmann, A. NO2 and NH3 Sensing Characteristics of Inkjet Printing Graphene Gas Sensors. Sensors 2019, 19, 3379. [Google Scholar] [CrossRef] [Green Version]
- Rodner, M.; Puglisi, D.; Ekeroth, S.; Helmersson, U.; Shtepliuk, I.; Yakimova, R.; Skallberg, A.; Uvdal, K.; Schütze, A.; Eriksson, J. Graphene Decorated with Iron Oxide Nanoparticles for Highly Sensitive Interaction with Volatile Organic Compounds. Sensors 2019, 19, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovsianytskyi, O.; Nam, Y.S.; Tsymbalenko, O.; Lan, P.T.; Moon, M.W.; Lee, K.B. Highly Sensitive Chemiresistive H2S Gas Sensor Based on Graphene Decorated with Ag Nanoparticles and Charged Impurities. Sens. Actuators B Chem. 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, C.; Cai, W.; Li, H.; Ji, H.; Kholmanov, I.; Wu, Y.; Piner, R.D.; Ruoff, R.S. Detection of Sulfur Dioxide Gas with Graphene Field Effect Transistor. Appl. Phys. Lett. 2012, 100, 163114. [Google Scholar] [CrossRef] [Green Version]
- Panda, D.; Nandi, A.; Datta, S.K.; Saha, H.; Majumdar, S. Selective Detection of Carbon Monoxide (CO) Gas by Reduced Graphene Oxide (RGO) at Room Temperature. RSC Adv. 2016, 6, 47337–47348. [Google Scholar] [CrossRef]
- Smith, A.D.; Elgammal, K.; Fan, X.; Lemme, M.C.; Delin, A.; Råsander, M.; Bergqvist, L.; Schröder, S.; Fischer, A.C.; Niklaus, F.; et al. Graphene-Based CO2 Sensing and Its Cross-Sensitivity with Humidity. RSC Adv. 2017, 7, 22329–22339. [Google Scholar] [CrossRef] [Green Version]
- Cadore, A.R.; Mania, E.; Alencar, A.B.; Rezende, N.P.; de Oliveira, S.; Watanabe, K.; Taniguchi, T.; Chacham, H.; Campos, L.C.; Lacerda, R.G. Enhancing the Response of NH3 Graphene-Sensors by Using Devices with Different Graphene-Substrate Distances. Sens. Actuators B Chem. 2018, 266, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.M.; Hwang, E.; Kim, D.; Lee, H. Mesoporous Non-Stacked Graphene-Receptor Sensor for Detecting Nerve Agents. Sci. Rep. 2016, 6, 33299. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-Based Gas Sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Minichino, M.; Mattoli, V.; Pucci, A. Chemical and Temperature Sensors Based on Functionalized Reduced Graphene Oxide. Chemosensors 2020, 8, 43. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Deokar, G.; Casanova-Cháfer, J.; Rajput, N.S.; Aubry, C.; Llobet, E.; Jouiad, M.; Costa, P.M.F.J. Wafer-Scale Few-Layer Graphene Growth on Cu/Ni Films for Gas Sensing Applications. Sens. Actuators B Chem. 2020, 305, 127458. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A Review on Chemiresistive Room Temperature Gas Sensors Based on Metal Oxide Nanostructures, Graphene and 2D Transition Metal Dichalcogenides. Microchim. Acta 2018, 185, 1–16. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W.; Cao, G.; Li, X.; Wang, X. A Study of Gas Sensing Behavior of Metal-Graphene Contact with Transfer Length Method. Appl. Phys. Lett. 2016, 108, 221604. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Bu, X.; Liu, W.; Cao, G.; Li, X.; Wang, X. Gas Sensing Performance of Graphene-Metal Contact after Thermal Annealing. Sens. Actuators B Chem. 2019, 282, 408–416. [Google Scholar] [CrossRef]
- Pereira, C.L.; Cadore, A.R.; Rezende, N.P.; Gadelha, A.; Soares, E.A.; Chacham, H.; Campos, L.C.; Lacerda, R.G. Reversible Doping of Graphene Field Effect Transistors by Molecular Hydrogen: The Role of the Metal/Graphene Interface. 2D Mater. 2019, 6, 025037. [Google Scholar] [CrossRef] [Green Version]
- Behi, S.; Bohli, N.; Casanova-Cháfer, J.; Llobet, E.; Abdelghani, A. Metal Oxide Nanoparticle-Decorated Few Layer Graphene Nanoflake Chemoresistors for the Detection of Aromatic Volatile Organic Compounds. Sensors 2020, 20, 3413. [Google Scholar] [CrossRef]
- Dey, A.; Chroneos, A.; Braithwaite, N.S.J.; Gandhiraman, R.P.; Krishnamurthy, S. Plasma Engineering of Graphene. Appl. Phys. Rev. 2016, 3, 021301. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.H.; Park, S.G.; Kwon, J.D.; Lee, Y.J.; Lee, K.H.; Lee, B.H.; et al. Chemical Sensing of 2D Graphene/MoS2 Heterostructure Device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef]
- Zamiri, G.; Haseeb, A.S.M.A. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials 2020, 13, 3311. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, Q.; Zhang, Z.; Dai, J.; Xing, G.; Li, S.; Huang, X.; Huang, W. Metal Halide Perovskites: Stability and Sensing-Ability. J. Mater. Chem. C 2018, 6, 10121–10137. [Google Scholar] [CrossRef]
- Kim, B.J.; Fabbri, E.; Abbott, D.F.; Cheng, X.; Clark, A.H.; Nachtegaal, M.; Borlaf, M.; Castelli, I.E.; Graule, T.; Schmidt, T.J. Functional Role of Fe-Doping in Co-Based Perovskite Oxide Catalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 5231–5240. [Google Scholar] [CrossRef] [Green Version]
- Fergus, J.W. Perovskite Oxides for Semiconductor-Based Gas Sensors. Sens. Actuators B Chem. 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Jellicoe, T.C.; Richter, J.M.; Glass, H.F.J.; Tabachnyk, M.; Brady, R.; Dutton, S.E.; Rao, A.; Friend, R.H.; Credgington, D.; Greenham, N.C.; et al. Synthesis and Optical Properties of Lead-Free Cesium Tin Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2016, 138, 2941–2944. [Google Scholar] [CrossRef] [Green Version]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-Free Halide Double Perovskites via Heterovalent Substitution of Noble Metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef] [Green Version]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An Extended Tolerance Factor Approach for Organic-Inorganic Perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef] [Green Version]
- Saparov, B.; Mitzi, D.B. Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, T.; Wang, Y.; Yang, W.; Lü, X. Pressure Responses of Halide Perovskites with Various Compositions, Dimensionalities, and Morphologies. Matter Radiat. Extrem. 2020, 5, 18201. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.H.; Ma, B. Low Dimensional Metal Halide Perovskites and Hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Baamran, K.S.; Tahir, M. Ni-Embedded TiO2-ZnTiO3 Reducible Perovskite Composite with Synergistic Effect of Metal/Support towards Enhanced H2 Production via Phenol Steam Reforming. Energy Convers. Manag. 2019, 200, 112064. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, Characterization and n-Propanol Sensing Properties of Perovskite-Type ZnSnO3 Nanospheres Based Gas Sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Reyes-Gómez, J.; Flores-Álvarez, J.M.; Guillén-Bonilla, H.; Olvera, M. de la, L.; Rodríguez Betancourtt, V.M.; Verde-Gómez, Y.; Guillén-Cervantes, A.; Santoyo-Salazar, J. Synthesis, Characterization and Sensitivity Tests of Perovskite-Type LaFeO3 Nanoparticles in CO and Propane Atmospheres. Ceram. Int. 2016, 42, 18821–18827. [Google Scholar] [CrossRef]
- Michel, C.R.; Martínez-Preciado, A.H.; López-Mena, E.R.; Elías-Zuñiga, A.; Cayetano-Castro, N.; Ceballos-Sanchez, O. Improvement of the Gas Sensing Response of Nanostructured LaCoO3 by the Addition of Ag Nanoparticles. Sens. Actuators B Chem. 2017, 246, 181–189. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Zhang, J.; Li, K.; Lv, T.; Shen, K.; Zhu, Z.; Liu, Q. Facile Lotus-Leaf-Templated Synthesis and Enhanced Xylene Gas Sensing Properties of Ag-LaFeO3 Nanoparticles. J. Mater. Chem. C 2018, 6, 6138–6145. [Google Scholar] [CrossRef]
- Szafraniak, B.; Fuśnik, Ł.; Xu, J.; Gao, F.; Brudnik, A.; Rydosz, A. Semiconducting Metal Oxides: SrTiO3, BaTiO3 and BaSrTiO3 in Gas-Sensing Applications: A Review. Coatings 2021, 11, 185. [Google Scholar] [CrossRef]
- Xiao, H.; Xue, C.; Song, P.; Li, J.; Wang, Q. Preparation of Porous LaFeO3 Microspheres and Their Gas-Sensing Property. Appl. Surf. Sci. 2015, 337, 65–71. [Google Scholar] [CrossRef]
- Xue, X.Y.; Chen, Y.J.; Wang, Y.G.; Wang, T.H. Synthesis and Ethanol Sensing Properties of ZnSnO3 Nanowires. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, J. Enhanced Ethanol Gas Sensing Performance of ZnO Nanoflowers Decorated with LaMnO3 Perovskite Nanoparticles. Mater. Lett. 2018, 216, 196–198. [Google Scholar] [CrossRef]
- Sharma, N.; Kushwaha, H.S.; Sharma, S.K.; Sachdev, K. Fabrication of LaFeO3 and RGO-LaFeO3 Microspheres Based Gas Sensors for Detection of NO2 and CO. RSC Adv. 2020, 10, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, T.; Wang, Y.; Hahn, Y.-B. SrTiO3/Al2O3-Graphene Electron Transport Layer for Highly Stable and Efficient Composites-Based Perovskite Solar Cells with 20.6% Efficiency. Adv. Energy Mater. 2020, 10, 1903369. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Borland, W.J.; Maria, J.-P. Enhanced Dielectric and Crystalline Properties in Ferroelectric Barium Titanate Thin Films. Adv. Funct. Mater. 2007, 17, 1199–1203. [Google Scholar] [CrossRef]
- Li, W.; Jacobs, R.; Morgan, D. Predicting the Thermodynamic Stability of Perovskite Oxides Using Machine Learning Models. Comput. Mater. Sci. 2018, 150, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Arandiyan, H.; Wang, Y.; Sun, H.; Rezaei, M.; Dai, H. Ordered Meso- and Macroporous Perovskite Oxide Catalysts for Emerging Applications. Chem. Commun. 2018, 54, 6484–6502. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Sackmann, A.; Weimar, U.; Bârsan, N. A Highly Selective Sensor to Acetylene and Ethylene Based on LaFeO3. Sens. Actuators B Chem. 2020, 303, 127204. [Google Scholar] [CrossRef]
- Lin, H.J.; Baltrus, J.P.; Gao, H.; Ding, Y.; Nam, C.Y.; Ohodnicki, P.; Gao, P.X. Perovskite Nanoparticle-Sensitized Ga2O3 Nanorod Arrays for CO Detection at High Temperature. ACS Appl. Mater. Interfaces 2016, 8, 8880–8887. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, H.J.; Gao, H.; Lu, X.; Nam, C.Y.; Gao, P.X. Perovskite-Sensitized β-Ga2O3 nanorod Arrays for Highly Selective and Sensitive NO2detection at High Temperature. J. Mater. Chem. A 2020, 8, 10845–10854. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.; Zhang, P.; Hu, J. High Sensing Properties of 3 Wt % Pd-Doped SmFe1- XMgxO3 Nanocrystalline Powders to Acetone Vapor with Ultralow Concentrations under Light Illumination. ACS Appl. Mater. Interfaces 2018, 10, 15558–15564. [Google Scholar] [CrossRef]
- Queraltó, A.; Graf, D.; Frohnhoven, R.; Fischer, T.; Vanrompay, H.; Bals, S.; Bartasyte, A.; Mathur, S. LaFeO3 Nanofibers for High Detection of Sulfur-Containing Gases. ACS Sustain. Chem. Eng. 2019, 7, 6023–6032. [Google Scholar] [CrossRef]
- Palimar, S.; Kaushik, S.D.; Siruguri, V.; Swain, D.; Viegas, A.E.; Narayana, C.; Sundaram, N.G. Investigation of Ca Substitution on the Gas Sensing Potential of LaFeO3 Nanoparticles towards Low Concentration SO2 Gas. Dalt. Trans. 2016, 45, 13547–13555. [Google Scholar] [CrossRef]
- Cao, E.; Wang, H.; Wang, X.; Yang, Y.; Hao, W.; Sun, L.; Zhang, Y. Enhanced Ethanol Sensing Performance for Chlorine Doped Nanocrystalline LaFeO3 Powders by Citric Sol-Gel Method. Sens. Actuators B Chem. 2017, 251, 885–893. [Google Scholar] [CrossRef]
- Cao, E.; Wu, A.; Wang, H.; Zhang, Y.; Hao, W.; Sun, L. Enhanced Ethanol Sensing Performance of Au and Cl Comodified LaFeO3 Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 1541–1551. [Google Scholar] [CrossRef]
- Yin, Y.; Li, F.; Zhang, N.; Ruan, S.; Zhang, H.; Chen, Y. Improved Gas Sensing Properties of Silver-Functionalized ZnSnO3 Hollow Nanocubes. Inorg. Chem. Front. 2018, 5, 2123–2131. [Google Scholar] [CrossRef]
- Wei, W.; Guo, S.; Chen, C.; Sun, L.; Chen, Y.; Guo, W.; Ruan, S. High Sensitive and Fast Formaldehyde Gas Sensor Based on Ag-Doped LaFeO3 nanofibers. J. Alloys Compd. 2017, 695, 1122–1127. [Google Scholar] [CrossRef]
- Tie, Y.; Ma, S.Y.; Pei, S.T.; Zhang, Q.X.; Zhu, K.M.; Zhang, R.; Xu, X.H.; Han, T.; Liu, W.W. Pr Doped BiFeO3 Hollow Nanofibers via Electrospinning Method as a Formaldehyde Sensor. Sens. Actuators B Chem. 2020, 308, 127689. [Google Scholar] [CrossRef]
- Teresita, V.M.; Manikandan, A.; Josephine, B.A.; Sujatha, S.; Antony, S.A. Electromagnetic Properties and Humidity-Sensing Studies of Magnetically Recoverable LaMgxFe1−xO3−δ Perovskites Nano-Photocatalysts by Sol-Gel Route. J. Supercond. Nov. Magn. 2016, 29, 1691–1701. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, M.; Li, T.; Zhang, Y.; Zou, H. Super-Fast Response Humidity Sensor Based on La0.7Sr0.3MnO3 Nanocrystals Prepared by PVP-Assisted Sol-Gel Method. Sens. Actuators B Chem. 2018, 258, 527–534. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath Analysis by Nanostructured Metal Oxides as Chemo-Resistive Gas Sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, M.; Ma, S.; Wang, P.; Zhang, G.; Chen, W.; Jiao, H.; Liu, L.; Xu, X. Enhanced Acetone Sensor Based on Au Functionalized In-Doped ZnSnO3 Nanofibers Synthesized by Electrospinning Method. J. Colloid Interface Sci. 2019, 543, 285–299. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A. The Rapid Evolution of Highly Efficient Perovskite Solar Cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- García, T.; García-Aboal, R.; Albero, J.; Atienzar, P.; García, H. Vapor-Phase Photocatalytic Overall Water Splitting Using Hybrid Methylammonium Copper and Lead Perovskites. Nanomaterials 2020, 10, 960. [Google Scholar] [CrossRef]
- Peng, Y.; Albero, J.; Álvarez, E.; García, H. Hybrid Benzidinium Lead Iodide Perovskites with a 1D Structure as Photoinduced Electron Transfer Photocatalysts. Sustain. Energy Fuels 2019, 3, 2356–2360. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bo, R.; Barugkin, C.; Zheng, J.; Ma, Q.; Huang, S.; Ho-Baillie, A.W.Y.; Catchpole, K.R.; Tricoli, A. Superior Self-Powered Room-Temperature Chemical Sensing with Light-Activated Inorganic Halides Perovskites. Small 2018, 14, 1702571. [Google Scholar] [CrossRef] [PubMed]
- Kakavelakis, G.; Gagaoudakis, E.; Petridis, K.; Petromichelaki, V.; Binas, V.; Kiriakidis, G.; Kymakis, E. Solution Processed CH3NH3PbI3-XClx Perovskite Based Self-Powered Ozone Sensing Element Operated at Room Temperature. ACS Sens. 2018, 3, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Zhang, Y.; Zhong, H.; Wang, X.; Xiao, H.; Chen, Y.; Li, X. High-Performance Room-Temperature NO2 Sensors Based on CH3NH3PbBr3 Semiconducting Films: Effect of Surface Capping by Alkyl Chain on Sensor Performance. J. Phys. Chem. Solids 2019, 129, 270–276. [Google Scholar] [CrossRef]
- Bao, C.; Yang, J.; Zhu, W.; Zhou, X.; Gao, H.; Li, F.; Fu, G.; Yu, T.; Zou, Z. A Resistance Change Effect in Perovskite CH3NH3PbI3 Films Induced by Ammonia. Chem. Commun. 2015, 51, 15426–15429. [Google Scholar] [CrossRef]
- Gagaoudakis, E.; Panagiotopoulos, A.; Maksudov, T.; Moschogiannaki, M.; Katerinopoulou, D.; Kakavelakis, G.; Kiriakidis, G.; Binas, V.; Kymakis, E.; Petridis, K. Self-Powered, Flexible and Room Temperature Operated Solution Processed Hybrid Metal Halide p-Type Sensing Element for Efficient Hydrogen Detection. J. Phys. Mater. 2020, 3, 014010. [Google Scholar] [CrossRef]
- Brintakis, K.; Gagaoudakis, E.; Kostopoulou, A.; Faka, V.; Argyrou, A.; Binas, V.; Kiriakidis, G.; Stratakis, E. Ligand-Free All-Inorganic Metal Halide Nanocubes for Fast, Ultra-Sensitive and Self-Powered Ozone Sensors. Nanoscale Adv. 2019, 1, 2699–2706. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Yuan, W.; Qian, L.; Chen, S.; Shi, G. High-Performance Gas Sensors Based on a Thiocyanate Ion-Doped Organometal Halide Perovskite. Phys. Chem. Chem. Phys. 2017, 19, 12876–12881. [Google Scholar] [CrossRef]
- Nur’aini, A.; Oh, I. Volatile Organic Compound Gas Sensors Based on Methylammonium Lead Iodide Perovskite Operating at Room Temperature. RSC Adv. 2020, 10, 12982–12987. [Google Scholar] [CrossRef] [Green Version]
- Jiao, W.; He, J.; Zhang, L. Synthesis and High Ammonia Gas Sensitivity of (CH3NH3)PbBr3-XIx Perovskite Thin Film at Room Temperature. Sens. Actuators B Chem. 2020, 309, 127786. [Google Scholar] [CrossRef]
- Lin, F.; Li, F.; Lai, Z.; Cai, Z.; Wang, Y.; Wolfbeis, O.S.; Chen, X. MnII-Doped Cesium Lead Chloride Perovskite Nanocrystals: Demonstration of Oxygen Sensing Capability Based on Luminescent Dopants and Host-Dopant Energy Transfer. ACS Appl. Mater. Interfaces 2018, 10, 23335–23343. [Google Scholar] [CrossRef]
- García-Aboal, R.; García, H.; Remiro-Buenamañana, S.; Atienzar, P. Expanding the Photoresponse of Multidimensional Hybrid Lead Bromide Perovskites into the Visible Region by Incorporation of Subphthalocyanine. Dalt. Trans. 2021, 50, 6100–6108. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Kelly, T.L. In Situ Studies of the Degradation Mechanisms of Perovskite Solar Cells. EcoMat 2020, 2, e12025. [Google Scholar] [CrossRef]
- Ava, T.T.; Al Mamun, A.; Marsillac, S.; Namkoong, G. A Review: Thermal Stability of Methylammonium Lead Halide Based Perovskite Solar Cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced Thermal Conductivity of Epoxy-Graphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef]
- Ghosh, A.; Rao, K.V.; George, S.J.; Rao, C.N.R. Noncovalent Functionalization, Exfoliation, and Solubilization of Graphene in Water by Employing a Fluorescent Coronene Carboxylate. Chem. A Eur. J. 2010, 16, 2700–2704. [Google Scholar] [CrossRef]
- Khan, R.; Nishina, Y. Covalent Functionalization of Carbon Materials with Redox-Active Organic Molecules for Energy Storage. Nanoscale 2021, 13, 36–50. [Google Scholar] [CrossRef]
- Park, J.; Yan, M. Covalent Functionalization of Graphene with Reactive Intermediates. Acc. Chem. Res. 2013, 46, 181–189. [Google Scholar] [CrossRef]
- Bottari, G.; Ángeles Herranz, M.; Wibmer, L.; Volland, M.; Rodríguez-Pérez, L.; Guldi, D.M.; Hirsch, A.; Martín, N.; D’Souza, F.; Torres, T. Chemical Functionalization and Characterization of Graphene-Based Materials. Chem. Soc. Rev. 2017, 46, 4464–4500. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Liu, D.; Xiao, Y.; Dai, L. Functionalization of Graphene Materials by Heteroatom-Doping for Energy Conversion and Storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 121–132. [Google Scholar] [CrossRef]
- Ullah, S.; Shi, Q.; Zhou, J.; Yang, X.; Ta, H.Q.; Hasan, M.; Ahmad, N.M.; Fu, L.; Bachmatiuk, A.; Rümmeli, M.H. Advances and Trends in Chemically Doped Graphene. Adv. Mater. Interfaces 2020, 7, 2000999. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of Functionalization, Structure and Properties of Graphene/Polymer Composite Fibers. Compos. Part A Appl. Sci. Manuf. 2016, 87, 29–45. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Antidormi, A.; Roche, S.; Colombo, L. Impact of Oxidation Morphology on Reduced Graphene Oxides upon Thermal Annealing. J. Phys Mater. 2020, 3, 15011. [Google Scholar] [CrossRef] [Green Version]
- Alsharaeh, E.; Ahmed, F.; Aldawsari, Y.; Khasawneh, M.; Abuhimd, H.; Alshahrani, M. Novel Synthesis of Holey Reduced Graphene Oxide (HRGO) by Microwave Irradiation Method for Anode in Lithium-Ion Batteries. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Qi, X.; Hao, G.; Ren, L.; Zhong, J. In-Situ Investigation of Graphene Oxide under UV Irradiation: Evolution of Work Function. AIP Adv. 2015, 5, 67154. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Sato, S. Photochemical Reduction of Graphene Oxide (GO) by Femtosecond Laser Irradiation. In Laser-based Micro- and Nanoprocessing X; SPIE: Bellingham, WA, USA, 2016; Volume 9736, p. 973617. [Google Scholar]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (RGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [Green Version]
- Some, S.; Kim, Y.; Yoon, Y.; Yoo, H.; Lee, S.; Park, Y.; Lee, H. High-Quality Reduced Graphene Oxide by a Dual-Function Chemical Reduction and Healing Process. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.; Zhu, J.; Chen, J.; Han, Y.; Yang, S.; Ding, B.; Zhang, X. Electrochemical Reduction of Graphene Oxide and Its Electrochemical Capacitive Performance. J. Solid State Electrochem. 2013, 17, 2857–2863. [Google Scholar] [CrossRef]
- Chin, S.J.; Doherty, M.; Vempati, S.; Dawson, P.; Byrne, C.; Meenan, B.J.; Guerra, V.; McNally, T. Solvothermal Synthesis of Graphene Oxide and Its Composites with Poly(ϵ-Caprolactone). Nanoscale 2019, 11, 18672–18682. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [Green Version]
- Song, N.J.; Chen, C.M.; Lu, C.; Liu, Z.; Kong, Q.Q.; Cai, R. Thermally Reduced Graphene Oxide Films as Flexible Lateral Heat Spreaders. J. Mater. Chem. A 2014, 2, 16563–16568. [Google Scholar] [CrossRef]
- Feng, M.; Feng, H. Effect of Reducing Agent on the Chemical Reduction of Graphene Oxides. J. Nanosci. Nanotechnol. 2013, 13, 937–941. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Y.; Huang, K. Advances in Microwave-Assisted Production of Reduced Graphene Oxide. Front. Chem. 2019, 7, 355. [Google Scholar] [CrossRef] [Green Version]
- Petridis, C.; Kakavelakis, G.; Kymakis, E. Renaissance of Graphene-Related Materials in Photovoltaics Due to the Emergence of Metal Halide Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 1030–1061. [Google Scholar] [CrossRef]
- Casanova-Chafer, J.; Garcia-Aboal, R.; Atienzar, P.; Llobet, E. The Role of Anions and Cations in the Gas Sensing Mechanisms of Graphene Decorated with Lead Halide Perovskite Nanocrystals. Chem. Commun. 2020, 56, 8956–8959. [Google Scholar] [CrossRef]
- Wen, X.; Wu, J.; Gao, D.; Lin, C. Interfacial Engineering with Amino-Functionalized Graphene for Efficient Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 13482–13487. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zuo, W.W.; Wang, Q.; Wang, K.L.; Zhuo, M.P.; Köbler, H.; Halbig, C.E.; Eigler, S.; Yang, Y.G.; Gao, X.Y.; et al. Ultrathin Nanosheets of Oxo-Functionalized Graphene Inhibit the Ion Migration in Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902653. [Google Scholar] [CrossRef] [Green Version]
- Butz, B.; Dolle, C.; Halbig, C.E.; Spiecker, E.; Eigler, S. Highly Intact and Pure Oxo-Functionalized Graphene: Synthesis and Electron-Beam-Induced Reduction. Angew. Chem. Int. Ed. 2016, 55, 15771–15774. [Google Scholar] [CrossRef]
- Wang, Z.; Eigler, S.; Ishii, Y.; Hu, Y.; Papp, C.; Lytken, O.; Steinrück, H.P.; Halik, M. A Facile Approach to Synthesize an Oxo-Functionalized Graphene/Polymer Composite for Low-Voltage Operating Memory Devices. J. Mater. Chem. C 2015, 3, 8595–8604. [Google Scholar] [CrossRef] [Green Version]
- Enhessari, M.; Salehabadi, A. Perovskites-Based Nanomaterials for Chemical Sensors. In Progresses in Chemical Sensor; InTech: London, UK, 2016. [Google Scholar]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Dubale, A.A.; Hwang, B.J. Organometal Halide Perovskite Solar Cells: Degradation and Stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; Van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [Green Version]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; Van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Casanova-Cháfer, J.; García-Aboal, R.; Atienzar, P.; Llobet, E. Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature. Sensors 2019, 19, 4563. [Google Scholar] [CrossRef] [Green Version]
- Bouclé, J.; Herlin-Boime, N. The Benefits of Graphene for Hybrid Perovskite Solar Cells. Synth. Met. 2016, 222, 3–16. [Google Scholar] [CrossRef]
- O’Keeffe, P.; Catone, D.; Paladini, A.; Toschi, F.; Turchini, S.; Avaldi, L.; Martelli, F.; Agresti, A.; Pescetelli, S.; Del Rio Castillo, A.E.; et al. Graphene-Induced Improvements of Perovskite Solar Cell Stability: Effects on Hot-Carriers. Nano Lett. 2019, 19, 684–691. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ottesen, M.; Wiedmann, S.; Wharmby, M.; Küchler, R.; Yuan, H.; Debroye, E.; Steele, J.A.; Martens, J.; Hussey, N.E.; et al. Tracking Structural Phase Transitions in Lead-Halide Perovskites by Means of Thermal Expansion. Adv. Mater. 2019, 31, 1900521. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Chen, Y.; Xiao, Y.Y.; Sun, J.; Zhang, X.; Han, C.B.; Gao, H.; Zhang, Y.; Yan, H. Effect of Temperature on the Performance of Perovskite Solar Cells. J. Mater. Sci. Mater. Electron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Ricciardella, F.; Vollebregt, S.; Polichetti, T.; Miscuglio, M.; Alfano, B.; Miglietta, M.L.; Massera, E.; Di Francia, G.; Sarro, P.M. Effects of Graphene Defects on Gas Sensing Properties towards NO2 Detection. Nanoscale 2017, 9, 6085–6093. [Google Scholar] [CrossRef] [Green Version]
- Garg, R.; Dutta, N.; Choudhury, N. Work Function Engineering of Graphene. Nanomaterials 2014, 4, 267–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Lu, Y.; Xu, W.; Mu, H.; Chen, C.; Qiao, H.; Song, J.; Li, S.; Sun, B.; et al. Hybrid Graphene-Perovskite Phototransistors with Ultrahigh Responsivity and Gain. Adv. Opt. Mater. 2015, 3, 1389–1396. [Google Scholar] [CrossRef]
- Vickers, E.T.; Graham, T.A.; Chowdhury, A.H.; Bahrami, B.; Dreskin, B.W.; Lindley, S.; Naghadeh, S.B.; Qiao, Q.; Zhang, J.Z. Improving Charge Carrier Delocalization in Perovskite Quantum Dots by Surface Passivation with Conductive Aromatic Ligands. ACS Energy Lett. 2018, 3, 2931–2939. [Google Scholar] [CrossRef]
- Chen, J.; Messing, M.E.; Zheng, K.; Pullerits, T. Cation-Dependent Hot Carrier Cooling in Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2019, 141, 3532–3540. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All Inorganic Halide Perovskites Nanosystem: Synthesis, Structural Features, Optical Properties and Optoelectronic Applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent Advances in Graphene-Based Humidity Sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova-Cháfer, J.; Navarrete, E.; Noirfalise, X.; Umek, P.; Bittencourt, C.; Llobet, E. Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors 2018, 19, 113. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.H.; Adjokatse, S.; Wei, H.; Yang, J.; Blake, G.R.; Huang, J.; Even, J.; Loi, M.A. Ultrahigh Sensitivity of Methylammonium Lead Tribromide Perovskite Single Crystals to Environmental Gases. Sci. Adv. 2016, 2, e1600534. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Chafer, J.; Garcia-Aboal, R.; Atienzar, P.; Bittencourt, C.; Llobet, E. Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept. Chemosensors 2021, 9, 215. https://doi.org/10.3390/chemosensors9080215
Casanova-Chafer J, Garcia-Aboal R, Atienzar P, Bittencourt C, Llobet E. Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept. Chemosensors. 2021; 9(8):215. https://doi.org/10.3390/chemosensors9080215
Chicago/Turabian StyleCasanova-Chafer, Juan, Rocio Garcia-Aboal, Pedro Atienzar, Carla Bittencourt, and Eduard Llobet. 2021. "Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept" Chemosensors 9, no. 8: 215. https://doi.org/10.3390/chemosensors9080215
APA StyleCasanova-Chafer, J., Garcia-Aboal, R., Atienzar, P., Bittencourt, C., & Llobet, E. (2021). Perovskite@Graphene Nanohybrids for Breath Analysis: A Proof-of-Concept. Chemosensors, 9(8), 215. https://doi.org/10.3390/chemosensors9080215







