Bi2S3/rGO Composite Based Electrochemical Sensor for Ascorbic Acid Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Synthesis of Bi2S3/rGO Nanocomposites
2.4. Sensor Fabrication
3. Results
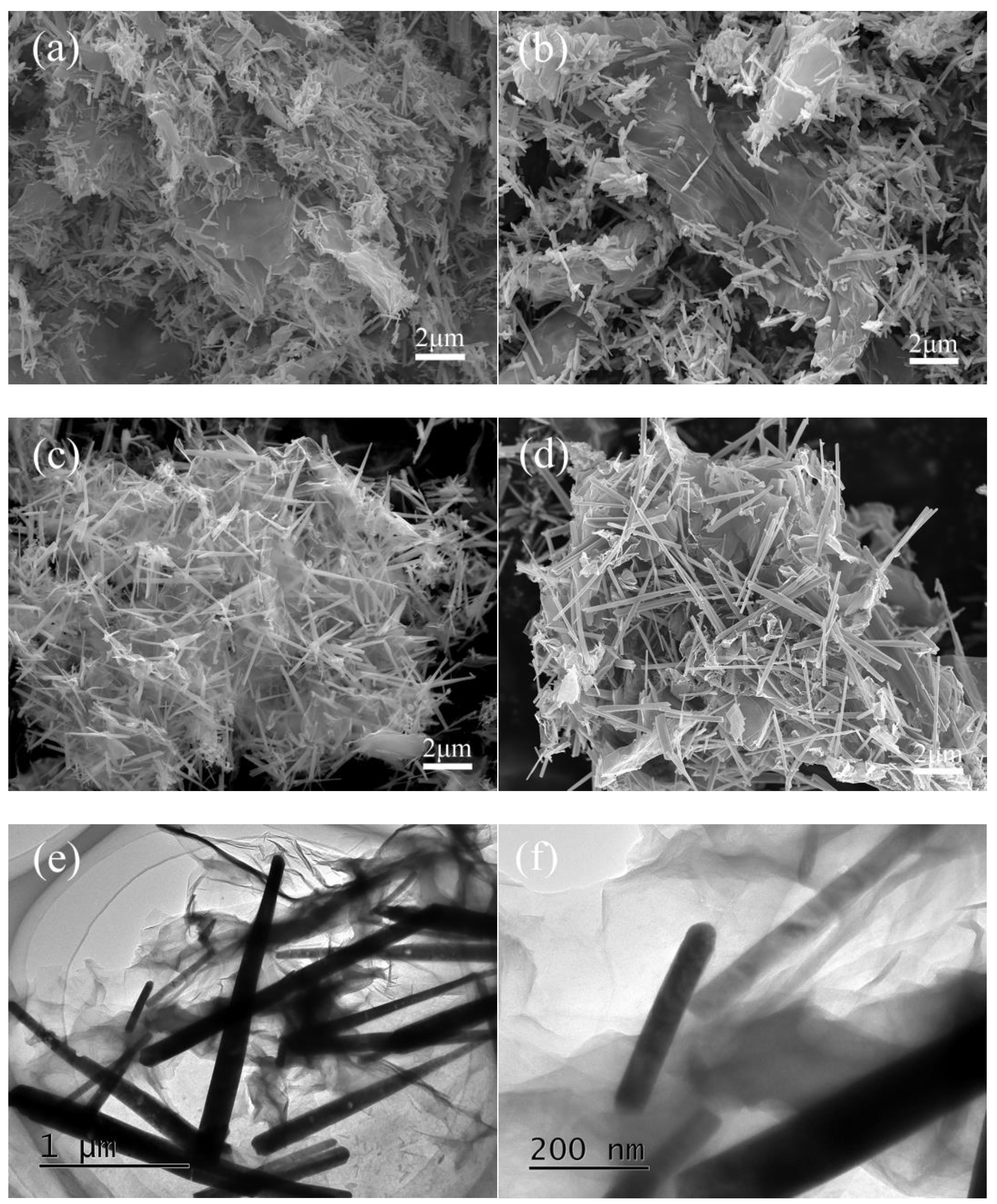
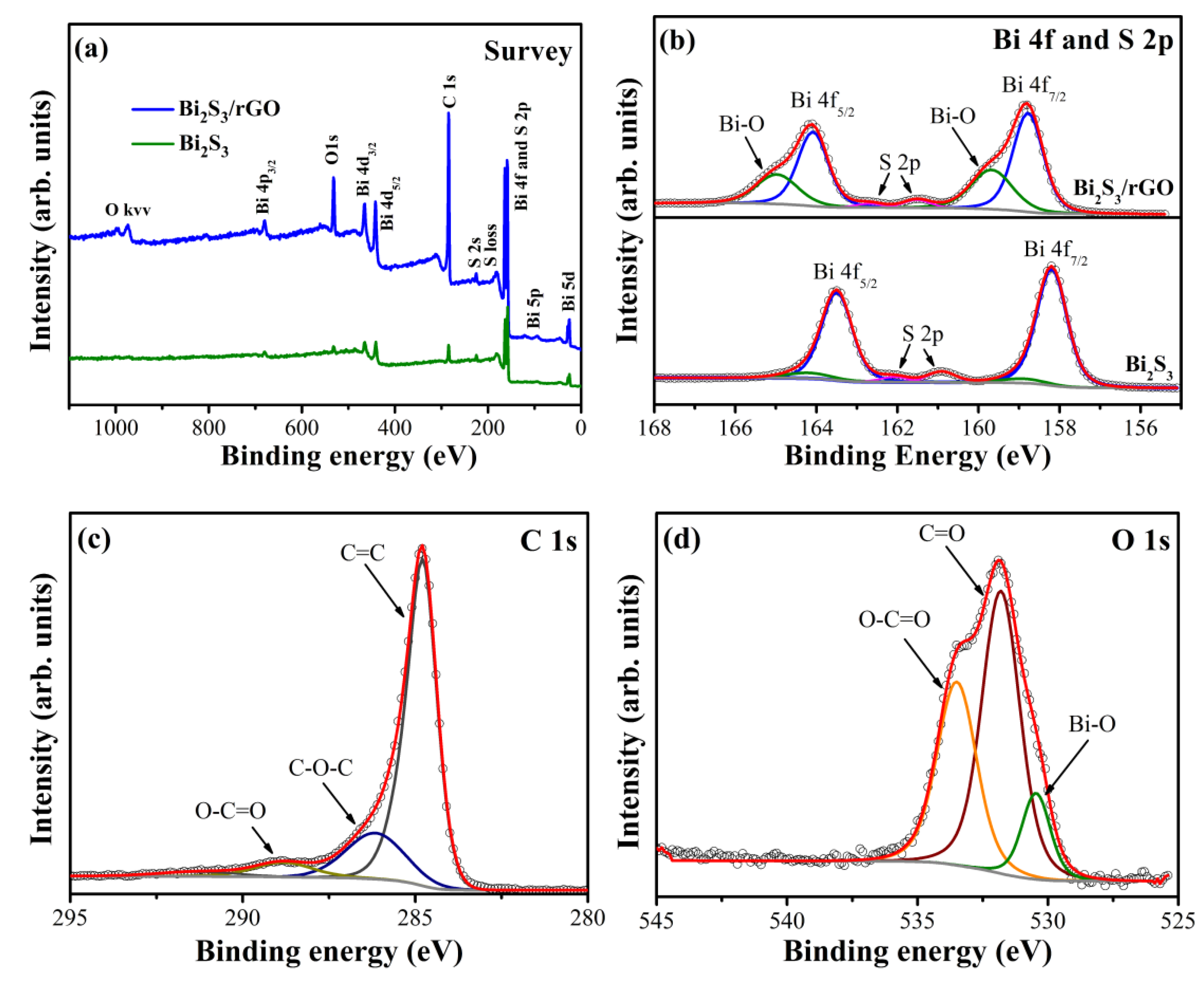
3.1. Characterization of Bi2S3/rGO
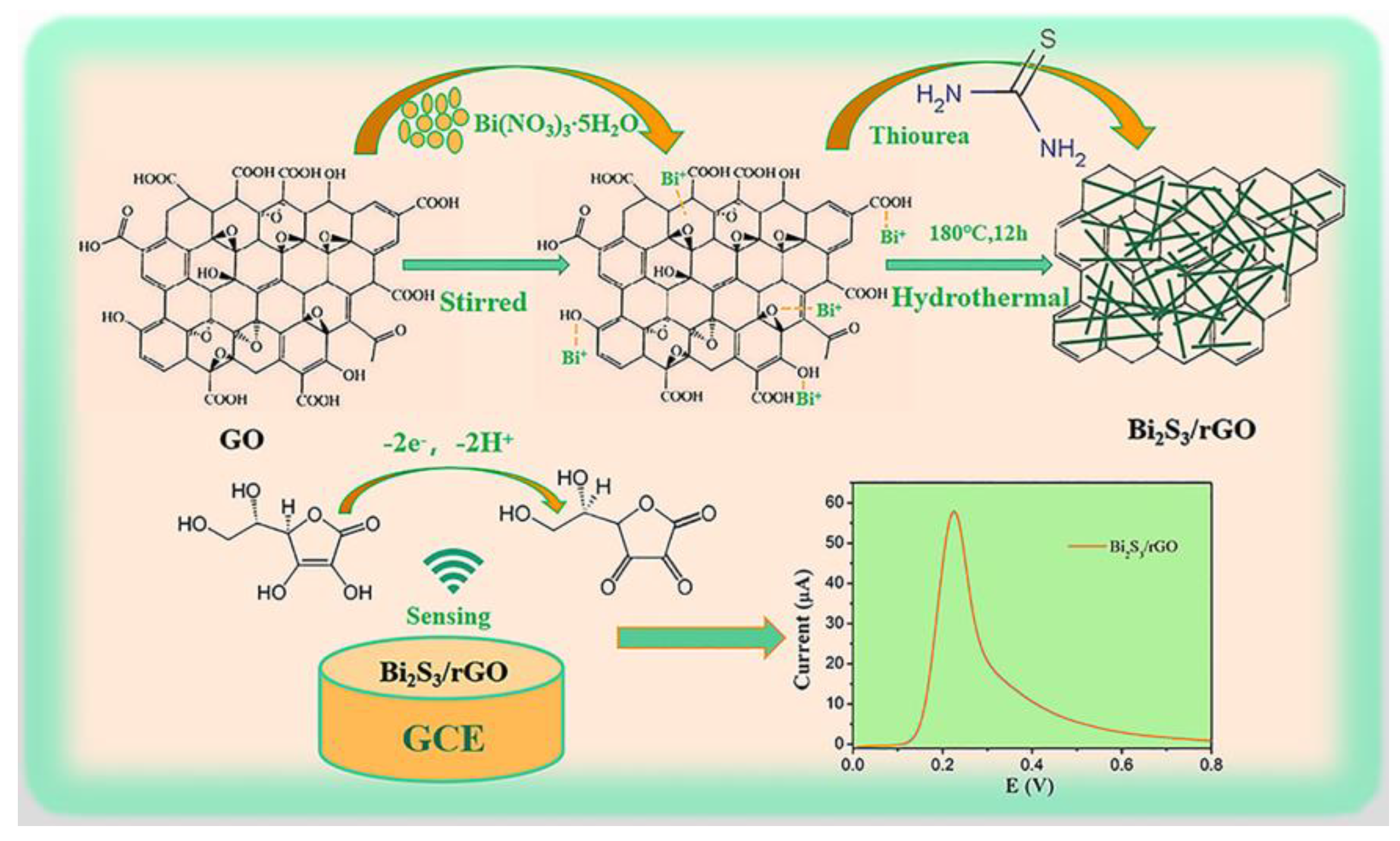
3.2. Formation Mechanism of Bi2S3/rGO
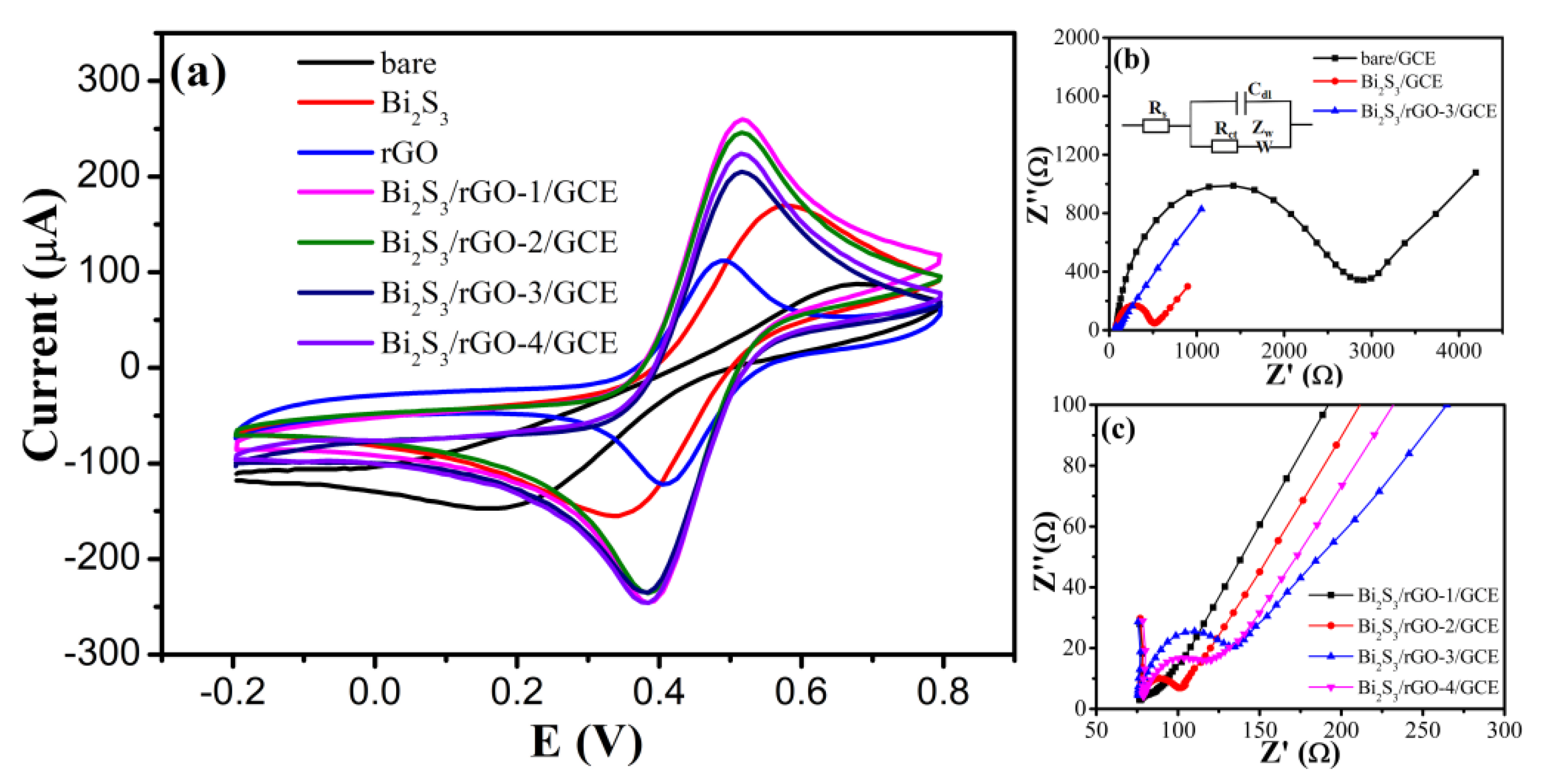
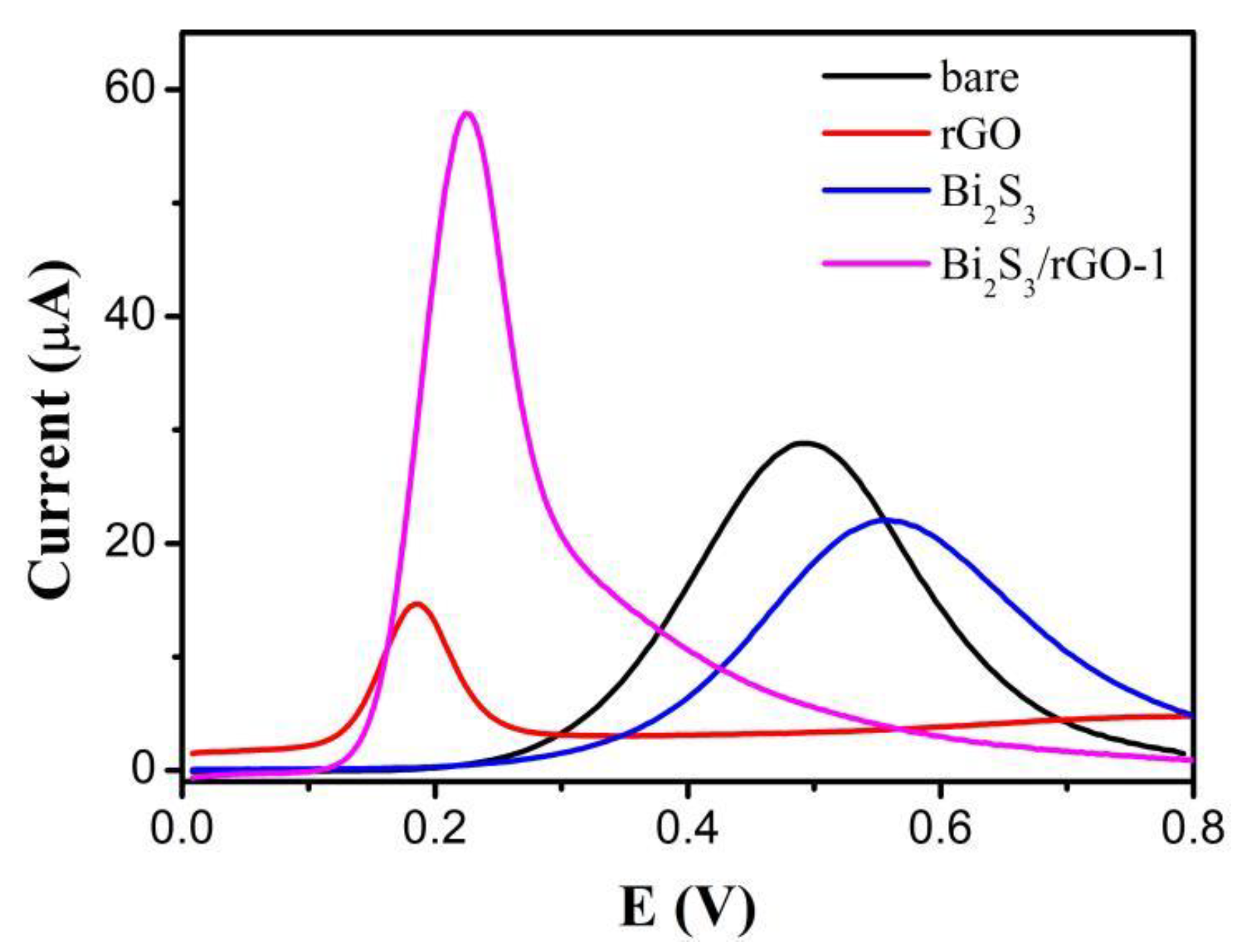
3.3. Bi2S3/rGO Electrochemical Evaluation
3.4. Optimization of Detection Conditions
3.4.1. Effect of Solution pH
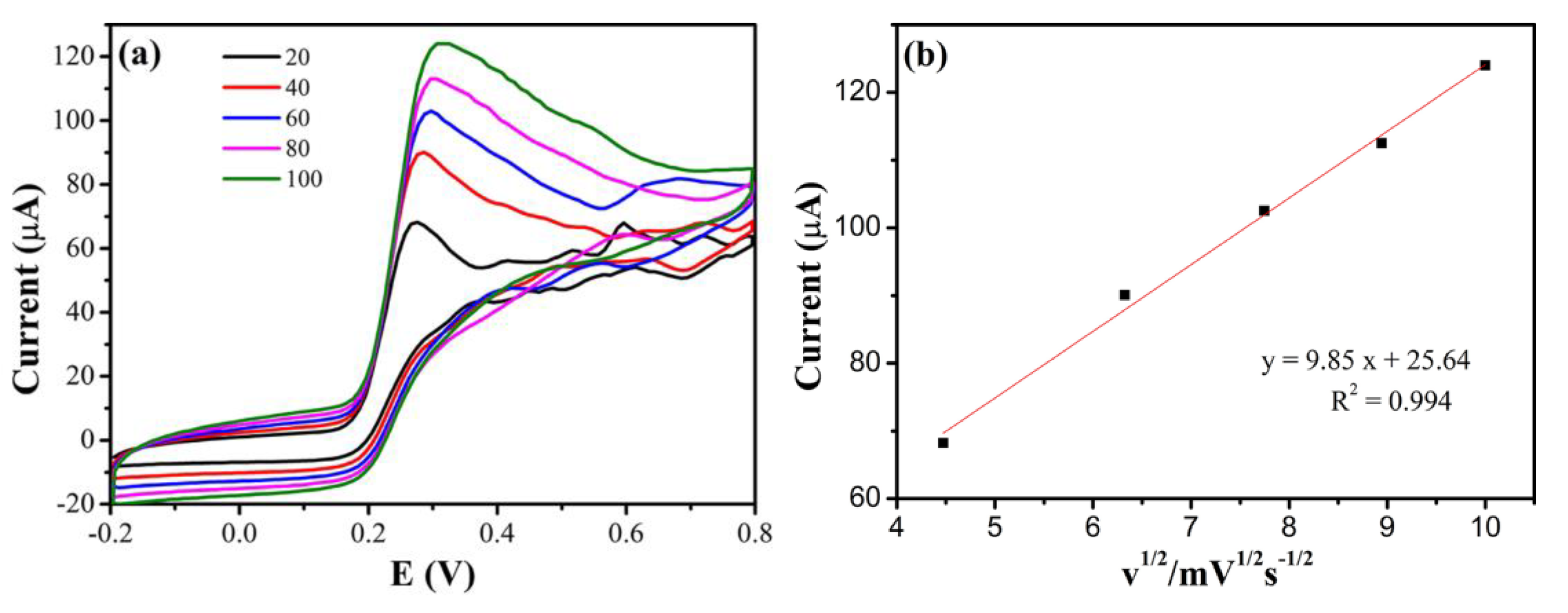
3.4.2. Effect of Scan Rate
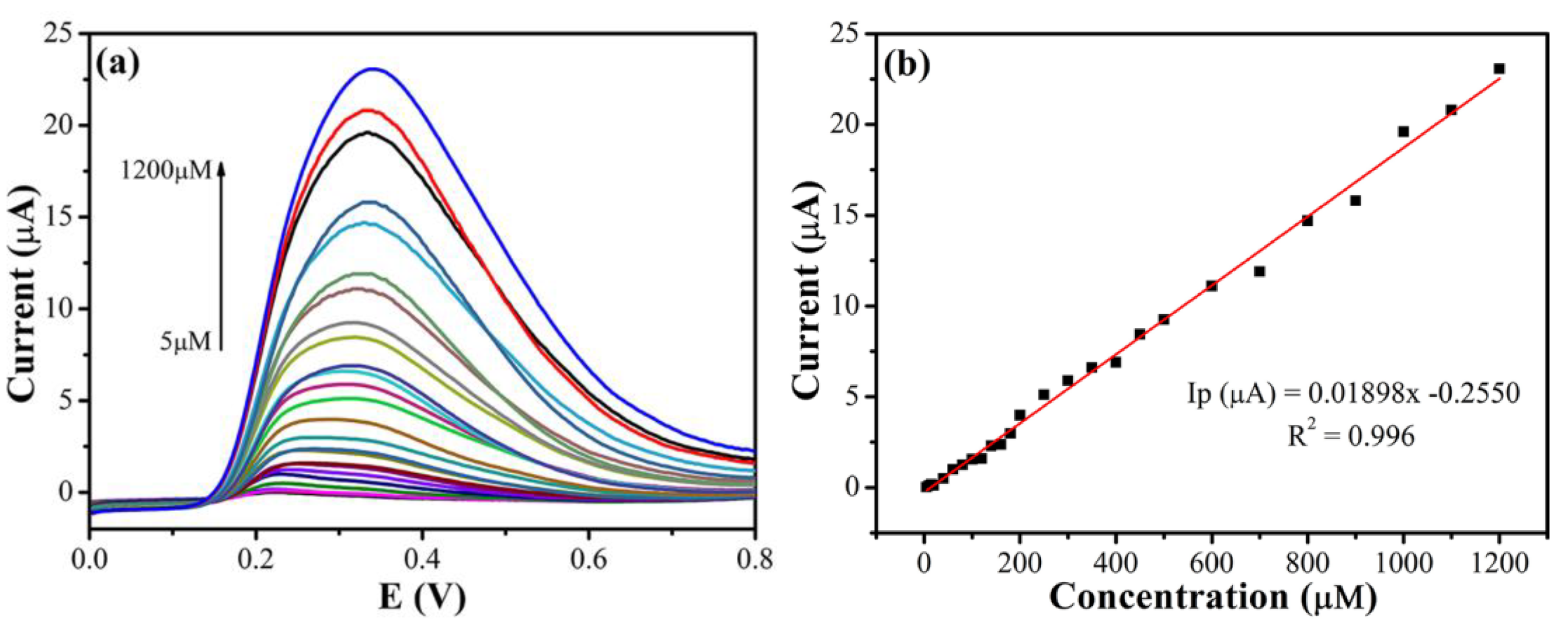
3.5. Analytical Sensing Performance
3.6. Reproducibility and Stability
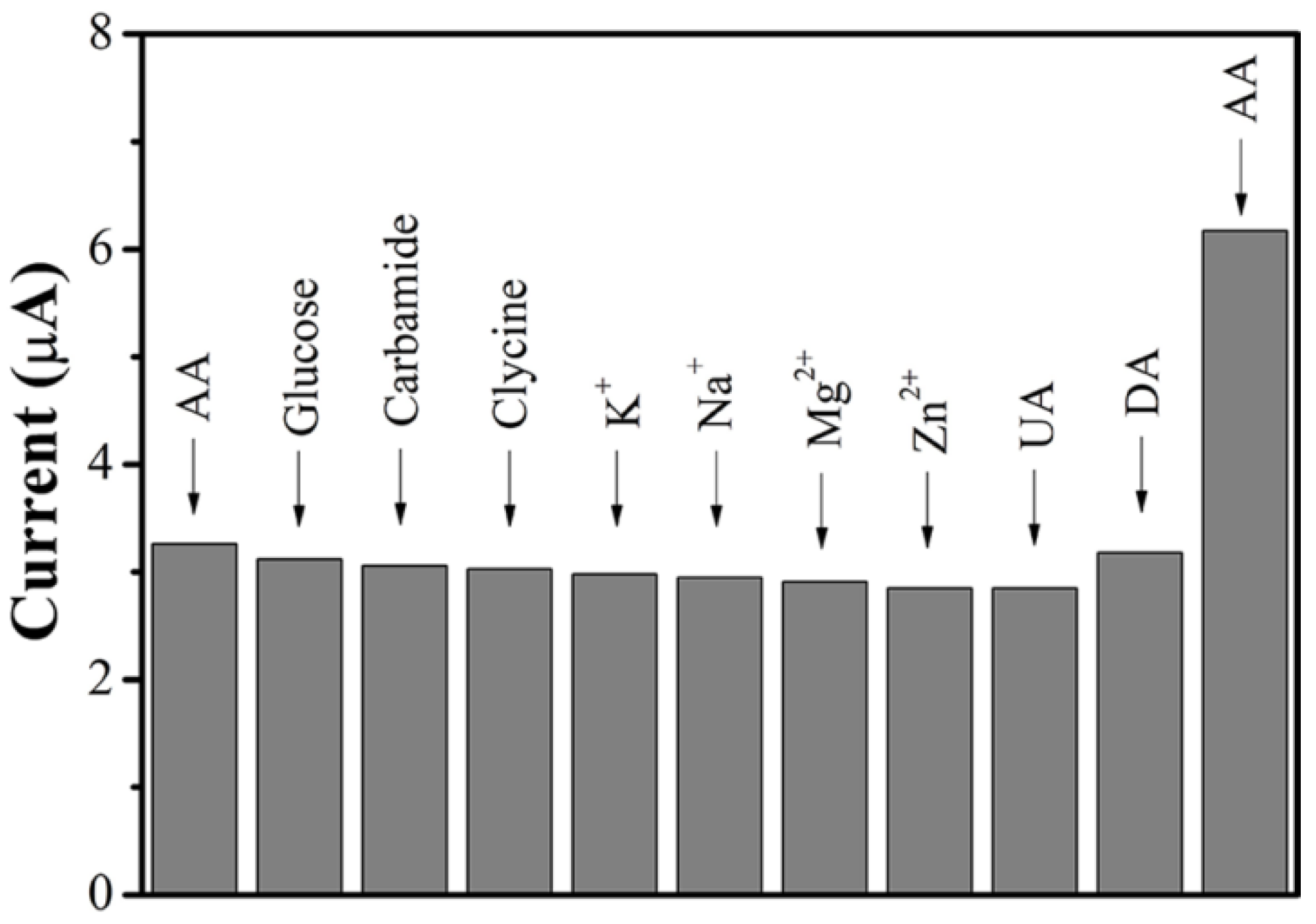
3.7. Interference Effect
3.8. AA Analytical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, H.J.; Yang, D.Q.; Tu, Y.F.; Yan, J.L. Turn-on fluorescence detection of ascorbic acid with gold nanolcusters. Talanta 2017, 165, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ling, J.; Zhang, X.Q.; Zhang, L.Y.; Cao, Q.E.; Ding, Z.T. A rapid, sensitive and selective colorimetric method for detection of ascorbic acid. Sens. Actuat B Chem. 2015, 221, 708–716. [Google Scholar] [CrossRef]
- Granero, A.M.; Pierini, G.D.; Robledo, S.N.; Di Nezio, M.S.; Fernandez, H.; Zon, M.A. Simultaneous determination of ascorbic and uric acids and dopamine in human serum samples using three-way calibration with data from square wave voltammetry. Microchem. J. 2016, 129, 205–212. [Google Scholar] [CrossRef]
- Uzun, D.; Gunduzalp, A.B.; Hasdemir, E. Selective determination of dopamine in the presence of uric acid and ascorbic acid by N, N′-bis(indole-3-carboxaldimine)-1,2-diaminocyclohexane thin film modified glassy carbon electrode by differential pulse voltammetry. J. Electroanal Chem. 2015, 747, 68–76. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Kumar, A.S. Reductive cleavage of methyl orange under formation of a redox-active hydroquinone/polyaniline nanocomposite on an electrode modified with MWCNTs, and its application to flow injection analysis of ascorbic acid at low potential and neutral pH value. Microchim. Acta 2017, 184, 3255–3264. [Google Scholar] [CrossRef]
- Sena, M.M.; Fernandes, J.C.B.; Rover, L.; Poppi, R.J.; Kubota, L.T. Application of two- and three-way chemometric methods in the study of acetylsalicylic acid and ascorbic acid mixtures using ultraviolet spectrophotometry. Anal. Chim. Acta 2000, 409, 159–170. [Google Scholar] [CrossRef]
- Hossu, A.M.; Cristiana, R.; Ioniţă, I.; Magearu, V. Determination of the vitamin D in pharmaceuticals by high performance liquid chromatography. Rev. Chim. Buchar. Orig. Ed. 2004, 55, 788–790. [Google Scholar]
- Zhang, H.; Huang, F.; Xu, S.; Xia, Y.; Huang, W.; Li, Z. Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid. Electrochem. Commun. 2013, 30, 46–50. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, X.; Huo, Z.H.; He, X.L.; Liang, Y.; Xu, M.T. Electrochemical detection of dopamine in the presence of ascorbic acid using PVP/graphene modified electrodes. Talanta 2012, 97, 557–562. [Google Scholar] [CrossRef]
- Li, L.; Zhang, P.; Li, Z.; Li, D.; Han, B.; Tu, L.; Li, B.; Wang, Y.; Ren, L.; Yang, P.; et al. CuS/Prussian blue core-shell nanohybrid as an electrochemical sensor for ascorbic acid detection. Nanotechnology 2019, 30, 325501. [Google Scholar] [CrossRef]
- Li, F.; Tang, C.; Liu, S.; Ma, G. Development of an electrochemical ascorbic acid sensor based on the incorporation of a ferricyanide mediator with a polyelectrolyte–calcium carbonate microsphere. Electrochim. Acta 2010, 55, 838–843. [Google Scholar] [CrossRef]
- Sivanesan, A.; Kannan, P.; Abraham John, S. Electrocatalytic oxidation of ascorbic acid using a single layer of gold nanoparticles immobilized on 1,6-hexanedithiol modified gold electrode. Electrochim. Acta 2007, 52, 8118–8124. [Google Scholar] [CrossRef]
- Qian, L.; Gao, Q.; Song, Y.; Li, Z.; Yang, X. Layer-by-layer assembled multilayer films of redox polymers for electrocatalytic oxidation of ascorbic acid. Sens. Actuators B Chem. 2005, 107, 303–310. [Google Scholar] [CrossRef]
- Kul, D.; Ghica, M.E.; Pauliukaite, R.; Brett, C.M. A novel amperometric sensor for ascorbic acid based on poly (Nile blue A) and functionalised multi-walled carbon nanotube modified electrodes. Talanta 2013, 111, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Feng, Y.; Yang, L.; Du, Z. Electrochemical behavior of graphene doped carbon paste electrode and its application for sensitive determination of ascorbic acid. Sens. Actuators B Chem. 2011, 157, 110–114. [Google Scholar] [CrossRef]
- Gao, J.; He, P.; Yang, T.T.; Zhou, L.H.; Wang, X.J.; Chen, S.X.; Lei, H.; Zhang, H.; Jia, B.; Liu, J.F. Electrodeposited NiO/graphene oxide nanocomposite: An enhanced voltammetric sensing platform for highly sensitive detection of uric acid, dopamine and ascorbic acid. J. Electroanal Chem. 2019, 852. [Google Scholar] [CrossRef]
- Konstantatos, G.; Levina, L.; Tang, J.; Sargent, E.H. Sensitive Solution-Processed Bi2S3 Nanocrystalline Photodetectors. Nano Lett. 2008, 8, 4002–4006. [Google Scholar] [CrossRef]
- Kumar, B.G.; Srinivas, B.; Muralidharan, K. Photo-responsive Bi2S3 nanoflakes: Synthesis and device fabrication at ambient conditions. Mater. Res. Bull. 2017, 89, 108–115. [Google Scholar] [CrossRef]
- Yang, X.Y.; Tian, S.S.; Li, R.; Wang, W.; Zhou, S.M. Use of single-crystalline Bi2S3 nanowires as room temperature ethanol sensor synthesized by hydrothermal approach. Sens. Actuat B-Chem. 2017, 241, 210–216. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Mitra, K.; Sarkar, A.; Saha, N.; Ghosh, A.B.; Adhikary, B. Single source precursor approach to the synthesis of Bi2S3 nanoparticles: A new amperometric hydrogen peroxide biosensor. Sens. Actuators B Chem. 2014, 192, 578–585. [Google Scholar] [CrossRef]
- Bernechea, M.; Miller, N.C.; Xercavins, G.; So, D.; Stavrinadis, A.; Konstantatos, G. Solution-processed solar cells based on environmentally friendly AgBiS2 nanocrystals. Nat. Photonics 2016, 10, 521. [Google Scholar] [CrossRef]
- Gao, F.; Song, J.; Zhang, B.; Tanaka, H.; Gao, F.; Qiu, W.W.; Wang, Q.X. Synthesis of core-shell structured Au@Bi2S3 nanorod and its application as DNA immobilization matrix for electrochemical biosensor construction. Chin. Chem. Lett. 2020, 31, 181–184. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.R.; Yue, H.Y.; Song, S.S.; Huang, S.; Gao, X.; Chen, H.T.; Wu, P.F.; Zhang, T.; Wang, Z.Z. Simultaneous electrochemical determination of dopamine and uric acid based on MoS2 nanoflowers-graphene/ITO electrode. Microchem. J. 2020, 154. [Google Scholar]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. Morphology-controlled synthesis of Bi2S3 nanorods-reduced graphene oxide composites with high-performance for electrochemical detection of dopamine. Sens. Actuators B Chem. 2018, 257, 936–943. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Park, S.K.; Woo, S.; Lee, S.; Seong, C.Y.; Piao, Y. Design and tailoring of three-dimensional graphene-Vulcan carbon-Bi2S3 ternary nanostructures for high-performance lithium-ion-battery anodes. Rsc. Adv. 2015, 5, 52687–52694. [Google Scholar] [CrossRef]
- Lambert, T.N.; Luhrs, C.C.; Chavez, C.A.; Wakeland, S.; Brumbach, M.T.; Alam, T.M. Graphite oxide as a precursor for the synthesis of disordered graphenes using the aerosol-through-plasma method. Carbon 2010, 48, 4081–4089. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.Y.; Yan, X.Y.; Gu, Y.; Liu, W.L.; Tang, L.; Zheng, B.; Li, Y.R.; Chen, R.X.; Zhang, Z.Q. Tremella-like graphene-Au composites used for amperometric determination of dopamine. Analyst 2015, 140, 1913–1920. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Tian, Y.F.; Zhao, W.; Liu, X.Q.; Yang, J.B. A facile way to fabricate graphene sheets on TiO2 nanotube arrays for dye-sensitized solar cell applications. J. Mater. Sci. 2014, 49, 7991–7999. [Google Scholar] [CrossRef]
- Heshmatynezhad, L.; Jamali-Sheini, F.; Monshi, A. UV-assisted sonochemical synthesis and optoelectrical properties of Bi2S3/rGO nanocomposites. Ceram. Int. 2019, 45, 13923–13933. [Google Scholar] [CrossRef]
- Deshpande, M.P.; Sakariya, P.N.; Bhatt, S.V.; Garg, N.; Patel, K.; Chaki, S.H. Characterization of Bi2S3 nanorods prepared at room temperature. Mat. Sci. Semicon Proc. 2014, 21, 180–185. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gote, G.H.; Bhopale, S.R.; More, M.A.; Late, D.J. Realization of Efficient Field Emitter Based on Reduced Graphene Oxide-Bi2S3 Heterostructures. Phys. Status Solidi A 2019, 216, 1900121. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Li, Q.D.; Wang, X.P.; Yang, W.; Zhou, L.; Mai, L.Q. Heterostructured Bi2S3-Bi2O3 Nanosheets with a Built-In Electric Field for Improved Sodium Storage. Acs Appl. Mater. Inter. 2018, 10, 7201–7207. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Karikalan, N.; Chen, S.-M.; Sundaresan, P.; Karthik, R. Synergistic activity of single crystalline bismuth sulfide and sulfur doped graphene towards the electrocatalysis of tryptophan. J. Catal. 2018, 367, 252–263. [Google Scholar] [CrossRef]
- Wang, X.T.; Lv, R.; Wang, K. Synthesis of ZnO@ZnS-Bi2S3 core-shell nanorod grown on reduced graphene oxide sheets and its enhanced photocatalytic performance. J. Mater. Chem A 2014, 2, 8304–8313. [Google Scholar]
- Lim, S.P.; Pandikumar, A.; Lim, Y.S.; Huang, N.M.; Lim, H.N. In-situ electrochemically deposited polypyrrole nanoparticles incorporated reduced graphene oxide as an efficient counter electrode for platinum-free dye-sensitized solar cells. Sci Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, G.D.; Lu, X.F.; Lei, J.Y.; Yang, L.; Wang, C. Facile and controlled synthesis of bismuth sulfide nanorods-reduced graphene oxide composites with enhanced supercapacitor performance. Electrochim. Acta 2015, 154, 24–30. [Google Scholar] [CrossRef]
- He, H.Y. Facile synthesis of Bi2S3 nanocrystalline-modified TiO2: Fe nanotubes hybrids and their photocatalytic activities in dye degradation. Part. Sci. Technol. 2017, 35, 410–417. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A Green Approach to the Synthesis of Graphene Nanosheets. Acs. Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Xie, J.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Facile synthesis of ultrafine CoSn2 nanocrystals anchored on graphene by one-pot route and the improved electrochemical Li-storage properties. New J. Chem. 2013, 37, 474–480. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.Y.; Liu, P.; Li, W. Chemical fabrication and electrochemical performance of Bi2S3-nanorods charged reduced graphene oxide. Mater. Lett. 2015, 161, 774–777. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tian, G.H.; Mao, G.J.; Li, R.; Xiao, Y.T.; Han, T.R. Facile synthesis of well-dispersed Bi2S3 nanoparticles on reduced graphene oxide and enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 378, 231–238. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S.; Wichitpanya, S.; Daengduang, S.; Puttota, S. Electrochemical sensor based on PANI/MnO2-Sb2O3 nanocomposite for selective simultaneous voltammetric determination of ascorbic acid and acetylsalicylic acid. J. Electroanal Chem. 2016, 782, 192–201. [Google Scholar] [CrossRef]
- Tian, H.L.; Fan, H.Q.; Ma, J.W.; Liu, Z.Y.; Ma, L.T.; Lei, S.H.; Fang, J.W.; Long, C.B. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef]
- Ray, C.; Dutta, S.; Roy, A.; Sahoo, R.; Pal, T. Redox mediated synthesis of hierarchical Bi2O3/MnO2 nanoflowers: A non-enzymatic hydrogen peroxide electrochemical sensor. Dalton T 2016, 45, 4780–4790. [Google Scholar] [CrossRef]
- Cabrita, J.F.; Ferreira, V.C.; Monteiro, O.C. Titanate nanofibers sensitized with nanocrystalline Bi2S3 as new electrocatalytic materials for ascorbic acid sensor applications. Electrochim. Acta 2014, 135, 121–127. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Bahrani, S.; Ramakrishna, S.; Babapoor, A.; Chiang, W.H. Coupled graphene oxide with hybrid metallic nanoparticles as potential electrochemical biosensors for precise detection of ascorbic acid within blood. Anal. Chim. Acta 2020, 1107, 183–192. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.Q.; Chen, S.H.; Zhang, Y.; Hu, F.X.; Zhang, M.H. Non-covalent iron (III)-porphyrin functionalized multi-walled carbon nanotubes for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Electrochim. Acta 2012, 62, 109–115. [Google Scholar] [CrossRef]
- Saleh Mohammadnia, M.; Marzi Khosrowshahi, E.; Naghian, E.; Homayoun Keihan, A.; Sohouli, E.; Plonska-Brzezinska, M.E.; Ali Sobhani, N.; Rahimi-Nasrabadi, M.; Ahmadi, F. Application of carbon nanoonion-NiMoO4-MnWO4 nanocomposite for modification of glassy carbon electrode: Electrochemical determination of ascorbic acid. Microchem. J. 2020, 159, 105470. [Google Scholar] [CrossRef]
- Hsu, S.C.; Cheng, H.T.; Wu, P.X.; Weng, C.J.; Santiago, K.S.; Yeh, J.M. Electrochemical Sensor Constructed Using a Carbon Paste Electrode Modified with Mesoporous Silica Encapsulating PANI Chains Decorated with GNPs for Detection of Ascorbic Acid. Electrochim. Acta 2017, 238, 246–256. [Google Scholar] [CrossRef]
- Huang, H.P.; Yue, Y.F.; Chen, Z.Z.; Chen, Y.N.; Wu, S.Z.; Liao, J.S.; Liu, S.J.; Wen, H.R. Electrochemical sensor based on a nanocomposite prepared from TmPO4 and graphene oxide for simultaneous voltammetric detection of ascorbic acid, dopamine and uric acid. Microchim. Acta 2019, 186, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Z.; Li, G. Progress on the development of DNA-mediated metal nanomaterials for environmental and biological analysis. Chin. Chem. Lett. 2019, 30, 285–291. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Liu, Y.; Xin, G.; Wang, M.; Zhang, Z.; Liu, Z. Electrochemical sensor based on a three dimensional nanostructured MoS2 nanosphere-PANI/reduced graphene oxide composite for simultaneous detection of ascorbic acid, dopamine, and uric acid. Rsc. Adv. 2019, 9, 2997–3003. [Google Scholar] [CrossRef] [Green Version]
- de Faria, L.V.; Lisboa, T.P.; de Farias, D.M.; Araujo, F.M.; Machado, M.M.; de Sousa, R.A.; Matos, M.A.C.; Munoz, R.A.A.; Matos, R.C. Direct analysis of ascorbic acid in food beverage samples by flow injection analysis using reduced graphene oxide sensor. Food Chem. 2020, 319, 126509. [Google Scholar] [CrossRef]
- Liu, H.; Guo, K.; Lv, J.; Gao, Y.; Duan, C.; Deng, L.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of horseradish peroxidase immobilized on porous Co3O4 nanosheets and reduced graphene oxide composite modified electrode. Sens. Actuators B Chem. 2017, 238, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.H.; Liu, C.D.; Wang, Q.W.; Wang, X.H.; Wang, S.T. Electrochemical sensor based on an electrode modified with porous graphitic carbon nitride nanosheets (C3N4) embedded in graphene oxide for simultaneous determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Shams, A. Silver-filled MWCNT nanocomposite as a sensing element for voltammetric determination of sulfamethoxazole. Anal. Chim. Acta 2018, 1039, 51–58. [Google Scholar] [CrossRef] [PubMed]




| Modified Materials | LOD (μM) | Linear Range (μM) | Reference (μA mM−1 cm−2) | Reference |
|---|---|---|---|---|
| Bi2S3/TNF | - | 1000–10,000 | 38 | [51] |
| GO/TmPO4 | 39 | 100–1000 | — | [56] |
| NP-Cu/RGO/GCE | 0.09 | 0.5–30 | — | [57] |
| MoS2-PANI/rGO | 22.2 | 50–8000 | 48.2 | [58] |
| rGO/GCE | 4.7 | 65–2530 | — | [59] |
| Pd NWs/GCE | 0.2 | 25–900 | 166.5 | [60] |
| PCN/ GO | 3.7 | 30–3000 | 380 | [61] |
| Bi2S3/rGO | 2.94 | 5–1200 | 268.84 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Vitamin C tablets | 0 | <LOD | - | - |
| 200 | 203.11 | 101.55 | 2.25 | |
| 300 | 297.42 | 98.26 | 1.58 | |
| 400 | 388.57 | 97.14 | 3.04 | |
| 500 | 487.62 | 97.42 | 2.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, C.; Li, H.; Zhou, S.; Li, G.; Wang, C.; Snyders, R.; Bittencourt, C.; Li, W. Bi2S3/rGO Composite Based Electrochemical Sensor for Ascorbic Acid Detection. Chemosensors 2021, 9, 190. https://doi.org/10.3390/chemosensors9080190
Qu C, Li H, Zhou S, Li G, Wang C, Snyders R, Bittencourt C, Li W. Bi2S3/rGO Composite Based Electrochemical Sensor for Ascorbic Acid Detection. Chemosensors. 2021; 9(8):190. https://doi.org/10.3390/chemosensors9080190
Chicago/Turabian StyleQu, Chengling, He Li, Shuang Zhou, Guodong Li, Cheng Wang, Rony Snyders, Carla Bittencourt, and Wenjiang Li. 2021. "Bi2S3/rGO Composite Based Electrochemical Sensor for Ascorbic Acid Detection" Chemosensors 9, no. 8: 190. https://doi.org/10.3390/chemosensors9080190
APA StyleQu, C., Li, H., Zhou, S., Li, G., Wang, C., Snyders, R., Bittencourt, C., & Li, W. (2021). Bi2S3/rGO Composite Based Electrochemical Sensor for Ascorbic Acid Detection. Chemosensors, 9(8), 190. https://doi.org/10.3390/chemosensors9080190






