Abstract
A simple, rapid, and environmentally-friendly spectrophotometric method for nitrite detection was developed. Detection was based on a redox reaction with iodide ions in an acidic condition. The reaction was evaluated by detecting the increase in absorbance of the colored product of iodine at 362 nm wavelength. To obtain a good spectrophotometric performance, the iodide ions concentration, hydrochloric acid concentration, and reaction time were optimized. In the optimal condition, the developed spectrophotometric method provided a linear range of 0.0625 to 4.00 mg L−1 (r = 0.9985), reaction time for 10 min, a limit of detection of 25 µg L−1, and a limit of quantitation of 85 µg L−1. This method showed good repeatability (RSD < 9.21%), high sample throughput (9 samples min−1), and good accuracy (recovery = 88 ± 2 to 99.5 ± 0.4%). The method has the potential to be used in crime scene investigations as a rapid screening test for gunshot residue detection via nitrite detection.
1. Introduction
The gunshot residue (GSR) is the product of primer detonation, gunpowder combustion, and emitted particles of cartridge, projectile, and gun barrel. Deposition of GSR on surfaces in the vicinity of a shooting [,,] made it useful forensic evidence in investigations of criminal use of firearms, aiding suspect identification, predicting firing distance, differentiating between suicide and homicide, and also indicating a bullet hole [,]. In routine forensic analysis, GSR is identified through morphological and elemental analysis using scanning electron microscopy coupled with energy dispersive X-ray analysis []. Additionally, inductively coupled plasma-mass spectrometry [] and atomic absorption spectroscopy [] were also reported in analyzing GSR. These techniques had demonstrated good accuracy and high sensitivity; however, these techniques could also be limited by their respective cost, time, availability, and accessibility of instrumentation, as well as the need for well-trained operators [,]. Therefore, a fast, inexpensive, and simple screening technique for determining GSR could benefit the forensic science community prior to the application of specific confirmatory techniques.
Nitrite ion is one of the most common inorganic compounds upon primer detonation and gunpowder combustion [,]. Detection of nitrite ions can provide valuable forensic information in the preliminary analysis of GSR on any related evidence, including the shooter, cartridge case, firearm, and surfaces nearby a shooting activity. The detection and measurement of nitrite could be employed as a screening method for the presence of GSR. Literatures reported various techniques for determination of nitrite, including electrochemical methods [,], ion chromatography [,], electrophoresis [,], and spectrophotometry [,]. Among these techniques, the spectrophotometry technique received increasing attention due to its simplicity, convenience, low cost, and good detection limit [,,]. During the forensic investigation of firearms-related cases, suspects are frequently approached, and suspected GSR samples are collected, commonly from the hands, face, or clothing. In such cases, the forensic evidence is not limited to one sample, but several samples recovered from various sites of collection. Apart from that, the numbers of suspected GSR samples would be greatly increased if more suspects are identified. Given this, a fast screening procedure to determine the presence of GSR via the detection of nitrite would be needed, prior to the application of confirmatory SEM-EDX analysis. Spectrophotometric analysis in 96-well microplate read mode could be utilized, in which it enabled multi-sample analyses and low consumption of chemical reagent. Moreover, the interpretation of the absorbance values captured by the instrumentation through a microplate reader and directly stored in the computer would also provide quick and accurate data [].
Most spectrophotometric techniques for the detection of nitrite were based on dye formation via a diazo-coupling reaction to produce the end product allowable for determination, and typically an aromatic amine was used. Aromatic amines employed for this purpose included p-rosaniline [], 3-nitroaniline [], p-nitroaniline [], 1-amino-4-naphtalenesulfonic acid [], p-aminobenzoic acid [], 4-aminobenzotrifluoride [], 4-aminophenylmercaptoacetic acid [], safranin [], and sulfanilamide []. However, these substances often possess some drawbacks, including the diazotization temperature, coupling time, and pH dependence. Furthermore, these techniques frequently employ large volumes of amines, and sometimes they are carcinogenic and toxic [,].
This present work reports a simple, inexpensive, and environmentally-friendly spectrophotometric method for the determination of nitrite based on a redox reaction with iodide ions in an acidic condition. The method was optimized utilizing a UV/Vis 96-well microplate spectrophotometer and its analytical performance was evaluated in terms of its linearity, limit of detection (LOD), the limit of quantification (LOQ), repeatability, accuracy, and interference-effect. Finally, as a proof-of-concept for the application, we investigated the performance of the developed spectrophotometric method by applying sodium nitrite powder to various substrate surfaces, such as hands, clothes, tables, phones, notebook computers, and tiled floors. It is hoped that the developed method can be used to screen large numbers of suspected GSR samples, serving as a useful determination method complementing the SEM-EDX analysis in forensic laboratories.
2. Materials and Methods
2.1. Reagents and Apparatus
Sodium nitrite (NaNO2) was purchased from Ajax Chemical Finechem Pty Ltd. (New South Wales, Australia). Potassium iodide (KI) and hydrochloric acid (HCl, 37%) were obtained from Merck KGaA (Darmstadt, Germany). Deionized water (18.2 MΩ cm) (BarnsteadTM Easy PureTM II water purification system, Thermo ScientificTM, Waltham, MA, USA) was employed for the preparation of all solutions. Stock nitrite standard solutions were prepared at a concentration level of 1000 mg L−1. Working solutions were prepared through dilution of a pre-determined volume of the stock nitrite standard solution with distilled water. A UV/Vis microplate spectrophotometer (Thermo Fisher Scientific, Multiskan TM, GO, USA) was utilized in this study and the analytical procedure was controlled by Skanlt software 3.2.
2.2. Analytical Procedure
A few milliliters of nitrite ions solution were transferred into a microtube which contained 0.020 mol L−1 of hydrochloric acid and 0.050 mol L−1 of iodide ion solution. When a color change was observed by the naked eye, the mixture solution was dropped into wells of a 96-well microplate and mixed for 10 min. The absorbances detected by UV/Vis microplate spectrophotometer at a wavelength of 362 nm against a blank solution were recorded for each analysis. Note that the blank solution was prepared in a similar procedure but without nitrite ions. Figure 1 illustrates the scheme for the detection of nitrate, comprising the preparation, incubation, and measurement steps.
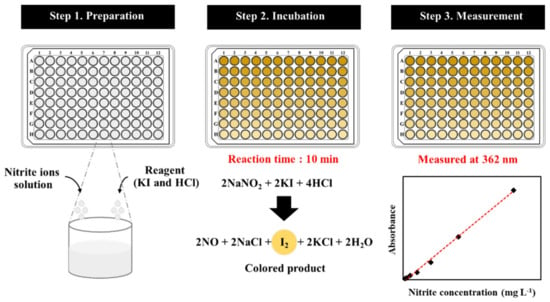
Figure 1.
The scheme for the detection of nitrite.
2.3. Optimization
In order to obtain good performance of the developed spectrophotometric method for nitrite detection, three operational conditions were optimized, including the iodide ions concentrations (0.040, 0.050, 0.060, 0.070, 0.080, 0.090, and 0.10 mol L−1), the concentrations of hydrochloric acid (0.010, 0.020, 0.030, 0.040, 0.050, 0.060, and 0.070 mol L−1), and the reaction time (2, 4, 6, 8, 10, 12, 14, and 16 min).
2.4. Analytical Performance
The linearity, LOD, LOQ, interference effect, precision, and accuracy of the developed spectrophotometric method were investigated. The linear range was investigated from 0 to 4.00 mg L−1. The LOD and LOQ were calculated based on 3 and 10 standard deviations of the intercept divided by the slope of the calibration curve, respectively. The precision of analytical methods was defined in terms of repeatability, which was expressed as a percentage of the relative standard deviation (%RSD) from the three replicate analyses. The intra-day and inter-day assays were studied with standard nitrite ions solutions at seven different concentrations (0.0625, 0.125, 0.250, 0.500, 1.00, 2.00, and 4.00 mg L−1). The accuracy was expressed as percent recovery, which was evaluated by analyzing the known standard nitrite ions solution concentrations against the established calibration curve.
2.5. Interference Effect
To check the selectivity of the developed spectrophotometric method for the determination of nitrite, including urea, Zn2+, Ni2+, Mg2+, Ba2+, Pb2+, Fe2+, NO3−, SO42−, and Cl− were used to investigate potential interferences found in gunshot residue. Tolerance limits were defined as the maximum concentration that resulted in less than ±5% variation and determined by adding different concentrations of these interfering substances to a testing solution containing 2 mg L−1 of nitrite ions concentration under the optimal conditions.
2.6. Application for Nitrite Sensing
To simulate the real case situations, nitrite samples were generated by putting sodium nitrite powder onto different substrate surfaces, namely hands, clothes, tables, telephones, notebook computers, and tiled floors. Samples were collected from substrates by carefully wiping with a cotton swab (15 cm size L, United Medicine Instruments Co., Ltd., Bangkok, Thailand). Each cotton swab was inserted into a microtube containing hydrochloric acid and iodide ions solution. The obtained solution was then incubated for 10 min and detected at 362 nm by UV/Vis microplate spectrophotometer, as demonstrated in Figure 1. The absorbance values generated for each analysis were recorded and compared.
3. Results and Discussion
3.1. Absorption Spectra
In an acidic condition, the iodide ion (I−) and nitrite ion (NO2−) functioned as a reducing and oxidizing agent, respectively. Iodide ion (I−) was oxidized to iodine (I2) by nitrite ion (NO2−), meanwhile nitrite ion (NO2−) was reduced to nitric oxide by iodide ion (I−) (2NaNO2 + 2KI + 4HCl → I2 + 2NO + 2NaCl + 2KCl + 2H2O). Upon the chemical reaction, the color of the mixture solution was changed from colorless to yellow, as observed by the naked eye. Such change was due to the oxidation of iodide ion (I−) to iodine (I2) [,,]. To obtain a good absorption by iodine, the incident wavelength of the spectrophotometer was adjusted from 300 to 500 nm, and Figure 2 shows the absorption spectra with and without the presence of nitrite. It was found that the maximum absorbance was at a wavelength of 362 nm [], and therefore this wavelength was selected for further experiments.
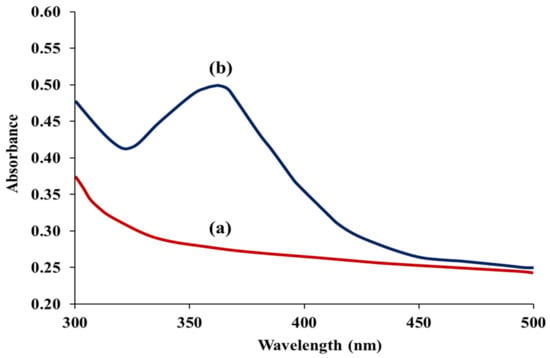
Figure 2.
Absorption spectra of the proposed method (a) in the absence and (b) in the presence of 0.600 mg L−1 nitrite.
3.2. Optimal Conditions
3.2.1. Concentration of Iodide Ions
The effect of the concentration levels of iodide ions on the absorbance of iodine was studied from 0.040 to 0.10 mol L−1 (Figure 3A). The absorbance increased with an increase in the iodide ion concentration from 0.040 to 0.050 mol L−1. At concentration levels greater than 0.050 mol L−1 up to 0.10 mol L−1, changes in absorbance were nearly negligible. Therefore, for subsequent experiments, a concentration of 0.050 mol L−1 of iodide ion was selected.
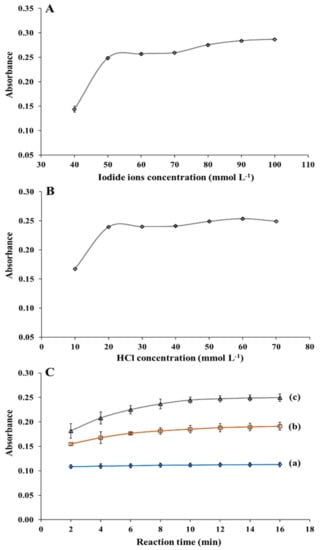
Figure 3.
Absorbance (0.600 mg L−1 nitrite) of the developed spectrophotometric method with (A) different iodide ions concentration (HCl concentration, 50 mmol L−1; reaction time, 10 min) and (B) different HCl concentration (iodide ions concentration, 50 mmol L−1; reaction time, 10 min). (C) Effect of reaction time on absorbance at nitrite ions concentrations of (a) 0.200, (b) 0.400, and (c) 0.600 mg L−1 (iodide ions concentration, 50 mmol L−1; HCl concentration, 20 mmol L−1).
3.2.2. Concentration of Hydrochloric Acid
The acidic condition is an important factor affecting the redox reaction of iodide ions with nitrite ions. The effect of concentration levels of hydrochloric acid on the signal was evaluated between 0.010 to 0.070 mol L−1 (Figure 3B). The absorbance was found to increase from 0.010 to 0.020 mol L−1, and no significant changes in their absorbance values with further increase in the concentration. Therefore, 0.020 mol L−1 hydrochloric acid was chosen as the optimum condition for further nitrite ions determination.
3.2.3. Reaction Time
To obtain a short analysis time with good absorption performance, the influence of dynamic reaction time was investigated by plotting the reaction time versus absorbance for different nitrite ions concentrations (Figure 3C). Absorbance increased with reaction time and reached saturation at 10 min, and remained constant after that. The nitrite ions were no longer available for conversion leading to the completion of the reaction []. Therefore, the reaction time of 10 min was chosen as the optimal condition.
3.3. Analytical Performance
3.3.1. Linearity, Limit of Detection, and Limit of Quantification
The analytical performance of the spectrophotometric method for nitrite ions detection was studied under optimal conditions. The results showed that the absorbance was increased linearly with the increasing concentration of nitrite ions from 0.0625 mg L−1 to 4.00 mg L−1 (Figure 4A). The linear regression equation was determined as y = (0.353 ± 0.009)x − (0.037 ± 0.003) at a correlation coefficient of 0.9985, where y and x are the absorbance values at 362 nm and the concentration level of nitrite ions at the unit of mg L−1, respectively. The detection and quantitation limits were respectively determined at 25 and 85 µg L−1, calculated based on 3 and 10 standard deviations of the intercept divided by the slope of the calibration curve. The spectrophotometric method was compared with previously reported methods [,,,,,,,,,] for the detection of nitrite ions in Table 1. Different reagents had been utilized to detect the presence of nitrite ions. Although some works are wide linear range and low LOD, they require fluorescent material and complicated procedure and can only analyze one sample at a time. Our spectrophotometric method allowed the detection in short duration which can be detected in 9 nitrite samples in 1 min. Furthermore, the developed method was simple, and more environment-friendly without involving the use of organic solvents.
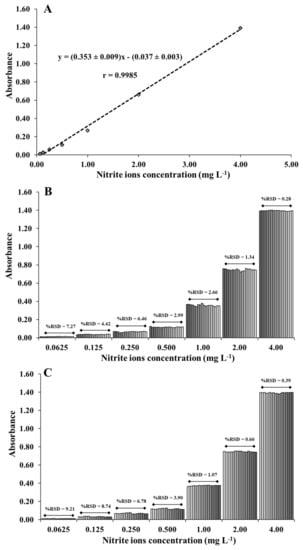
Figure 4.
(A) The calibration plot between absorbance and concentration of nitrite ions. The repeatability of (B) intra-day and (C) inter-day assays.

Table 1.
Comparison of the analytical performance of previously reported methods and the developed spectrophotometric method for the determination of nitrite ions.
3.3.2. Repeatability
To evaluate the precision of the method, the repeatability of intra-day and inter-day assays was studied with standard nitrite ions solutions at concentrations of 0.0625, 0.125, 0.250, 0.500, 1.00, 2.00, and 4.00 mg L−1. For the intra-day assay, the relative standard deviations (RSDs) of all concentration levels were reported in the range of 0.28% to 7.27% (six sets of measurements were investigated for each concentration (n = 3), giving 18 measurements for each concentration) (Figure 4B). For the inter-day assay, the measurements were repeated on six consecutive days, yielding relative standard deviations between 0.39% to 9.21% (Figure 4C). The repeatability of intra-day and inter-day assays were acceptable where respective RSD of 15%, 11%, and 7.3% were suggested for the concentration levels of 0.1 mg L−1, 1 mg L−1, and 10 mg L−1 according to the AOAC guidelines []. These results demonstrated the good precision of the spectrophotometric method.
3.3.3. Interference Study
To investigate the interference effect of common interfering substances in gunshot residue, including trinitrotoluene (TNT), dinitrobenzene (DNB), dinitrotoluene (DNT), urea, Zn2+, Ni2+, Mg2+, Ba2+, Pb2+, Fe2+, NO3−, SO42−, and Cl− were tested. The tolerance limit and %relative error for each interference were calculated and determined. The tolerance limit is defined as the ratio of the highest concentration in a mixture of nitrite that causes a relative error below ±5% change in the absorbance signal in relation to the concentration of nitrite ions at 2 mg L−1 under the optimal conditions. Table 2 demonstrates the experimental results, and it showed that 5000-fold concentration of urea, Zn2+, Ni2+, Mg2+, Ba2+, SO42−, and Cl−, 150-fold of Fe2+ and 50-fold of NO3−, TNT, DNB, DNT, and Pb2+ did not interfere with nitrite determination. These results suggested the applicability of the method in the determination of nitrite, particularly in suspected GSR samples.

Table 2.
Tolerance ratio of interferences on the determination of 2 mg L−1 nitrite.
3.4. Application for Nitrite Sensing
The developed spectrophotometric method was employed to detect the presence of nitrite on different substrate surfaces (hands, clothes, tables, telephones, notebook computers, and tiled floors), and their respective recovery percentages were determined. The recoveries of nitrite detection were ranged from 88 ± 2 to 99.5 ± 0.4% for all samples (Table 3), which were acceptable according to AOAC guidelines []. Furthermore, the developed method can be employed to detect up to 96 nitrite samples within 10 min, allowing rapid analysis for more effective forensic investigation and identification.

Table 3.
Recovery analysis of nitrite samples on different surfaces (n = 3).
4. Conclusions
A spectrophotometric method for nitrite detection was successfully demonstrated based on a redox reaction with iodide ions in an acidic condition. The developed method incorporates a microtiter plate assay, enabling rapid, and multi-sample analyses, as well as low and environmentally friendly reagent consumption. Under the optimal conditions established in this study, the proposed method exhibited a wide linear range from 0.0625 to 4.00 mg L−1 (r = 0.9985), rapid analysis (9 sample min−1), low detection limit (25 µg L−1), low quantitation limit (85 µg L−1), good repeatability (RSD < 9.21%), good recovery (88 ± 2 to 99.5 ± 0.4%), and good anti-interference performance. Future development of this simple and rapid spectrophotometric method for detecting nitrite in real gunshot residue samples could be useful for forensic investigators screening a large number of suspected GSR samples.
Author Contributions
Conceptualization, Y.T., J.J., K.S., V.K., A.P., A.T., K.H.C., A.F.L.A., and W.L.; methodology, Y.T., J.J., K.S., V.K., A.P., A.T., and W.L.; validation, Y.T., J.J., K.S., V.K., A.P., A.T., K.H.C., A.F.L.A., and W.L.; formal analysis, Y.T., J.J., K.S., V.K., A.P., A.T., K.H.C., A.F.L.A., and W.L.; writing—original draft preparation, Y.T., K.S., and W.L.; writing—review and editing, Y.T., J.J., K.S., V.K., A.P., A.T., K.H.C., A.F.L.A., and W.L.; project administration, W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Prince of Songkla University and the Ministry of Higher Education, Science, Research and Innovation under the Reinventing University Project (grant number REV64043). The financial support from the Thailand Research Fund (TRF) and Prince of Songkla University (grant no. RSA 6280081), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), the Center of Excellence for Trace Analysis and Biosensor (TAB-CoE), Faculty of Science, Prince of Songkla University, Hat Yai, Thailand are gratefully acknowledged. Thanks also to Mr. Thomas Duncan Coyne, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, Thailand for assistance with the English.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Thomas Duncan Coyne for his assistance with the English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalby, O.; Butler, D.; Birkett, J.W. Analysis of gunshot residue and associated materials—A review. J. Forensic Sci. 2010, 55, 924–943. [Google Scholar] [CrossRef] [PubMed]
- Garofano, L.; Capra, M.; Ferrari, F.; Bizzaro, G.P.; Di Tullio, D.; Dell’Olio, M.; Ghitti, A. Gunshot residue: Further studies on particles of environmental and occupational origin. Forensic Sci. Int. 1999, 103, 1–21. [Google Scholar] [CrossRef]
- Saverio Romolo, F.; Margot, P. Identification of gunshot residue: A critical review. Forensic Sci. Int. 2001, 119, 195–211. [Google Scholar] [CrossRef]
- Lucena, M.A.M.; Ordonez, C.; Weber, I.T.; Torre, M.; Garcia-Ruiz, C.; Lopez-Lopez, M. Investigation of the use of luminescent markers as gunshot residue indicators. Forensic Sci. Int. 2017, 280, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Goudsmits, E.; Sharples, G.P.; Birkett, J.W. Preliminary classification of characteristic organic gunshot residue compounds. Sci. Justice 2016, 56, 421–425. [Google Scholar] [CrossRef]
- Brozek-Mucha, Z. On the prevalence of gunshot residue in selected populations—An empirical study performed with SEM-EDX analysis. Forensic Sci. Int. 2014, 237, 46–52. [Google Scholar] [CrossRef]
- Turillazzi, E.; Di Peri, G.P.; Nieddu, A.; Bello, S.; Monaci, F.; Neri, M.; Pomara, C.; Rabozzi, R.; Riezzo, I.; Fineschi, V. Analytical and quantitative concentration of gunshot residues (Pb, Sb, Ba) to estimate entrance hole and shooting-distance using confocal laser microscopy and inductively coupled plasma atomic emission spectrometer analysis: An experimental study. Forensic Sci. Int. 2013, 231, 142–149. [Google Scholar] [CrossRef]
- Aliste, M.; Chavez, L.G. Analysis of gunshot residues as trace in nasal mucus by GFAAS. Forensic Sci. Int. 2016, 261, 14–18. [Google Scholar] [CrossRef]
- Goudsmits, E.; Sharples, G.P.; Birkett, J.W. Recent trends in organic gunshot residue analysis. Trac. Trends Anal. Chem. 2015, 74, 46–57. [Google Scholar] [CrossRef]
- Aksoy, C.; Bora, T.; Senocak, N.; Aydin, F. A new method to reduce false positives due to antimony in detection of gunshot residues. Forensic Sci. Int. 2015, 250, 87–90. [Google Scholar] [CrossRef]
- Maitre, M.; Kirkbride, K.P.; Horder, M.; Roux, C.; Beavis, A. Current perspectives in the interpretation of gunshot residues in forensic science: A review. Forensic Sci. Int. 2017, 270, 1–11. [Google Scholar] [CrossRef]
- Petraco, N.; Yander, M.; Sardone, J. A method for the quantitative determination of nitrites in gunshot residue cases. Forensic Sci. Int. 1981, 18, 85–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, M.; He, H.; Cai, Z.; Gao, N.; He, C.; Chang, G.; Wang, X.; He, Y. Highly sensitive nitrite sensor based on AuNPs/RGO nanocomposites modified graphene electrochemical transistors. Biosens. Bioelectron. 2019, 146, 111751. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef]
- Murray, E.; Roche, P.; Harrington, K.; McCaul, M.; Moore, B.; Morrin, A.; Diamond, D.; Paull, B. Low cost 235 nm ultra-violet light-emitting diode-based absorbance detector for application in a portable ion chromatography system for nitrite and nitrate monitoring. J. Chromatogr. A 2019, 1603, 8–14. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.Q.; Zhu, Y. A novel ion chromatography cycling-column-switching system for the determination of low-level chlorate and nitrite in high salt matrices. J. Hazard. Mater. 2012, 235, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.B.; Moreira, R.C.; de Oliveira Tavares, M.G.; Coltro, W.K.T. Monitoring of nitrite, nitrate, chloride and sulfate in environmental samples using electrophoresis microchips coupled with contactless conductivity detection. Talanta 2016, 147, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Troška, P.; Chudoba, R.; Danč, L.; Bodor, R.; Horčičiak, M.; Tesařová, E.; Masár, M. Determination of nitrite and nitrate in cerebrospinal fluid by microchip electrophoresis with microsolid phase extraction pre-treatment. J. Chromatogr. B 2013, 930, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Mašić, A.; Santos, A.T.L.; Etter, B.; Udert, K.M.; Villez, K. Estimation of nitrite in source-separated nitrified urine with UV spectrophotometry. Water Res. 2015, 85, 244–254. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Y.; Fan, C.; Wang, J.; Yang, Y. Development of a cloud point extraction and spectrophotometry-based microplate method for the determination of nitrite in human urine and blood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Senra-Ferreiro, S.; Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Griess micro-assay for the determination of nitrite by combining fibre optics-based cuvetteless UV-vis micro-spectrophotometry with liquid-phase microextraction. Anal. Chim Acta 2010, 668, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Okab, R.A.; Syed, A.A. Novel reactions for simple and sensitive spectrophotometric determination of nitrite. Talanta 2007, 72, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Fat’hi, M.R.; Hatami, A. Indirect cloud point extraction and spectrophotometric determination of nitrite in water and meat products. Microchem. J. 2012, 104, 22–25. [Google Scholar] [CrossRef]
- Raman, V.; Dabbas, M.S. Some observations on the use of P-rosaniline hydrochloride/phloroglucinol for the spectrophotometric determination of nitrite. Microchem. J. 1989, 40, 242–245. [Google Scholar] [CrossRef]
- Rathore, H.P.S.; Tiwari, S.K. Spectrophotometric determination of nitrite in polluted waters using 3-nitroaniline. Anal. Chim. Acta 1991, 242, 225–228. [Google Scholar] [CrossRef]
- Tsao, F.-P.; Underwood, A.L. Spectrophotometric determination of nitrite with p-nitroaniline and 2-methyl-8-quinolinol in hexadecyl-trimethylammonium bromide solution. Anal. Chim. Acta 1982, 136, 129–134. [Google Scholar] [CrossRef]
- Sulaiman, S.T.; Al-Nuri, I.J. Coulometric microdetermination of nitrite via diazotization of 1-amino-4-naphthalenesulfonic acid. Microchem. J. 1986, 33, 112–115. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Xue, Z.; Shinger, M.I.; Abdu, H.I.; Xiong, L.; Shan, D.; Lu, X. A simple yet sensitive colorimetric nitrite ions assay based on diazotization with p-Aminobenzoic and coupling with phloroglucinol in acidic medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Amin, D. Determination of nitrite ion using the reaction with 4-aminobenzotrifluoride and 1-naphthol. Analyst 1986, 111, 1335–1337. [Google Scholar] [CrossRef]
- Tarafder, P.K.; Rathore, D.P.S. Spectrophotometric determination of nitrite in water. Analyst 1988, 113, 1073–1076. [Google Scholar] [CrossRef]
- Filik, H.; Giray, D.; Ceylan, B.; Apak, R. A novel fiber optic spectrophotometric determination of nitrite using Safranin O and cloud point extraction. Talanta 2011, 85, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shi, W.J.; Yang, N.F. Study and spectrophotometric determination of nitrite with sulfanilamide and N-phenyl J-Acid system. Guang Pu Xue Yu Guang Pu Fen Xi 2007, 27, 573–576. [Google Scholar]
- Nouroozi, S.; Mirshafian, R. Flow injection kinetic spectrophotometric method for the determination of trace amounts of nitrite. Talanta 2009, 79, 1149–1153. [Google Scholar] [CrossRef]
- Shanmugam, R.; Sathiyanarayanan, K.; Kumar, A.S. A Simple Colorimetric Screening of Nitrite Using Iodide in an Acidic pH Solution. Austin J. Anal. Pharm. Chem. 2014, 1, 1–3. [Google Scholar]
- Miura, Y.; Hamada, H. Ion chromatography of nitrite at the ppb level with photometric measurement of iodine formed by post-column reaction of nitrite with iodide. J. Chromatogr. A 1999, 850, 153–160. [Google Scholar] [CrossRef]
- Grgur, B.N.; Gvozdenović, M.M.; Stevanović, J.S.; Jugović, B.Z.; Trišović, L.T. Electrochemical oxidation of iodide in aqueous solution. Chem. Eng. J. 2006, 124, 47–54. [Google Scholar] [CrossRef]
- Ozmen, H.; Polat, F.; Cukurovali, A. Spectrophotometric Determination of Nitrite in Water Samples with 4-(1-Methyl-1-Mesitylcyclobutane-3-yl) -2-Aminothiazole. Anal. Lett. 2006, 39, 823–833. [Google Scholar] [CrossRef]
- Afkhami, A.; Masahi, S.; Bahram, M. Spectrophotometric Determination of Nitrite Based on Its Reaction with p-Nitroaniline in the Presence of Diphenylamine in Micellar Media. Bull. Korean Chem. Soc. 2004, 25, 1009–1011. [Google Scholar]
- Zatar, N.A.; Abu-Eid, M.A.; Eid, A.F. Spectrophotometric determination of nitrite and nitrate using phosphomolybdenum blue complex. Talanta 1999, 50, 819–826. [Google Scholar] [CrossRef]
- Satake, M.; Wang, G.-F. Spectrophotometric determination of nitrite in natural waters using diazotization-coupling method with column preconcentration on naphthalene supported with ion-pair of tetradecyldimethylbenzyl-ammonium and iodide. Fresenius J. Anal. Chem. 1997, 357, 433–438. [Google Scholar] [CrossRef]
- Rastegarzadeh, S.; Kalantaripour, M. Development of an optical redox chemical sensor for nitrite determination. Química Nova 2012, 35, 766–770. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Ma, Q.; Wang, C.-H.; Hu, X.; Gao, Y.; Wang, H.; Qin, D. A Simple Fluorescence sensor for detecting of Nitrite (NO2-) in Real Samples using Water-dispersible Graphite-like Carbon Nitride (w-g-C3N4) Nanomaterials. New J. Chem. 2017, 41, 7171–7176. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.; Yang, C.; Xu, J.; Liu, H.; Zhu, B. A colorimetric fluorescent probe for rapid and specific detection of nitrite. Luminescence 2019, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Badiee, H.; Zanjanchi, M.; Zamani, A.; Fashi, A. Solvent stir bar microextraction technique with three-hollow fiber configuration for trace determination of nitrite in river water samples. Environ. Sci. Pollut. Res. 2019, 26, 32967–32976. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Appendix F: Guidelines for Standard Method Performance Requirements. AOAC Int. 2016, 1–18. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).