A Potentiometric Electronic Tongue as a Discrimination Tool of Water-Food Indicator/Contamination Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Inoculum Preparation
2.2. Growth Conditions and Biomass Recovery
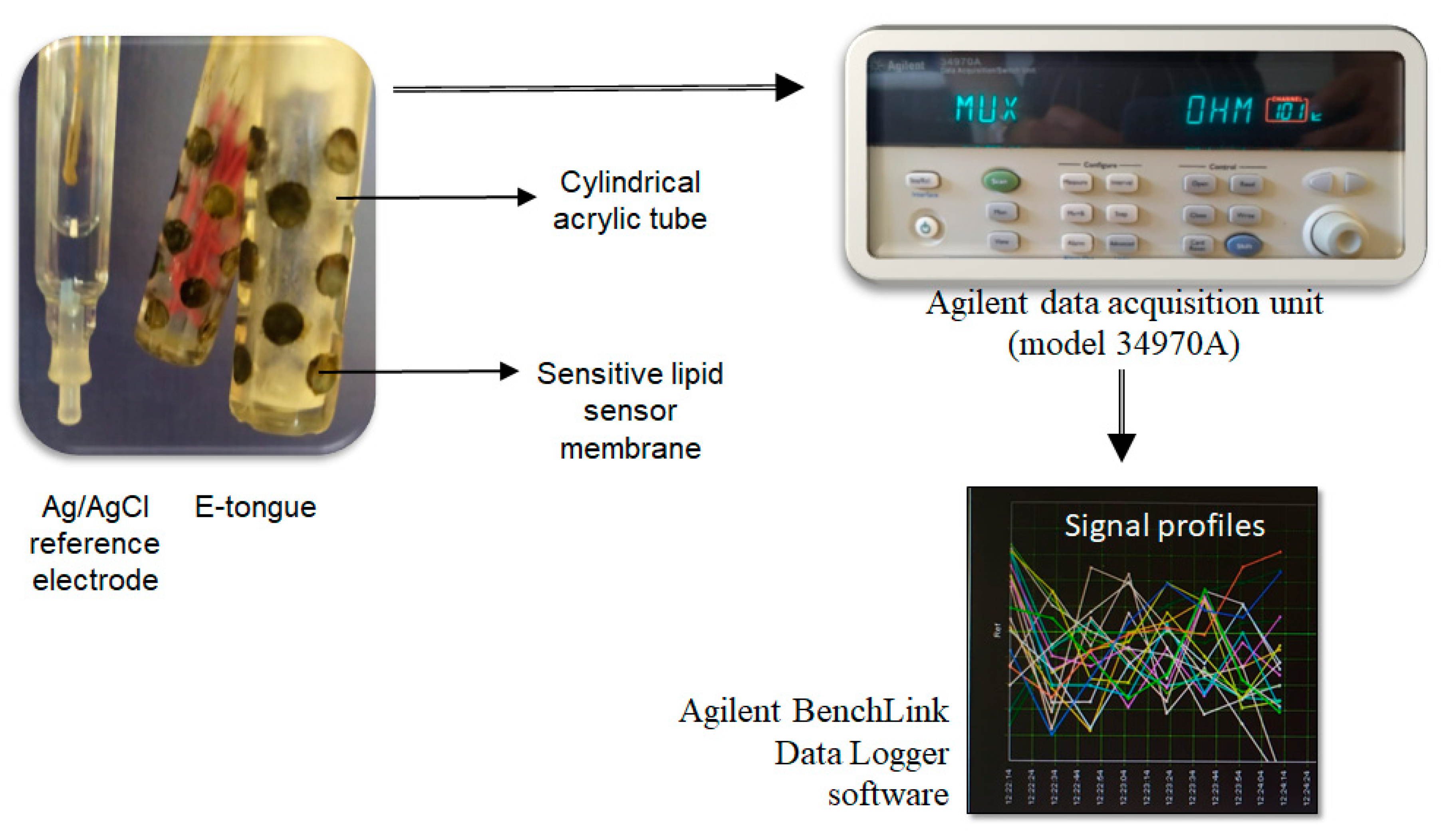
2.3. E-Tongue Apparatus
2.4. E-Tongue Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Biomass Determination by Dry Weight
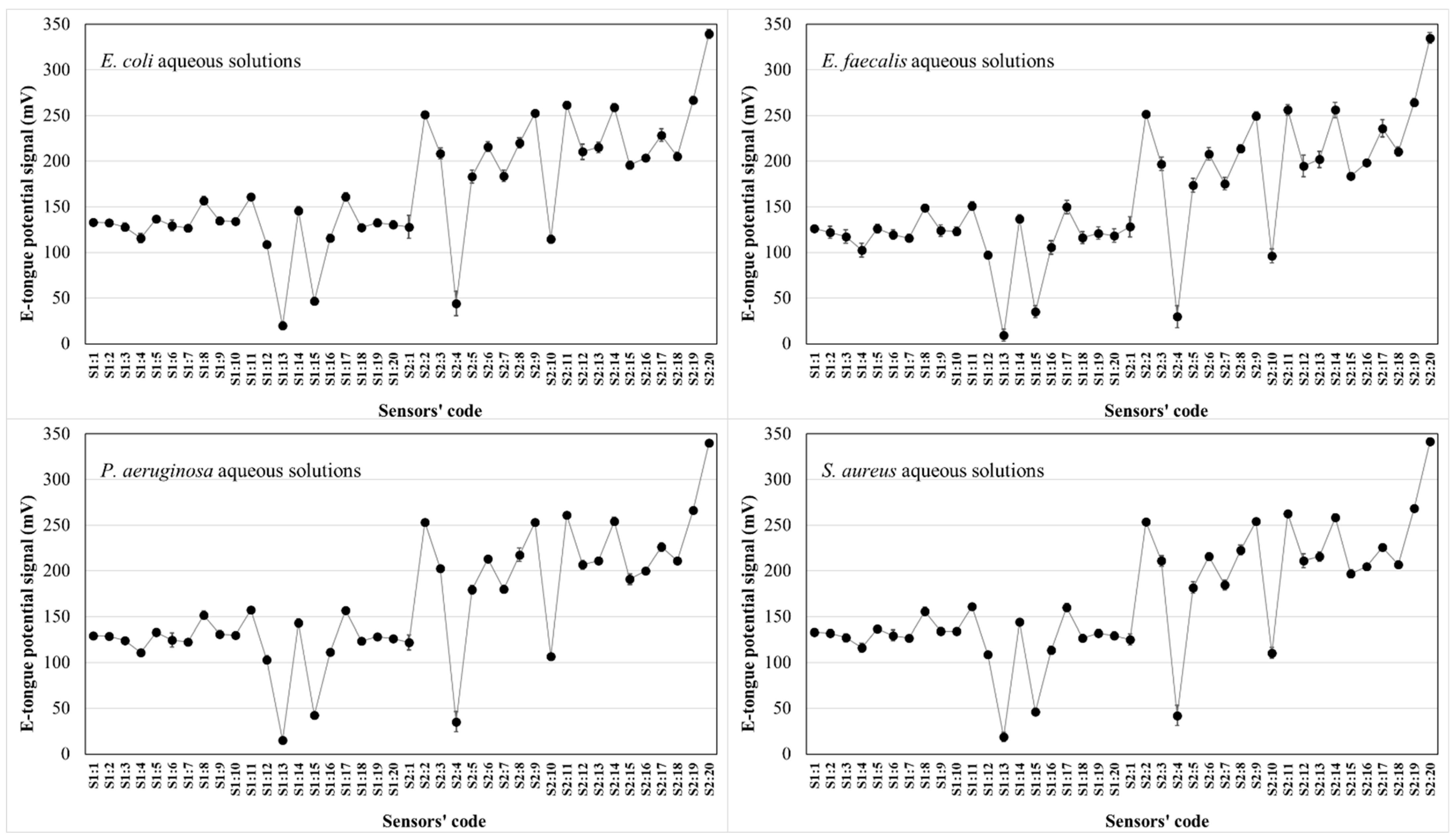
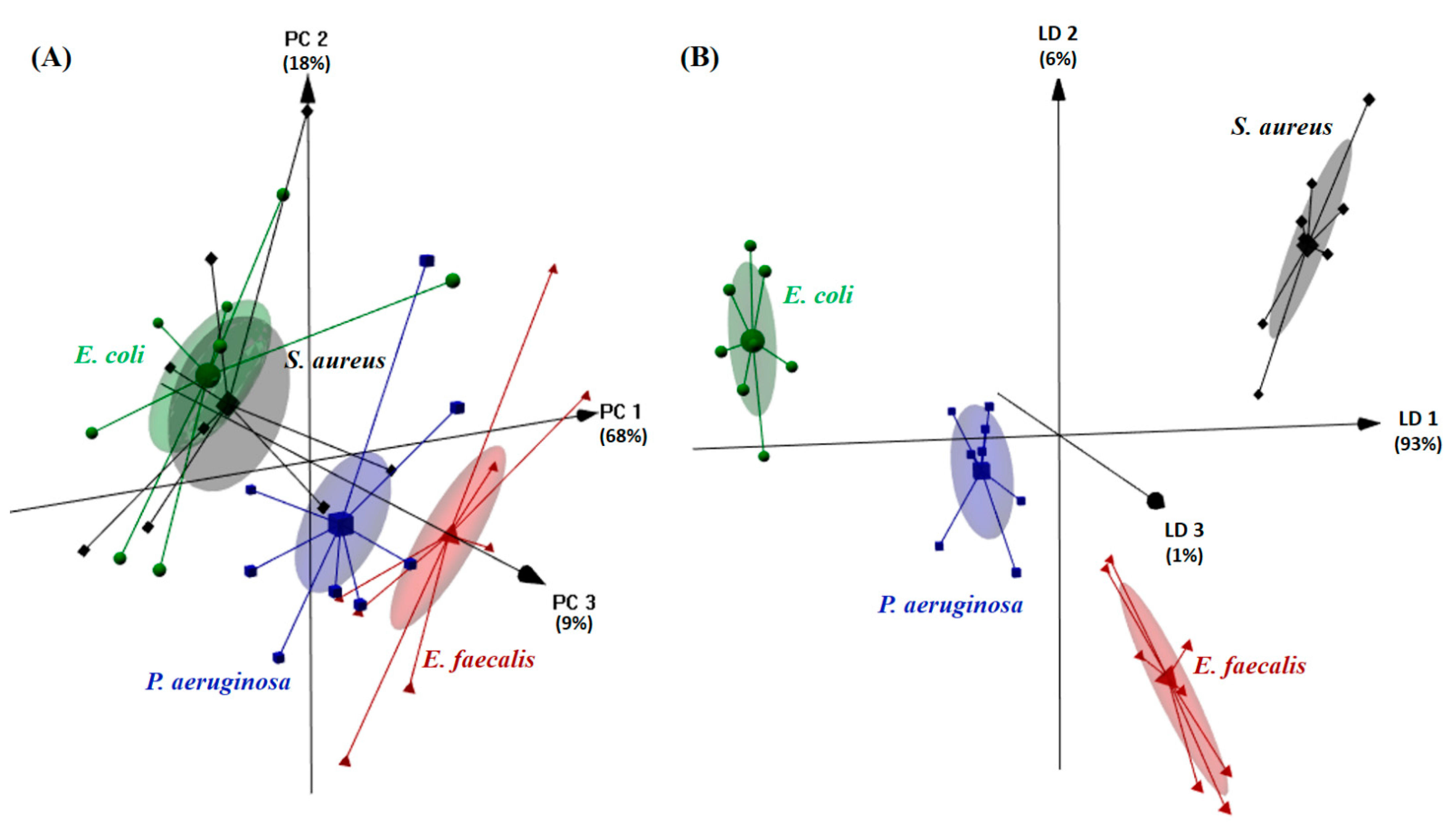
3.2. Microorganism Recognition and Differentiation Based on E-Tongue Potentiometric Profiles
3.3. Microorganism Quantification Based on E-Tongue Potentiometric Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Rodríguez, F.; González-García, P.; Valero, A.; Hernández, M.; Rodríguez-Lázaro, D. Impact of the prevalence of different pathogens on the performance of sampling plans in lettuce products. Int. J. Food Microbiol. 2014, 184, 69–73. [Google Scholar] [CrossRef]
- Pierson, M.; Zink, D.; Smoot, L. Indicator Microorganisms and Microbiological Criteria. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; Doyle, M., Beuchat, L., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 69–85. [Google Scholar]
- Codex Alimentarius Commission—FAO/WHO. Codex Alimentarius; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- European Union. Commission Regulation (EC) 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2015, L338, 1–26. [Google Scholar]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns—An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Ahmed, A.B.H.; Abbassi, M.S.; Rojo-Bezares, B.; Ruiz-Roldán, L.; Dhahri, R.; Mehri, I.; Sáenz, Y.; Hassen, A. Characterization of Pseudomonas aeruginosa isolated from various environmental niches: New STs and occurrence of antibiotic susceptible “high-risk clones”. Int. Environ. Health Res. 2020, 30, 643–652. [Google Scholar] [CrossRef]
- Stellato, G.; Utter, D.R.; Voorhis, A.; de Angelis, M.; Eren, A.M.; Ercolini, D. A Few Pseudomonas Oligotypes Dominate in the Meat and Dairy Processing Environment. Front. Microbiol. 2017, 2, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croughs, P.D.; Klaassen, C.H.W.; van Rosmalen, J.; Maghdid, D.M.; Boers, S.A.; Hays, J.P.; Goessens, W.H.F. Unexpected mechanisms of resistance in Dutch Pseudomonas aeruginosa isolates collected during 14 years of surveillance. Int. J. Antimicrob. Agents 2018, 52, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 26 March 2021).
- European Union. Commission Directive (EU) 2015/1787 of 6 October 2015 on the quality of water intended for human consumption. Off. J. Eur. Union 2015, L260, 6–17. [Google Scholar]
- Gracias, K.S.; McKillip, J.L. A review of conventional detection and enumeration methods for pathogenic bacteria in food. Can. J. Microbiol. 2004, 50, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Ali, Y.M.; Sim, R.B.; Schwaeble, W.; Shaaban, M.I. Enterococcus faecalis Escapes Complement-Mediated Killing via Recruitment of Complement Factor H. J. Infect. Dis. 2019, 220, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, E.; Forouzandeh, M. Designing a new biosensor “DNA ELISA” to detect Escherichia coli using genomic DNA and comparison of this method to PCR-ELISA. J. Enzyme Inhib. Med. Chem. 2018, 33, 722–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; He, J.; Cao, X.; Huang, K.; Luo, Y.; Xu, W. Development of a double-antibody sandwich ELISA for rapid detection of Bacillus Cereus in food. Sci. Rep. 2016, 6, 16092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordana-Lluch, E.; Giménez, M.; Quesada, M.D.; Ausina, V.; Martró, E. Improving the diagnosis of bloodstream infections: PCR coupled with mass spectrometry. BioMed Res. Int. 2014, 2014, 501214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Deng, Y.; Bai, Y.; Xu, D.; Chen, E.; Wu, H.; Li, B.; Gao, L. Rapid and simultaneous detection of Salmonella, Shigella, and Staphylococcus aureus in fresh pork using a multiplex real-time PCR assay based on immunomagnetic separation. Food Cont. 2014, 42, 87–93. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Zheng, S.; Liu, Y.; He, Z.; Luo, F. Surface plasmon resonance immunosensor for fast, highly sensitive, and in situ detection of the magnetic nanoparticles-enriched Salmonella enteritidis. Sens. Actuators B Chem. 2016, 230, 191–198. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Apetrei, C.; Lozano, J.; Anyogu, A. Potential use of electronic noses, electronic tongues and biosensors as multisensor systems for spoilage examination in foods. Trends Food Sci. Technol. 2018, 80, 71–92. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Yu, Y.-N.; Abbod, M.F.; Shih, C.-H.; Shieh, J.-S. Using artificial neural network to predict a variety of pathogenic microorganisms. Sens. Mater. 2020, 32, 2375–2385. [Google Scholar]
- Capuano, R.; Paba, E.; Mansi, A.; Marcelloni, A.M.; Chiominto, A.; Proietto, A.R.; Zampetti, E.; Macagnano, A.; Lvova, L.; Catini, A.; et al. Aspergillus species discrimination using a gas sensor array. Sensors 2020, 20, 4004. [Google Scholar] [CrossRef]
- Martínez-Bisbal, M.C.; Carbó Mestre, N.; Martínez-Máñez, R.; Bauzá, J.; Alcañiz Fillol, M. Microalgae degradation follow up by voltammetric electronic tongue, impedance spectroscopy and NMR spectroscopy. Sens. Actuators B Chem. 2019, 281, 44–52. [Google Scholar] [CrossRef]
- Söderström, C.; Borén, H.; Winquist, F.; Krantz-Rülcker, C. Use of an electronic tongue to analyze mold growth in liquid media. Int. J. Food Microbiol. 2003, 83, 253–261. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, X.; Dou, W.; Tian, S.; Deng, S.; Shi, J. Use of the smart tongue to monitor mold growth and discriminate between four mold species grown in liquid media. Anal. Chim. Acta 2011, 690, 240–247. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Zhang, X. Monitoring of quality and storage time of unsealed pasteurized milk by voltammetric electronic tongue. Electrochim. Acta 2013, 88, 231–239. [Google Scholar] [CrossRef]
- Gomez, J.K.C.; Acevedo, C.M.D.; Garcia, R.O. Application of an E-Tongue and E-nose for a rapid E. coli detection in a drinking water treatment plant. In Proceedings of the ISOEN 2019—18th International Symposium on Olfaction and Electronic Nose, Fukuoka, Japan, 26–29 May 2019. [Google Scholar]
- Ruiz-Rico, M.; Fuentes, A.; Masot, R.; Alcañiz, M.; Fernández-Segovia, I.; Barat, J.M. Use of the voltammetric tongue in fresh cod (Gadus morhua) quality assessment. Innov. Food Sci. Emerg. Technol. 2013, 18, 256–263. [Google Scholar] [CrossRef]
- Söderström, C.; Winquist, F.; Krantz-Rülcker, C. Recognition of six microbial species with an electronic tongue. Sens. Actuators B Chem. 2003, 89, 248–255. [Google Scholar] [CrossRef]
- Söderström, C.; Rudnitskaya, A.; Legin, A.; Krantz-Rülcker, C. Differentiation of four Aspergillus species and one Zygosaccharomyces with two electronic tongues based on different measurement techniques. J. Biotechnol. 2005, 119, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Jianping, S.; Jing, Z.; Yuming, G.; Zhiming, X. The compare of microbial electronic tongue data based on direct and two-stage processing. Appl. Mech. Mater. 2010, 20, 331–336. [Google Scholar]
- Gil, L.; Barat, J.M.; Escriche, I.; Garcia-Breijo, E.; Martínez-Máñez, R.; Soto, J. An electronic tongue for fish freshness analysis using a thick-film array of electrodes. Microchim. Acta 2008, 163, 121–129. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Teye, E.; Haiyang, G. Quantitative Analysis of Fish Microbiological Quality Using Electronic Tongue Coupled with Nonlinear Pattern Recognition Algorithms. J. Food Saf. 2015, 35, 336–344. [Google Scholar] [CrossRef]
- Al Ramahi, R.; Zaid, A.N.; Abu-Khalaf, N. Evaluating the potential use of electronic tongue in early identification and diagnosis of bacterial infections. Infect. Drug Resist. 2019, 12, 2445–2451. [Google Scholar] [CrossRef] [Green Version]
- Abu-Khalaf, N.; Rumaila, B.A. Electronic tongue and box-PCR for categorization of different Fusarium strains. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 121–128. [Google Scholar]
- Saylan, Y.; Erdem, O.; Cihangir, N.; Denizli, A. Detecting fingerprints of waterborne bacteria on a sensor. Chemosensors 2019, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Singh, M.; Kim, S.J.; Schaefer, J. Characterization of the tertiary structure of the peptidoglycan of Enterococcus faecalis. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, S.M.; De Pedro, M.A.; Cava, F.; Huang, K.C. HPLC analyses of bacterial cell wall composition. Mol. Microbiol. 2013, 89, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalen, V.R.; Peschel, A.; Sorge, N.M.V. Wall Teichoic Acid in Staphylococcus aureus Host Interaction. Trends Microbiol. 2020, 28, 985–996. [Google Scholar] [CrossRef]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste sensor: Electronic tongue with lipid membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the amount of bitter substances adsorbed onto lipid/polymer membrane and the electric response of taste sensors. Sensors 2014, 14, 16274–16286. [Google Scholar] [CrossRef]
- Yasuura, M.; Shen, Q.; Tahara, Y.; Yatabe, R.; Toko, K. Development and investigation of a sweetness sensor for sugars—Effect of lipids. Sens. Mater. 2015, 27, 351–358. [Google Scholar]
- Sharma, G.; Kumar, S.; Kumar, A.; Sharma, A.; Kumar, R.; Kaur, R.; Bhondekar, A.P. Development of Lipid Membrane Based Taste Sensors for Electronic Tongue. Procedia Comput. Sci. 2015, 70, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Veloso, A.C.A.; Dias, L.G.; Rodrigues, N.; Pereira, J.A.; Peres, A.M. Sensory intensity assessment of olive oils using an electronic tongue. Talanta 2016, 146, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Slim, S.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Oueslati, S.; Peres, A.M. Application of an electronic tongue for Tunisian olive oils’ classification according to olive cultivar or physicochemical parameters. Eur. Food Res. Technol. 2017, 243, 1459–1470. [Google Scholar] [CrossRef]
- Arca, V.C.; Peres, A.M.; Machado, A.A.; Bona, E.; Dias, L.G. Sugars’ quantifications using a potentiometric electronic tongue with cross-selective sensors: Influence of an ionic background. Chemosensors 2019, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-M.; Kwon, H.-C.; Xu, B.; Jung, D.; Han, M.; Kwon, D.-H.; Kang, S.-W. Taste sensor based on the floating gate structure of a lateral double-diffused metal-oxide semiconductor. Sens. Actuators B Chem. 2020, 308, 127661. [Google Scholar] [CrossRef]
- Wu, X.; Miyake, K.; Tahara, Y.; Fujimoto, H.; Iwai, K.; Narita, Y.; Hanzawa, T.; Kobayashi, T.; Kakiuchi, M.; Ariki, S.; et al. Quantification of bitterness of coffee in the presence of high-potency sweeteners using taste sensors. Sens. Actuators B Chem. 2020, 309, 127784. [Google Scholar] [CrossRef]
- Gregersen, T. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 1978, 5, 123–127. [Google Scholar] [CrossRef]
- Rodrigues, N.; Marx, I.M.G.; Casal, S.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Application of an electronic tongue as a single-run tool for olive oils’ physicochemical and sensory simultaneous assessment. Talanta 2019, 197, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, L.A.; Peres, A.M.; Veloso, A.C.A.; Reis, F.S.; Vilas-Boas, M.; Machado, A.A.S.C. An electronic tongue taste evaluation: Identification of goat milk adulteration with bovine milk. Sens. Actuators B Chem. 2009, 136, 209–217. [Google Scholar] [CrossRef]
- Bertsimas, D.; Tsitsiklis, J. Simulated annealing. Stat. Sci. 1993, 8, 10–15. [Google Scholar] [CrossRef]
- Cadima, J.; Cerdeira, J.O.; Minhoto, M. Computational aspects of algorithms for variable selection in the context of principal components. Comput. Stat. Data Anal. 2004, 47, 225–236. [Google Scholar] [CrossRef]
- Kirkpatrick, S.; Gelatt, C.D.; Vecchi, M.P. Optimization by simulated annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef]
- Roig, B.; Thomas, O. Rapid estimation of global sugars by UV photodegradation and UV spectrophotometry. Anal. Chim. Acta 2003, 477, 325–329. [Google Scholar] [CrossRef]
- Roig, B.; Thomas, O. UV monitoring of sugars during wine making. Carbohydr. Res. 2003, 338, 79–83. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, R.B. Modern Applied Statistics with S—Statistics and Computing, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Association Française de Normalisation (AFNOR). Recueil des Normes Françaises, Qualité de l’Eau, 2nd ed.; Association Française de Normalisation (AFNOR): Paris, France, 1997; Volume 1, p. 293. [Google Scholar]

| Sensor Code | Plasticizer (~32%) | Additive (~3%) | |
|---|---|---|---|
| 1st Array | 2nd Array | ||
| S1:1 | S2:1 | Bis(1-butylpentyl) adipate | Octadecylamine |
| S1:2 | S2:2 | Oleyl alcohol | |
| S1:3 | S2:3 | Methyltrioctylammonium chloride | |
| S1:4 | S2:4 | Oleic acid | |
| S1:5 | S2:5 | Dibutyl sebacate | Octadecylamine |
| S1:6 | S2:6 | Oleyl alcohol | |
| S1:7 | S2:7 | Methyltrioctylammonium chloride | |
| S1:8 | S2:8 | Oleic acid | |
| S1:9 | S2:9 | 2-nitrophenyl-octyl ether | Octadecylamine |
| S1:10 | S2:10 | Oleyl alcohol | |
| S1:11 | S2:11 | Methyltrioctylammonium chloride | |
| S1:12 | S2:12 | Oleic acid | |
| S1:13 | S2:13 | Tris(2-ethylhexyl) phosphate | Octadecylamine |
| S1:14 | S2:14 | Oleyl alcohol | |
| S1:15 | S2:15 | Methyltrioctylammonium chloride | |
| S1:16 | S2:16 | Oleic acid | |
| S1:17 | S2:17 | Dioctyl phenylphosphonate | Octadecylamine |
| S1:18 | S2:18 | Oleyl alcohol | |
| S1:19 | S2:19 | Methyltrioctylammonium chloride | |
| S1:20 | S2:20 | Oleic acid | |
| Microorganism | Concentration Range (mg/mL) a | E-Tongue-MLR-SA Models b | ||||
|---|---|---|---|---|---|---|
| N° of Sensors c | Determination Coefficient (R2) | Root-Mean-Square Error (RMSE, mg/mL) | ||||
| LOO-CV d | Repeated K-Fold-CV e | LOO-CV d | Repeated K-Fold-CV e | |||
| E. coli | [0.083, 3.203] | 15 f | 0.996 | 0.993 ± 0.008 | 0.054 | 0.076 ± 0.036 |
| P. aeruginosa | [0.079, 2.820] | 14 g | 0.998 | 0.998 ± 0.002 | 0.028 | 0.032 ± 0.014 |
| E. faecalis | [0.070, 2.485] | 13 h | 0.996 | 0.993 ± 0.011 | 0.041 | 0.048 ± 0.019 |
| S. aureus | [0.148, 3.143] | 12 i | 0.994 | 0.993 ± 0.005 | 0.062 | 0.072 ± 0.030 |
| Microorganism | LOO-CV a | ||||
| R2 | Slope | Slope CI c | Intercept (mg/mL) | Intercept CI d (mg/mL) | |
| E. coli | 0.996 | 0.992 | [0.967, 1.018] | 0.0046 | [−0.0291, 0.0382] |
| P. aeruginosa | 0.998 | 0.999 | [0.982, 1.017] | −0.0007 | [−0.0190, 0.0176] |
| E. faecalis | 0.996 | 0.991 | [0.965, 1.017] | 0.0058 | [−0.0184, 0.0300] |
| S. aureus | 0.994 | 0.986 | [0.955, 1.018] | 0.0152 | [−0.0270, 0.0574] |
| Microorganism | Repeated K-fold-CV b | ||||
| R2 | Slope | Slope CI c | Intercept (mg/mL) | Intercept CI d (mg/mL) | |
| E. coli | 0.990 | 1.000 | [0.988, 1.012] | 0.0012 | [−0.0145, 0.0173] |
| P. aeruginosa | 0.997 | 1.006 | [0.999, 1.012] | −0.0026 | [−0.0096, 0.0044] |
| E. faecalis | 0.993 | 0.991 | [0.981, 1.001] | 0.0052 | [−0.0042, 0.0146] |
| S. aureus | 0.990 | 0.984 | [0.972, 0.995] | 0.0212 | [0.0053,0.0371] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghrissi, H.; Veloso, A.C.A.; Marx, Í.M.G.; Dias, T.; Peres, A.M. A Potentiometric Electronic Tongue as a Discrimination Tool of Water-Food Indicator/Contamination Bacteria. Chemosensors 2021, 9, 143. https://doi.org/10.3390/chemosensors9060143
Ghrissi H, Veloso ACA, Marx ÍMG, Dias T, Peres AM. A Potentiometric Electronic Tongue as a Discrimination Tool of Water-Food Indicator/Contamination Bacteria. Chemosensors. 2021; 9(6):143. https://doi.org/10.3390/chemosensors9060143
Chicago/Turabian StyleGhrissi, Hiba, Ana C. A. Veloso, Ítala M. G. Marx, Teresa Dias, and António M. Peres. 2021. "A Potentiometric Electronic Tongue as a Discrimination Tool of Water-Food Indicator/Contamination Bacteria" Chemosensors 9, no. 6: 143. https://doi.org/10.3390/chemosensors9060143
APA StyleGhrissi, H., Veloso, A. C. A., Marx, Í. M. G., Dias, T., & Peres, A. M. (2021). A Potentiometric Electronic Tongue as a Discrimination Tool of Water-Food Indicator/Contamination Bacteria. Chemosensors, 9(6), 143. https://doi.org/10.3390/chemosensors9060143









