Compact Miniaturized Bioluminescence Sensor Based on Continuous Air-Segmented Flow for Real-Time Monitoring: Application to Bile Salt Hydrolase (BSH) Activity and ATP Detection in Biological Fluids
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
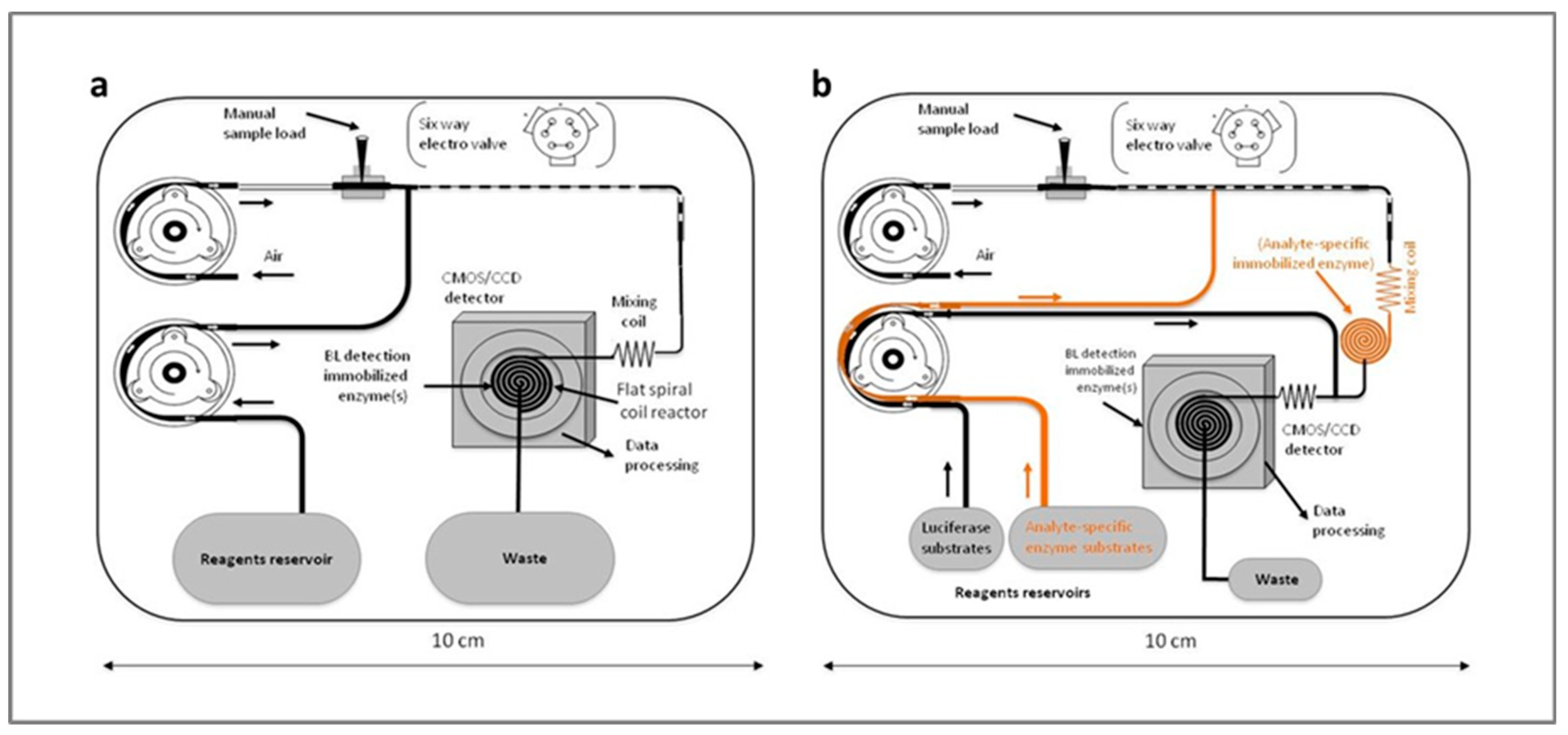
2.2. Apparatus
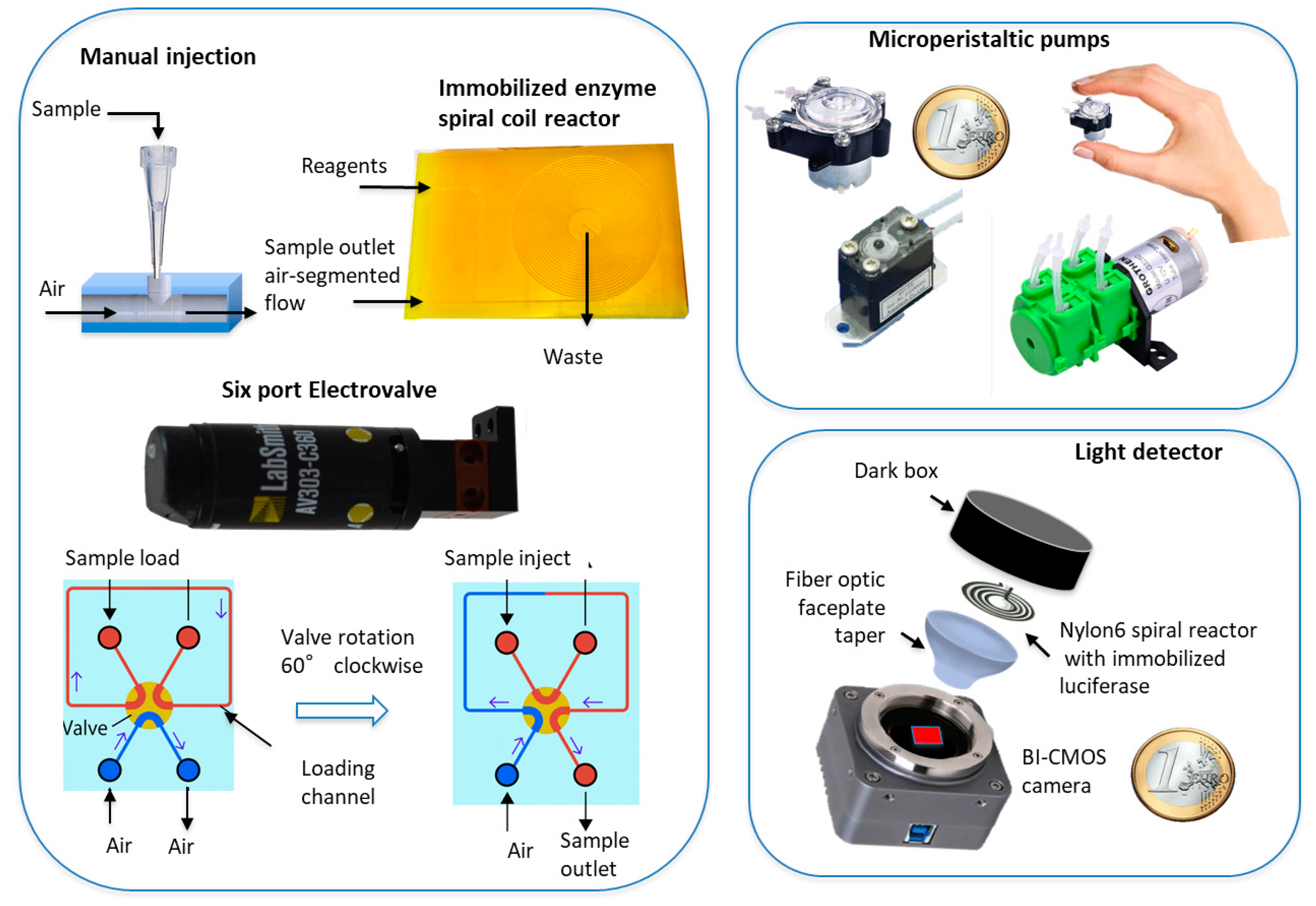
2.2.1. Peristaltic Pumps
2.2.2. Sample Injection and Reagent Delivery
2.2.3. Firefly Luciferase Immobilization on Nylon 6
2.2.4. Light Imaging Detector
2.3. Microdialysis
2.4. ATP Analysis
2.5. BSH Determination
2.5.1. BSH BL Probes
2.5.2. Synthesis of Bile Acid-Aminoluciferin-CDCA and Stable Amidated CDCA BSH Substrates
2.5.3. BSH Enzymatic Activity
2.5.4. Analysis of the Aminoluciferin Probe and Amidated BA Metabolism by HPLC-ES-MS/MS
2.5.5. BSH Ex Vivo Studies
3. Results and Discussion
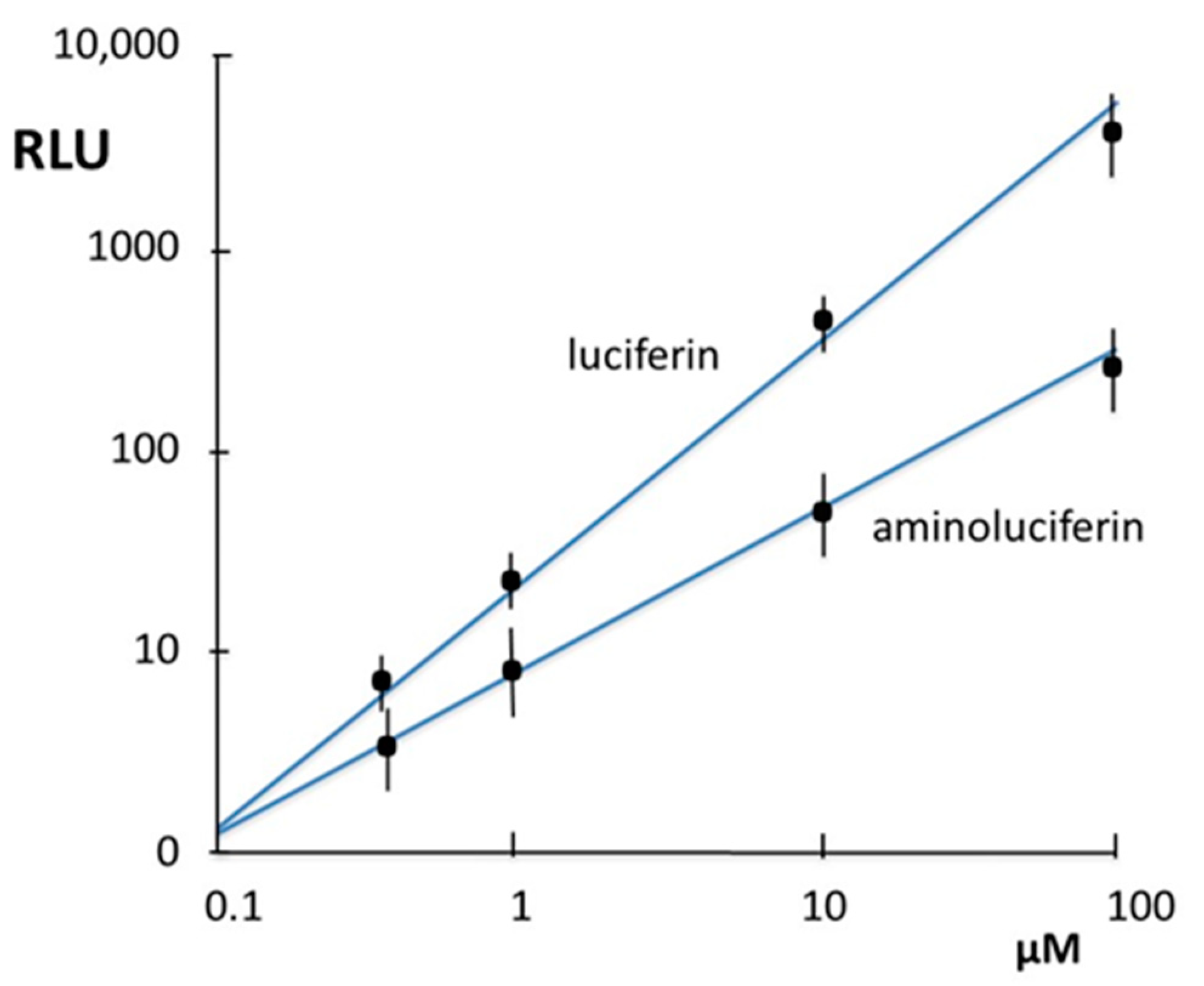
3.1. Properties of Nylon 6 Immobilized Luciferase
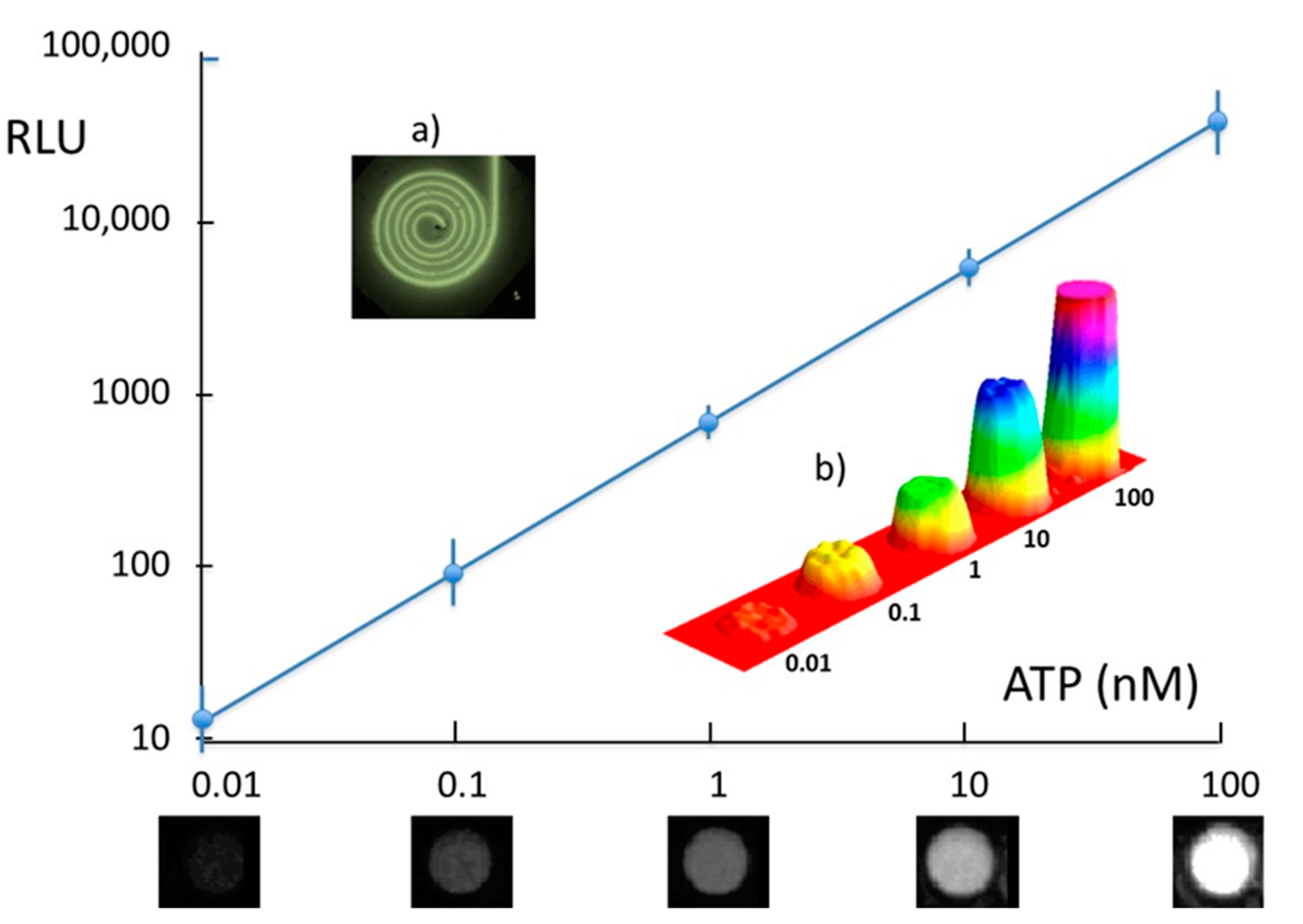
3.2. ATP Detection
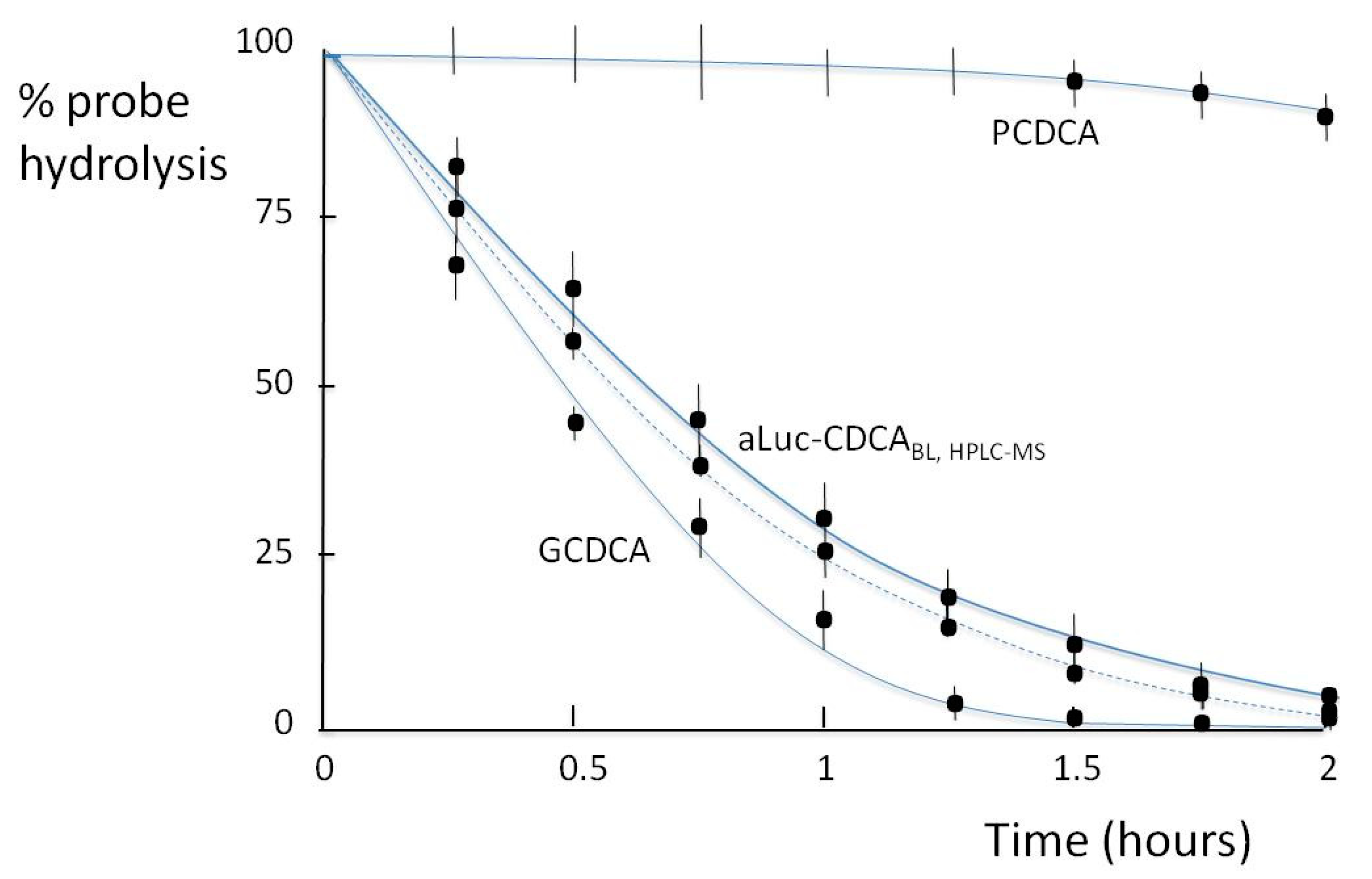
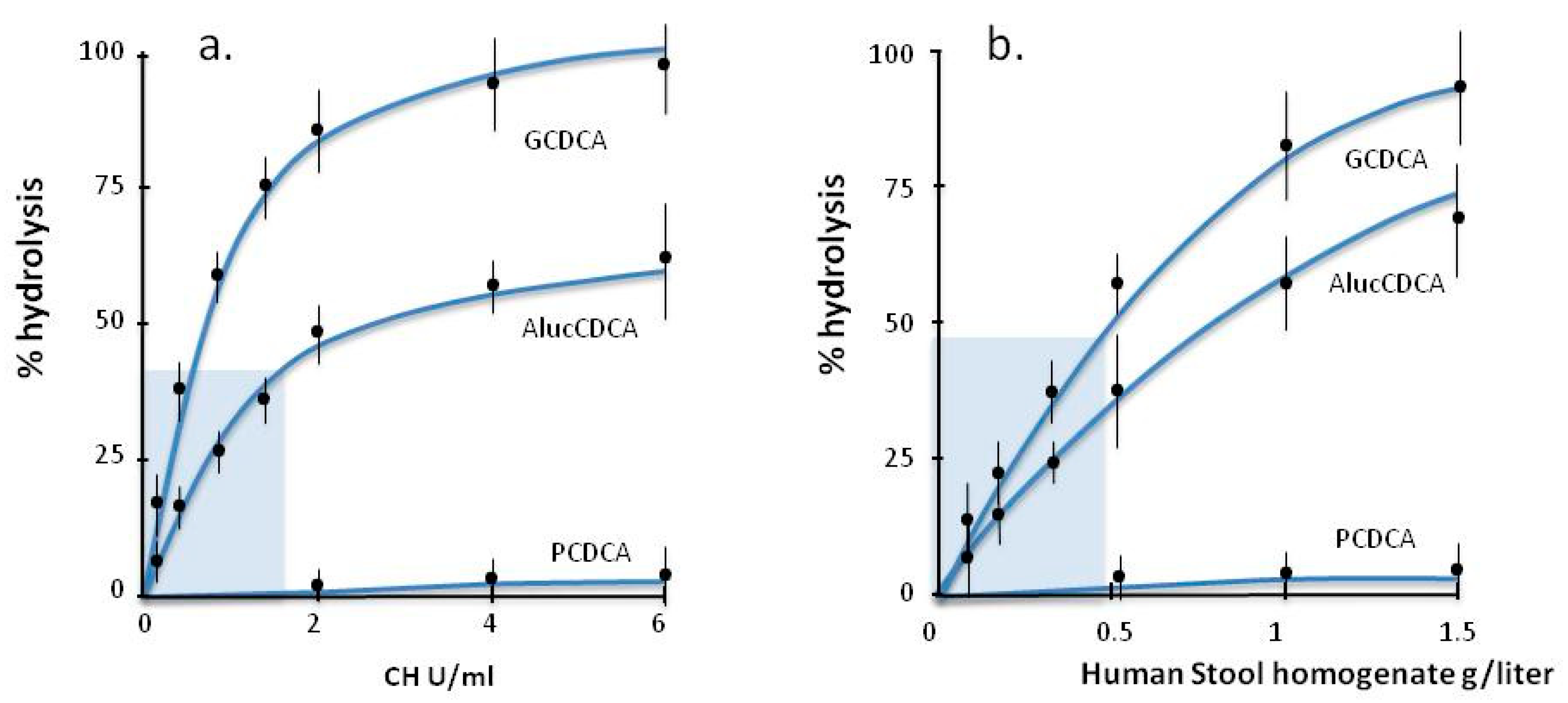
3.3. BSH Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Trojanowicz, M. Flow analysis as advanced branch of flow chemistry. Mod. Chem. Appl. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Gomes, P.R.B.; Barreto, I.S.; Carvalho, L.L.; Pinheiro, H.A.; de Lima, H.S.; Silva, E.F.; Filho, V.E.M.; Fernandes, R.N. An approach of the historical aspects, the advantages and disadvantages of automated analyzers: Analysis in segmented flow (SFA) the Flow Analyzer-batch (FBA). J. Chem. Pharm. Res. 2015, 7, 901–906. [Google Scholar]
- Timofeeva, I.I.; Vakha, C.S.; Bulatova, A.V.; Worsfold, P.J. Flow analysis with chemiluminescence detection: Recent advances and applications. Talanta 2018, 179, 246–270. [Google Scholar]
- Ruzicka, J.; Hansen, E.H. Flow injection analyses: Part I. A new concept of fast continuous flow analysis. Anal. Chim. Acta 1975, 78, 145–157. [Google Scholar] [CrossRef]
- Skeggs, L.T. An automatic method for colorimetric analysis. Am. J. Clin. Pathol. 1957, 28, 311–322. [Google Scholar] [CrossRef]
- Roda, A.; Girotti, S.; Ghini, S.; Grigolo, B.; Carrea, G.; Bovara, R. Continous-flow determination of primary bile acids, by bioluminescence, with use of nylon-immobilized bacterial enzymes. Clin. Chem. 1984, 30, 206–220. [Google Scholar] [CrossRef]
- Carrea, G.; Bovara, R.; Mazzola, G.; Girotti, S.; Roda, A.; Ghini, S. Bioluminescent continous-flow assay of adenosine 5’-triphosphate using firefly luciferase immobilized on nylon tube. Anal. Chem. 1986, 58, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Girotti, S.; Ghini, S.; Carrea, G.; Bovara, R.; Roda, A.; Budini, R. Bioluminescent flow sensor for d-(−)-lactate. Anal. Chim. Acta 1991, 255, 259–268. [Google Scholar] [CrossRef]
- Girotti, S.; Ferri, E.; Ghini, S.; Budini, R.; Carrea, G.; Bovara, R.; Piazzi, R.; Merighi, R.; Roda, A. Bioluminescent flow sensor for L-phenylalanine determination in serum. Talanta 1993, 40, 425–430. [Google Scholar] [CrossRef]
- Girotti, S.; Roda, A.; Ghini, S.; Grignolo, B.; Carrea, G.; Bovara, R. Continuous flow analysis of NADH using bacterial bioluminescent enzymes immobilized on nylon enzymes, flow analysis. Anal. Lett. 1984, 17, 1–12. [Google Scholar] [CrossRef]
- Alatawi, F.S.; Elsayed, N.H.; Monier, M. Immobilization of horseradish peroxidase on modified nylon-6 fiber. ChemistrySelect 2020, 5, 6841–6850. [Google Scholar] [CrossRef]
- Roda, A.; Girotti, S.; Ghini, S.; Carrea, G. Coupled reactions for the determination of analytes and enzymes based on the use of luminescence. J. Biolumin. Chemilumin. 1989, 4, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Girotti, S.; Roda, A.; Ghini, S.; Piacentini, A.; Carrea, G.; Bovara, R.; Angellotti, M. Luminescent flow-sensors: Properties and automation. Ann. Chim. 1987, 77, 625–636. [Google Scholar]
- Kim, H.; Jung, Y.; Doh, I.-J.; Lozano-Mahecha, R.A.; Applegate, B.; Bae, E. Smartphone-based low light detection for bioluminescence application. Sci. Rep. 2017, 7, 40203. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Shirane, S.; Matsuda, T.; Nagayama, K.; Nagai, T. Smartphone-based portable bioluminescence imaging system enabling observation at various scales from whole mouse body to organelle. Sensors 2020, 20, 7166. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.M.; Zangheri, M.; Lopreside, A.; Marchegiani, E.; Montali, L.; Simoni, P.B.; Roda, A. Precision medicine, bioanalytics and nanomaterials: Toward a new generation of personalized portable diagnostics. Analyst 2020, 145, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAc Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Rong, G.; Corrie, S.R.; Clark, H.A. In vivo biosensing: Progress and perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhu, B.; Rong, G.; Sawan, M. Towards wearable and implantable continuous drug monitoring: A review. J. Pharm. Anal. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Dulay, S.; Miserere, S.; Pla, L.; Marin, S.B.; Parra, J.; Eixarch, E.; Illa, M.; Mir, M.; Samitier, J. Micro-needle implantable electrochemical oxygen sensor: Ex-vivo and in-vivo studies. Biosens. Bioelectron. 2020, 153, 112028. [Google Scholar] [CrossRef]
- Ligon, W.V. Mass spectrometric determination of dipeptides after formation of a surface active derivative. Anal. Chem. 1986, 58, 485–487. [Google Scholar] [CrossRef]
- Ungersted, U. Microdialysis-a new bio- analytical sampling technique. Curr. Separat. 1986, 7, 43–46. [Google Scholar]
- Ungersted, U.; Hallstriim, A. In vivo micro-dialysis—A new approach to the analysis of neurotransmitters in the brain. Life Sci. 1987, 41, 861–864. [Google Scholar] [CrossRef]
- Roda, A.; Girotti, S.; Grigolo, B.; Ghini, S.; Carrea, G.; Bovara, R.; Zini, I.; Grimaldi, R. Microdialysis and luminescent probe: Analytical and clinical aspects. Biosens. Bioelectron. 1991, 6, 21–29. [Google Scholar] [CrossRef]
- Nandia, P.; Luntea, S.M. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: A review. Anal. Chim. Acta 2009, 651, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of brain microdialysis. Curr. Protoc. Neurosci. 2009, 47, 7.1.1–7.1.28. [Google Scholar] [CrossRef]
- Lietsche, J.; Gorka, J.; Hardt, S.; Karas, M.; Klein, J. Custom-made microdialysis probe design. J. Vis. Exp. 2015, 101, e53048. [Google Scholar] [CrossRef] [PubMed]
- van der Mierden, S.; Savelyev, S.A.; IntHout, J.; de Vries, R.B.M.; Leenaars, C.H.C. Intracerebral microdialysis of adenosine and adenosine monophosphate-a systematic review and meta-regression analysis of baseline concentrations. J. Neurochem. 2018, 147, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Abel, L.; Gibson, G.E.; Dienel, G.A. Handbook of Neurochemistry and Molecular Neurobiology, 3rd ed.; Abel, L., Gibson, G.E., Dienel, G.A., Eds.; Springer Science & Business Media: New York, NY, USA, 2007. [Google Scholar]
- Lada, M.W.; Kennedy, R.T. Quantitative in vivo monitoring of primary amines in rat caudate nucleus using microdialysis coupled by a flow-gated interface to capillary electrophoresis with laser-induced fluorescence detection. Anal. Chem. 1996, 68, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Lee, B.H. Bile salt hydrolases: Structure and function, substrate preference, and inhibitor development. Protein Sci. 2018, 27, 1742–1754. [Google Scholar] [CrossRef]
- Khodakivskyi, P.V.; Lauber, C.L.; Yevtodiyenko, A.; Bazhin, A.A.; Bruce, S.; Ringel-Kulka, T.; Ringel, Y.; Bétrisey, B.; Torres, J.; Hu, J.; et al. Noninvasive imaging and quantification of bile salt hydrolase activity: From bacteria to humans. Sci. Adv. 2021, 7, eaaz9857. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Cerrè, C.; Manetta, A.C.; Cainelli, G.; Umani-Ronchi, A.; Panunzio, M. Synthesis and physicochemical, biological, and pharmacological properties of new bile acids amidated with cyclic amino acids. J. Med. Chem. 1996, 39, 2270–2276. [Google Scholar] [CrossRef]
- Venturoni, F.; Gioiello, A.; Sardella, R.; Natalini, B.; Pellicciari, R. Continuous flow synthesis and scale-up of glycine- and taurine-conjugated bile salts. Org. Biomol. Chem. 2012, 10, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Batta, A.K.; Salen, G.; Shefer, S. Substrate specificity of cholylglycine hydrolase for the hydrolysis of bile acid conjugates. J. Biol. Chem. 1984, 259, 15036–15039. [Google Scholar] [CrossRef]
- Lilienau, J.; Schteingart, C.D.; Hofmann, A.F. Physicochemical and physiological properties of cholyl-sarcosine: A potential replacement detergent for bile acid deficiency states in the small intestine. J. Clin. Investig. 1992, 89, 420–431. [Google Scholar] [CrossRef][Green Version]
- Roda, A.; Grigolo, B.; Aldini, R.; Simoni, P.; Pellicciari, R.; Natalini, B.; Balducci, R. Bile acids with a cyclopropyl-containing side chain. IV. Physicochemical and biological properties of the four diastereoisomers of 3R,7â-dihydroxy-22,23-methylene-5â-cholan-24-oic acid. J. Lipid Res. 1987, 28, 1384–1397. [Google Scholar] [CrossRef]
- Yoon, Y.B.; Hagey, L.R.; Hofmann, A.F.; Gurantz, D.; Michelotti, E.L.; Steinbach, J.H. Effect of side-chain shortening on the physiologic properties of bile acids: Hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology 1986, 90, 837–852. [Google Scholar] [CrossRef]
- Roda, A.; Gioacchini, A.M.; Cerrè, C.; Baraldini, M. High-performance liquid chromatographic-electrospray mass spectrometric analysis of bile acids in biological fluids. J. Chromatogr. B 1995, 665, 281–294. [Google Scholar] [CrossRef]
- Hornby, W.E.; Goldstein, L. Methods in Enzymology; Mosbach, K., Ed.; Academic Press: New York, NY, USA, 1976; Volume 44, pp. 118–134. [Google Scholar]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef]
- Grigolo, B.; Roda, A.; Girotti, S.; Ghini, S.; Carrea, G.; Bovara, R.; Zini, I.; Grimaldi, R. Real time analysis of ATP in vivo using a micropdialysis and bioluminescent probe. Chim. Oggi Chem. Today 1990, 8, 19–21. [Google Scholar]
- Velasquez, S.; Prevedel, L.; Valdebenito, S.; Gorska, A.M.; Golovko, M.; Khanb, N.; Geiger, J.; Eugenin, E.A. Circulating levels of ATP is a biomarker of HIV cognitive impairment. EBioMedicine 2020, 51, 102503. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Knol, J.; Garssen, J.; van Bergenhenegouwen, J. The gut microbiota as a therapeutic target in IBD and metabolic disease: A role for the bile acid receptors FXR and TGR5. Microorganisms 2015, 3, 641–666. [Google Scholar] [CrossRef]
- Das, P.; Marcišauskas, S.; Ji, B.; Nielsen, J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genom. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Girotti, S.; Cascione, M.L.; Ghini, S.; Carrea, G.; Bovara, R.; Roda, A.; Motta, R.; Petilino, R. Bioluminescence flow sensor for determination of creatine kinase activity in blood. Anal. Chim. Acta 1989, 227, 29–36. [Google Scholar] [CrossRef]

| Body Fluid | ATP Conc Nmol/L | ||||
|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | Min | |
| Jugular vein | 12 | 10 | 11.5 | 12 | |
| Cerebrospinal fluid | 800 | 745 | 873 | 701 | |
| Caudate nucleus | 9 | 11 | 9 | 12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, A.; Greco, P.; Simoni, P.; Marassi, V.; Moroni, G.; Gioiello, A.; Roda, B. Compact Miniaturized Bioluminescence Sensor Based on Continuous Air-Segmented Flow for Real-Time Monitoring: Application to Bile Salt Hydrolase (BSH) Activity and ATP Detection in Biological Fluids. Chemosensors 2021, 9, 122. https://doi.org/10.3390/chemosensors9060122
Roda A, Greco P, Simoni P, Marassi V, Moroni G, Gioiello A, Roda B. Compact Miniaturized Bioluminescence Sensor Based on Continuous Air-Segmented Flow for Real-Time Monitoring: Application to Bile Salt Hydrolase (BSH) Activity and ATP Detection in Biological Fluids. Chemosensors. 2021; 9(6):122. https://doi.org/10.3390/chemosensors9060122
Chicago/Turabian StyleRoda, Aldo, Pierpaolo Greco, Patrizia Simoni, Valentina Marassi, Giada Moroni, Antimo Gioiello, and Barbara Roda. 2021. "Compact Miniaturized Bioluminescence Sensor Based on Continuous Air-Segmented Flow for Real-Time Monitoring: Application to Bile Salt Hydrolase (BSH) Activity and ATP Detection in Biological Fluids" Chemosensors 9, no. 6: 122. https://doi.org/10.3390/chemosensors9060122
APA StyleRoda, A., Greco, P., Simoni, P., Marassi, V., Moroni, G., Gioiello, A., & Roda, B. (2021). Compact Miniaturized Bioluminescence Sensor Based on Continuous Air-Segmented Flow for Real-Time Monitoring: Application to Bile Salt Hydrolase (BSH) Activity and ATP Detection in Biological Fluids. Chemosensors, 9(6), 122. https://doi.org/10.3390/chemosensors9060122








