Recent Microfluidic Innovations for Sperm Sorting
Abstract
1. Introduction
2. Microfluidic Sperm-Sorting Techniques
2.1. Passive Methods
2.1.1. Geometry and Chip Design
2.1.2. Rheotaxis
2.1.3. Fluid Flows
| Sorting Strategy | Parameter(s) | Advantages | Disadvantages | Significance | Ref. |
|---|---|---|---|---|---|
| Geometry | Swimming behavior of sperms, micro-pillar arrays | -Noninvasive -Reduced complexity of structural features -Mimics filtering characteristics of female reproductive tract | -Complicated chip fabrication process due to complex high-aspect-ratio geometry | -Morphology: 5-fold enhancement -Nuclear Maturity: 3-fold enhancement -DNA integrity: 2–4-fold enhancement -Throughput: 99% -Working time: 10 min | [62] |
| -Velocity shear gradient -Hydrodynamic profile of fluid micro-confinement | -Simple working procedure | -Complicated chip design and fabrication due to complex high-aspect-ratio geometry | -Retrieval efficiency: 44% increased -Throughput: 80% -Optimized flow rate: 0.7 µL/min | [64] | |
| -Hydrodynamic profile of fluid within the channel -Fluid flow mechanics -Shear rate butterfly-shape structure | -Mimics the variable width of the junctions within the female reproductive tract -Simple chip design and fabrication | -Accumulation of a large population of sperms in front of the stricture leads to reduced efficiency of sorting highly motile sperms | -Highly progressive motile sperms swim to the fertilized site -Non-motile and slow sperms accumulate in front of the stricture | [65] | |
| Rheotaxis | -Rheotactic behavior of sperms -Corrals inside microchannels -Flow rate | -Adding sperm retainer | -Complicated chip fabrication due to complex high-aspect-ratio geometry | -Throughput: 100% -Residence time: 45 min | [70] |
| -Fluid flow -Rheotactic behavior of sperms -Gravity | -Automated procedure -Fast sorting -Eliminate the use of additional tools, such as a pump -Simple chip design and fabrication | -Misses some of the potentially high-quality sperms due to the rapid pace | -Optimized delay time between semen injection and suctioning motile sperms: 80 s -Highest figures of motility indexes are mean velocity: 8.94%, motility percentage: 32.58%, motile sperm rate: 21.99% | [71] | |
| -Fluid velocity inside the channel -Designing a diffuser-type channel | -Simple chip design and fabrication -Performance based on continuity equation in fluid dynamics | -Imprecise collection of sorted sperms in appropriate region | -Throughput: 8.6 × 105 sperms/min -Working time: 10 min -%Motility: 82.24% -Motile sperm rate: 53.10% | [72] | |
| Fluid Flow | -Three different parallel laminar flows -Variable semen flow rate - Ability of sperms to cross streamlines in laminar flow | -Mimic viscous environment of female reproductive tract -Simple chip design and fabrication | -Missing some of potentially high-quality sperms due to time dependency of migration in laminar fluid | -Sperm activity: 95.7% | [76] |
| -Diffuser-type channel -Fluid dynamics production -Enabling cross-passage of sperms through laminar flow streamline | -Continuity equation in fluid dynamics | -Complicated chip design and fabrication due to complex high-aspect-ratio geometry | -Motility pattern of more functional sperms: sinusoidal trajectory pattern -DNA integrity: 95% -DNA fragmentation: 18.4–21.9% | [77] |

2.2. Active Methods
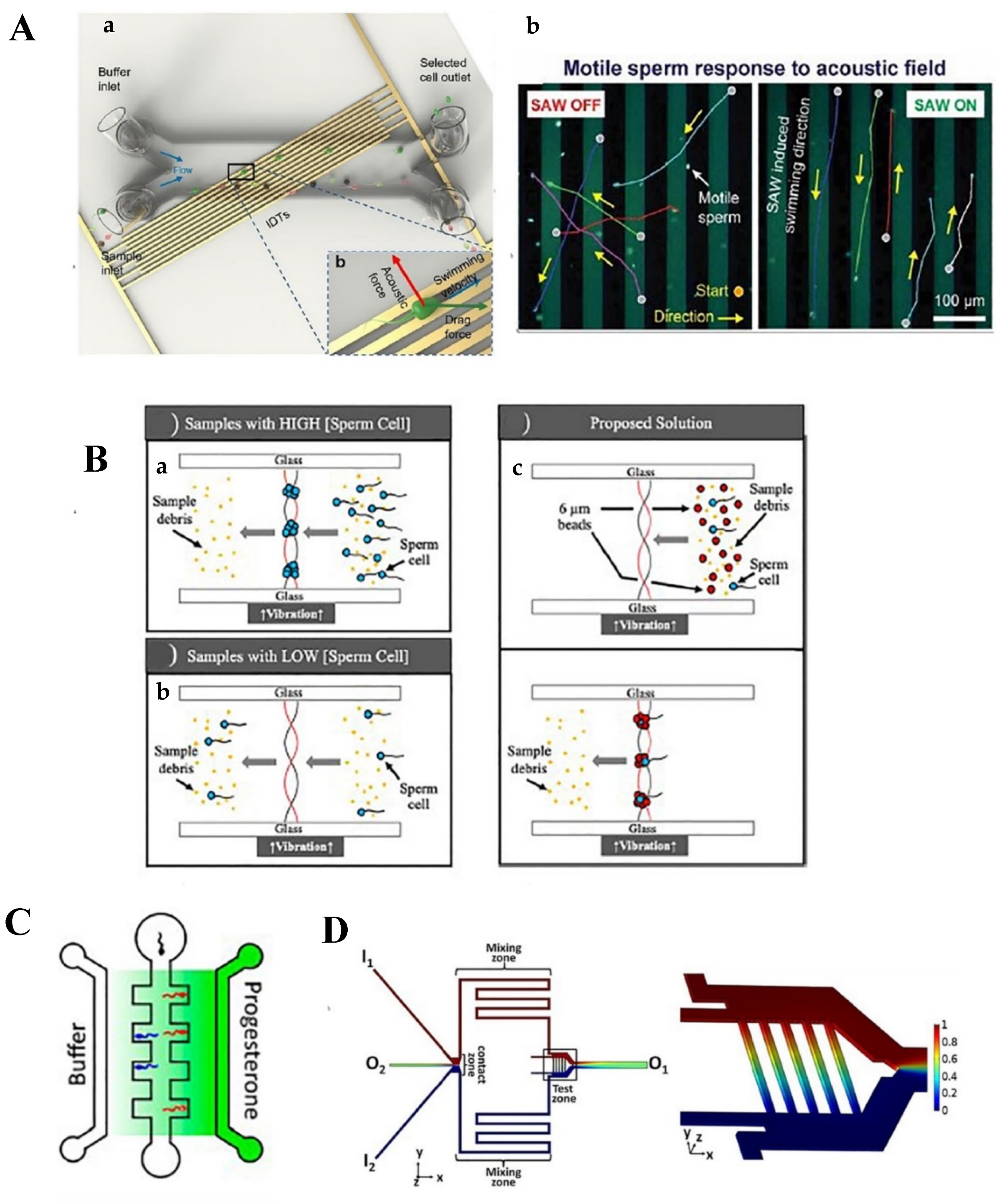
2.2.1. Acoustic Waves
2.2.2. Chemotaxis and Thermotaxis
| Sorting Strategy | Parameter(s) | Advantages | Disadvantages | Significance | Ref. |
|---|---|---|---|---|---|
| Acoustic waves | -Surface acoustic wave -Sperm size -Motility pattern | -External sorting -Precise control of sperm selection process | -Invasive -Need for additional equipment | -Operation time: 50 min -Throughput: 60,000 sperms/cycle -Vitality: 50% -Progressive motility: 60% -DNA integrity: >38% -Swimming velocity: 64% | [78] |
| -Bulk acoustic wave -Pressure distribution through the fluid -Addition of polystyrene beads | -Isolates scarce number of sperms from female DNA samples | -Lower power compared to surface acoustic wave -Invasive -Need for additional equipment | -Operation time: 15 min -Particle size of polystyrene beads: equal to sperms -Isolation efficiency: 85% | [84] | |
| Chemotaxis | -Progesterone gradient concentration -Sperms’ chemoattractant behavior | -Noninvasive -Biomimetic strategy -Flow-free | -Low efficiency | -Sperms chemotactic ratio: 1.41 | [87] |
| -Ach 1 and rat oviductal fluid gradient concentration -Sperms’ chemoattractant behavior | -Uniform gradient -Stationary fluidic environment -Biomimetic strategy -Eliminate rheotactic and chemokinetic behavior of sperms as selection criteria | -Low efficiency | -Improved number of entered sperms by increasing ACh concentration: 20% -Sperm population with chemotactic behavior in ACh-rich environment: 8.5% -Sperm population with chemotactic behavior in oviductal fluidic environment: 6.6% | [90] | |
| Chemotaxis and thermotaxis | -ACh gradient concentration -Temperature gradient -Sperms’ chemoattractant and thermoattractant behavior | -Flow-free -Biomimetic strategy | -Complicated chip design and fabrication due to complex high-aspect-ratio geometry -Need of additional structural features | -Optimized temperature gradient: 0.154 °C/mm from 35 to 37 °C | [91] |
2.3. Point-of-Care (PoC) Microfluidic Devices for Sperm Sorting
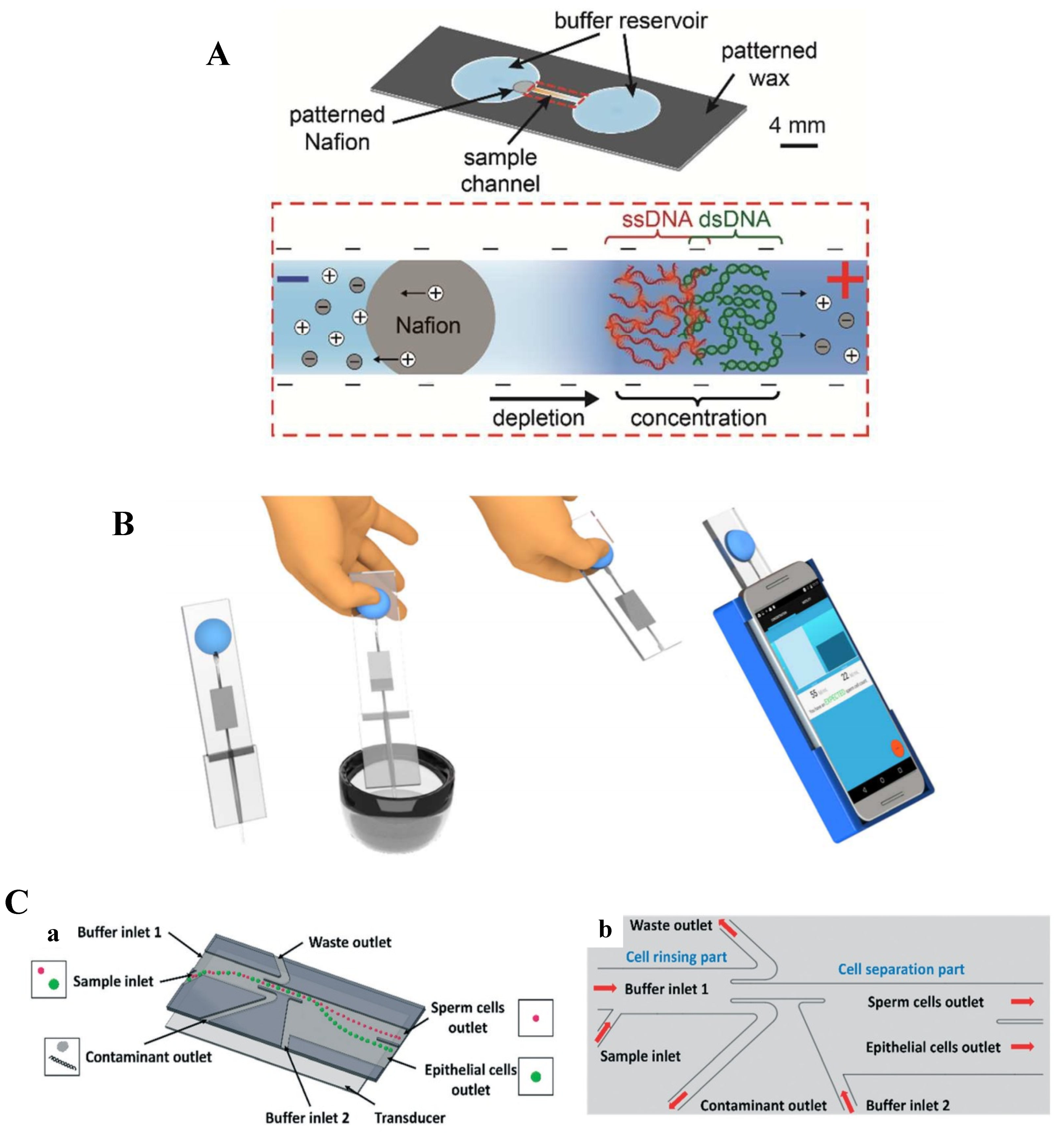
3. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuel, R.; Feng, H.; Jafek, A.; Despain, D.; Jenkins, T.; Gale, B. Microfluidic—Based sperm sorting & analysis for treatment of male infertility. Transl. Androl. Urol. 2018, 7, S336. [Google Scholar]
- Ortseifen, V.; Viefhues, M.; Wobbe, L.; Grünberger, A. Microfluidics for Biotechnology: Bridging Gaps to Foster Microfluidic Applications. Front. Bioeng. Biotechnol. 2020, 8, 1324. [Google Scholar] [CrossRef]
- Nikshad, A.; Aghlmandi, A.; Safaralizadeh, R.; Aghebati-Maleki, L.; Warkiani, M.E.; Khiavi, F.M.; Yousefi, M. Advances of microfluidic technology in reproductive biology. Life Sci. 2021, 265, 118767. [Google Scholar] [CrossRef]
- Sequeira, R.C.; Criswell, T.; Atala, A.; Yoo, J.J. Microfluidic systems for assisted reproductive technologies: Advantages and potential applications. Tissue Eng. Regen. Med. 2020, 17, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shoaie, N.; Jahanpeyma, F.; Zhao, J.; Azimzadeh, M.; Al−Jamal, K.T. Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview. Biosens. Bioelectron. 2020, 161. [Google Scholar] [CrossRef] [PubMed]
- Manafi, N.; Shokri, F.; Achberger, K.; Hirayama, M.; Mohammadi, M.H.; Noorizadeh, F.; Hong, J.; Liebau, S.; Tsuji, T.; Quinn, P.M.J.; et al. Organoids and organ chips in ophthalmology. Ocul. Surf. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, S.N.; Azimzadeh, M.; Ozsoz, M. Electrochemical biosensors for cancer biomarkers detection: Recent advances and challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Ahmed, I.; Akram, Z.; Bule, M.H.; Iqbal, H.M.N. Advancements and potential applications of microfluidic approaches—A review. Chemosensors 2018, 6, 46. [Google Scholar] [CrossRef]
- Tzouanas, C.; Lim, J.S.Y.; Wen, Y.; Thiery, J.P.; Khoo, B.L. Microdevices for non-invasive detection of bladder cancer. Chemosensors 2017, 5, 30. [Google Scholar] [CrossRef]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Hosseini, M.; Verplancke, R.; Schaubroeck, D.; Van Put, S.; Razi, F.; De Keersmaecker, M.; Adriaens, A.; et al. A Multiplexed microfluidic platform for bone marker measurement: A Proof-of-concept. Micromachines 2017, 8, 133. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Marzano, G.; Chiriacò, M.S.; Primiceri, E.; Dell’Aquila, M.E.; Ramalho-Santos, J.; Zara, V.; Ferramosca, A.; Maruccio, G. Sperm selection in assisted reproduction: A review of established methods and cutting-edge possibilities. Biotechnol. Adv. 2020, 40, 107498. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Aflatoonian, B. From stem cells to spermatozoa and back. Soc. Reprod. Fertil. Suppl. 2007, 65, 19–32. [Google Scholar]
- Cardona Barberán, A.; Boel, A.; Vanden Meerschaut, F.; Stoop, D.; Heindryckx, B. Diagnosis and treatment of male infertility-related fertilization failure. J. Clin. Med. 2020, 9, 3899. [Google Scholar] [CrossRef]
- Omidi, M.; Aflatoonian, B.; Tahajjodi, S.S.; Khalili, M.A. Attempts for generation of embryonic stem cells from human embryos following in vitro embryo twinning. Stem Cells Dev. 2019, 28, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Aflatoonian, B.; Ruban, L.; Jones, M.; Aflatoonian, R.; Fazeli, A.; Moore, H.D. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum. Reprod. 2009, 24, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Akyash, F.; Aflatoonian, R.; Golzadeh, J.; Tahajjodi, S.; Moore, H.; Aflatoonian, B. Testicular sperm extraction derived cells conditioned medium as an in vitro niche supports germ cells development from human embryonic stem cells. In Proceedings of the 35th Annual Meeting of the European Society for Human Reproduction and Embryology, Vienna, Austria, 23–26 June 2019. [Google Scholar]
- Adib, M.; Seifati, S.M.; Ashkezari, M.D.; Akyash, F.; Khoradmehr, A.; Aflatoonian, B. Effect of human testicular cells conditioned medium on in vitro maturation and morphology of mouse oocytes. Int. J. Fertil. Steril. 2020, 14, 176–184. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E. Approaches and technologies in male fertility preservation. Int. J. Mol. Sci. 2020, 21, 5471. [Google Scholar] [CrossRef]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L.R. Sperm selection in natural conception: What can we learn from Mother Nature to improve assisted reproduction outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, M.; Martinez, C.A.; Wright, D.; Rodriguez-Martinez, H. Does the act of copulation per se, without considering seminal deposition, change the expression of genes in the porcine female genital tract? Int. J. Mol. Sci. 2020, 21, 5477. [Google Scholar] [CrossRef]
- Lin, D.; Ran, J.; Zhu, S.; Quan, S.; Ye, B.; Yu, A.; Kang, Y.; Lin, Y. Effect of GOLPH3 on cumulus granulosa cell apoptosis and ICSI pregnancy outcomes. Sci. Rep. 2017, 7, 7863. [Google Scholar] [CrossRef]
- Tahajjodi, S.; Farashahi Yazd, E.; Aflatoonian, R.; Agharahimi, A.; Hajizadeh-Tafti, F.; Akyash, F.; Moore, H.; Aflatoonian, B. Cumulus cells conditioned medium as an in vitro niche for differentiation of human embryonic stem cells to female germ cells. In Proceedings of the 35th Annual Meeting of the European Society for Human Reproduction and Embryology, Vienna, Austria, 23–26 June 2019. [Google Scholar]
- Tahajjodi, S.S.; Yazd, E.F.; Agha-Rahimi, A.; Aflatoonian, R.; Khalili, M.A.; Mohammadi, M.; Aflatoonian, B. Biological and physiological characteristics of human cumulus cells in adherent culture condition. Int. J. Reprod. Biomed. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Jin, R.; Bao, J.; Tang, D.; Liu, F.; Wang, G.; Zhao, Y.; Bai, G.; Liu, Y.; Wang, Y.; Liu, L.; et al. Outcomes of intracytoplasmic sperm injection using the zona pellucida-bound sperm or manually selected sperm. J. Assist. Reprod. Genet. 2016, 33, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Khalili, M.A.; Salehi-Abargouei, A.; Rezvani, M.E.; Aflatoonian, B. Use of zona pellucida-bound sperm as a natural selection in improvement of ICSI outcomes: A systematic review and meta-analysis. Andrologia 2020, 7, e14022. [Google Scholar]
- Fitzpatrick, J.L.; Lüpold, S. Sexual selection and the evolution of sperm quality. Mol. Hum. Reprod. 2014, 20, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.; Lewis, S.; Morroll, D. Sperm quality and its relationship to natural and assisted conception: British Fertility Society Guidelines for practice. Hum. Fertil. 2013, 16, 175–193. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Martini, F.O.; McLachlan, R.; Oates, R.D.; van der Poel, S.; John, B.S.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef]
- Nosrati, R.; Vollmer, M.; Eamer, L.; San Gabriel, M.C.; Zeidan, K.; Zini, A.; Sinton, D. Rapid selection of sperm with high DNA integrity. Lab Chip 2014, 14, 1142–1150. [Google Scholar] [CrossRef]
- Nosrati, R.; Graham, P.J.; Zhang, B.; Riordon, J.; Lagunov, A.; Hannam, T.G.; Escobedo, C.; Jarvi, K.; Sinton, D. Microfluidics for sperm analysis and selection. Nat. Rev. Urol. 2017, 14, 707–730. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, T.L.; Yang, J. A novel microfluidic device for selecting human sperm to increase the proportion of morphologically normal, motile sperm with uncompromised DNA integrity. Anal. Methods 2015, 7, 5981–5988. [Google Scholar] [CrossRef]
- de Wagenaar, B.; Berendsen, J.T.W.; Bomer, J.G.; Olthuis, W.; van den Berg, A.; Segerink, L.I. Microfluidic single sperm entrapment and analysis. Lab Chip 2015, 15, 1294–1301. [Google Scholar] [CrossRef]
- Shirota, K.; Yotsumoto, F.; Itoh, H.; Obama, H.; Hidaka, N.; Nakajima, K.; Miyamoto, S. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil. Steril. 2016, 105, 315–321.e1. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Sakkas, D. Sperm selection methods in the 21. Biol. Reprod. 2019, 101, 1076–1082. [Google Scholar] [CrossRef]
- Sharma, S.; Venzac, B.; Burgers, T.; Le Gac, S.; Schlatt, S. Microfluidics in male reproduction: Is ex vivo culture of primate testis tissue a future strategy 2 for ART or toxicology research? Mol. Hum. Reprod. 2020, 26, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Lai, D.; Takayama, S.; Smith, G.D. Thinking big by thinking small: Application of microfluidic technology to improve ART. Lab Chip 2013, 13, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, Q.; Ma, R.; Xie, L.; Qiu, T.; Wang, L.; Mitchelson, K.; Wang, J.; Huang, G.; Qiao, J.; et al. Integration of single oocyte trapping, in vitrofertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip 2010, 10, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, Z.; Zhang, Y.; Chen, Z.; Niu, D.; Cao, Y.; He, X. A microfluidic perfusion approach for on-chip characterization of the transport properties of human oocytes. Lab Chip 2017, 17, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.G.; Haubert, K.; Beebe, D.J.; Ferguson, C.E.; Wheeler, M.B. Reduction of polyspermic penetration using biomimetic microfluidic technology during in vitro fertilization. Lab Chip 2005, 5, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.C.; Wheeler, M.B.; Beebe, D.J. A microfluidic method for removal of the zona pellucida from mammalian embryos. Lab Chip 2005, 5, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.C.; Beebe, D.J.; Wheeler, M.B. Removal of cumulus from mammalian zygotes using microfluidic techniques. Biomed. Microdevices 2001, 3, 219–224. [Google Scholar] [CrossRef]
- Schuster, T.G.; Cho, B.; Keller, L.M.; Takayama, S.; Smith, G.D. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod. Biomed. Online 2003, 7, 75–81. [Google Scholar] [CrossRef]
- Beebe, D.J.; Wheeler, M.; Zeringue, H.C.; Walters, E.; Raty, S. Microfluidic technology for assisted reproduction. Theriogenology 2002, 57, 125–135. [Google Scholar] [CrossRef]
- McCormack, M.C.; McCallum, S.; Behr, B. A novel microfluidic device for male subfertility screening. J. Urol. 2006, 175, 2223–2227. [Google Scholar] [CrossRef]
- Koyama, S.; Amarie, D.; Soini, H.A.; Novotny, M.V.; Jacobson, S.C. Chemotaxis assays of mouse sperm on microfluidic devices. Anal. Chem. 2006, 78, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Khimji, I.; Gurkan, U.A.; Safaee, H.; Catalano, P.N.; Keles, H.O.; Kayaalp, E.; Demirci, U. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab Chip 2011, 11, 2535–2540. [Google Scholar] [CrossRef]
- Lopez-Garcia, M.d.C.; Monson, R.L.; Haubert, K.; Wheeler, M.B.; Beebe, D.J. Sperm motion in a microfluidic fertilization device. Biomed. Microdevices 2008, 10, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Schuster, T.G.; Zhu, X.; Chang, D.; Smith, G.D.; Takayama, S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Huang, Z.-W.; Tsai, F.-S.; Chen, C.-Y.; Lin, C.-M.; Wo, A.M. Analysis of sperm concentration and motility in a microfluidic device. Microfluid. Nanofluidics 2011, 10, 59–67. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chiang, T.-C.; Lin, C.-M.; Lin, S.-S.; Jong, D.-S.; Tsai, V.F.-S.; Hsieh, J.-T.; Wo, A.M. Sperm quality assessment via separation and sedimentation in a microfluidic device. Analyst 2013, 138, 4967–4974. [Google Scholar] [CrossRef]
- Xiao, S.; Riordon, J.; Simchi, M.; Lagunov, A.; Hannam, T.; Jarvi, K.; Nosrati, R.; Sinton, D. FertDish: Microfluidic sperm selection-in-a-dish for intracytoplasmic sperm injection. Lab Chip 2021, 21, 775–783. [Google Scholar] [CrossRef]
- Knowlton, S.M.; Sadasivam, M.; Tasoglu, S. Microfluidics for sperm research. Trends Biotechnol. 2015, 33, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Takeo, T.; Watanabe, H.; Kondoh, G.; Nakagata, N. Successful selection of mouse sperm with high viability and fertility using microfluidics chip cell sorter. Sci. Rep. 2020, 10, 8862. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Jalalian, L.; Ribeiro, S.; Ona, K.; Demirci, U.; Cedars, M.I.; Rosen, M.P. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum. Reprod. 2018, 33, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Phiphattanaphiphop, C.; Leksakul, K.; Phatthanakun, R.; Khamlor, T. A novel microfluidic chip-based sperm-sorting device constructed using design of experiment method. Sci. Rep. 2020, 10, 17143. [Google Scholar] [CrossRef] [PubMed]
- Riordon, J.; Tarlan, F.; You, J.B.; Zhang, B.; Graham, P.J.; Kong, T.; Wang, Y.; Lagunov, A.; Hannam, T.; Jarvi, K.; et al. Two-dimensional planar swimming selects for high DNA integrity sperm. Lab Chip 2019, 19, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, R.-R.; Yin, T.; Zou, W.; Tang, Y.; Ding, J.; Yang, J. Generation of gradients on a microfluidic device: Toward a high-throughput investigation of spermatozoa chemotaxis. PLoS ONE 2015, 10, e0142555. [Google Scholar] [CrossRef]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Shiddiky, M.J.A.; Nguyen, N. Advances in microfluidics-based assisted reproductive technology: From sperm sorter to reproductive system-on-a-chip. Adv. Biosyst. 2017, 9, 1700197. [Google Scholar] [CrossRef]
- Riffell, J.A.; Zimmer, R.K. Sex and flow: The consequences of fluid shear for sperm-egg interactions. J. Exp. Biol. 2007, 210, 3644–3660. [Google Scholar] [CrossRef]
- Chinnasamy, T.; Kingsley, J.L.; Inci, F.; Turek, P.J.; Rosen, M.P.; Behr, B.; Tüzel, E.; Demirci, U. Guidance and self-sorting of active swimmers: 3D periodic arrays increase persistence length of human sperm selecting for the fittest. Adv. Sci. 2018, 5, 1700531. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Strünker, T. Signaling in sperm: More different than similar. Trends Cell Biol. 2016, 27, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.B.; Chen, C. Microfluidic retention of progressively motile zebrafish sperms. Lab Chip 2019, 19, 4033–4042. [Google Scholar] [CrossRef]
- Zaferani, M.; Palermo, G.D.; Abbaspourrad, A. Strictures of a microchannel impose fierce competition to select for highly motile sperm. Sci. Adv. 2019, 5, eaav2111. [Google Scholar] [CrossRef]
- Suarez, S.S.; Wu, M. Microfluidic devices for the study of sperm migration. Mol. Hum. Reprod. 2017, 23, 227–234. [Google Scholar] [CrossRef][Green Version]
- Miki, K.; Clapham, D.E. Rheotaxis guides mammalian sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef]
- Eisenbach, M.; Giojalas, L.C. Sperm guidance in mammals—An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006, 7, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.K.; Chen, P.C.; Lin, Y.N.; Wang, C.W.; Pan, L.C.; Tseng, F.G. High-throughput flowing upstream sperm sorting in a retarding flow field for human semen analysis. Analyst 2017, 142, 938–944. [Google Scholar] [CrossRef]
- Zaferani, M.; Cheong, S.H.; Abbaspourrad, A. Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. Proc. Natl. Acad. Sci. USA 2018, 115, 8272–8277. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; An, T.; Lee, D.; Kim, B. Gravity and rheotaxis based sperm sorting device employing a cam-actuated pipette mechanism. Rev. Sci. Instrum. 2019, 90. [Google Scholar] [CrossRef]
- Hwang, B.; Lee, D.; Hwang, S.J.; Baek, J.H.; Kim, B. Rheotaxis Based High-Throughput Motile Sperm Sorting Device. Int. J. Precis. Eng. Manuf. 2019, 20, 1037–1045. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, H.; Zhang, B.; Liu, R. A PMMA-based microfluidic device for human sperm evaluation and screening on swimming capability and swimming persistence. Micromachines 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lu, C.Y.; Wang, I.W.; Yao, D.J. Motility-driven sperm-sorting microfluidic chip with little cell damage for oligozoospermia patients. Sens. Mater. 2020, 32, 2585–2596. [Google Scholar] [CrossRef]
- Huang, H.Y.; Huang, P.W.; Yao, D.J. Enhanced efficiency of sorting sperm motility utilizing a microfluidic chip. Microsyst. Technol. 2017, 23, 305–312. [Google Scholar] [CrossRef]
- Nagata, M.P.B.; Endo, K.; Ogata, K.; Yamanaka, K.; Egashira, J.; Katafuchi, N.; Yamanouchi, T.; Matsuda, H.; Goto, Y.; Sakatani, M.; et al. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc. Natl. Acad. Sci. USA 2018, 115, E3087–E3096. [Google Scholar] [CrossRef]
- Gai, J.; Nosrati, R.; Neild, A. High DNA integrity sperm selection using surface acoustic waves. Lab Chip 2020, 20, 4262–4272. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics, 3rd ed.; Artech: Norwood, MA, USA, 2019; ISBN 9781630813659. [Google Scholar]
- Lin, S.C.S.; Mao, X.; Huang, T.J. Surface Acoustic Wave (SAW) acoustophoresis: Now and beyond. Lab Chip 2012, 12, 2766–2770. [Google Scholar] [CrossRef]
- Neild, A.; Oberti, S.; Dual, J. Design, modeling and characterization of microfluidic devices for ultrasonic manipulation. Sens. Actuators B Chem. 2007, 121, 452–461. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Clark, C.P.; Xu, K.; Scott, O.; Hickey, J.; Tsuei, A.C.; Jackson, K.; Landers, J.P. Acoustic trapping of sperm cells from mock sexual assault samples. Forensic Sci. Int. Genet. 2019, 41, 42–49. [Google Scholar] [CrossRef]
- Xu, K.; Clark, C.P.; Poe, B.L.; Lounsbury, J.A.; Nilsson, J.; Laurell, T.; Landers, J.P. Isolation of a low number of sperm cells from female DNA in a glass-PDMS-glass microchip via bead-assisted acoustic differential extraction. Anal. Chem. 2019, 91, 2186–2191. [Google Scholar] [CrossRef]
- Bahat, A.; Eisenbach, M. Sperm thermotaxis. Mol. Cell. Endocrinol. 2006, 252, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cerezales, S.; Laguna-Barraza, R.; De Castro, A.C.; Sánchez-Calabuig, M.J.; Cano-Oliva, E.; De Castro-Pita, F.J.; Montoro-Buils, L.; Pericuesta, E.; Fernández-González, R.; Gutiérrez-Adán, A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 2018, 8, 2902. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, J.T.W.; Kruit, S.A.; Atak, N.; Willink, E.; Segerink, L.I. Flow-free microfluidic device for quantifying chemotaxis in spermatozoa. Anal. Chem. 2020, 92, 3302–3306. [Google Scholar] [CrossRef]
- EL-sherry, T.M.; Abdel-Ghani, M.A.; Mahmoud, G.B.; Ezzat, A.A. Kisspeptin injection improved the semen characteristics and sperm rheotaxis in Ossimi ram. Reprod. Domest. Anim. 2020, 55, 240–247. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Qiu, T.; Xie, L.; Chen, W.; Liu, R.; Lu, Y.; Mitchelson, K.; Wang, J.; Qiao, J.; et al. The construction of an interfacial valve-based microfluidic chip for thermotaxis evaluation of human sperm. Biomicrofluidics 2014, 8, 024102. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Sontakke, S.; Deekshith, K.; Parte, P.; Jadhav, S. Chemotactic behavior of spermatozoa captured using a microfluidic chip. Biomicrofluidics 2018, 12, 024112. [Google Scholar] [CrossRef]
- Ko, Y.J.; Maeng, J.H.; Hwang, S.Y.; Ahn, Y. Design, fabrication, and testing of a microfluidic device for thermotaxis and chemotaxis assays of sperm. SLAS Technol. 2018, 23, 507–515. [Google Scholar] [CrossRef]
- Kanakasabapathy, M.K.; Sadasivam, M.; Singh, A.; Preston, C.; Thirumalaraju, P.; Venkataraman, M.; Bormann, C.L.; Draz, M.S.; Petrozza, J.C.; Shafiee, H. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 2017, 7863, eaai7863. [Google Scholar] [CrossRef]
- Dimitriadis, I.; Bormann, C.L.; Kanakasabapathy, M.K.; Thirumalaraju, P.; Id, H.K.; Yogesh, V.; Gudipati, N. Automated smartphone-based system for measuring sperm viability, DNA fragmentation, and hyaluronic binding assay score. PLoS ONE 2019, 14, e0212562. [Google Scholar] [CrossRef]
- Nosrati, R.; Gong, M.M.; Gabriel, M.C.S.; Pedraza, C.E.; Zini, A.; Sinton, D. Paper-based quantification of male fertility potential. Clin. Chem. 2016, 62, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Huang, H.W.; Chen, M.C.; Chen, Y.; Cheng, C.M. Relationship between porcine sperm motility and sperm enzymatic activity using paper-based devices. Sci. Rep. 2017, 7, 46213. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Yang, C.Y.; Wen, Y.C.; Chang, T.C.; Matsuura, K.; Chen, Y.; Cheng, C.M. Point-of-care semen analysis of patients with infertility via smartphone and colorimetric paper-based diagnostic device. Bioeng. Transl. Med. 2021, 6, e10176. [Google Scholar] [CrossRef]
- Nosrati, R.; Gong, M.M.; San Gabriel, M.C.; Zini, A.; Sinton, D. Paper-based sperm DNA integrity analysis. Anal. Methods 2016, 8, 6260–6264. [Google Scholar] [CrossRef]
- Kim, Y.; Chun, K. New disposable microfluidic chip without evaporation effect for semen analysis in clinics and homes. Microsyst. Technol. 2020, 26, 647–655. [Google Scholar] [CrossRef]
- Sun, K.; Wang, H.; Wang, L.; Lu, Y.; Liu, R.; Liu, P.; Cheng, J. A portable sperm cell purification instrument based on continuous flow acoustophoretic separation of sperm cells for on-site forensic sample pretreatment. Lab Chip 2021, 21, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Banejad, A.; Passandideh-Fard, M.; Niknam, H.; Mirshojaeian Hosseini, M.J.; Mousavi Shaegh, S.A. Design, fabrication and experimental characterization of whole-thermoplastic microvalves and micropumps having micromilled liquid channels of rectangular and half-elliptical cross-sections. Sens. Actuators A Phys. 2020, 301, 111713. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Pourmand, A.; Nabavinia, M.; Avci, H.; Tamayol, A.; Mostafalu, P.; Ghavifekr, H.B.; Aghdam, E.N.; Dokmeci, M.R.; Khademhosseini, A.; et al. Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sens. Actuators B Chem. 2018, 255, 100–109. [Google Scholar] [CrossRef]
- Annabestani, M.; Shaegh, A.M.; Esmaeili-Dokht, P.; Fardmanesh, M. An intelligent machine learning-based sheath-free microfluidic impedance flow cytometer. In Proceedings of the 2020 10th International Conference on Computer and Knowledge Engineering (ICCKE), Mashhad, Iran, 29–30 October 2020; pp. 284–288. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodamoradi, M.; Rafizadeh Tafti, S.; Mousavi Shaegh, S.A.; Aflatoonian, B.; Azimzadeh, M.; Khashayar, P. Recent Microfluidic Innovations for Sperm Sorting. Chemosensors 2021, 9, 126. https://doi.org/10.3390/chemosensors9060126
Khodamoradi M, Rafizadeh Tafti S, Mousavi Shaegh SA, Aflatoonian B, Azimzadeh M, Khashayar P. Recent Microfluidic Innovations for Sperm Sorting. Chemosensors. 2021; 9(6):126. https://doi.org/10.3390/chemosensors9060126
Chicago/Turabian StyleKhodamoradi, Maedeh, Saeed Rafizadeh Tafti, Seyed Ali Mousavi Shaegh, Behrouz Aflatoonian, Mostafa Azimzadeh, and Patricia Khashayar. 2021. "Recent Microfluidic Innovations for Sperm Sorting" Chemosensors 9, no. 6: 126. https://doi.org/10.3390/chemosensors9060126
APA StyleKhodamoradi, M., Rafizadeh Tafti, S., Mousavi Shaegh, S. A., Aflatoonian, B., Azimzadeh, M., & Khashayar, P. (2021). Recent Microfluidic Innovations for Sperm Sorting. Chemosensors, 9(6), 126. https://doi.org/10.3390/chemosensors9060126







