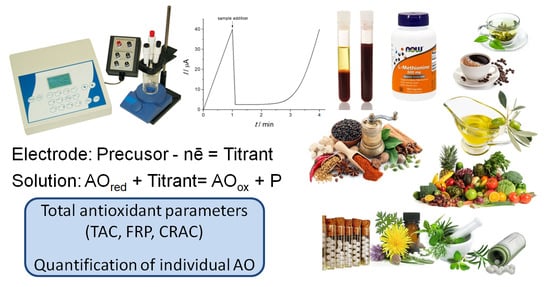
Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis
Abstract
1. Introduction
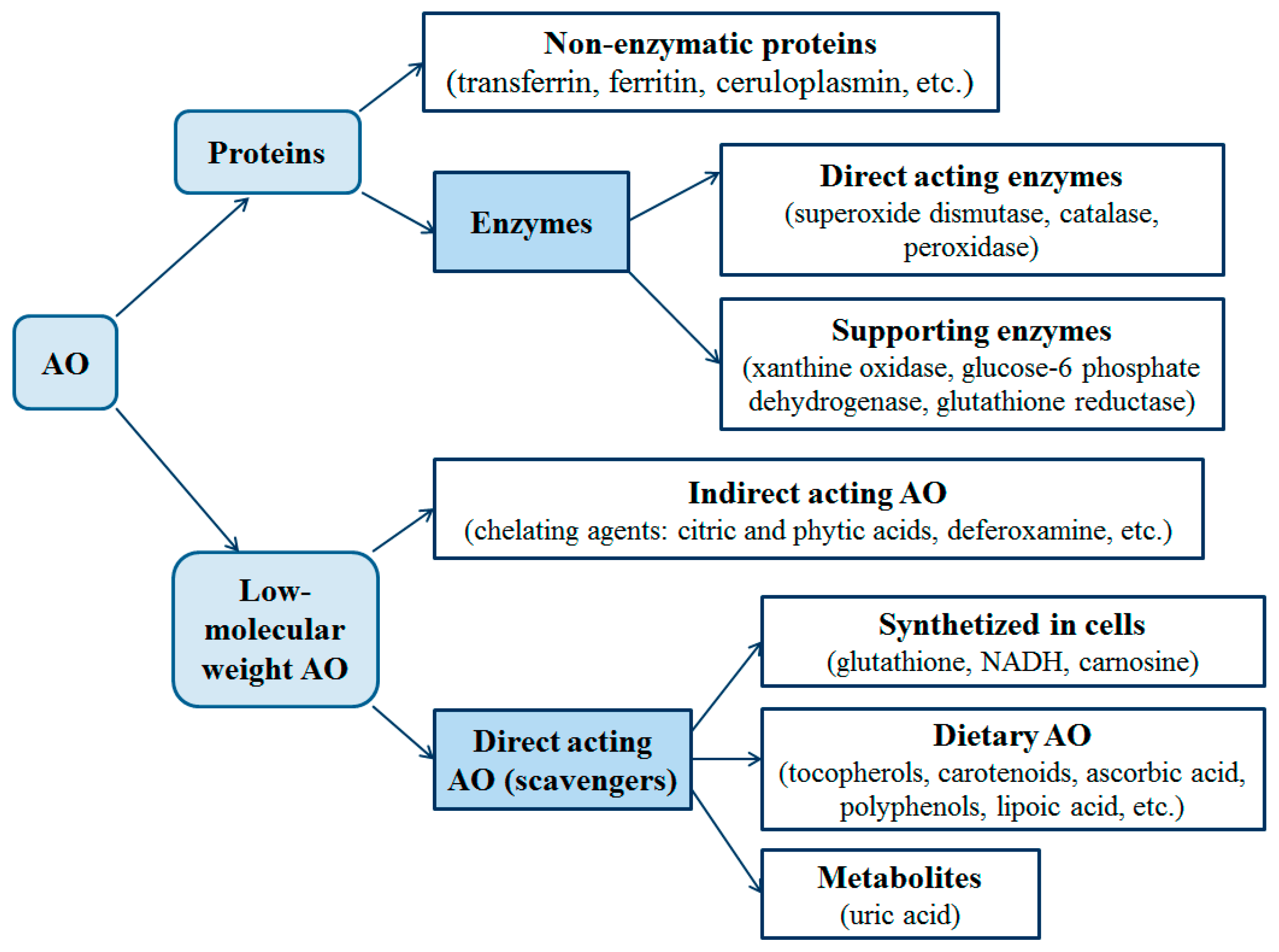
1.1. Antioxidants: Definition, Classification and Properties
- Prevention of the formation of reactive oxygen and nitrogen species and their neutralization via subsequent reactions. Chelating agents, proteins and catalase act in such a way;
- Termination of cascade reactions of reactive oxygen and nitrogen species generation and scavenging of free radicals. This mechanism is used by low-molecular weight AOs, for example, α-tocopherol, ascorbic acid, polyphenols;
- Restoration of damage to macromolecules caused by exposure to reactive oxygen species and their derivatives with the help of enzymatic systems.
1.2. Electrochemical Sensor Systems for Antioxidants Analysis
2. Coulometric Sensor Systems
- No necessity to use standard solutions and a calibration graph (the method is absolute);
- The absence of a titrant standardization step as far as the quantity of the electrogenerated titrant is determined by the quantity of electricity;
- The possibility to use unstable reagents for which standard solutions cannot be prepared due to their high reactivity;
- The absence of solution dilution during titration;
- Small volume of the sample;
- Microamounts of analyte can be quantified;
- The possibility of the quantification of electrochemically inert compounds by a chemical reaction with the titrant;
- High accuracy and reproducibility of the determination;
- Easiness of automation;
- Rapidity (the titration time does not exceed 5 min);
- Simplicity;
- Wide range of analytes and samples to be studied.
3. Constant-Current Coulometry with Electrogenerated Titrants in Antioxidants Analysis
3.1. Electrogeneration of Titrants–Oxidants and Their Properties
3.2. Quantification of Individual Antioxidants
3.3. Evaluation of Total Antioxidant Parameters
4. Coulometric Detection of Antioxidants in Chromatography and Flow Systems
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 77. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Azzi, A.; Davies, K.J.A.; Kelly, F. Free radical biology—terminology and critical thinking. FEBS Lett. 2004, 558, 3–6. [Google Scholar] [CrossRef]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Budnikov, G.K.; Ziyatdinova, G.K. Antioxidants as analytes in analytical chemistry. J. Anal. Chem. 2005, 60, 600–613. [Google Scholar] [CrossRef]
- Granot, E.; Kohen, R. Oxidative stress in childhood—in health and disease states. Clin. Nutr. 2004, 23, 3–11. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. Electroanalysis of antioxidants in pharmaceutical dosage forms: State-of-the-art and perspectives. Mon. Chem. 2015, 146, 741–753. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, V.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Diplock, A.T. Antioxidants and free radical scavengers. In Free Radical Damage and Its Control, 1st ed.; Rise-Evans, C.A., Burdon, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 28, pp. 113–130. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.M.; Ross, S.A. Beta-carotene increases lung cancer incidence in cigarette smokers. Nutr. Rev. 1996, 54, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Zhu, M.; Phillipson, J.D.; Greengrass, P.M.; Bowery, N.E.; Cai, Y. Plant polyphenols: Biologically active compounds or non-selective binders to protein. Phytochemistry 1997, 44, 441–447. [Google Scholar] [CrossRef]
- Syndge, R.L.M. Interactions of polyphenols with proteins in plants and plant products. Qual. Plant. 1975, 24, 337–350. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical methods to evaluate the antioxidant activity and capacity of foods: A review. Electroanalysis 2021. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Kazakov, Y.E. Electrochemical hybrid methods and sensors for antioxidant/oxidant activity monitoring and their use as a diagnostic tool of oxidative stress: Future perspectives and challenges. Chemosensors 2020, 8, 90. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical methods for total antioxidant capacity and its main contributors determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Chronoamperometric estimation of cognac and brandy antioxidant capacity using MWNT modified glassy carbon electrode. Talanta 2014, 125, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Tarasov, A.V.; Kazakov, Y.E.; Vidrevich, M.B. Platinum electrode regeneration and quality control method for chronopotentiometric and chronoamperometric determination of antioxidant activity of biological fluids. J. Electroanal. Chem. 2018, 808, 14–20. [Google Scholar] [CrossRef]
- Zikos, N.; Karaliota, A.; Liouni, M. Chronoamperometry as a tool for the evaluation of antioxidant properties of red wines. J. Anal. Chem. 2011, 66, 859–864. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Kozlova, E.V.; Budnikov, H.C. Chronoamperometric evaluation of the antioxidant capacity of tea on a polyquercetin-modified electrode. J. Anal. Chem. 2017, 72, 382–389. [Google Scholar] [CrossRef]
- de Queiroz Ferreira, R.; Avaca, L.A. Electrochemical determination of the antioxidant capacity: The ceric reducing/antioxidant capacity (CRAC) assay. Electroanalysis 2008, 20, 1323–1329. [Google Scholar] [CrossRef]
- Oliveira, R.; Bento, F.; Sella, C.; Thouin, L.; Amatore, C. Direct electroanalytical method for alternative assessment of global antioxidant capacity using microchannel electrodes. Anal. Chem. 2013, 85, 9057–9063. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Os’kina, K.S.; Ziganshina, E.R.; Budnikov, H.C. Chronoamperometric determination of synthetic phenolic antioxidants in Brij® 35 micellar medium. J. Anal. Chem. 2015, 70, 1501–1506. [Google Scholar] [CrossRef]
- Gotoh, M.; Hirose, H.; Ishikawa, T.; Nakamura, H.; Yokoyama, K. Establishment of ferricyanide chronoamperometric total antioxidant capacity assay employing a carbon screen-printed disposable microchip —fundamental study using vegetable extraction. Sens. Mater. 2015, 27, 825–838. [Google Scholar] [CrossRef][Green Version]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Chronocoulometry of wine on multi-walled carbon nanotube modified electrode: Antioxidant capacity assay. Food Chem. 2016, 196, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Kozlova, E.; Morozova, E.; Budnikov, H. Chronocoulometric method for the evaluation of antioxidant capacity of medicinal plant tinctures. Anal. Methods 2018, 10, 4995–5003. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric study of antioxidant activity: Development and prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Ziganshina, E.; Budnikov, H. Surfactant media for constant-current coulometry. Application for the determination of antioxidants in pharmaceuticals. Anal. Chim. Acta 2012, 744, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Abdullin, I.F.; Turova, E.N.; Budnikov, G.K. Coulometric determination of the antioxidant capacity of tea extracts using electrogenerated bromine. J. Anal. Chem. 2001, 56, 557–559. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Turova, E.N.; Gaisina, G.K.; Budnikov, G.K. Use of electrogenerated bromine for estimating the total antioxidant capacity of plant raw materials and plant-based medicinal preparations. J. Anal. Chem. 2002, 57, 557–560. [Google Scholar] [CrossRef]
- Hauser, P.C. Coulometry. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 234–240. [Google Scholar] [CrossRef]
- Scholz, E. Karl Fischer Titration: Determination of Water; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Milner, G.W.C.; Phillips, G. Coulometry in Analytical Chemistry; Pergamon Press: Oxford, UK, 1967; 220p. [Google Scholar] [CrossRef]
- Qurashi, M.M.; Štulík, K.; Zýka, J. The suitability of various electrode materials for the electrogeneration of halogens in constant-current coulometry. Anal. Lett. 1973, 6, 435–439. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Budnikov, G.K. Coulometric analysis of organic compounds (review). Ind. Lab. 1998, 64, 1–11. [Google Scholar]
- Zozulya, A.P. Kulonometricheskii Analiz (Coulometric Analysis). In Russian; Khimiya: Moscow, Russia, 1965. [Google Scholar]
- Kostromin, A.I.; Badretdinova, G.Z.; Abdullin, I.F. Electrogeneration of iodine compounds in acetic-acid for use in coulometric analysis. J. Anal. Chem. Ussr 1983, 38, 662–665. [Google Scholar]
- Ziyatdinova, G.K.; Budnikov, G.K.; Pogorel’tsev, V.I. Electrochemical determination of lipoic acid. J. Anal. Chem. 2004, 59, 288–290. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Direct determination of captopril using electrogenerated halogens for pharmaceuticals quality control. Eurasian J. Anal. Chem. 2007, 2, 84–92. [Google Scholar]
- Ziyatdinova, G.K.; Budnikov, G.K.; Lapin, A.A. Direct determination of hypoxen and its analogs by galvanostatic coulometry. J. Anal. Chem. 2007, 62, 260–262. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Grigor’eva, L.V.; Budnikov, G.K. Coulometric determination of sulfur-containing amino acids using halogens as oxidizing titrants. J. Anal. Chem. 2007, 62, 1176–1179. [Google Scholar] [CrossRef]
- Budnikov, G.K.; Ziyatdinova, G.K.; Valitova, Y.R. Electrochemical determination of glutathione. J. Anal. Chem. 2004, 59, 573–576. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Turova, E.N.; Ziyatdinova, G.K.; Budnikov, G.K. Determination of fat-soluble antioxidants by galvanostatic coulometry using electrogenerated oxidants. J. Anal. Chem. 2002, 57, 730–732. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Khuzina, A.A.; Budnikov, H.C. Reactions of phenolic antioxidants with electrogenerated hexacyanoferrate(III) ions and their use in vegetable oils analysis. J. Anal. Chem. 2013, 68, 80–85. [Google Scholar] [CrossRef]
- Xu, L.X.; Liu, A.R.; Zhang, X.Q. Coulometric titration of total flavonoids in Sophora japonica L. Yao Xue Xue Bao 1989, 24, 755–758. [Google Scholar]
- Ziyatdinova, G.K.; Nizamova, A.M.; Budnikov, G.K. Galvanostatic coulometry in the analysis of natural polyphenols and its use in pharmacy. J. Anal. Chem. 2010, 65, 1176–1180. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Salikhova, I.R.; Budnikov, H.C. Reactions of cognac antioxidants with electrogenerated oxidizers. Uchenye Zapiski Kazanskogo Universiteta. Seriya Estestv. Nauk. 2013, 155, 78–86. [Google Scholar]
- Abdullin, I.F.; Turova, E.N.; Parshakova, Y.V.; Budnikov, G.K.; Gogolashvili, E.L. Determination of ionol by voltammetry and coulometric titration. J. Anal. Chem. 2002, 57, 248–252. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Gainetdinova, A.A.; Budnikov, G.K. Reactions of synthetic phenolic antioxidants with electrogenerated titrants and their analytical applications. J. Anal. Chem. 2010, 65, 929–934. [Google Scholar] [CrossRef]
- Harris, S.; Gonzales, J.; Melaku, S.; Dabke, R.B. Feasibility of performing concurrent coulometric titrations using a multicompartment electrolysis cell. ACS Omega 2019, 4, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.; Gebeyehu, Z.; Griffin, K.; Dabke, R.B. Volumetric titrations using electrolytically generated reagents for the determination of ascorbic acid and iron in dietary supplement tablets: An undergraduate laboratory experiment. J. Chem. Educ. 2014, 91, 898–901. [Google Scholar] [CrossRef]
- Evlash, V.; Gubsky, S.; Aksonova, E.; Borisova, A.; Zhelezniak, Z. Determination of ascorbic acid amount in gelatin aqueous solutions by galvanostatic coulometry using electrogenerated bromine. Ind. Technol. Eng. 2016, 18, 22–31. [Google Scholar]
- Abdullin, I.F.; Bakanina, Y.N.; Turova, E.N.; Budnikov, G.K. Determination of uric acid by voltammetry and coulometric titration. J. Anal. Chem. 2001, 56, 453–456. [Google Scholar] [CrossRef]
- Budnikov, G.K.; Ziyatdinova, G.K.; Gil’metdinova, D.M. Determination of some liposoluble antioxidants by coulometry and voltammetry. J. Anal. Chem. 2004, 59, 654–658. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Kapoor, S.; Naik, D.B. One- and two-electron oxidation reactions of trolox by peroxynitrite. Chem. Res. Toxicol. 2001, 14, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.K.; Budnikov, H.C.; Pogorel’tzev, V.I.; Ganeev, T.S. The application of coulometry for total antioxidant capacity determination of human blood. Talanta 2006, 68, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinova, D.D.; Ziyatdinova, G.K.; Semenov, V.V.; Pakhalina, I.A.; Kolochkova, E.V. Clastogenesis and aneugenesis in children with cerebral palsy. Bull. Exp. Biol. Med. 2005, 139, 596–599. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C.; Pogorel’tzev, V.I. Electrochemical determination of the total antioxidant capacity of human plasma. Anal. Bioanal. Chem. 2005, 381, 1546–1551. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Voloshin, A.V.; Gilmutdinov, A.K.; Budnikov, H.C.; Ganeev, T.S. Application of constant-current coulometry for estimation of plasma total antioxidant capacity and its relationship with transition metal contents. J. Pharm. Biomed. Anal. 2006, 40, 958–963. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C.; Pogorel’tzev, V.I. Determination of total antioxidant capacity of human plasma from patients with lung diseases using constant-current coulometry. Eurasian J. Anal. Chem. 2006, 1, 19–30. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Turova, E.N.; Budnikov, G.K.; Ziyatdinova, G.K.; Gajsina, G.K. Electrogenerated bromine-reagent for determination of antioxidant capacity of juices and extracts. Zavod. Lab. Diagn. Mater. 2002, 68, 12–15. [Google Scholar]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Coulometric titration with electrogenerated oxidants as a tool for evaluation of cognac and brandy antioxidant properties. Food Chem. 2014, 150, 80–86. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Ultrasound-assisted micellar extraction of phenolic antioxidants from spices and antioxidant properties of the extracts based on coulometric titration data. Anal. Methods 2016, 8, 7150–7157. [Google Scholar] [CrossRef]
- Nizamova, A.M.; Ziyatdinova, G.K.; Budnikov, G.K. Electrogenerated bromine as a coulometric reagent for the estimation of the bioavailability of polyphenols. J. Anal. Chem. 2011, 66, 301–309. [Google Scholar] [CrossRef]
- Gubsky, S.; Artamonova, M.; Shmatchenko, N.; Piliugina, I.; Aksenova, E. Determination of total antioxidant capacity in marmalade and marshmallow. East. Eur. J. Enterp. Technol. 2016, 4, 43–50. [Google Scholar] [CrossRef]
- Mazur, L.; Gubsky, S.; Dorohovych, A.; Labazov, M. Antioxidant properties of candy caramel with plant extracts. Ukr. Food J. 2018, 7, 7–21. [Google Scholar] [CrossRef]
- Siano, F.; Picariello, G.; Vasca, E. Coulometrically determined antioxidant capacity (CDAC) as a possible parameter to categorize extra virgin olive oil. Food Chem. 2021, 354, 129564. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Nizamova, A.; Budnikov, H. Novel coulometric approach to evaluation of total free polyphenols in tea and coffee beverages in presence of milk proteins. Food Anal. Methods 2011, 4, 334–340. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Nguyen Cong, F.; Budnikov, H.C. Assessment of the antioxidant properties of micellar spice extracts by galvanostatic coulometry with electrogenerated hexacyanoferrate(III) ions. J. Anal. Chem. 2015, 70, 974–982. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Skorobogatova, N.; Chibisova, M.; Budnikov, H. New electrochemistry-based approaches to brandy quality evaluation using antioxidant parameters. Food Anal. Methods 2015, 8, 1794–1803. [Google Scholar] [CrossRef]
- Sontag, G.; Pinto, M.I.; Noronha, J.P.; Burrows, H.D. Analysis of food by high performance liquid chromatography coupled with coulometric detection and related techniques: A review. J. Agric. Food Chem. 2019, 67, 4113–4144. [Google Scholar] [CrossRef]
- Hicks, M.B.; Salituro, L.; Mangion, I.; Schafer, W.; Xiang, R.; Gonga, X.; Welch, C.J. Assessment of coulometric array electrochemical detection coupled with HPLC-UV for the absolute quantitation of pharmaceuticals. Analyst 2017, 142, 525–536. [Google Scholar] [CrossRef]
- Zerzaňová, A.; Žižkovský, V.; Kučera, R.; Klimeš, J.; Jesenský, I.; Dohnal, J.; Barrón, D. Using of HPLC coupled with coulometric detector for the determination of biotin in pharmaceuticals. J. Pharm. Biomed. Anal. 2007, 45, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K. Review: The application of liquid chromatography electrochemical detection for the determination of drugs of abuse. Separations 2016, 3, 28. [Google Scholar] [CrossRef]
- Trojanowicz, M. Recent developments in electrochemical flow detections—A review: Part II. Liquid chromatography. Anal. Chim. Acta 2011, 688, 8–35. [Google Scholar] [CrossRef]
- Mika, J.; Barek, J.; Zima, J.; Dejmkova, H. New flow-through coulometric detector with renewable working electrode material for flow injection analysis and HPLC. Electrochim. Acta 2015, 154, 397–403. [Google Scholar] [CrossRef]
- Dejmková, H.; Baroch, M.; Krejčová, M.; Barek, J.; Zima, J. Coulometric detector based on carbon felt. Appl. Mater. Today 2017, 9, 482–486. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Nurmi, T. Application of coulometric electrode array detectionto the analysis of isoflavonoids and lignans. J. Pharm. Biomed. Anal. 2006, 41, 149–1507. [Google Scholar] [CrossRef] [PubMed]
- Cortina-Puig, M.; Gallart-Ayala, H.; Lacorte, S. Liquid chromatography coupled to electrochemical detection and mass spectrometry for the determination of phenolic compounds in food and beverages. Curr. Anal. Chem. 2012, 8, 436–455. [Google Scholar] [CrossRef]
- Riman, D.; Prodromidis, M.I.; Jirovsky, D.; Hrbac, J. Low-cost pencil graphite-based electrochemical detector for HPLC with near-coulometric efficiency. Sens. Actuators B 2019, 296, 126618. [Google Scholar] [CrossRef]
- Careri, M.; Elviri, L.; Mangia, A.; Musci, M. Spectrophotometric and coulometric detection in the high-performance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. J. Chromatogr. A 2000, 881, 449–460. [Google Scholar] [CrossRef]
- Hájek, T.; Škeříková, V.; Česla, P.; Vyňuchalová, K.; Jandera, P. Multidimensional LC × LC analysis of phenolic and flavone natural antioxidants with UV-electrochemical coulometric and MS detection. J. Sep. Sci. 2008, 31, 3309–3328. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, X.; Wen, J.; Zhou, T.; Fan, G. Simultaneous fingerprint, quantitative analysis and anti-oxidative based screening of components in Rhizoma Smilacis Glabrae using liquid chromatography coupled with charged aerosol and coulometric array detection. J. Chromatogr. B 2017, 1049–1050, 41–50. [Google Scholar] [CrossRef]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Beňová, B.; Hájek, T. Utilization of coulometric array detection in analysis of beverages and plant extracts. Procedia Chem. 2010, 2, 92–100. [Google Scholar] [CrossRef][Green Version]
- Kahoun, D.; Řezková, S.; Veškrnová, K.; Královský, J.; Holčapek, M. Determination of phenolic compounds and hydroxymethylfurfural in meads using high performance liquid chromatography with coulometric-array and UV detection. J. Chromatogr. A 2008, 1202, 19–33. [Google Scholar] [CrossRef]
- Cantalapiedra, A.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Sensitive and selective determination of phenolic compounds from aromatic plants using an electrochemical detection coupled with HPLC method. Phytochem. Anal. 2014, 25, 247–254. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Schultheiss, G.; Frank, J.; Rimbach, G. A validated method for the determination of selected phenolics in olive oil using high-performance liquid chromatography with coulometric electrochemical detection and a fused-core column. Food Chem. 2013, 138, 1663–1669. [Google Scholar] [CrossRef]
- Petrus, K.; Schwartz, H.; Sontag, G. Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 2555–2563. [Google Scholar] [CrossRef]
- Takeda, H.; Shibuya, T.; Yanagawa, K.; Kanoh, H.; Takasaki, M. Simultaneous determination of α-tocopherol and α-tocopherolquinone by high-performance liquid chromatography and coulometric detection in the redox mode. J. Chromatogr. A 1996, 722, 287–294. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Nguyen, M.L.; Sander, L.C.; Rock, C.L.; Schwartz, S.J. Analysis of lycopene geometrical isomers in biological microsamples by liquid chromatography with coulometric array detection. J. Chromatogr. B 2001, 760, 289–299. [Google Scholar] [CrossRef]
- Durrani, A.I.; Schwartz, H.; Schmid, W.; Sontag, G. α-Lipoic acid in dietary supplements: Development and comparison of HPLC-CEAD and HPLC-ESI-MS methods. J. Pharm. Biomed. Anal. 2007, 45, 694–699. [Google Scholar] [CrossRef]
- Sechovcová, S.; Královcová, P.; Kanďár, R.; Ventura, K. The issue of HPLC determination of endogenous lipoic acid in human plasma. Biomed. Chromatogr. 2018, 32, e4172. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Rimbach, G.; Frank, J.; Esatbeyoglu, T. Rapid method for glutathione quantitation using high-performance liquid chromatography with coulometric electrochemical detection. J. Agric. Food Chem. 2014, 62, 402–408. [Google Scholar] [CrossRef]
- Kanďár, R.; Žáková, P.; Marková, M.; Lotková, H.; Kučera, O.; Červinková, Z. Determination of glutathione and glutathione disulfide in human whole blood using HPLC with coulometric detection: A comparison with fluorescence detection. Collect. Czech. Chem. Commun. 2011, 76, 277–294. [Google Scholar] [CrossRef]
- Khamanga, S.M.; Walker, R.B. The use of experimental design in the development of an HPLC–ECD method for the analysis of captopril. Talanta 2011, 83, 1037–1049. [Google Scholar] [CrossRef]
- Gazdik, Z.; Zitka, O.; Petrlova, J.; Adam, V.; Zehnalek, J.; Horna, A.; Reznicek, V.; Beklova, M.; Kizek, R. Determination of vitamin C (ascorbic acid) using high performance liquid chromatography coupled with electrochemical detection. Sensors 2008, 8, 7097–7112. [Google Scholar] [CrossRef]
- Langer, S.; Lodge, J.K. Determination of selected water-soluble vitamins using hydrophilic chromatography: A comparison of photodiode array, fluorescence, and coulometric detection, and validation in a breakfast cereal matrix. J. Chromatogr. B 2014, 960, 73–81. [Google Scholar] [CrossRef]
- Li, H.; Tu, H.; Wang, Y.; Levine, M. Vitamin C in mouse and human red blood cells: An HPLC assay. Anal. Biochem. 2012, 426, 109–117. [Google Scholar] [CrossRef]
- Skrinjar, M.; Kolar, M.H.; Jelsek, N.; Hras, A.R.; Bezjak, M.; Knez, Z. Application of HPLC with electrochemical detection for the determination of low levels of antioxidants. J. Food Comp. Anal. 2007, 20, 539–545. [Google Scholar] [CrossRef]
- Catapano, M.C.; Protti, M.; Fontana, T.; Mandrioli, R.; Mladěnka, P.; Mercolini, L. An original HPLC method with coulometric detection to monitor hydroxyl radical generation via Fenton chemistry. Molecules 2019, 24, 3066. [Google Scholar] [CrossRef]
- Morozova, K.; Rodríguez-Buenfil, I.; López-Domínguez, C.; Ramírez-Sucre, M.; Ballabio, D.; Scampicchio, M. Capsaicinoids in chili habanero by flow injection with coulometric array detection. Electroanalysis 2019, 31, 844–850. [Google Scholar] [CrossRef]
- Oney Montalvo, J.E.; Morozova, K.; Ferrentino, G.; Ramírez Sucre, M.O.; Rodríguez Buenfil, I.M.; Scampicchio, M. Effects of local environmental factors on the spiciness of habanero chili peppers (Capsicum chinense Jacq.) by coulometric electronic tongue. Eur. Food Res. Technol. 2021, 247, 101–110. [Google Scholar] [CrossRef]
- Kongwong, P.; Morozova, K.; Ferrentino, G.; Poonlarp, P.; Scampicchio, M. Rapid determination of the antioxidant capacity of lettuce by an e-tongue based on flow injection coulometry. Electroanalysis 2018, 30, 230–237. [Google Scholar] [CrossRef]
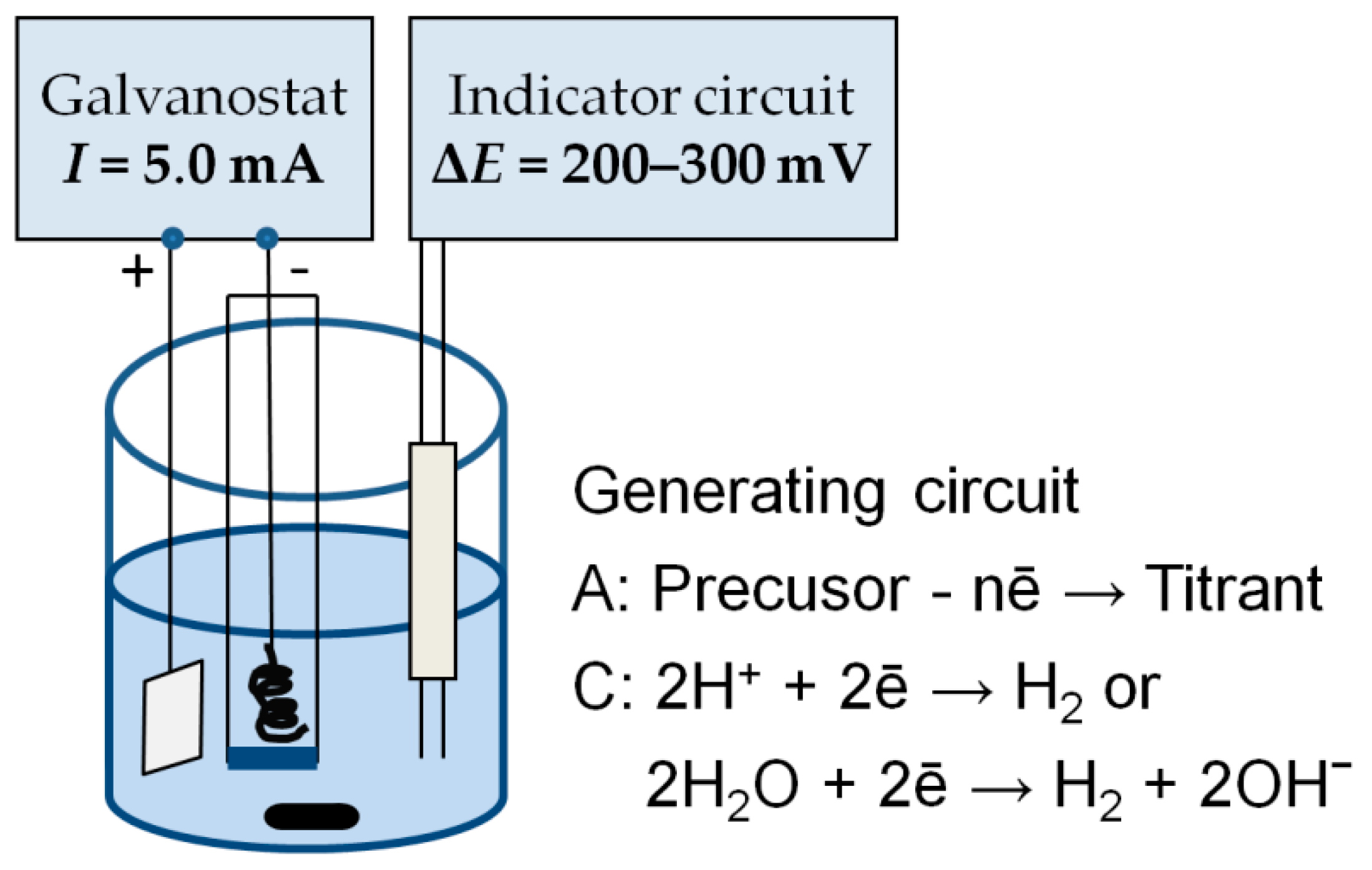
| Titrant | Electrode | Precursor and Electrolyte | Anodic Process | Cathodic Process |
|---|---|---|---|---|
| Cl2 | Pt | 0.2 M KCl in 0.1 M H2SO4 | 2Cl− − 2ē → Cl2 | 2H+ + 2ē → H2 |
| Pt | 0.2 M HCl in 0.1 M HClO4 in acetonitrile | 2Cl− − 2ē → Cl2 | 2H+ + 2ē → H2 | |
| Br2 | Pt | 0.2 M KBr in 0.1 M H2SO4 | 2Br− − 2ē → Br2 | 2H+ + 2ē → H2 |
| Pt | 0.2 M (C2H5)4NBr in 0.1 M HClO4 in acetonitrile | 2Br− − 2ē → Br2 | 2H+ + 2ē → H2 | |
| I2 | Pt | 0.1 M KI in acetate buffer pH 3.56 | 2I− − 2ē → I2 | 2H+ + 2ē → H2 |
| [Fe(CN)6]3− | Pt | 0.1 M K4Fe(CN)6 in 2 M KOH | [Fe(CN)6]4− − ē → [Fe(CN)6]3− | 2H2O + 2ē → H2 + 2OH− |
| Ce4+ | Pt | 0.1 M Ce(NO3)3 in 3 M H2SO4 | Ce3+ − ē → Ce4+ | 2H+ + 2ē → H2 |
| Antioxidant | Titrant | Number of Electrons | Sample | Ref. |
|---|---|---|---|---|
| S-containing AO | ||||
| Lipoic acid | Cl2 | 10 | Tablets | [47] |
| Br2 | 4 | |||
| I2 | 4 | |||
| Captopril | Cl2 | 6 | Tablets | [48] |
| Br2 | 6 | |||
| I2 | 2 | |||
| Sodium polydihydroxyphenylenethiosulfonate | Cl2 | 3 | Pharmaceutical substance | [49] |
| Br2 | 2 | |||
| Methionine | Cl2 | 4 | Tablets | [50] |
| Br2 | 2 | |||
| Glutathione | Cl2 | 6 | Human blood | [51] |
| Br2 | 6 | |||
| I2 | 2 | |||
| Liposoluble AO | ||||
| α-Tocopherol | Cl2 | 2 | Model solutions 2 | [52] |
| Br2 | 2 | |||
| [Fe(CN)6]3− | 1 | Vegetable oil | [53] | |
| Retinol | Cl2 | 6 | Model solutions | [52] |
| Br2 | 6 | |||
| Natural Phenolic AO | ||||
| Rutin | Br2 | 4 | Total flavonoids | [54] |
| [Fe(CN)6]3− | 4 | Pharmaceutical dosage forms | [55] | |
| Quercetin | [Fe(CN)6]3− | 5 | Pharmaceutical dosage forms | [55] |
| Dihydroquercetin | [Fe(CN)6]3− | 5 | Pharmaceutical dosage forms | [55] |
| Ellagic acid | Br2 | 4 | Model solutions | [56] |
| [Fe(CN)6]3− | 4 | |||
| Gallic acid | Br2 | 6 | Model solutions | [56] |
| [Fe(CN)6]3− | 4 | |||
| Syringaldehyde | Br2 | 2 | Model solutions | [56] |
| [Fe(CN)6]3− | 0 | |||
| Vanillin | Br2 | 2 | Model solutions | [56] |
| [Fe(CN)6]3− | 0 | |||
| Coniferaldehyde | Br2 | 5 | Model solutions | [56] |
| [Fe(CN)6]3− | 0 | |||
| Sterically Hindered Synthetic Phenols | ||||
| Ionol (BHT 1) | Cl2 | 2 | Mineral oil | [57] |
| [Fe(CN)6]3− | 2 | Vegetable oil | [53] | |
| BHT amino derivatives | [Fe(CN)6]3− | 2 | Model solutions | [53] |
| Irganox® 1081 | [Fe(CN)6]3− | 2 | Model solutions | [53] |
| Water-Soluble Synthetic Phenols | ||||
| Hydroquinone | Cl2 | 2 | Model solutions | [58] |
| Br2 | 2 | |||
| [Fe(CN)6]3− | 2 | |||
| Hydroquinone derivative | Cl2 | 12 | Model solutions | [58] |
| Br2 | 7 | |||
| [Fe(CN)6]3− | 4 | |||
| Catechol | Cl2 | 2 | Model solutions | [58] |
| Br2 | 2 | |||
| [Fe(CN)6]3− | 2 | |||
| Catechol derivatives | Cl2 | 9–12 | Model solutions | [58] |
| Br2 | 7 | |||
| [Fe(CN)6]3− | 4 | |||
| Pyrogallol | Cl2 | 2 | Model solutions | [58] |
| Br2 | 2 | |||
| [Fe(CN)6]3− | 2 | |||
| Pyrogallol derivatives | Cl2 | 2–8 | Model solutions | [58] |
| Br2 | 2–5 | |||
| [Fe(CN)6]3− | 2 | |||
| Others | ||||
| Ascorbic acid | I2 | 2 | Pharmaceutical dosage forms | [55] |
| Vitamin C dietary supplement | [59] | |||
| Dietary supplement tablets | [60] | |||
| Br2 | 2 | Gelatin | [61] | |
| [Fe(CN)6]3− | 2 | Pharmaceutical dosage forms | [55] | |
| Uric acid | Br2 | 2 | Model solutions | [62] |
| Antioxidant Parameter | Titrant | Sample | AO Major Contributors | Ref. |
|---|---|---|---|---|
| TAC | Br2 | Human blood from patients with different pathologies | S-containing amino acids, ascorbic and uric acids, catecholamines, serum albumin, porphyrins | [65,66] |
| Human plasma from patients with different pathologies | S-containing amino acids, ascorbic and uric acids, catecholamines, serum albumin | [65,66,67,68,69] | ||
| Tea extracts | Natural phenolic AOs, ascorbic acid | [38] | ||
| Plant raw materials and plant-based medicinal preparations | Natural phenolic AOs, ascorbic acid | [39] | ||
| Juices, balms and Rhodiola rosea L. extract | Natural phenolic AOs, ascorbic acid | [70] | ||
| Cognac and brandy | Ellagic and gallic acids, syring- and coniferaldehydes, vanillin, 5-hydroxymethylfurfural | [71] | ||
| Spices micellar extracts | Natural phenolic AOs, capsaicinoids, ascorbic acid | [72] | ||
| Tea and coffee | Natural phenolic AOs, ascorbic acid | [73] | ||
| Marmalade and marshmallow with plant additives | Ascorbic acid, natural phenolic AOs | [74] | ||
| Candy caramel with plant extracts | Ascorbic acid, natural phenolic AOs | [75] | ||
| Extra virgin olive oils | Phenolic compounds | [76] | ||
| FRP | [Fe(CN)6]3− | Tea and coffee | Natural phenolic AOs | [77] |
| Cognac and brandy | Ellagic and gallic acids | [71] | ||
| Spices micellar extracts | Natural phenolic AOs, ascorbic acid | [72,78] | ||
| CRAC | Ce4+ | Spices micellar extracts | Natural phenolic AOs, ascorbic acid | [72] |
| Antioxidant | Working Electrode | Potential, V | Limit of Detection | Sample | Ref. |
|---|---|---|---|---|---|
| Phenolic acids | PGDE | +1.0 | 0.4–1.1 | Model solutions 3 | [89] |
| Flavonoids | 2 PGE | −0.5, +0.8 | 0.13–1.84 μg mL−1 | Orange juice | [90] |
| Flavones | 8 PGE | +0.25–+0.9 | No data | Beer | [91] |
| 8 Marker polyphenols | 16 PGE | +0.05–+1.0 | No data | Rhizoma Smilacis Glabrae | [92] |
| Flavonols and phenolic acids | 4 PGE | +0.12, +0.36, +0.48, +0.60 | No data | Bilberry, lingonberry, cloudberry and sea-buckthorn berry | [93] |
| Phenolic AOs | 8 PGE | +0.2–+0.9 with 0.1 V step | 1.9–25.1 μg L−1 | Wines, meads and Japanese knotweed ’s roots | [94] |
| 25 Phenolic AOs | 8 PGE | +0.2–+0.9 with 0.1 V step | 4–29 μg L−1 | Meads | [95] |
| Vanillin | 2 PGE | +0.5 | 0.81 μg L−1 | Essential oils for aromatherapy and aromatic herbs for culinary | [96] |
| Eugenol | 3.0 μg L−1 | ||||
| Thymol | 3.1 μg L−1 | ||||
| Carvacrol | 1.4 μg L−1 | ||||
| Phenolic AOs | 4 PGE | +0.25–+0.75 | 0.03–1.70 ng mL−1 | Olive oils | [97] |
| Phenolic AOs | 8 PGE | +0.3, +0.4, +0.5, +0.65, +0.75, +0.8, +0.1, +0.2 | 1.6–8.3 μg kg−1 | Honey | [98] |
| α-Tocopherol | 4 PGE 1 | −0.45, −0.45, −0.45, +0.4 | 50 pg | Rat plasma or erythrocyte membrane | [99] |
| Tocopherols | PGDE 2 | +0.5 | 0.8–2.2 nM | Model solutions 3 | [89] |
| trans-Lycopene | 8 PGE | +0.2–+0.62 with 0.060 V step | 50 fmol per 20 μL injection | Human plasma, buccal mucosal cells, prostate and cervical tissue biopsies | [100] |
| α-Lipoic acid | 2 PGE | +0.3–+0.7 | 0.005 μg mL−1 | Dietary supplements | [101] |
| 2 PGE | +0.45, +0.59 | 1.85 nM | Human plasma | [102] | |
| Reduced glutathione | 4 PGE | +0.25–+0.80 | 15 fmol | Cultured hepatocytes | [103] |
| 2 PGE | +0.75, +0.95 | 2.1 μM | Human blood | [104] | |
| Captopril | 2 PGE | +0.60, +0.95 | 0.6 μg mL−1 | Captopril tablets | [105] |
| Ascorbic acid | 8 PGE | +0.1–+0.4 | 90 nM | Celaskon tablets, orange, apple and human serum | [106] |
| 16 PGE | +0.03 | 164 ng mL−1 | Model solution 3 | [107] | |
| 2 PGE | 0.0, +0.25 | 0.025 μM | Mouse and human red blood cells | [108] | |
| Carnosic acid | 2 PGE | -0.1, +0.45 | 1.387 μg L−1 | Meat and meat products | [109] |
| Antioxidant | Detector | Limit of Detection | Ref. |
|---|---|---|---|
| Eugenol | UV | 62 μg mL−1 | [96] |
| Amperometric | 9.7 μg mL−1 | ||
| Coulometric | 3.0 μg mL−1 | ||
| Vanillin | UV | 15 μg mL−1 | |
| Amperometric | 12 μg mL−1 | ||
| Coulometric | 0.81 μg mL−1 | ||
| Thymol | UV | 41 μg mL−1 | |
| Amperometric | 17 μg mL−1 | ||
| Coulometric | 1.4 μg mL−1 | ||
| Carvacrol | UV | 55 μg mL−1 | |
| Amperometric | 13 μg mL−1 | ||
| Coulometric | 3.1 μg mL−1 | ||
| α-Lipoic acid | UV | 0.025 μg mL−1 | [101] |
| Coulometric | 0.005 μg mL−1 | ||
| Pyridoxal | Fluorescent | 97.2 ng mL−1 | [107] |
| Coulometric | 6.6 ng mL−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziyatdinova, G.; Budnikov, H. Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis. Chemosensors 2021, 9, 91. https://doi.org/10.3390/chemosensors9050091
Ziyatdinova G, Budnikov H. Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis. Chemosensors. 2021; 9(5):91. https://doi.org/10.3390/chemosensors9050091
Chicago/Turabian StyleZiyatdinova, Guzel, and Herman Budnikov. 2021. "Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis" Chemosensors 9, no. 5: 91. https://doi.org/10.3390/chemosensors9050091
APA StyleZiyatdinova, G., & Budnikov, H. (2021). Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis. Chemosensors, 9(5), 91. https://doi.org/10.3390/chemosensors9050091