Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal
Abstract
1. Introduction
2. Optimization Requirements for MOFs-Based Mercury Detection/Removal
- A.
- Selection of suitable organic linkers that can form MOFs with designated metal nodes with greater surface area to interact with mercury analogous in the environment. Similarly, organic linkers with side chains, such as thiol (-SH; which shows the greater affinity to Hg2+, Hg0 and CH3Hg+), can be chosen for effective removal and detection mercury [55].
- B.
- Selection of suitable metal nodes that can form MOFs with good stability in aqua/non-aqua solvents and afford large surface area for analyte (Hg2+) adsorption or collision for improving signal detection or quantification [56].
- C.
- Selection of appropriate synthetic tactics/conditions to afford high yield over impurities. Similarly, the selection of suitable solvent for sensory studies also needs attention [18].
- D.
- Many MOFs are well known coordinating polymers with porous nano/micro-structures [27], the design for capturing mercury requires more attention with respect to linkers, nodes, adsorbing ability, and opto-electronic properties.
- E.
- In the case of removal of mercury from environmental samples, design and development of MOFs with high adsorption efficiency and stability in aqua medium need more attention and optimization for improvement [57].
- F.
- To avoid the interfering effect from competing species, a unique MOF design with selectivity only to the mercury analogous must require optimization either by modulation of organic linkers or metal nodes or by tuning the opto-electronic properties [58].
- G.
- Post-modification of MOFs with certain materials to form composited structures towards mercury detection/removal also requires optimization for authorized applicability [59].
3. Synthetic Tactics Involved in MOFs Construction
- (1)
- Diffusion method: This is a tactic that involves gradual conveyance of various species into interaction and can be sub-divided into (i) solvent liquid diffusion method, which takes place between precipitant solvent and product in the solvent and leads to crystallization at interface via gradual diffusion; (ii) gradual diffusion of reactants by adjusting the physical barriers, such as placing two reactant vials with different sizes to form MOFs [60,61].
- (2)
- (3)
- Microwave method: In this tactic, solution containing small metal oxide particles is treated with microwave to raise temperature so that nano-sized metal crystals can be generated and leads to MOFs formation with controlled shape and size [63]. Contrary to other synthetic methods, microwave technology is a promising tactic with reduced reaction time and less processing energy consumption to have control over MOF properties. It is able to easily produce MOFs and MOF-hybrids in an isolated manner [64]. For example, Le et al. developed the mesoporous MOF-MIL-100 (Fe) via microwave-assisted continuous flow synthesis [by reacting iron(III) chloride hexahydrate (FeCl3⋅6H2O), 1,3,5-benzenetricarboxylic acid (H3BTC)] to support the construction of Cu(I) modified adsorbents towards CO/CO2 separation [65].
- (4)
- Electrochemical method: This tactic is generally used in the industry to produce MOFs in bulk. Contrary to solvothermal synthesis, this method has the advantage of quick synthesis at low temperature and also avoids usage of anionic metal salts, such as metallic nitrates [66]. However, fine tuning in applied voltage is required to attain better results towards designed MOFs.
- (5)
- Mechanochemical method: Contrast to traditional way of synthesis (dissolving, heating, and stirring chemicals in a solution), this method is environmentally friendly for synthesizing MOFs via mechanical forces, such as grinding and ball milling at ambient temperature without any solvent consumption. Moreover, certain number of MOFs can be obtained in a short time (10–20 min). This method is also noted as a technique at the interface of mechanical engineering and chemistry [67].
- (6)
- Sonochemistry method: This is a quick synthesis tactic reported for producing MOFs in an environmentally friendly manner via treating the reaction mixture with high energy ultrasound force (10–20 MHz with upper limit of human hearing). During this process, dissolution of the starting materials can be enhanced, thereby becoming a special research topic for scientists for producing MOFs in bulk [68].
- (7)
- Post-synthetic modifications: Apart from the aforementioned tactics, the designated MOFs can be synthesized via post-synthetic modifications, such as ligand exchange, metal exchange, opening of the coordinating sites, etc [69].
4. MOFs in Optical Detection of Hg2+
5. Metal Coordinated Polymers as Luminescent Hg2+ Sensors
6. MOFs Holding Composites for Optical Recognition of Hg2+
7. MOFs in Electrochemical Recognition of Hg2+
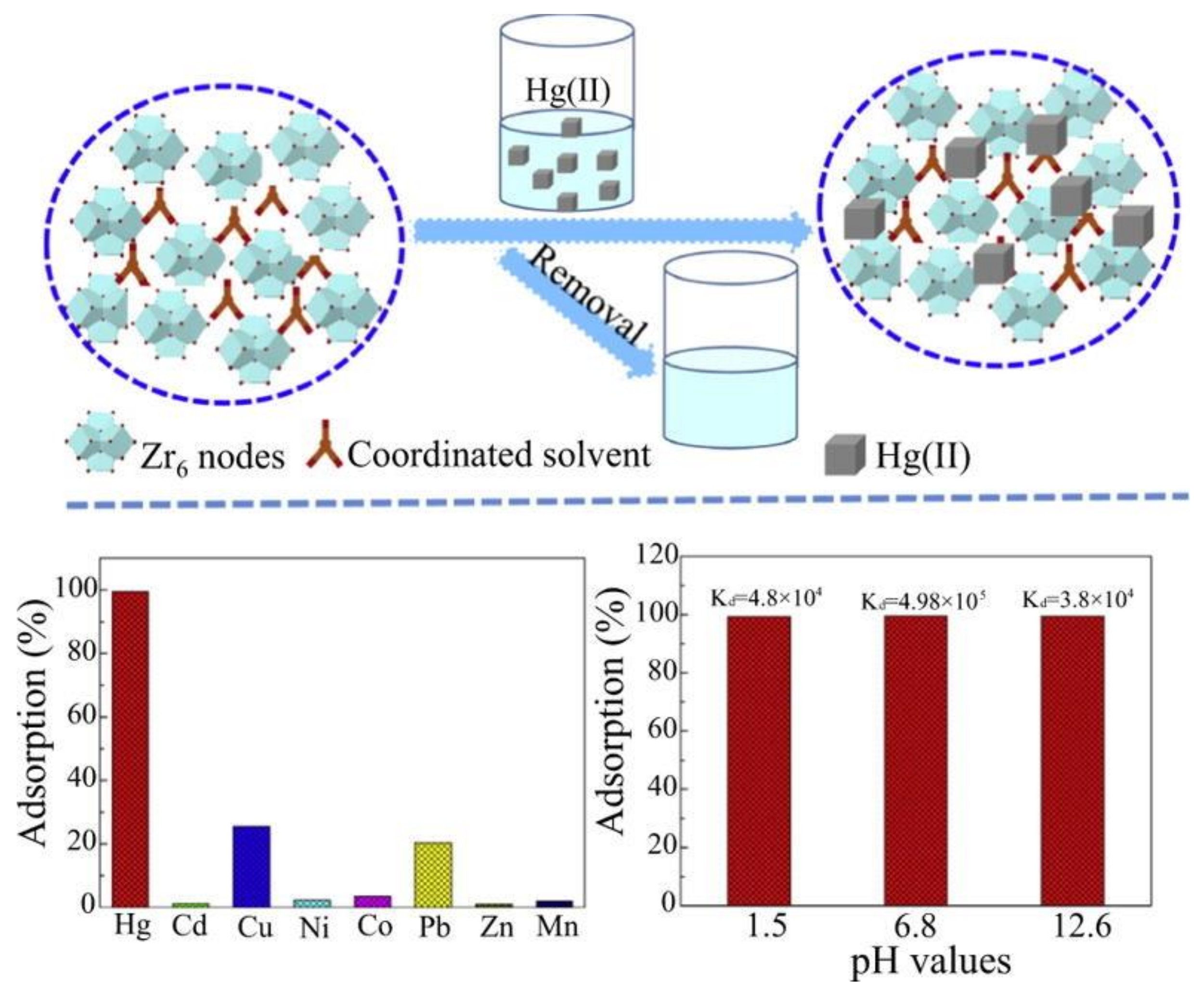
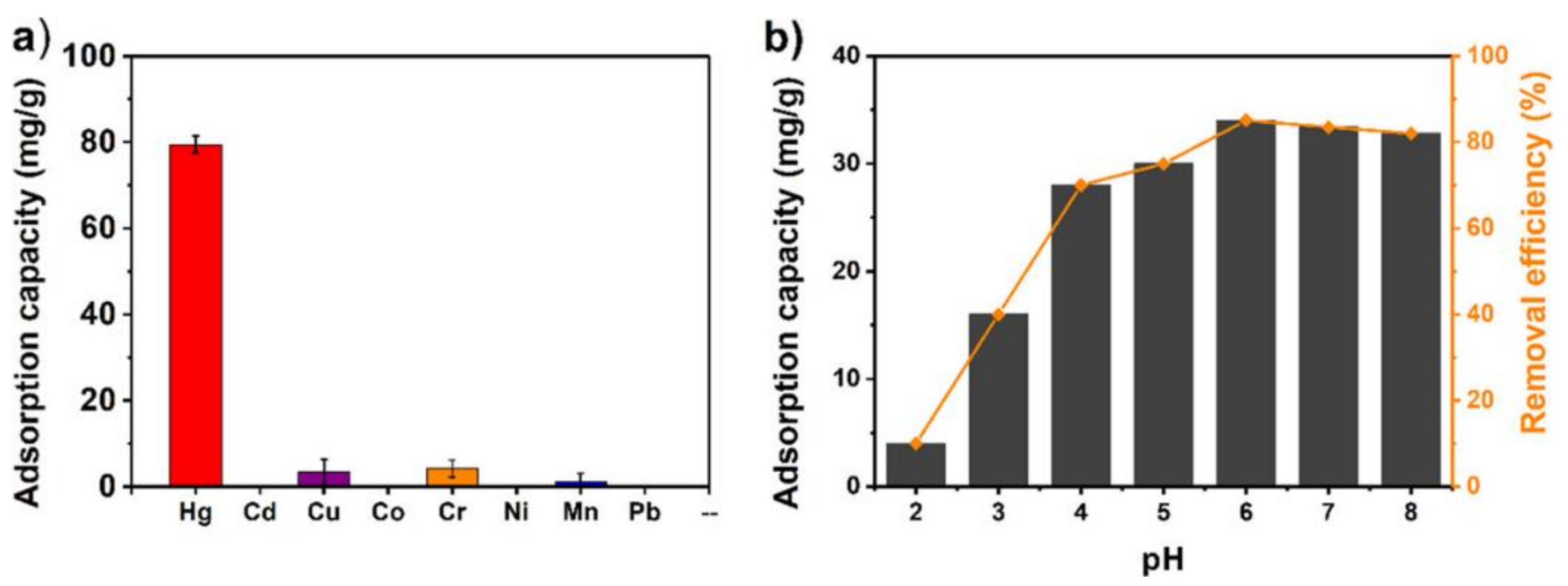
8. MOFs in Removal of Hg2+
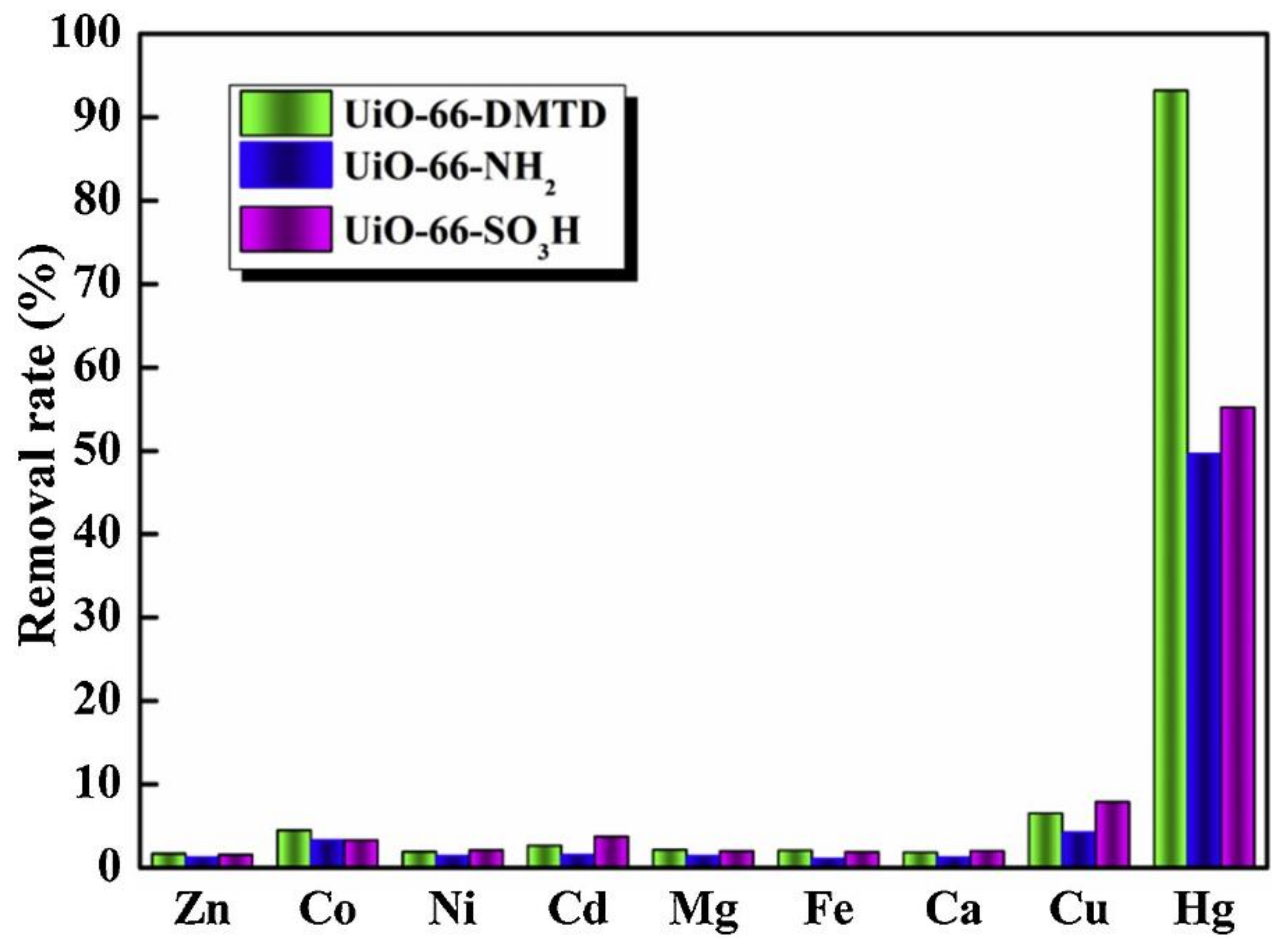
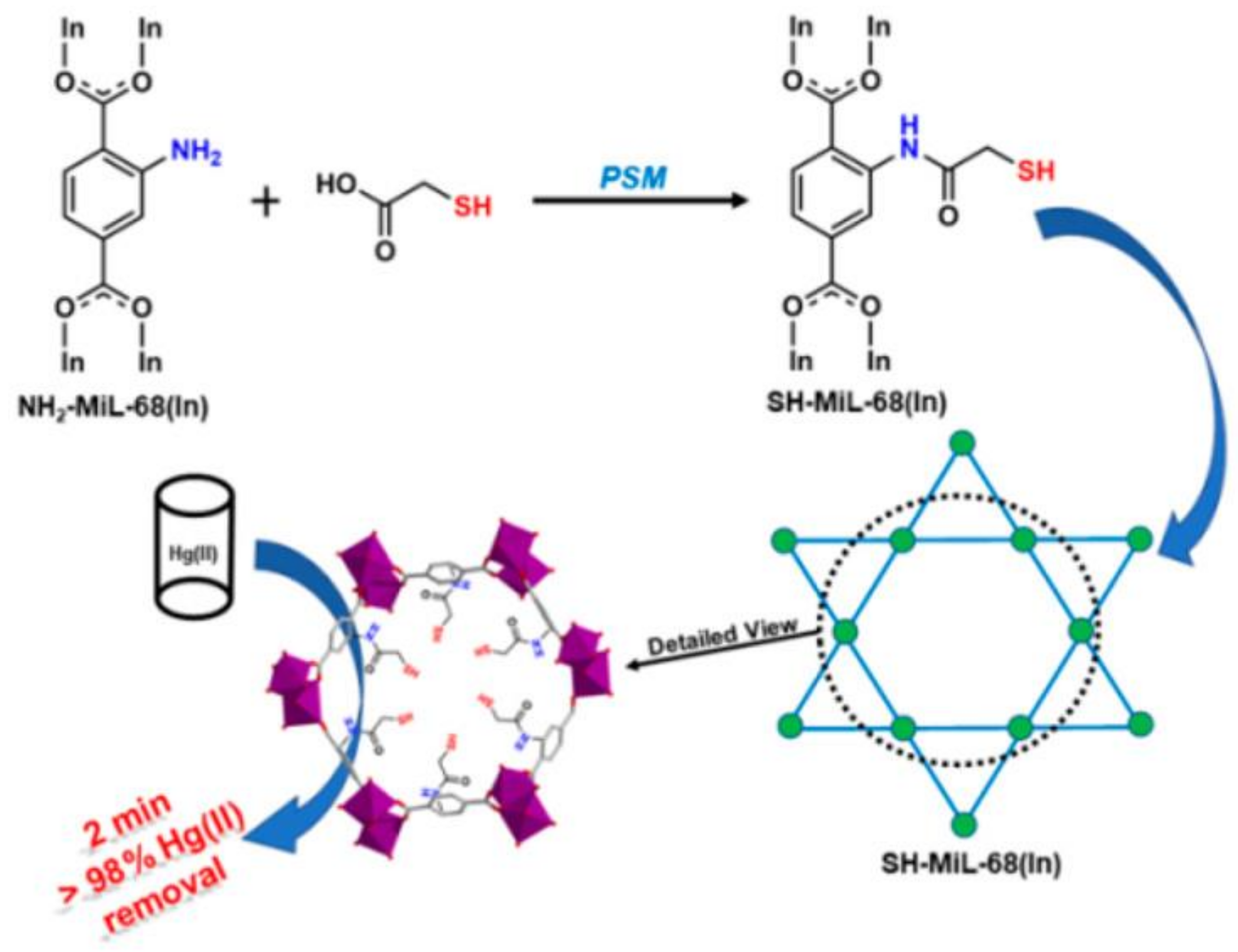
9. MOFs Comprised Composites for Hg2+ Removal
10. MOFs and Its Analogous in Elemental Mercury (Hg0) Adsorption
11. MOFs for Simultaneous Detection and Removal of Hg2+
12. Advantages
- The majority of MOFs and their derivatives detect or adsorb the Hg species in aqueous media, therefore, MOFs-based detection and removal experiments could sustain the eco-friendly process via decontaminating the toxic mercury from aquatic environment.
- Due to the porous nano/micro structural features, MOFs can be tuned towards encapsulation of specific Hg species, which could be further enhanced by post-synthetic modification or loading of specific groups, such as thiols (-SH).
- MOFs and their analogous have the advantage of recognition of multiple analytes, including Hg2+, via variations of detection conditions, masking agents, and analyte concentrations.
- MOFs can act as probes towards recognition and removal of Hg species through many tactics, such as optical, electrochemical, photoelectrochemical, etc. Thus, they are noted as materials with exceptional advantages.
- By tuning the compositions to adjust the specific porous surface, many composites comprised of MOFs have unique advantages of capturing Hg species in the presence of other interfering analytes.
- MOFs mediated Hg detection/removal process can be further extended towards recognition of specific bio-analytes, such as glutathione, cysteine, and thiol containing species.
13. Limitations
- Synthesis of designated MOFs and their analogous is still considered a tough task due to certain limitations, such as possible co-adduct formation, suitable tactics, reaction conditions, solvent, etc.
- Though MOFs display high sensitivity via fluorescence quenching or enhancement, however, many of them are consisted of toxic metals, such as Al, Cr, Zr, Lanthanides, etc. Therefore, bioimaging or biological assays of Hg2+ by these MOFs are restricted.
- MOFs with free thiol (-SH) containing organic linkers also showed selectivity to Pb2+, Cd2+, and Ag+, thereby limiting high selectivity towards Hg species via certain interfering effect.
- Majority of MOFs-based Hg2+ adsorption or removal studies were limited by many factors, such as MOFs concentration, structural stability, porosity, pH, time, operating temperature, suitable eluent, etc. Those factors require further attention.
- Design and development of certain MOFs comprised composites are also limited by the multiple complicated procedures, which not only increase the cost of the processes but also restrict the commercialization of materials.
- Complete characterizations of the Hg assay and removal processes also requires many costly instruments, such as scanning electron microscopy (SEM), powder-X-ray diffraction analyzer (PXRD), elemental analyzer, thermogravimetric analyzer (TGA), etc., which limits future research towards development of MOFs-based materials for mercury remediations.
- Finally, adsorption capacities of a few MOFs were found to be affected by the multiple interference effects and physical/chemical stability of MOFs during the Hg assays in real samples. Thus, much focus is anticipated to address this problem.
14. Conclusions and Perspectives
- A standardized cost-effective synthetic and purification technique must be proposed to develop MOFs for mercury detection and removal towards reliable environmental remediation.
- Luminescent MOFs of low toxicity must be developed by using less toxic metal nodes and organic linkers for detecting the presence of Hg2+ ions in biological samples.
- Fewer reports are available for MOFs-based Hg2+ detection via fluorescent enhancement, thereby such designs require more attention in the future.
- The underlying mechanisms of MOFs-based electrochemical detection of Hg2+ is not entirely clear, which require more experiments and investigations.
- The T-Hg2+-T coordination-based Hg2+ detection has already been stabilized in many MOF-DNA hybrid systems. It could be extended towards removal studies, which is currently incomplete.
- Many MOFs-based mercury detection and removal studies still lack evidence to strongly support the proposed mechanisms. This may require more attention.
- Following parameters, such as MOFs concentrations, pH, time, eluent solvent, and temperature, must be carefully examined and controlled for mercury removal, which complicate the separation processes. Therefore, development of MOFs that can adsorb Hg under less optimal conditions is becoming important.
- Development of MOFs for detection and removal of CH3Hg+ and Hg0 is currently less reported, thereby requiring much attention.
- Many free thiol (-SH) containing MOFs have been reported for Hg2+ removal. Thus “state-of-the-art” procedure with certain well-known MOFs (such as UIO-66) must be developed towards commercialization.
- Efficiency of post-synthetic modified MOFs over the unmodified one for mercury removal is still under debate, therefore, more evidence must be provided to support the PSM strategy.
- Interaction between Hg to Se produces the water insoluble and stable HgSe as an adduct, which improves the recyclability of the MOF probe. Thus, such design should be a future-focused research.
- Some MOFs also demonstrated moderately adsorption of other ions other than efficient mercury removal. This problem needs to be overcome for effective Hg separation in real samples.
- There are not many studies available which report both detection and removal of Hg2+ ions. This kind of studies are able to achieve great impact towards environmental remediation and require more attention.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.-H.; Shetaya, W.H.; Osterwalder, S. Determination of (Bio)-available mercury in soils: A review. Environ. Pollut. 2020, 263, 114323. [Google Scholar] [CrossRef]
- Botasini, S.; Heijo, G.; Méndez, E. Toward decentralized analysis of mercury (II) in real samples. A critical review on nanotechnology-based methodologies. Anal. Chim. Acta 2013, 800, 1–11. [Google Scholar] [CrossRef]
- Xie, Z.-J.; Bao, X.-Y.; Peng, C.-F. Highly Sensitive and Selective Colorimetric Detection of Methylmercury Based on DNA Functionalized Gold Nanoparticles. Sensors 2018, 18, 2679. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-modified diamond nanoparticles applied in cellular imaging and Hg2+ ions detection. Appl. Surf. Sci. 2019, 465, 340–350. [Google Scholar] [CrossRef]
- Aderinto, S.O. Fluorescent, colourimetric, and ratiometric probes based on diverse fluorophore motifs for mercuric(II) ion (Hg2+) sensing: Highlights from 2011 to 2019. Chem. Pap. 2020, 74, 3195–3232. [Google Scholar] [CrossRef] [PubMed]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Selid, P.D.; Xu, H.; Collins, E.M.; Face-Collins, M.S.; Zhao, J.X. Sensing Mercury for Biomedical and Environmental Monitoring. Sensors 2009, 9, 5446–5459. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2011, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Langford, N.J.; Ferner, R.E. Toxicity of mercury. J. Hum. Hypertens. 1999, 13, 651–656. [Google Scholar] [CrossRef]
- Rana, M.; Balcioglu, M.; Robertson, N.M.; Hizir, M.S.; Yumak, S.; Yigit, M.V. Low picomolar, instrument-free visual detection of mercury and silver ions using low-cost programmable nanoprobes. Chem. Sci. 2017, 8, 1200–1208. [Google Scholar] [CrossRef]
- Gauthama, B.; Narayana, B.; Sarojini, B.; Suresh, N.; Sangappa, Y.; Kudva, A.K.; Satyanarayana, G.; Raghu, S.V. Colorimetric “off–on” fluorescent probe for selective detection of toxic Hg2+ based on rhodamine and its application for in-vivo bioimaging. Microchem. J. 2021, 166, 106233. [Google Scholar] [CrossRef]
- Busairi, N.; Syahir, A. Recent Advances in Mercury Detection: Towards Enabling a Sensitive and Rapid Point-of-Check Measurement. J. Toxicol. Risk Assess 2018, 4, 010. [Google Scholar]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Luminescent Metal Nanoclusters for Potential Chemosensor Applications. Chemosensors 2017, 5, 36. [Google Scholar] [CrossRef]
- Shuai, H.; Xiang, C.; Qian, L.; Bin, F.; Xiaohui, L.; Jipeng, D.; Chang, Z.; Jiahui, L.; Wenbin, Z. Fluorescent sensors for detection of mercury: From small molecules to nanoprobes. Dyes Pigment. 2021, 187, 109125. [Google Scholar] [CrossRef]
- Dai, D.; Li, Z.; Yang, J.; Wang, C.; Wu, J.-R.; Wang, Y.; Zhang, D.; Yang, Y.-W. Supramolecular Assembly-Induced Emission Enhancement for Efficient Mercury(II) Detection and Removal. J. Am. Chem. Soc. 2019, 141, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Z.; Liu, Y.; Wei, J.; Liu, Q.; Ran, P.; Li, X. Fibrous strips decorated with cleavable aggregation-induced emission probes for visual detection of Hg2+. J. Hazard. Mater. 2020, 385, 121556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Li, Y.; Hu, T.; Chen, R.; Xiang, R.; Wang, Q.; Zeng, Y.; He, C. Novel thiol-functionalized covalent organic framework as adsorbent for simultaneous removal of BTEX and mercury (II) from water. Chem. Eng. J. 2020, 398, 125566. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Peng, J.; Du, Q.; He, H. Ratiometric fluorescence sensing of metal-organic frameworks: Tactics and perspectives. Coord. Chem. Rev. 2020, 404, 213113. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Metal ion detection using luminescent-MOFs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299. [Google Scholar] [CrossRef]
- Devaraj, M.; Sasikumar, Y.; Rajendran, S.; Ponce, L.C. Review—Metal Organic Framework Based Nanomaterials for Electrochemical Sensing of Toxic Heavy Metal Ions: Progress and Their Prospects. J. Electrochem. Soc. 2021, 168, 037513. [Google Scholar] [CrossRef]
- Vardali, S.C.; Manousi, N.; Barczak, M.; Giannakoudakis, D.A. Novel Approaches Utilizing Metal-Organic Framework Composites for the Extraction of Organic Compounds and Metal Traces from Fish and Seafood. Molecules 2020, 25, 513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, W.; Li, H.; Zhang, Q.; Liu, H. Sulfur-functionalized metal-organic frameworks: Synthesis and applications as advanced adsorbents. Coord. Chem. Rev. 2020, 408, 213191. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.H.; Jeoung, S.; Moon, H.R. Transformation of Metal–Organic Frameworks/Coordination Polymers into Functional Nanostructured Materials: Experimental Approaches Based on Mechanistic Insights. Acc. Chem. Res. 2017, 50, 2684–2692. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, W.; Cui, X.; Wang, Y. Recent Progress in Metal–Organic Frameworks and Their Derived Nanostructures for Energy and Environmental Applications. ChemSusChem 2017, 10, 1645–1663. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Sun, L.; Wang, L. Metal/metal oxide nanostructures derived from metal–organic frameworks. RSC Adv. 2014, 5, 7267–7279. [Google Scholar] [CrossRef]
- Heo, D.Y.; Do, H.H.; Ahn, S.H.; Kim, S.Y. Metal-Organic Framework Materials for Perovskite Solar Cells. Polymers 2020, 12, 2061. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Wu, G.; Huang, J.; Zang, Y.; He, J.; Xu, G. Porous Field-Effect Transistors Based on a Semiconductive Metal–Organic Framework. J. Am. Chem. Soc. 2017, 139, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, J.; Yin, J.; Li, N.; Bu, X.-H. Recent advances in luminescent metal-organic frameworks for chemical sensors. Sci. China Mater. 2019, 62, 1655–1678. [Google Scholar] [CrossRef]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter Metal–Organic Framework-Based Ratiometric Fluorescent Sensors. Adv. Mater. 2020, 32, 1805871. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Let, S.; Mandal, W.; Dutta, S.; Ghosh, S.K. Luminescent metal–organic frameworks (LMOFs) as potential probes for the recognition of cationic water pollutants. Inorg. Chem. Front. 2020, 7, 1801–1821. [Google Scholar] [CrossRef]
- Huang, S.-Z.; Liu, S.-S.; Zhang, H.-j.; Han, Z.; Zhao, G.; Dong, X.-Y.; Zang, S.-Q. Dual-Functional Proton-Conducting and pH-Sensing Polymer Membrane Benefiting from a Eu-MOF. ACS Appl. Mater. Interfaces 2020, 12, 28720–28726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-organic frameworks for virus detection. Biosens. Bioelectron. 2020, 169, 112604. [Google Scholar] [CrossRef]
- Gai, S.; Zhang, J.; Fan, R.; Xing, K.; Chen, W.; Zhu, K.; Zheng, X.; Wang, P.; Fang, X.; Yang, Y. Highly Stable Zinc-Based Metal-Organic Frameworks and Corresponding Flexible Composites for Removal and Detection of Antibiotics in Water. ACS Appl. Mater. Interfaces 2020, 12, 8650–8662. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Feng, D.; Yang, J.; Ma, X.; Wang, X.-Q. A turn-on luminescent probe for Fe3+ and ascorbic acid with logic gate operation based on a zinc(ii)-based metal–organic framework. New J. Chem. 2020, 44, 8728–8735. [Google Scholar] [CrossRef]
- Wu, R.-Z.; Yang, X.; Zhang, L.-W.; Zhou, P.-P. Luminescent lanthanide metal–organic frameworks for chemical sensing and toxic anion detection. Dalton Trans. 2017, 46, 9859–9867. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Dalapati, R.; Nandi, S.; Biswas, S. Post-synthetic modification of a metal-organic framework with a chemodosimeter for the rapid detection of lethal cyanide via dual emission. Dalton Trans. 2020, 49, 8684–8692. [Google Scholar] [CrossRef]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Mandal, S.C.; Pathak, B.; Neogi, S. Guest-Induced Ultrasensitive Detection of Multiple Toxic Organics and Fe3+ Ions in a Strategically Designed and Regenerative Smart Fluorescent Metal-Organic Framework. ACS Appl. Mater. Interfaces 2019, 11, 9042–9053. [Google Scholar] [CrossRef]
- Tchalala, M.R.; Bhatt, P.M.; Chappanda, K.N.; Tavares, S.R.; Adil, K.; Belmabkhout, Y.; Shkurenko, A.; Cadiau, A.; Heymans, N.; De Weireld, G.; et al. Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef]
- Rouhani, F.; Morsali, A. Goal-Directed Design of Metal-Organic Frameworks for HgII and PbII Adsorption from Aqueous Solutions. Chem. Eur. J. 2018, 24, 17170–17179. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Liu, B.; Chen, Y. Lanthanide Coordination Polymer Nanoparticles for Sensing of Mercury(II) by Photoinduced Electron Transfer. ACS Nano 2012, 6, 10505–10511. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.R. Preconcentration of mercury(II) using a thiol-functionalized metal-organic framework nanocomposite as a sorbent. Microchim. Acta 2014, 181, 435–444. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, B.; Zhu, S.; Xu, H.; Tian, L. UiO-66 and its Br-modified derivates for elemental mercury removal. J. Hazard. Mater. 2016, 320, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal–Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Fang, X.; Zong, B.; Mao, S. Metal-Organic Framework-Based Sensors for Environmental Contaminant Sensing. Nano-Micro Lett. 2018, 10, 1–19. [Google Scholar] [CrossRef]
- Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers 2020, 12, 691. [Google Scholar] [CrossRef]
- Yee, K.-K.; Reimer, N.; Liu, J.; Cheng, S.-Y.; Yiu, S.-M.; Weber, J.; Stock, N.; Xu, Z. Effective Mercury Sorption by Thiol-Laced Metal–Organic Frameworks: In Strong Acid and the Vapor Phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanoca-talysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Hu, M.-L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal–organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598–1632. [Google Scholar] [CrossRef]
- Dalapati, R.; Biswas, S. Post-synthetic modification of a metal-organic framework with fluorescent-tag for dual naked-eye sensing in aqueous medium. Sens. Actuators B Chem. 2017, 239, 759–767. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Al-Ghoul, M.; Issa, R.; Hmadeh, M. Synthesis, size and structural evolution of metal–organic framework-199 via a reaction–diffusion process at room temperature. CrystEngComm 2016, 19, 608–612. [Google Scholar] [CrossRef]
- Al Amery, N.; Abid, H.; Al-Saadi, S.; Wang, S.; Liu, S. Facile directions for synthesis, modification and activation of MOFs. Mater. Today Chem. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Klinowski, J.; Almeida Paz, F.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal-Organic Frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Thomas-Hillman, I.; Laybourn, A.; Dodds, C.; Kingman, S.W. Realising the environmental benefits of metal-organic frameworks: Recent advances in microwave synthesis. J. Mater. Chem. A 2018, 6, 11564–11581. [Google Scholar] [CrossRef]
- Le, V.N.; Kwon, H.T.; Vo, T.K.; Kim, J.-H.; Kim, W.-S.; Kim, J. Microwave-assisted continuous flow synthesis of mesoporous metal-organic framework MIL-100 (Fe) and its application to Cu(I)-loaded adsorbent for CO/CO2 separation. Mater. Chem. Phys. 2020, 253, 123278. [Google Scholar] [CrossRef]
- Li, W.-J.; Tu, M.; Cao, R.; Fischer, R.A. Metal-organic framework thin films: Electrochemical fabrication techniques and corresponding applications & perspectives. J. Mater. Chem. A 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ng, M.; Milner, P.J. Rapid mechanochemical synthesis of metal–organic frameworks using exogenous organic base. Dalton Trans. 2020, 49, 16238–16244. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Chapter 11—Sonochemical synthesis of MOFs. In Metal-Organic Frameworks for Bio-medical Applications; Mozafari, M., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 223–244. [Google Scholar]
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal-Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Zhao, W.; Gu, J. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase. RSC Adv. 2016, 6, 69807–69814. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, T.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. Highly sensitive and selective detection of mercury (II) based on a zirconium metal-organic framework in aqueous media. J. Solid State Chem. 2017, 253, 277–281. [Google Scholar] [CrossRef]
- Samanta, P.; Desai, A.V.; Sharma, S.; Chandra, P.; Ghosh, S.K. Selective Recognition of Hg2+ ion in Water by a Functionalized Metal–Organic Framework (MOF) Based Chemodosimeter. Inorg. Chem. 2018, 57, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxiong, Z.; Wenjun, Z.; Cuiliu, L.; Xiaohong, Q.; Chengyu, Z. Eu3+-Postdoped UIO-66-Type Metal-Organic Framework as a Luminescent Sensor for Hg2+ Detection in Aqueous Media. Inorg. Chem. 2019, 58, 3910–3915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, J.; Li, Y.; Zhuang, Q.; Gu, J. Zr-Based MOFs integrated with a chromophoric ruthenium complex for specific and reversible Hg2+sensing. Dalton Trans. 2018, 47, 5570–5574. [Google Scholar] [CrossRef]
- Li, W.-Y.; Yang, S.; Li, Y.-A.; Li, Q.-Y.; Guan, Q.; Dong, Y.-B. Synthesis of an MOF-based Hg2+-fluorescent probe via stepwise post-synthetic modification in a single-crystal-to-single-crystal fashion and its application in bioimaging. Dalton Trans. 2019, 48, 16502–16508. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.S.; Park, K.C.; Gupta, G.; Lee, C.Y. Colorimetric detection of heavy metal ions in water via metal-organic framework. Inorg. Chim. Acta 2019, 486, 69–73. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Liang, M.; Chen, G.; Kong, R.-M.; Xia, L.; Qu, F. A Boric Acid-Functionalized Lanthanide Metal-Organic Framework as a Fluorescence “Turn-on” Probe for Selective Monitoring of Hg2+ and CH3Hg+. Anal. Chem. 2020, 92, 3366–3372. [Google Scholar] [CrossRef]
- Xia, T.; Song, T.; Zhang, G.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. A Terbium Metal-Organic Framework for Highly Selective and Sensitive Luminescence Sensing of Hg2+ Ions in Aqueous Solution. Chem. A Eur. J. 2016, 22, 18429–18434. [Google Scholar] [CrossRef]
- Liu, X.; Lin, H.; Xiao, Z.; Fan, W.; Huang, A.; Wang, R.; Zhang, L.; Sun, D. Multifunctional lanthanide–organic frameworks for fluorescent sensing, gas separation and catalysis. Dalton Trans. 2016, 45, 3743–3749. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Masoomi, M.Y.; Morsali, A. Double Solvent Sensing Method for Improving Sensitivity and Accuracy of Hg(II) Detection Based on Different Signal Transduction of a Tetrazine-Functionalized Pillared Metal-Organic Framework. Inorg. Chem. 2017, 56, 9646–9652. [Google Scholar] [CrossRef]
- Wan, Y.; Zou, D.; Cui, Y.; Yang, Y.; Qian, G. A Zn based anionic metal-organic framework for trace Hg2+ ion detection. J. Solid State Chem. 2018, 266, 70–73. [Google Scholar] [CrossRef]
- Pankajakshan, A.; Kuznetsov, D.; Mandal, S. Ultrasensitive Detection of Hg(II) Ions in Aqueous Medium Using Zinc-Based Metal-Organic Framework. Inorg. Chem. 2019, 58, 1377–1381. [Google Scholar] [CrossRef]
- Khatun, A.; Panda, D.K.; Sayresmith, N.; Walter, M.G.; Saha, S. Thiazolothiazole-Based Luminescent Metal-Organic Frameworks with Ligand-to-Ligand Energy Transfer and Hg2+-Sensing Capabilities. Inorg. Chem. 2019, 58, 12707–12715. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Liu, J.; Zhou, Y.; Zhao, D.; Li, C. An acid-base resistant Zn-based metal-organic framework as a luminescent sensor for mercury(II). J. Solid State Chem. 2020, 283, 121153. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; Liu, Y.; Wang, J.; Li, Y.; Liu, W.; Wang, J. Cadmium-Based Metal-Organic Framework as a Highly Selective and Sensitive Ratiometric Luminescent Sensor for Mercury(II). Inorg. Chem. 2015, 54, 11046–11048. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-H.; Liu, D.-X.; Yang, K.-Q.; Dong, S.-J.; Li, W.; Wang, Y.-J. A new cluster-based metal-organic framework with triazine backbones for selective luminescent detection of mercury(II) ion. Inorg. Chem. Commun. 2018, 90, 61–64. [Google Scholar] [CrossRef]
- El Taher, B.J.; Sabouni, R.; Ghommem, M. Luminescent metal organic framework for selective detection of mercury in aqueous media: Microwave-based synthesis and evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125477. [Google Scholar] [CrossRef]
- Basaleh, A.S.; Sheta, S.M. Novel advanced nanomaterial based on ferrous metal-organic framework and its application as chemosensors for mercury in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 3153–3165. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Chen, C.; Chen, M.; Li, Z.; Wu, Y.; Zhu, S.; Peng, G. A hydrostable and bromine-functionalized manganese-organic framework with luminescence sensing of Hg2+ and antiferromagnetic properties. J. Solid State Chem. 2019, 269, 257–263. [Google Scholar] [CrossRef]
- Song, Y.; Fan, R.; Fan, J.; Xing, K.; Du, X.; Wang, P.; Yang, Y. Highly sensitive and selective fluorescent probes for Hg2+ in Ag(i)/Cu(ii) 3D supramolecular architectures based on noncovalent interactions. Dalton Trans. 2016, 45, 16422–16432. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.K.; Natarajan, S. Fluorescent Metal-Organic Frameworks for Selective Sensing of Toxic Cations (Tl3+, Hg2+) and Highly Oxidizing Anions ((CrO4)2−, (Cr2O7)2−, (MnO4)−). ChemPlusChem 2017, 82, 1153–1163. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, J.; Gao, X.; Ji, G.; Wang, D.; Liu, Z. A multi-chemosensor based on Zn-MOF: Ratio-dependent color transition detection of Hg (II) and highly sensitive sensor of Cr (VI). Sens. Actuators B Chem. 2018, 269, 164–172. [Google Scholar] [CrossRef]
- Fu, H.-R.; Zhao, Y.; Xie, T.; Han, M.-L.; Ma, L.-F.; Zang, S.-Q. Stable dye-encapsulated indium-organic framework as dual-emitting sensor for the detection of Hg2+/Cr2O72− and a wide range of nitro-compounds. J. Mater. Chem. C 2018, 6, 6440–6448. [Google Scholar] [CrossRef]
- Li, F.; Hong, Y.-S.; Zuo, K.-X.; Sun, Q.; Gao, E.-Q. Highly selective fluorescent probe for Hg2+ and MnO4− by the two-fold interpenetrating metal-organic framework with nitro functionalized linkers. J. Solid State Chem. 2019, 270, 509–515. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Liu, D.; Li, Y.-H.; Dong, G.-Y. Two new luminescent ternary Cd(II)-MOFs by regulation of aromatic dicarboxylate ligands used as efficient dual-responsive sensors for toxic metal ions in water. Polyhedron 2019, 159, 32–42. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Moussa, Z.; Prakasam, T.; Li, L.; Abiad, M.G.; Patra, D.; Hmadeh, M. Lanthanides based metal organic frameworks for luminescence sensing of toxic metal ions. J. Solid State Chem. 2020, 281, 121031. [Google Scholar] [CrossRef]
- Ren, M.; Wang, H.; Liu, Y.; Ma, Q.; Jia, W.; Liu, M.; Wang, H.; Lu, Y. Fluorescent Determination of Mercury (II) and Glutathione Using Amino-MIL-53(Al) Nanosheets. Anal. Lett. 2020, 53, 2700–2714. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Qin, C.; Shao, K.-Z.; Su, Z.-M. A fluorescent sensor for highly selective sensing of nitro explosives and Hg(ii) ions based on a 3D porous layer metal–organic framework. CrystEngComm 2016, 18, 4765–4771. [Google Scholar] [CrossRef]
- Gong, W.-J.; Ren, Z.-G.; Li, H.-X.; Zhang, J.-G.; Lang, J.-P. Cadmium(II) Coordination Polymers of 4-Pyr-poly-2-ene and Carboxylates: Construction, Structure, and Photochemical Double [2 + 2] Cycloaddition and Luminescent Sensing of Nitroaromatics and Mercury(II) Ions. Cryst. Growth Des. 2017, 17, 870–881. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Qu, Y.-H.; Fu, L.; Cui, G.-H. An unprecedented binodal (4,6)-connected Co(II) MOF as dual-responsive luminescent sensor for detection of acetylacetone and Hg2+ ions. Inorg. Chem. Commun. 2020, 118, 108013. [Google Scholar] [CrossRef]
- Sun, X.; Yang, P.; Hou, G.; Wei, J.; Wang, X.; Yang, D.; Zhang, X.; Dong, H.; Zhang, F. Luminescent Functionalised Supramolecular Coordination Polymers Based on an Aromatic Carboxylic Acid Ligand for Sensing Hg2+ Ions Aust. J. Chem. 2017, 70, 786–791. [Google Scholar]
- Li, Q.; Wang, C.; Tan, H.; Tang, G.; Gao, J.; Chen, C.-H. A turn on fluorescent sensor based on lanthanide coordination polymer nanoparticles for the detection of mercury(ii) in biological fluids. RSC Adv. 2016, 6, 17811–17817. [Google Scholar] [CrossRef]
- Li, W.-X.; Li, H.-X.; Li, H.-Y.; Chen, M.-M.; Shi, Y.-X.; Lang, J.-P. 1,4-Bis(2-(pyridin-4-yl)vinyl)naphthalene and Its Zinc(II) Coordination Polymers: Synthesis, Structural Characterization, and Selective Luminescent Sensing of Mercury(II) Ion. Cryst. Growth Des. 2017, 17, 3948–3959. [Google Scholar] [CrossRef]
- Zhang, S.-R.; Wang, W.; Xu, G.-J.; Yao, C.; Xu, Y.-H.; Su, Z.-M. A fluorescent sensor for selective, sensitive, and recyclable detection of mercury(II) in aqueous solution based on a zinc(II) coordination polymer. Inorg. Chem. Commun. 2018, 89, 73–77. [Google Scholar] [CrossRef]
- Gong, W.-J.; Yao, R.; Li, H.-X.; Ren, Z.-G.; Zhang, J.-G.; Lang, J.-P. Luminescent cadmium(ii) coordination polymers of 1,2,4,5-tetrakis(4-pyridylvinyl)benzene used as efficient multi-responsive sensors for toxic metal ions in water. Dalton Trans. 2017, 46, 16861–16871. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Han, X.X.; Hou, X.; Wang, J.-J.; Fu, F. Highly selective and sensitive detection of Hg2+, Cr2O72−, and nitrobenzene/2,4-dinitrophenol in water via two fluorescent Cd-CPs. New J. Chem. 2018, 42, 19844–19852. [Google Scholar] [CrossRef]
- Fang, Y.-M.; Ye, X.; Xia, L.; Dong, W.-W.; Zhao, J.; Li, D.-S. Four different dimensional Zn(II) coordination polymers as fluorescent sensor for detecting Hg2+, Cr2O72− in aqueous solution. J. Solid State Chem. 2018, 266, 181–188. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, Q.; Zhou, J.; Lin, X.; Reddy, R.C.K.; Yang, T.; Zhang, G. Five 3D lanthanide-based coordination polymers with 3,3,6T13 topology: Structures and luminescent sensor for Hg2+ and Pb2+ ions. J. Solid State Chem. 2019, 270, 339–345. [Google Scholar] [CrossRef]
- Rachuri, Y.; Parmar, B.; Bisht, K.K.; Suresh, E. Multiresponsive Adenine-Based Luminescent Zn(II) Coordination Polymer for Detection of Hg2+ and Trinitrophenol in Aqueous Media. Cryst. Growth Des. 2017, 17, 1363–1372. [Google Scholar] [CrossRef]
- Zhu, H.; Han, C.; Li, Y.-H.; Cui, G.-H. Two new coordination polymers containing long flexible bis(benzimidazole) ligand as luminescent chemosensors for acetylacetone and Hg(II) ions detection. J. Solid State Chem. 2020, 282, 121132. [Google Scholar] [CrossRef]
- Ravikumar, A.; Panneerselvam, P.; Morad, N. Metal-Polydopamine Framework as an Effective Fluorescent Quencher for Highly Sensitive Detection of Hg(II) and Ag(I) Ions through Exonuclease III Activity. ACS Appl. Mater. Interfaces 2018, 10, 20550–20558. [Google Scholar] [CrossRef]
- Huang, N.-H.; Liu, Y.; Li, R.-T.; Chen, J.; Hu, P.-P.; Young, D.J.; Chen, J.-X.; Zhang, W.-H. Sequential Ag+/biothiol and synchronous Ag+/Hg2+ biosensing with zwitterionic Cu2+-based metal–organic frameworks. Analyst 2020, 145, 2779–2788. [Google Scholar] [CrossRef]
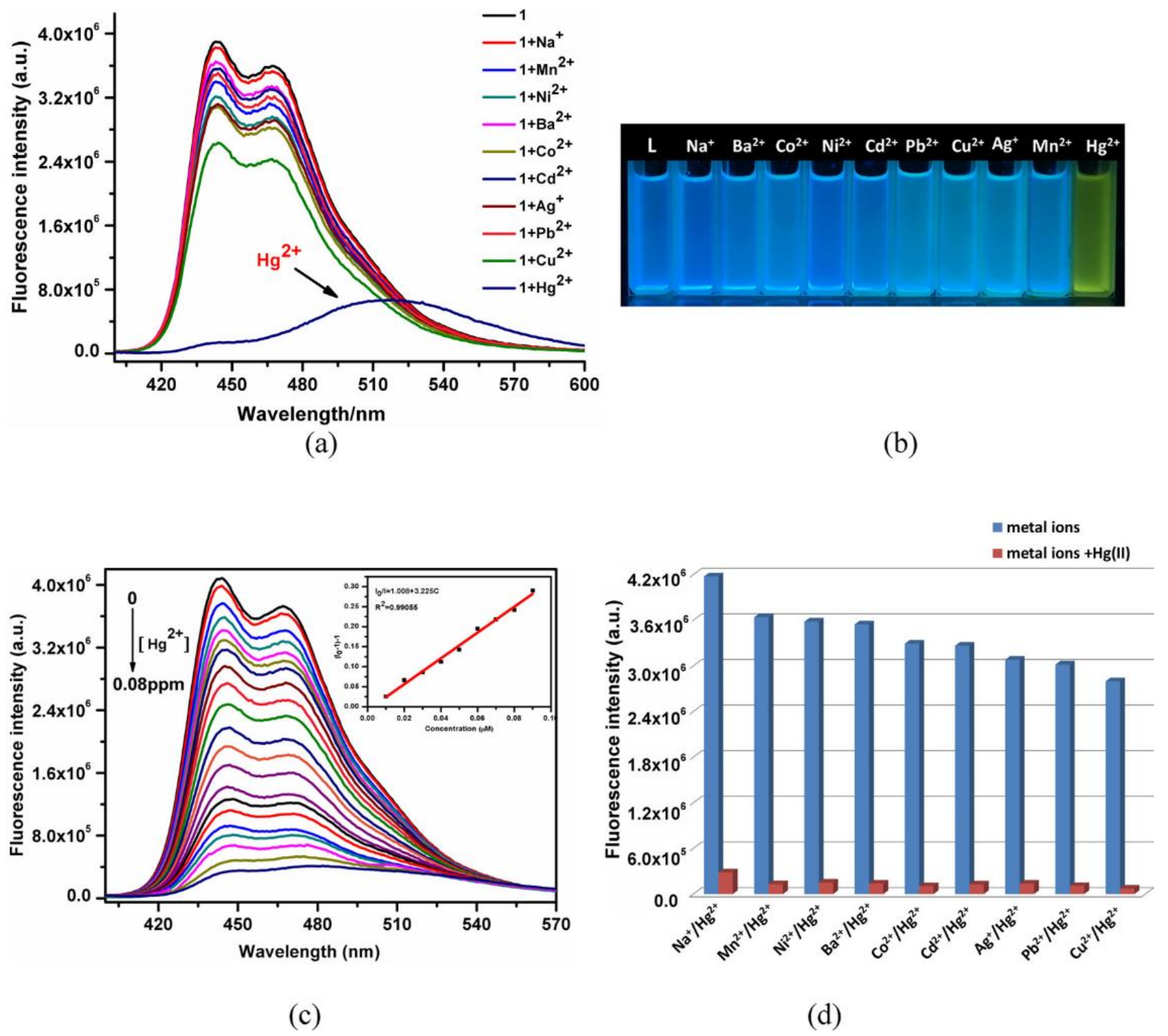
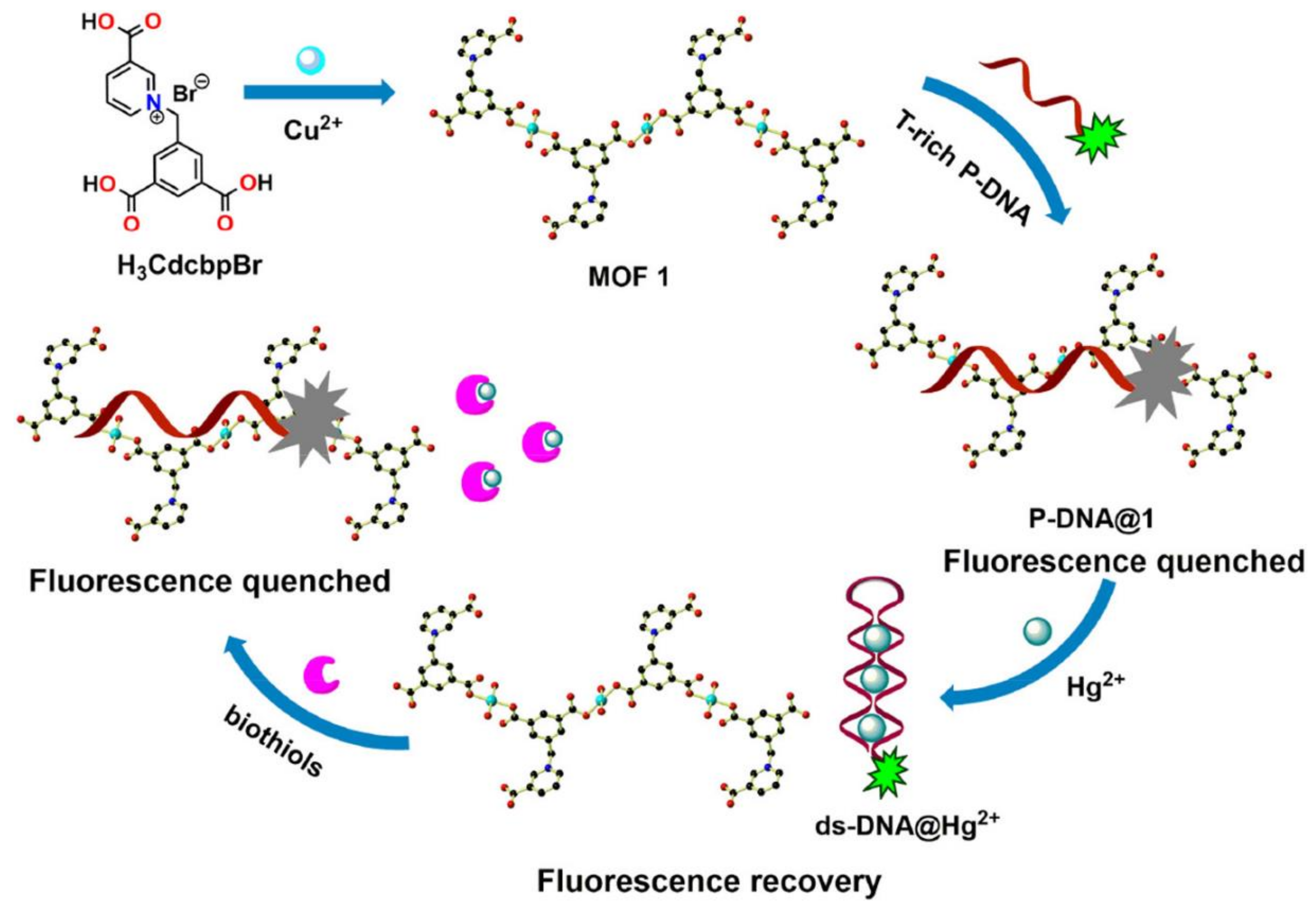
- Huang, N.-H.; Li, R.-T.; Fan, C.; Wu, K.-Y.; Zhang, Z.; Chen, J.-X. Rapid sequential detection of Hg2+ and biothiols by a probe DNA-MOF hybrid sensory system. J. Inorg. Biochem. 2019, 197, 110690. [Google Scholar] [CrossRef]
- Wu, L.-L.; Wang, Z.; Zhao, S.-N.; Meng, X.; Song, X.-Z.; Feng, J.; Song, S.-Y.; Zhang, H.-J. A Metal-Organic Framework/DNA Hybrid System as a Novel Fluorescent Biosensor for Mercury(II) Ion Detection. Chem. A Eur. J. 2015, 22, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, G.; Tan, H. Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Microchim. Acta 2018, 185, 475. [Google Scholar] [CrossRef]
- Wu, F.; Ye, J.; Cao, Y.; Wang, Z.; Miao, T.; Shi, Q. Recent advances in fluorescence sensors based on DNA-MOF hybrids. Luminescence 2020, 35, 440–446. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, R.-T.; Wu, K.-Y.; Hu, P.-P.; Zhang, Z.; Huang, N.-H.; Zhang, W.-H.; Chen, J.-X. Experimental and theoretical validations of a one-pot sequential sensing of Hg2+ and biothiols by a 3D Cu-based zwitterionic metal−organic framework. Talanta 2020, 210, 120596. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Kong, F.; Zhao, C.-Q.; Ding, S.-N. Ratiometric fluorescent nanosensors for ultra-sensitive detection of mercury ions based on AuNCs/MOFs. Analyst 2019, 144, 2523–2530. [Google Scholar] [CrossRef]
- Govindaraju, S.; Puthiaraj, P.; Lee, M.-H.; Yun, K. Photoluminescent AuNCs@UiO-66 for Ultrasensitive Detection of Mercury in Water Samples. ACS Omega 2018, 3, 12052–12059. [Google Scholar] [CrossRef] [PubMed]
- Marieeswaran, M.; Panneerselvam, P. A magnetic nanoscale metal-organic framework (MNMOF) as a viable fluorescence quencher material for ssDNA and for the detection of mercury ions via a novel quenching-quenching mechanism. RSC Adv. 2020, 10, 3705–3714. [Google Scholar] [CrossRef]
- Hu, P.-P.; Liu, N.; Wu, K.-Y.; Zhai, L.-Y.; Xie, B.-P.; Sun, B.; Duan, W.-J.; Zhang, W.-H.; Chen, J.-X. Successive and Specific Detection of Hg2+ and I– by a DNA@MOF Biosensor: Experimental and Simulation Studies. Inorg. Chem. 2018, 57, 8382–8389. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Guo, L.; Chen, G.; Kong, R.; Qu, F.; Xia, L. Colorimetric detection of Hg(ii) based on the gold amalgam-triggered reductase mimetic activity in aqueous solution by employing AuNP@MOF nanoparticles. Analyst 2020, 145, 1362–1367. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Pan, Q.; Zhang, X.; Shao, W.; Quan, C.; Li, J. An Au@NH2-MIL-125(Ti)-based multifunctional platform for colorimetric detections of biomolecules and Hg2+. J. Mater. Chem. B 2020, 8, 114–124. [Google Scholar] [CrossRef]
- Guo, M.; Chi, J.; Li, Y.; Waterhouse, G.I.N.; Ai, S.; Hou, J.; Li, X. Fluorometric determination of mercury(II) based on dual-emission metal-organic frameworks incorporating carbon dots and gold nanoclusters. Microchim. Acta 2020, 187, 534. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, W.; Cao, J.; Wu, Y. On-site, rapid and visual determination of Hg2+ and Cu2+ in red wine by ratiometric fluorescence sensor of metal-organic frameworks and CdTe QDs. Food Chem. 2020, 328, 127119. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Inorganic-Diverse Nanostructured Materials for Volatile Organic Compound Sensing. Sensors 2021, 21, 633. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Zhu, M.; Xu, Y.; Guo, Z.; Shi, J.; Han, E.; Zou, X.; Wang, D. Electrochemical DNA sensor for inorganic mercury(II) ion at attomolar level in dairy product using Cu(II)-anchored metal-organic framework as mimetic catalyst. Chem. Eng. J. 2020, 383, 123182. [Google Scholar] [CrossRef]
- Salandari-Jolge, N.; Ensafi, A.A.; Rezaei, B. Ultra-sensitive electrochemical aptasensor based on zeolitic imidazolate framework-8 derived Ag/Au core-shell nanoparticles for mercury detection in water samples. Sensors Actuators B Chem. 2021, 331, 129426. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Jiang, Y.; Wang, X.; Guo, Z.; Shi, J.; Zou, X.; Han, E. Simple electrochemical sensing for mercury ions in dairy product using optimal Cu2+-based metal-organic frameworks as signal reporting. J. Hazard. Mater. 2020, 400, 123222. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, K.; Wang, A.; Lyu, F.; Ge, J.; Zhang, L.; Zhang, H.; Su, W.; Hou, Y.-L.; Zhou, C.; et al. High selective detection of mercury (II) ions by thioether side groups on metal-organic frameworks. Anal. Chim. Acta 2019, 1081, 51–58. [Google Scholar] [CrossRef]
- Qing, M.; Chen, S.; Xie, S.; Tang, Y.; Zhang, J.; Yuan, R. Encapsulation and Release of Recognition Probes Based on a Rigid Three-Dimensional DNA “Nanosafe-box” for Construction of a Electrochemical Biosensor. Anal. Chem. 2020, 92, 1811–1817. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Zhan, Y.; Singh, V.; Van Hung, T.; Nam, N.D. A novel highly efficient and ultrasensitive electro-chemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework. J. Hazard. Mater. 2020, 399, 123042. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Pournara, A.; Manos, M.; Spanopoulos, I.; Kanatzidis, M.; Tziotzi, T.; Petkov, V.; Margariti, A.; Oikonomopoulos, P.; et al. 3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca-MOF sorbent for the determination of Hg(II). Sens. Actuators B Chem. 2020, 321, 128508. [Google Scholar] [CrossRef]
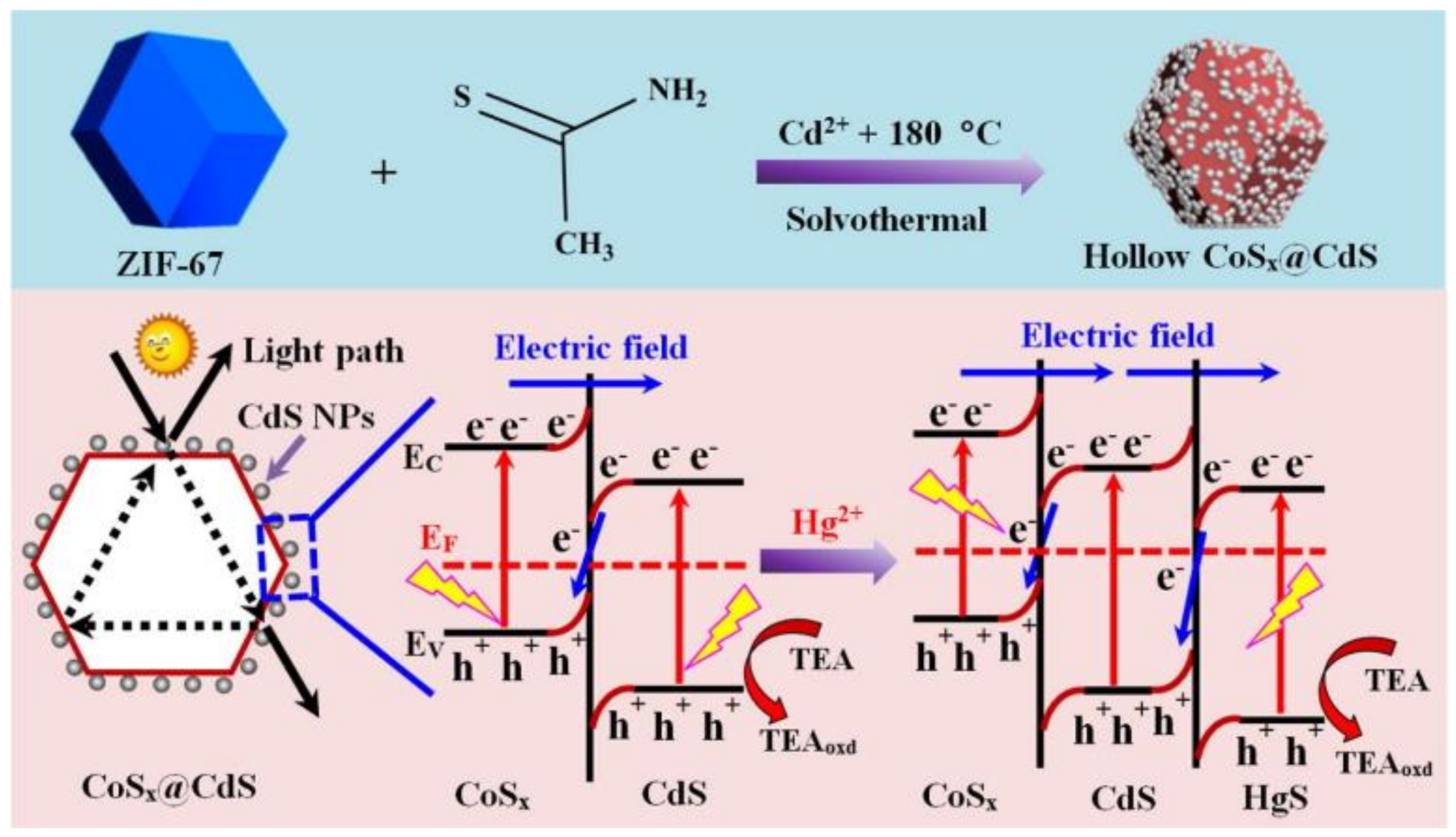
- Zhang, L.; Feng, L.; Li, P.; Chen, X.; Jiang, J.; Zhang, S.; Zhang, C.; Zhang, A.; Chen, G.; Wang, H. Direct Z-scheme photocatalyst of hollow CoSx@CdS polyhedron constructed by ZIF-67-templated one-pot solvothermal route: A signal-on photoelectrochemical sensor for mercury (II). Chem. Eng. J. 2020, 395, 125072. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ye, Z. Recent Progress in Heavy Metal Ion Decontamination Based on Metal-Organic Frameworks. Nanomaterials 2020, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Kahkha, M.R.; Daliran, S.; Oveisi, A.R.; Kaykhaii, M.; Sepehri, Z. The Mesoporous Porphyrinic Zirconium Metal-Organic Framework for Pipette-Tip Solid-Phase Extraction of Mercury from Fish Samples Followed by Cold Vapor Atomic Absorption Spectrometric Determination. Food Anal. Methods 2017, 10, 2175–2184. [Google Scholar] [CrossRef]
- Hasankola, Z.S.; Rahimi, R.; Shayegan, H.; Moradi, E.; Safarifard, V. Removal of Hg2+ heavy metal ion using a highly stable mesoporous porphyrinic zirconium metal-organic framework. Inorg. Chim. Acta 2020, 501, 119264. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Alsaedi, A.; Hayat, T.; Chen, C. Synthesis of highly porous inorganic adsorbents derived from metal-organic frameworks and their application in efficient elimination of mercury(II). J. Colloid Interface Sci. 2018, 517, 61–71. [Google Scholar] [CrossRef]
- Leus, K.; Perez, J.P.H.; Folens, K.; Meledina, M.; Van Tendeloo, G.; Du Laing, G.; Van Der Voort, P. UiO-66-(SH)2 as stable, selective and regenerable adsorbent for the removal of mercury from water under environmentally-relevant conditions. Faraday Discuss. 2017, 201, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Ai, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups. Chem. Eng. J. 2018, 354, 790–801. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Fang, J.; Wei, C.; Liu, G. Post-functionalization of UiO-66-NH2 by 2,5-Dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(II) in water. J. Hazard. Mater. 2019, 368, 42–51. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.; Shao, P.; Yang, J.; Sun, D. Thiol-Functionalized Zr-Based Metal-Organic Framework for Capture of Hg(II) through a Proton Exchange Reaction. ACS Sustain. Chem. Eng. 2018, 6, 8494–8502. [Google Scholar] [CrossRef]
- Li, M.-Q.; Wong, Y.-L.; Lum, T.-S.; Leung, K.S.-Y.; Lam, P.K.S.; Xu, Z. Dense thiol arrays for metal–organic frameworks: Boiling water stability, Hg removal beyond 2 ppb and facile crosslinking. J. Mater. Chem. A 2018, 6, 14566–14570. [Google Scholar] [CrossRef]
- Yang, P.; Shu, Y.; Zhuang, Q.; Li, Y.; Gu, J. A robust MOF-based trap with high-density active alkyl thiol for the super-efficient capture of mercury. Chem. Commun. 2019, 55, 12972–12975. [Google Scholar] [CrossRef]
- Lin, D.; Liu, X.; Huang, R.; Qi, W.; Su, R.; He, Z. One-pot synthesis of mercapto functionalized Zr-MOFs for the enhanced removal of Hg2+ ions from water. Chem. Commun. 2019, 55, 6775–6778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, Z.; Wang, S.; Zhang, L.; Zhou, Y. Design of l-Cysteine Functionalized UiO-66 MOFs for Selective Adsorption of Hg(II) in Aqueous Medium. ACS Appl. Mater. Interfaces 2019, 11, 46973–46983. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiong, W.; Feng, X.; Cheng, G.; Shi, L.; Chen, D.; Zhang, Y. Highly recyclable cysteamine-modified acid-resistant MOFs for enhancing Hg (II) removal from water. Environ. Technol. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- He, Y.; Hou, Y.-L.; Wong, Y.-L.; Xiao, R.; Li, M.-Q.; Hao, Z.; Huang, J.; Wang, L.; Zeller, M.; He, J.; et al. Improving stability against desolvation and mercury removal performance of Zr(iv)-carboxylate frameworks by using bulky sulfur functions. J. Mater. Chem. A 2017, 6, 1648–1654. [Google Scholar] [CrossRef]
- Chen, S.; Feng, F.; Li, S.; Li, X.-X.; Shu, L. Metal-organic framework DUT-67 (Zr) for adsorptive removal of trace Hg2+ and CH3Hg+ in water. Chem. Speciat. Bioavailab. 2018, 30, 99–106. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Liu, B.; Ma, J. A new synthesis and adsorption mechanism of ZrO2 based metal-organic frames for efficient removal of mercury ions from aqueous solution. Ceram. Int. 2019, 45, 15720–15724. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Li, J.Q.; Le Gong, L.; Feng, X.F.; Na Meng, L.; Zhang, L.; Meng, P.P.; Luo, M.B.; Luo, F. Using MOF-74 for Hg2+ removal from ultra-low concentration aqueous solution. J. Solid State Chem. 2017, 246, 16–22. [Google Scholar] [CrossRef]
- Wang, C.-M.; Lin, Y.-J.; Pan, M.-F.; Su, C.-K.; Lin, T.-Y. A Highly Stable Framework of Crystalline Zinc Phosphite with Selective Removal, Recovery, and Turn-On Sensing Abilities for Mercury Cations in Aqueous Solutions. Chem. Eur. J. 2018, 24, 9729–9734. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, L.; Gharib, M.; Morsali, A. The targeted design of dual-functional metal–organic frameworks (DF-MOFs) as highly efficient adsorbents for Hg2+ ions: Synthesis for purpose. Dalton Trans. 2019, 48, 17831–17839. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, G.; Wei, F.; Song, Q.; Tang, T.; Wang, X.; Hu, Q. Determination of Hg (II) in tea and mushroom samples based on metal-organic frameworks as solid phase extraction sorbents. Microporous Mesoporous Mater. 2016, 235, 204–210. [Google Scholar] [CrossRef]
- Mon, M.; Qu, X.; Ferrando-Soria, J.; Pellicer-Carreño, I.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Jansen, J.C.; Armentano, D.; Pardo, E. Fine-tuning of the confined space in microporous metal–organic frameworks for efficient mercury removal. J. Mater. Chem. A 2017, 5, 20120–20125. [Google Scholar] [CrossRef]
- Yazdi, M.N.; Yamini, Y.; Asiabi, H.; Alizadeh, A. A metal organic framework prepared from benzene-1,3,5-tricarboxylic acid and copper(II), and functionalized with various polysulfides as a sorbent for selective sorption of trace amounts of heavy metal ions. Microchim. Acta 2018, 185, 525. [Google Scholar] [CrossRef]
- Xu, W.-Q.; Lin, C.-C.; Qiu, Y.-X.; He, S.; Jiang, T.; Liu, X.-J.; Xie, L.-J.; Fuhr, O.; Fenske, D.; Jiang, J.-J. A Recoverable Complex with Nitrogen-Rich Double Rings for Hg(II) Sorption. ChemistrySelect 2018, 3, 7592–7595. [Google Scholar] [CrossRef]
- Xu, W.-Q.; He, S.; Liu, S.-J.; Liu, X.-H.; Qiu, Y.-X.; Liu, W.-T.; Jiang, L.-C.; Jiang, J.-J. Post-synthetic modification of a metal-organic framework based on 5-aminoisophthalic acid for mercury sorption. Inorg. Chem. Commun. 2019, 108, 107515. [Google Scholar] [CrossRef]
- Liang, L.; Chen, Q.; Jiang, F.; Yuan, D.; Qian, J.; Lv, G.; Xue, H.; Liu, L.; Jiang, H.-L.; Hong, M. In situ large-scale construction of sulfur-functionalized metal–organic framework and its efficient removal of Hg(ii) from water. J. Mater. Chem. A 2016, 4, 15370–15374. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; He, W.-W.; Li, S.-L.; Su, Z.-M.; Lan, Y.-Q. Introduction of Molecular Building Blocks to Improve the Stability of Metal–Organic Frameworks for Efficient Mercury Removal. Inorg. Chem. 2018, 57, 6118–6123. [Google Scholar] [CrossRef]
- Wang, C.; He, C.; Luo, Y.-H.; Su, S.; Wang, J.-Y.; Hong, D.-L.; He, X.-T.; Chen, C.; Sun, B.-W. Efficient mercury chloride capture by ultrathin 2D metal-organic framework nanosheets. Chem. Eng. J. 2020, 379, 122337. [Google Scholar] [CrossRef]
- Tian, J.; Shi, C.; Xiao, C.; Jiang, F.; Yuan, D.; Chen, Q.; Hong, M. Introduction of Flexibility into a Metal-Organic Framework to Promote Hg(II) Capture through Adaptive Deformation. Inorg. Chem. 2020, 59, 18264–18275. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Mondal, J.; Ortega-Castro, J.; Frontera, A.; Roy, P. A Ni-based MOF for selective detection and removal of Hg2+ in aqueous medium: A facile strategy. Dalton Trans. 2017, 46, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-P.; Zhang, K.; Zhang, P.-F.; Liu, W.-N.; Tong, W.-Q.; Hou, L.; Wang, Y.-Y. Thiol-Functionalized Pores via Post-Synthesis Modification in a Metal-Organic Framework with Selective Removal of Hg(II) in Water. Inorg. Chem. 2019, 58, 3409–3415. [Google Scholar] [CrossRef]
- Li, K.; Li, J.-J.; Zhao, N.; Xie, T.-T.; Di, B.; Xu, L.-L. Thioether-based recyclable metal-organic frameworks for selective and efficient removal of Hg2+ from water. Dalton Trans. 2019, 48, 17800–17809. [Google Scholar] [CrossRef]
- Han, Y.; Zheng, H.; Liu, K.; Wang, H.; Huang, H.; Xie, L.-H.; Wang, L.; Li, J.-R. In-Situ Ligand Formation-Driven Preparation of a Heterometallic Metal-Organic Framework for Highly Selective Separation of Light Hydrocarbons and Efficient Mercury Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 23331–23337. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Lloret, F.; Ferrando-Soria, J.; Martí-Gastaldo, C.; Armentano, D.; Pardo, E. Selective and Efficient Removal of Mercury from Aqueous Media with the Highly Flexible Arms of a BioMOF. Angew. Chem. Int. Ed. 2016, 55, 11167–11172. [Google Scholar] [CrossRef]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater. 2016, 221, 238–244. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, J.; Zhang, S.; Alsaedi, A.; Hayat, T.; Li, J.; Song, Y. Efficient removal of metal contaminants by EDTA modified MOF from aqueous solutions. J. Colloid Interface Sci. 2019, 555, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, F.; Morsali, A. Fast and Selective Heavy Metal Removal by a Novel Metal-Organic Framework Designed with In-Situ Ligand Building Block Fabrication Bearing Free Nitrogen. Chem. A Eur. J. 2018, 24, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Hakimifar, A.; Morsali, A. Urea-Based Metal-Organic Frameworks as High and Fast Adsorbent for Hg2+ and Pb2+ Removal from Water. Inorg. Chem. 2019, 58, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Bruno, R.; Tiburcio, E.; Viciano-Chumillas, M.; Kalinke, L.H.G.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Multivariate Metal-Organic Frameworks for the Simultaneous Capture of Organic and Inorganic Contaminants from Water. J. Am. Chem. Soc. 2019, 141, 13601–13609. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions: Synthesis, applications and adsorption mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Zhang, J.; Cheng, Y.; Zhang, C.; Fei, X.; Xian, Y. Platinum Nanoparticle Encapsulated Metal-Organic Frameworks for Colorimetric Measurement and Facile Removal of Mercury(II). ACS Appl. Mater. Interfaces 2017, 9, 40716–40725. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, D.; Huang, R.; Qi, W.; Su, R.; He, Z. Construction of a Mercapto-Functionalized Zr-MOF/Melamine Sponge Composite for the Efficient Removal of Oils and Heavy Metal Ions from Water. Ind. Eng. Chem. Res. 2020, 59, 13220–13227. [Google Scholar] [CrossRef]
- Liu, F.; Xiong, W.; Feng, X.; Shi, L.; Chen, D.; Zhang, Y. A novel monolith ZnS-ZIF-8 adsorption material for ultraeffective Hg (II) capture from wastewater. J. Hazard. Mater. 2019, 367, 381–389. [Google Scholar] [CrossRef]
- Nosike, E.I.; Jiang, Z.; Miao, L.; Akakuru, O.U.; Yuan, B.; Wu, S.; Zhang, Y.; Zhang, Y.; Wu, A. A novel hybrid nanoadsorbent for effective Hg2+ adsorption based on zeolitic imidazolate framework (ZIF-90) assembled onto poly acrylic acid capped Fe3O4 nanoparticles and cysteine. J. Hazard. Mater. 2020, 392, 122288. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. A designable magnetic MOF composite and facile coordination-based post-synthetic strategy for the enhanced removal of Hg2+ from water. J. Mater. Chem. A 2015, 3, 11587–11595. [Google Scholar] [CrossRef]
- Liang, L.; Liu, L.; Jiang, F.; Liu, C.; Yuan, D.; Chen, Q.; Wu, D.; Jiang, H.-L.; Hong, M. Incorporation of In2S3 Nanoparticles into a Metal-Organic Framework for Ultrafast Removal of Hg from Water. Inorg. Chem. 2018, 57, 4891–4897. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Jiang, J.; Li, Y.; Wan, X.; Ko, S. Highly selective removal of Hg 2+ and Pb2+ by thiol-functionalized Fe3O4@metal-organic framework core-shell magnetic microspheres. Appl. Surf. Sci. 2017, 413, 266–274. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal-Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef]
- Abdollahi, N.; Akbar Razavi, S.A.; Morsali, A.; Hu, M.-L. High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Liu, J. Theoretical prediction the removal of mercury from flue gas by MOFs. Fuel 2016, 184, 474–480. [Google Scholar] [CrossRef]
- Tang, H.; Fang, H.; Duan, Y.; Sholl, D.S. Predictions of Hg0 and HgCl2 Adsorption Properties in UiO-66 from Flue Gas Using Molecular Simulations. J. Phys. Chem. C 2019, 123, 5972–5979. [Google Scholar] [CrossRef]
- Zhao, S.; Mei, J.; Xu, H.; Liu, W.; Qu, Z.; Cui, Y.; Yan, N. Research of mercury removal from sintering flue gas of iron and steel by the open metal site of Mil-101(Cr). J. Hazard. Mater. 2018, 351, 301–307. [Google Scholar] [CrossRef]
- Dong, L.; Huang, Y.; Liu, L.; Liu, C.; Xu, L.; Zha, J.; Chen, H.; Liu, H. Investigation of Elemental Mercury Removal from Coal-Fired Boiler Flue Gas over MIL101-Cr. Energy Fuels 2019, 33, 8864–8875. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, S.; Qu, Z.; Yan, N. Cu-BTC as a novel material for elemental mercury removal from sintering gas. Fuel 2018, 217, 297–305. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, L.; Wang, Q.; Tariq, M.; Xue, Y.; Zhou, Z.; Sun, W.; Yang, J. Enhanced Hg0 removal via α-MnO2 anchored to MIL-96(Al). Appl. Surf. Sci. 2019, 483, 252–259. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Shen, B.; Hu, Z. MIL-100(Fe) supported Mn-based catalyst and its behavior in Hg0 removal from flue gas. J. Hazard. Mater. 2020, 381, 121003. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yang, J.; Feng, S.; Liu, X.; Zhao, J.; Qu, W.; Li, P.; Feng, Y.; Lee, P.-H.; et al. Nanosized Copper Selenide Functionalized Zeolitic Imidazolate Framework-8 (CuSe/ZIF-8) for Efficient Immobilization of Gas-Phase Elemental Mercury. Adv. Funct. Mater. 2019, 29, 1807191. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, W.; Qu, W.; Yang, Z.; Wang, J.; Zhang, M.; Li, H. Selenium Functionalized Metal–Organic Framework MIL-101 for Efficient and Permanent Sequestration of Mercury. Environ. Sci. Technol. 2019, 53, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, D.; Xu, H.; Mei, J.; Qu, Z.; Liu, P.; Cui, Y.; Yan, N. Combined effects of Ag and UiO-66 for removal of elemental mercury from flue gas. Chemosphere 2018, 197, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, B.; Shen, F.; Si, M.; Yuan, P. The behavior of the manganese-cerium loaded metal-organic framework in elemental mercury and NO removal from flue gas. Chem. Eng. J. 2017, 326, 551–560. [Google Scholar] [CrossRef]
- Rudd, N.D.; Wang, H.; Fuentes-Fernandez, E.M.A.; Teat, S.J.; Chen, F.; Hall, G.; Chabal, Y.J.; Li, J. Highly Efficient Luminescent Metal-Organic Framework for the Simultaneous Detection and Removal of Heavy Metals from Water. ACS Appl. Mater. Interfaces 2016, 8, 30294–30303. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Du, T.; Zhang, W.; Zhu, W.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Wang, J. NH2-MIL-53(Al) Metal–Organic Framework as the Smart Platform for Simultaneous High-Performance Detection and Removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.; El-Sewify, I.M.; Shahat, A.; Azzazy, H.M.E.; Khalil, M.M.H.; El-Shahat, M.F. Multiuse Al-MOF Chemosensors for Visual Detection and Removal of Mercury Ions in Water and Skin-Whitening Cosmetics. ACS Sustain. Chem. Eng. 2020, 8, 15097–15107. [Google Scholar] [CrossRef]
- Shahat, A.; Elsalam, S.A.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F.; Ramis-Ramos, G. Optical recognition and removal of Hg(II) using a new self-chemosensor based on a modified amino-functionalized Al-MOF. Sens. Actuators B Chem. 2017, 253, 164–172. [Google Scholar] [CrossRef]
- Esrafili, L.; Gharib, M.; Morsali, A. Selective detection and removal of mercury ions by dual-functionalized metal-organic frameworks: Design-for-purpose. New J. Chem. 2019, 43, 18079–18091. [Google Scholar] [CrossRef]
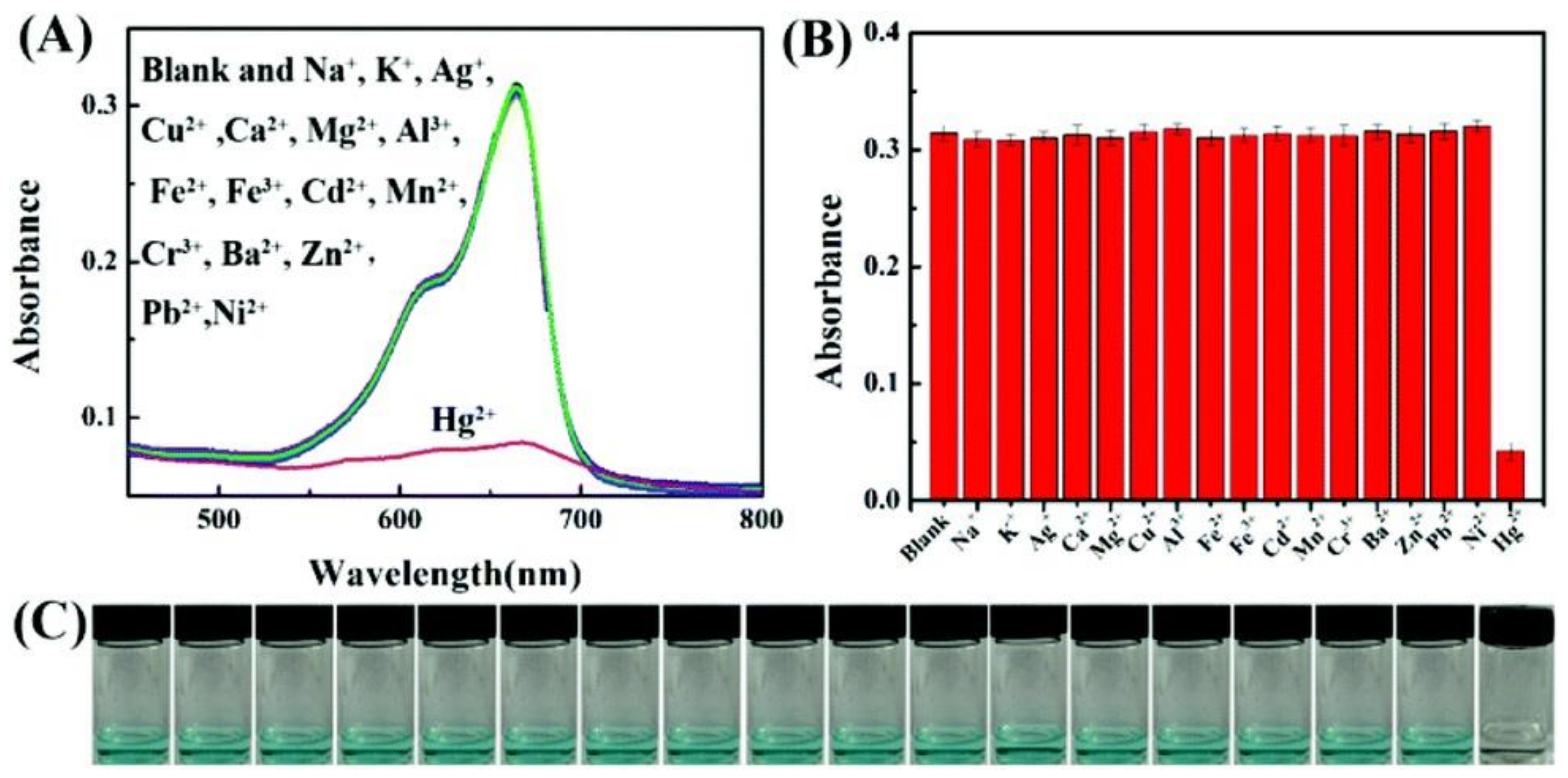
- Xiong, Y.; Su, L.; Yang, H.; Ye, F.; Zhang, P. Fabrication of copper sulfide using a Cu-based metal organic framework for the colorimetric determination and the efficient removal of Hg2+ in aqueous solutions. New J. Chem. 2015, 39, 9221–9227. [Google Scholar] [CrossRef]
- Moradi, E.; Rahimi, R.; Safarifard, V. Porphyrinic zirconium-based MOF with exposed pyrrole Lewis base site as an efficient fluorescence sensing for Hg2+ ions, DMF small molecule, and adsorption of Hg2+ ions from water solution. J. Solid State Chem. 2020, 286, 121277. [Google Scholar] [CrossRef]
- Yang, H.; Peng, C.; Han, J.; Song, Y.; Wang, L. Three-dimensional macroporous Carbon/Zr-2,5-dimercaptoterephthalic acid metal-organic frameworks nanocomposites for removal and detection of Hg(II). Sens. Actuators B Chem. 2020, 320, 128447. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Sun, K.W.; Wu, S.-P. A pH cooperative strategy for enhanced colorimetric sensing of Cr(III) ions using biocompatible L-glutamic acid stabilized gold nanoparticles. Microchem. J. 2021, 160, 105754. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-capped gold-copper nanoclusters for fluorometric determination and imaging of chromium(VI) and dopamine. Microchim. Acta 2019, 186, 788. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Chen, Y.-C.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device. Nanomaterials 2019, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Review on Nanomaterial-Based Melamine Detection. Chemosensors 2019, 7, 9. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Long, Z.; Hou, X. Fast and sensitive fluorescent and visual sensing of cysteine using Hg-metalated PCN-222. Microchem. J. 2019, 145, 68–73. [Google Scholar] [CrossRef]
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. https://doi.org/10.3390/chemosensors9050101
Shellaiah M, Sun K-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors. 2021; 9(5):101. https://doi.org/10.3390/chemosensors9050101
Chicago/Turabian StyleShellaiah, Muthaiah, and Kien-Wen Sun. 2021. "Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal" Chemosensors 9, no. 5: 101. https://doi.org/10.3390/chemosensors9050101
APA StyleShellaiah, M., & Sun, K.-W. (2021). Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors, 9(5), 101. https://doi.org/10.3390/chemosensors9050101






