Dual Transduction of H2O2 Detection Using ZnO/Laser-Induced Graphene Composites
Abstract
1. Introduction
2. Materials and Methods
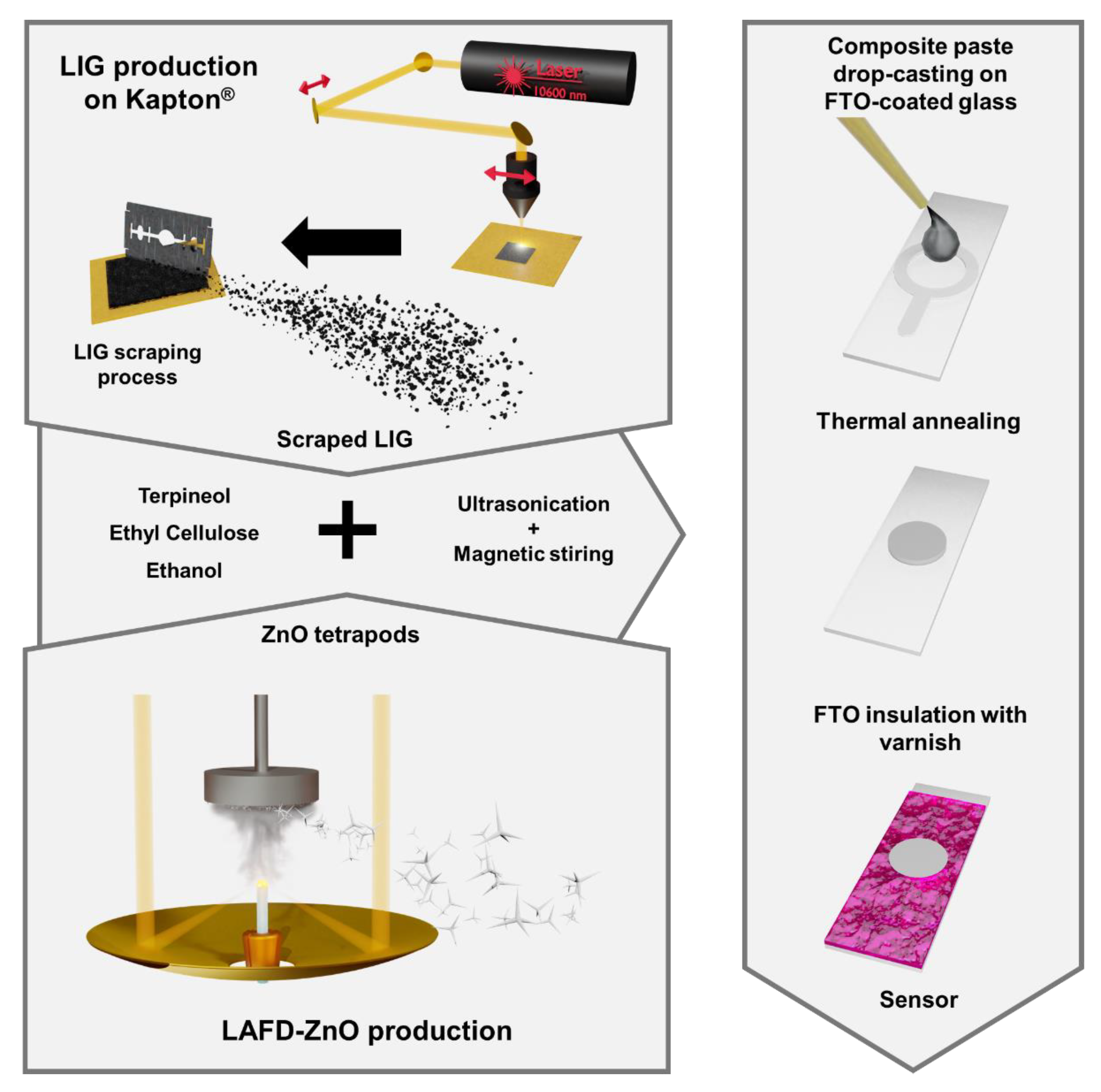
2.1. Materials’ Preparation
2.2. Material Characterization
2.3. H2O2 Sensing
3. Results and Discussion
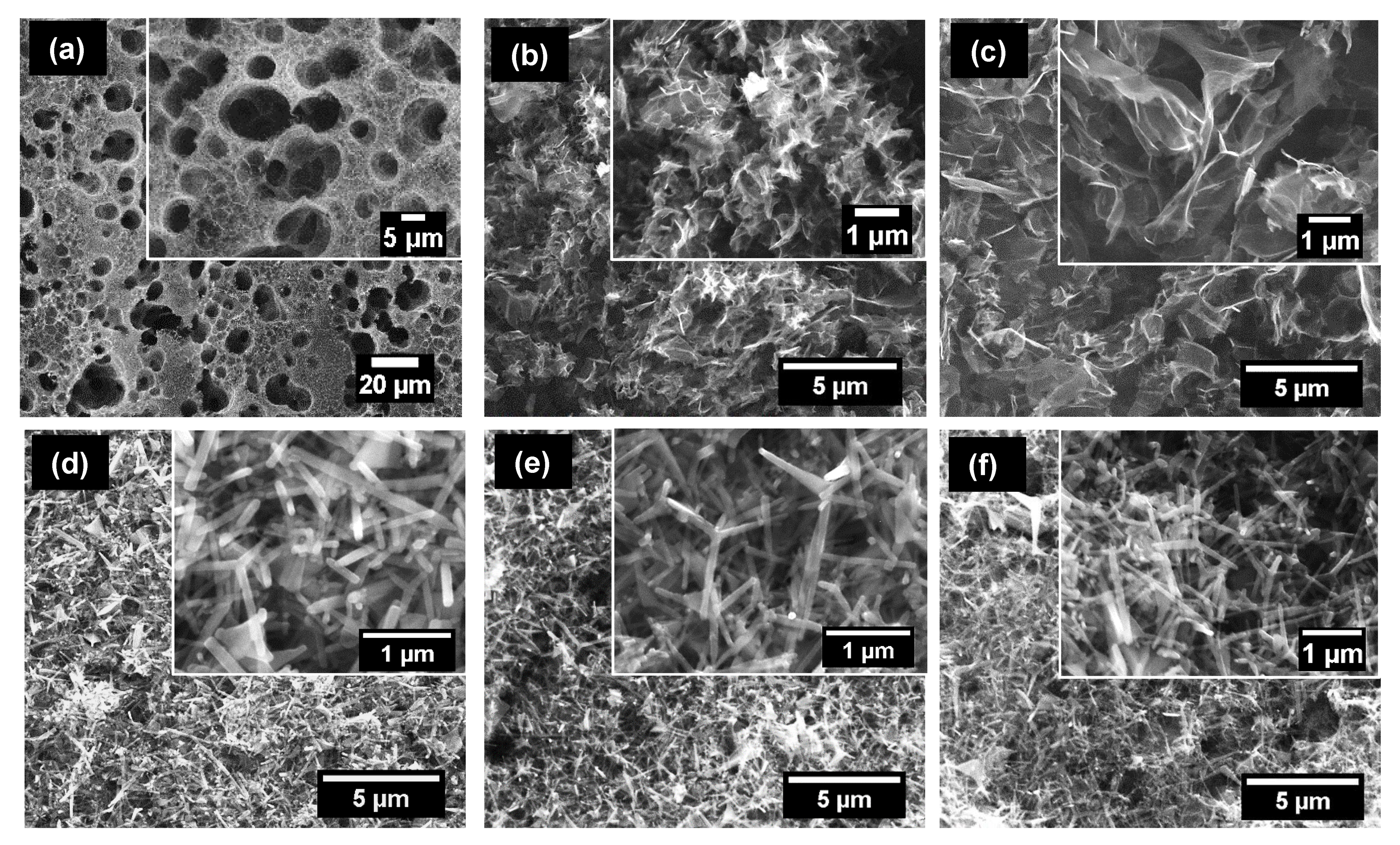
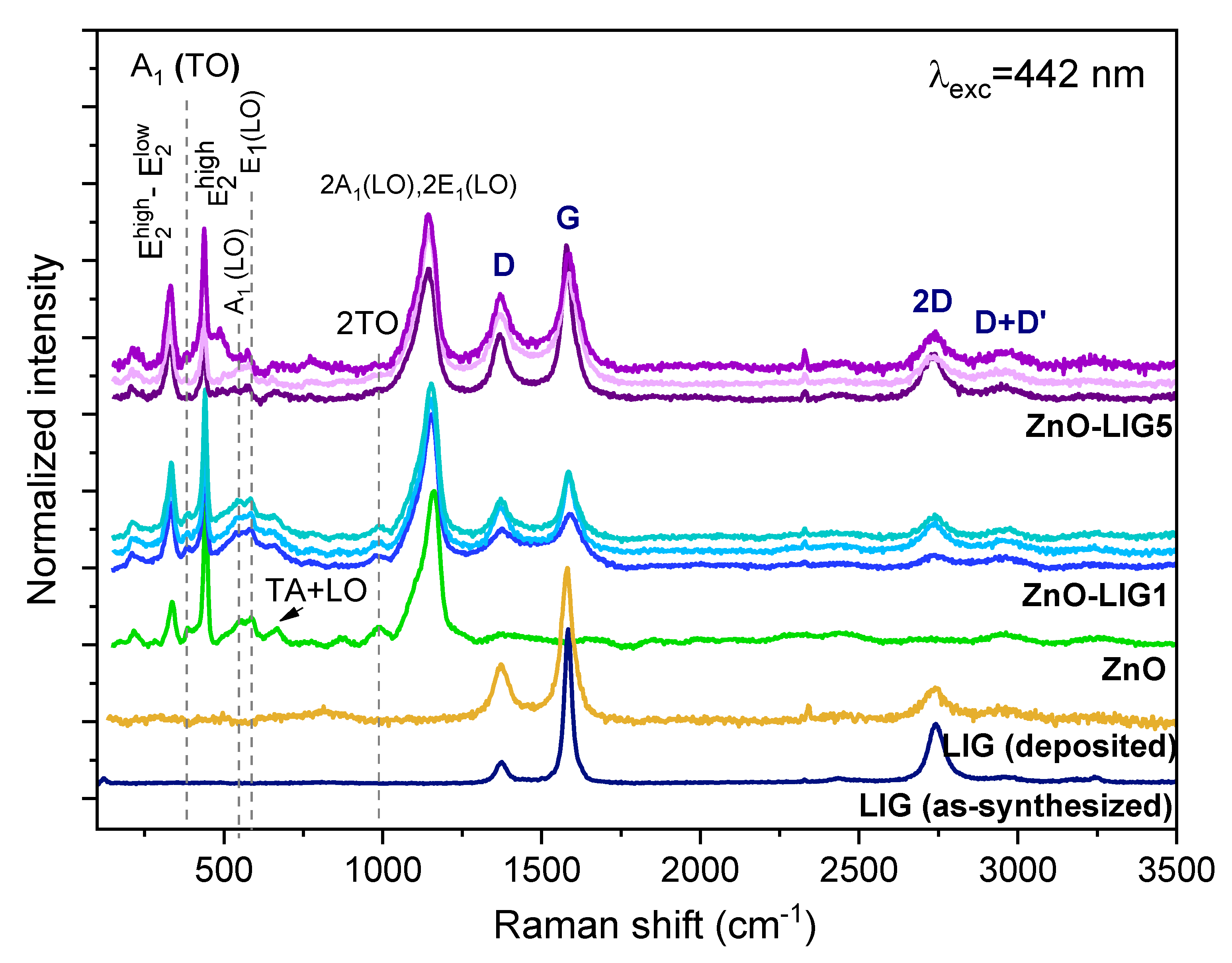
3.1. Morphological and Structural Analysis
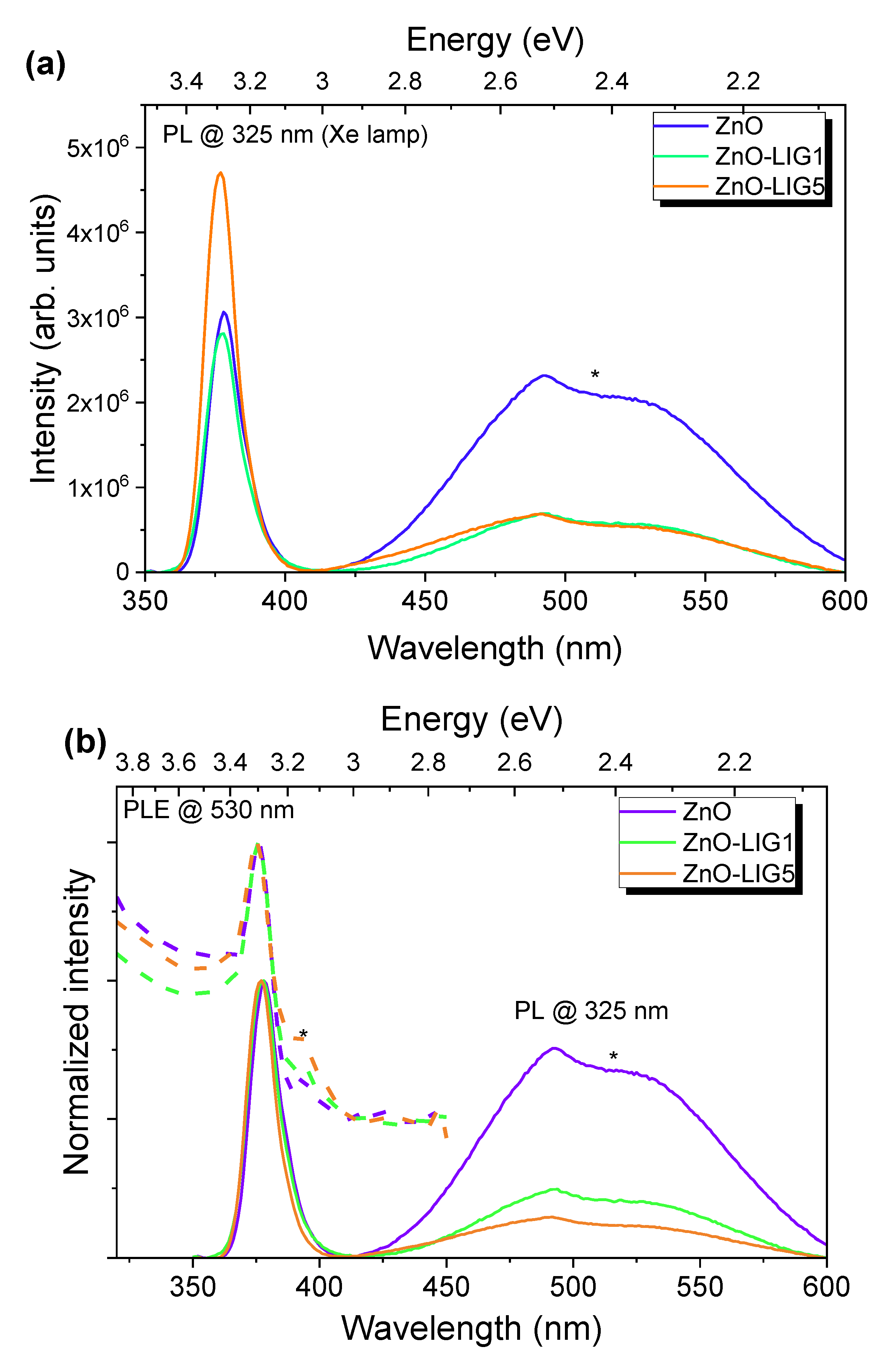
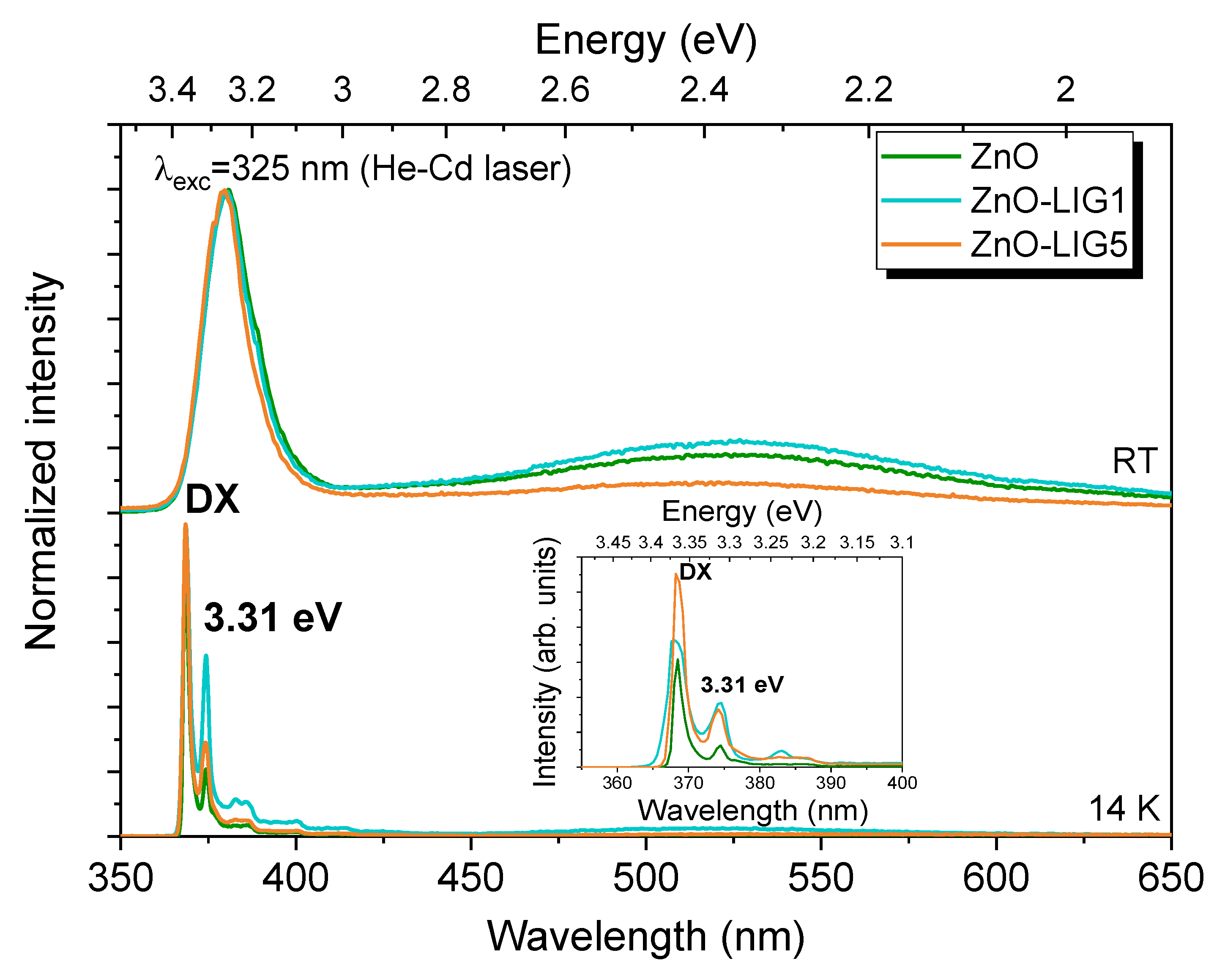
3.2. Photoluminescence Assessment
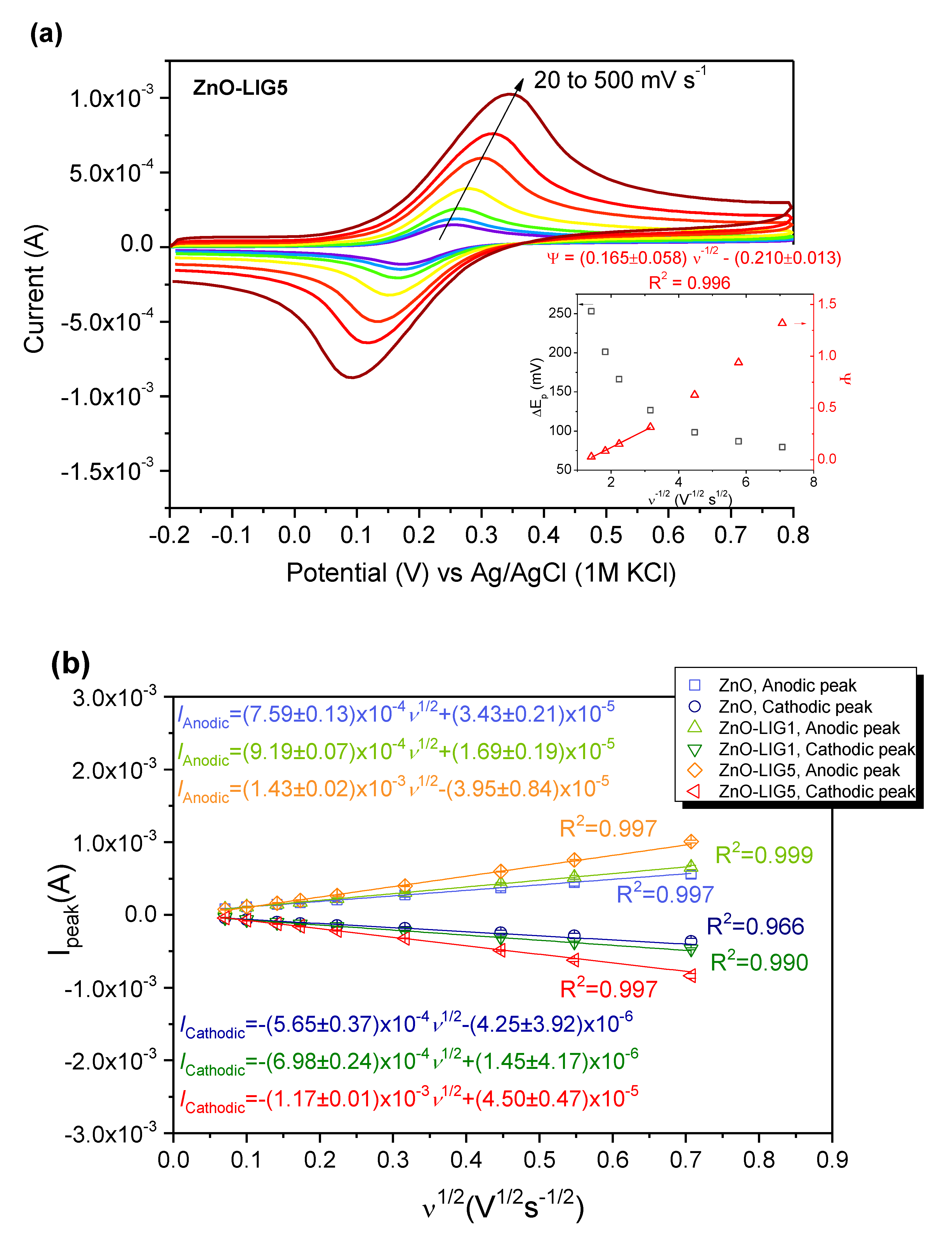
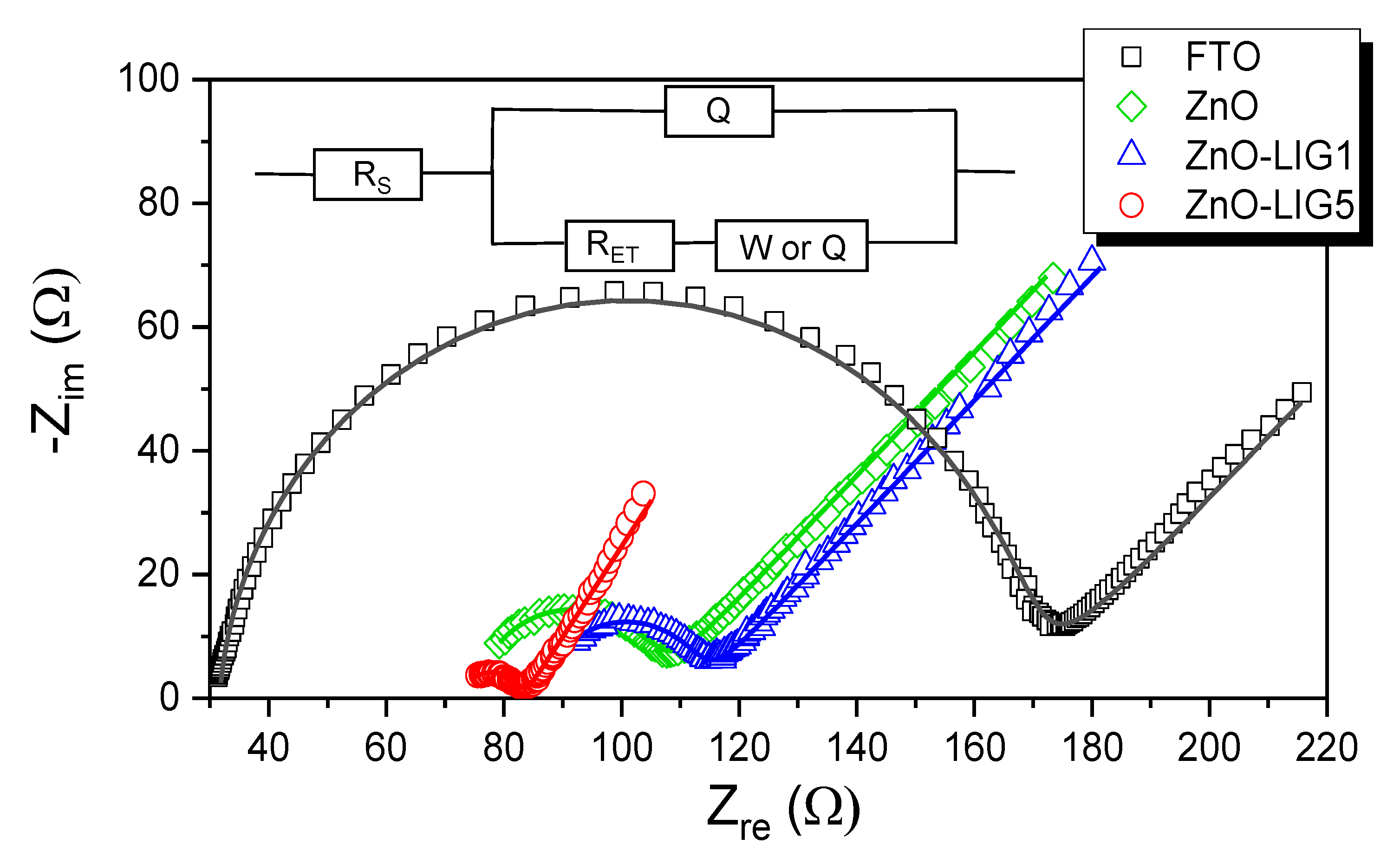
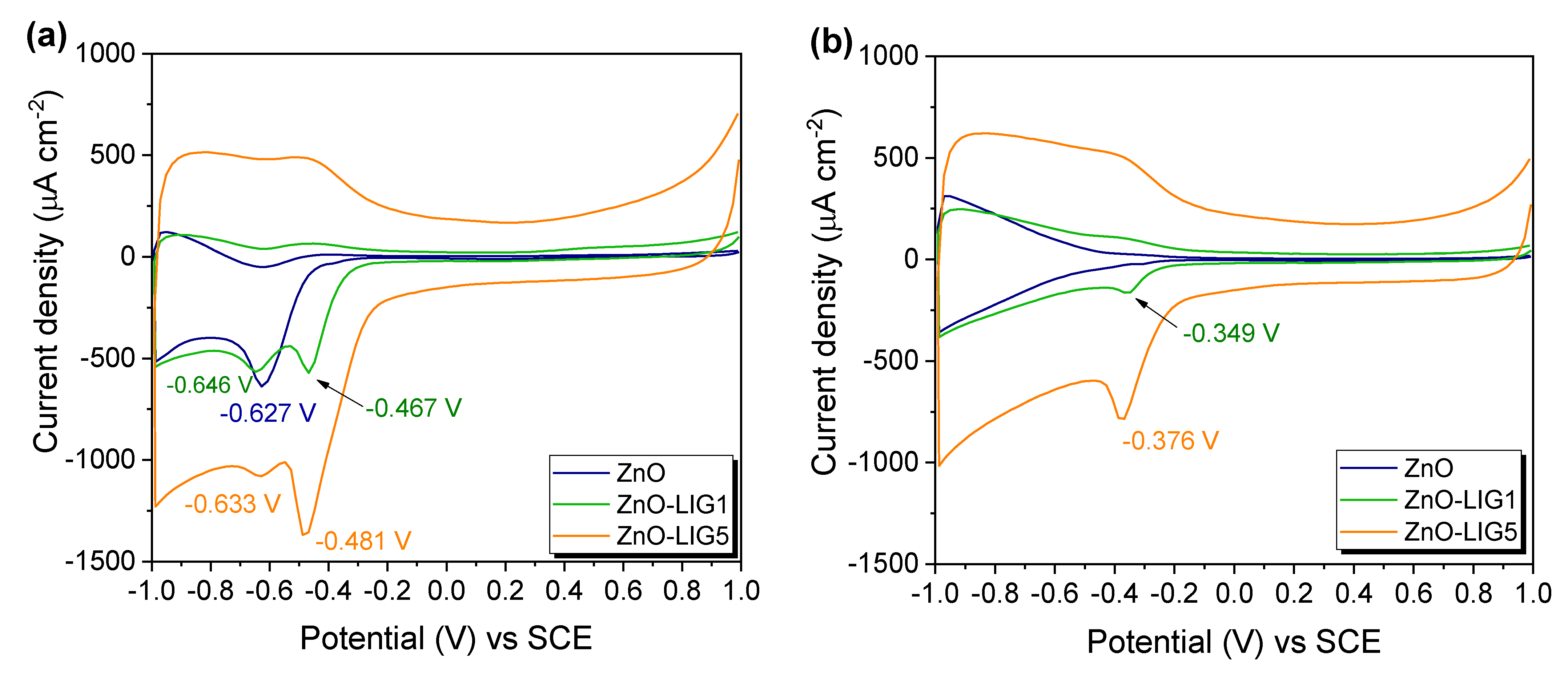
3.3. Electrochemical Evaluation
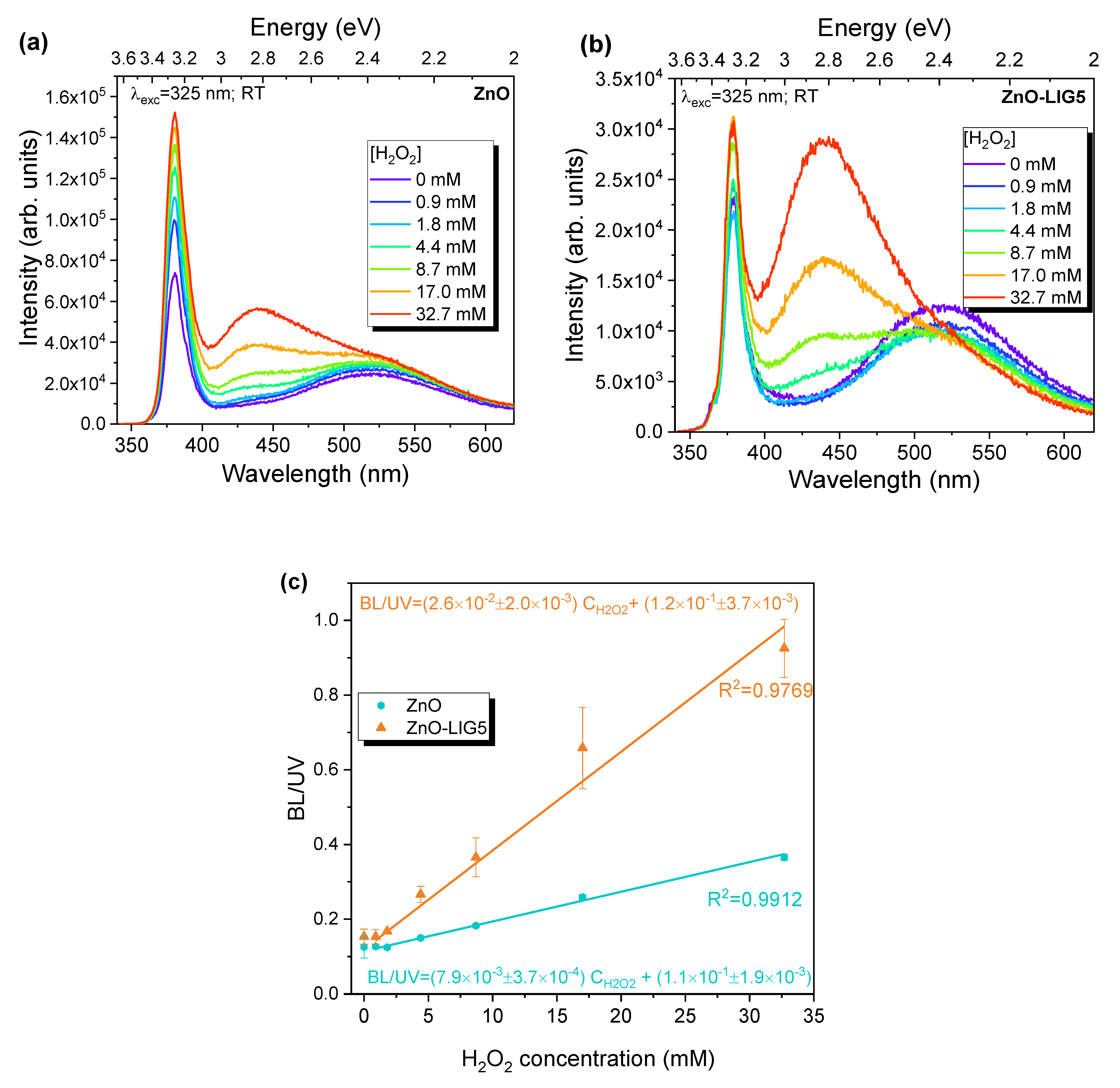
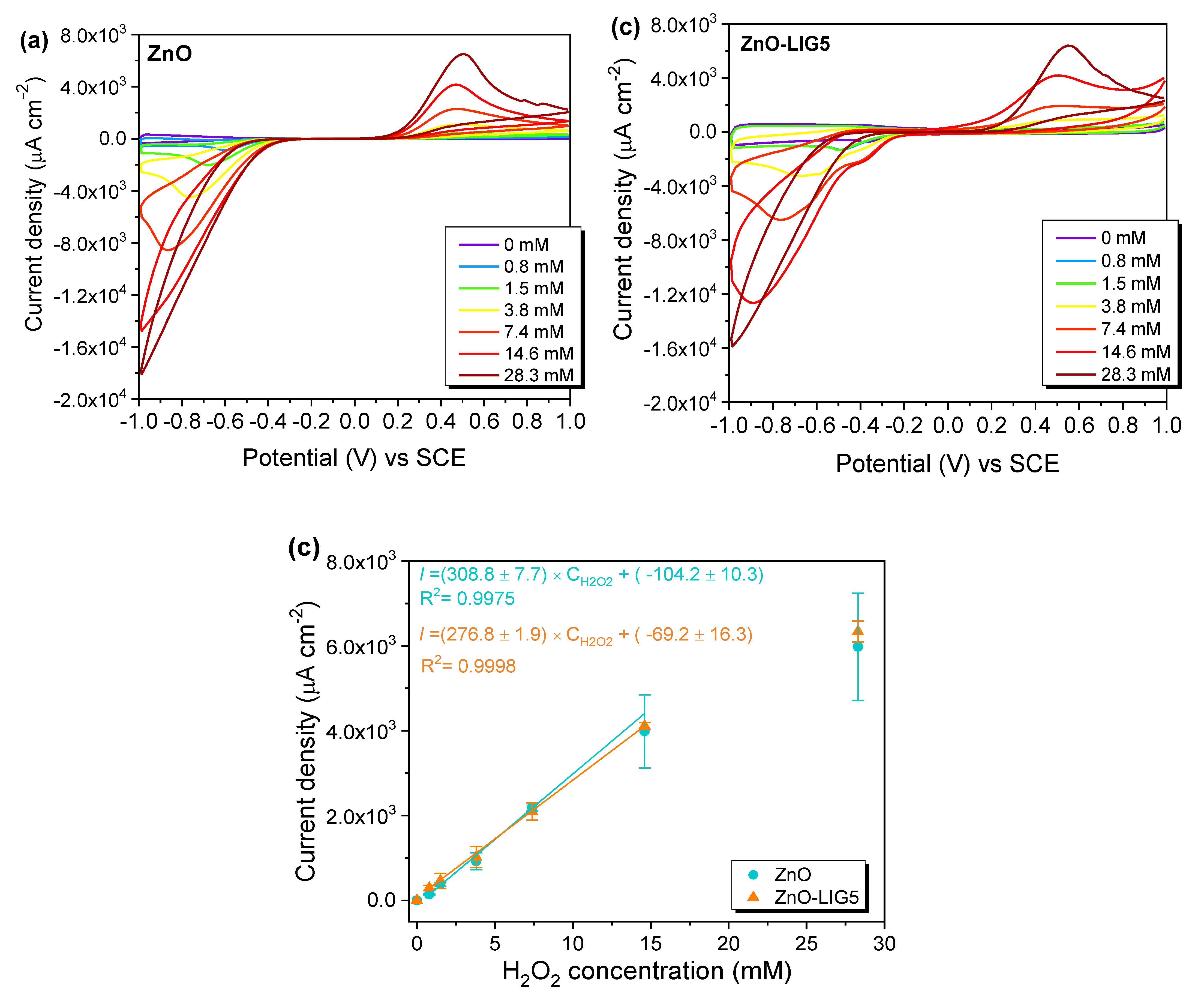
3.4. H2O2 Sensing via PL and CV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Rodrigues, J.; Ben Sedrine, N.; Correia, M.R.; Monteiro, T. Photoluminescence investigations of ZnO micro/nanostructures. Mater. Today Chem. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Rodrigues, J.; Pereira, S.O.; Santos, N.F.; Rodrigues, C.; Costa, F.M.; Monteiro, T. Insights on luminescence quenching of ZnO tetrapods in the detection of hCG. Appl. Surf. Sci. 2020, 527, 146813. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanostructures of zinc oxide. Mater. Today 2004, 7, 26–33. [Google Scholar] [CrossRef]
- Ozgür, U.; Hofstetter, D.; Morkoç, H. ZnO Devices and Applications: A Review of Current Status and Future Prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef] [PubMed]
- Smazna, D.; Rodrigues, J.; Shree, S.; Postica, V.; Neubüser, G.; Martins, A.F.; Ben Sedrine, N.; Jena, N.K.; Siebert, L.; Schütt, F.; et al. Buckminsterfullerene hybridized zinc oxide tetrapods: Defects and charge transfer induced optical and electrical response. Nanoscale 2018, 10, 10050–10062. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Smazna, D.; Ben Sedrine, N.; Nogales, E.; Adelung, R.; Mishra, Y.K.; Mendez, B.; Correia, M.R.; Monteiro, T. Probing surface states in C60 decorated ZnO microwires: Detailed photoluminescence and cathodoluminescence investigations. Nanoscale Adv. 2019, 1, 1516–1526. [Google Scholar] [CrossRef]
- Rodrigues, J.; Mata, D.; Pimentel, A.; Nunes, D.; Martins, R.; Fortunato, E.; Neves, A.J.; Monteiro, T.; Costa, F.M. One-step synthesis of ZnO decorated CNT buckypaper composites and their optical and electrical properties. Mater. Sci. Eng. B 2015, 195, 38–44. [Google Scholar] [CrossRef]
- Messina, M.M.; Coustet, M.E.; Ubogui, J.; Ruiz, R.; Saccone, F.D.; dos Santos Claro, P.C.; Ibañez, F.J. Simultaneous Detection and Photocatalysis Performed on a 3D Graphene/ZnO Hybrid Platform. Langmuir 2020, 36, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Laverde, N.M.; Pruna, A.; Busquets-Mataix, D.; Pullini, D. Graphene Oxide-Assisted Morphology and Structure of Electrodeposited ZnO Nanostructures. Materials 2020, 13, 365. [Google Scholar] [CrossRef]
- Rodrigues, J.; Mata, D.; Fernandes, A.J.S.; Neto, M.A.; Silva, R.F.; Monteiro, T.; Costa, F.M. ZnO nanostructures grown on vertically aligned carbon nanotubes by laser-assisted flow deposition. Acta Mater. 2012, 60, 5143–5150. [Google Scholar] [CrossRef]
- Pargoletti, E.; Hossain, U.H.; Di Bernardo, I.; Chen, H.; Tran-Phu, T.; Lipton-Duffin, J.; Cappelletti, G.; Tricoli, A. Room-temperature photodetectors and VOC sensors based on graphene oxide–ZnO nano-heterojunctions. Nanoscale 2019, 11, 22932–22945. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. ZnO conjugated graphene: An efficient sunlight driven photocatalyst for degradation of organic dyes. Mater. Res. Bull. 2020, 129, 110911. [Google Scholar] [CrossRef]
- Jung, J.; Jeong, J.R.; Lee, J.; Lee, S.H.; Kim, S.Y.; Kim, M.J.; Nah, J.; Lee, M.H. In situ formation of graphene/metal oxide composites for high-energy microsupercapacitors. Npg Asia Mater. 2020, 12, 50. [Google Scholar] [CrossRef]
- Víctor-Román, S.; García-Bordejé, E.; Hernández-Ferrer, J.; González-Domínguez, J.M.; Ansón-Casaos, A.; Silva, A.M.T.; Maser, W.K.; Benito, A.M. Controlling the surface chemistry of graphene oxide: Key towards efficient ZnO-GO photocatalysts. Catal. Today 2020, 357, 350–360. [Google Scholar] [CrossRef]
- Rafiee, Z.; Mosahebfard, A.; Sheikhi, M.H. High-performance ZnO nanowires-based glucose biosensor modified by graphene nanoplates. Mater. Sci. Semicond. Process. 2020, 115, 105116. [Google Scholar] [CrossRef]
- Samouco, A.; Marques, A.C.; Pimentel, A.; Martins, R.; Fortunato, E. Laser-induced electrodes towards low-cost flexible UV ZnO sensors. Flex. Print. Electron. 2018, 3, 044002. [Google Scholar] [CrossRef]
- Palanisamy, S.; Cheemalapati, S. Enzymatic glucose biosensor based on multiwalled carbon nanotubes-zinc oxide composite. Int. J. Electrochem. Sci. 2012, 7, 8394–8407. [Google Scholar]
- Zhao, Y.; Li, W.; Pan, L.; Zhai, D.; Wang, Y.; Li, L. ZnO-nanorods/graphene heterostructure: A direct electron transfer glucose biosensor. Sci. Rep. 2016, 6, 32327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jing, W.; Xu, Y.; Chen, Z.; Jiang, Z.; Wei, Z. Performance enhancement of ZnO nanorod-based enzymatic glucose sensor via reduced graphene oxide deposition and UV irradiation. Sens. Actuators B Chem. 2019, 284, 377–385. [Google Scholar] [CrossRef]
- Low, S.S.; Tan, M.T.T.; Loh, H.-S.; Khiew, P.S.; Chiu, W.S. Facile hydrothermal growth graphene/ZnO nanocomposite for development of enhanced biosensor. Anal. Chim. Acta 2016, 903, 131–141. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Yu, J.; Wang, S.; Ge, S.; Song, X. Application of ZnO/graphene and S6 aptamers for sensitive photoelectrochemical detection of SK-BR-3 breast cancer cells based on a disposable indium tin oxide device. Biosens. Bioelectron. 2014, 51, 413–420. [Google Scholar] [CrossRef]
- Liu, F.; Deng, W.; Zhang, Y.; Ge, S.; Yu, J.; Song, X. Application of ZnO quantum dots dotted carbon nanotube for sensitive electrochemiluminescence immunoassay based on simply electrochemical reduced Pt/Au alloy and a disposable device. Anal. Chim. Acta 2014, 818, 46–53. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Madhusudana Reddy, T.; Venu Gopal, T.; Venkataprasad, G.; Kotakadi, V.S.; Palakollu, V.N.; Karpoormath, R. A simple sonochemical assisted synthesis of nanocomposite (ZnO/MWCNTs) for electrochemical sensing of Epinephrine in human serum and pharmaceutical formulation. Colloids Surf. A Phys. Eng. Asp. 2020, 584, 124038. [Google Scholar] [CrossRef]
- Yue, H.Y.; Zhang, H.J.; Huang, S.; Lu, X.X.; Gao, X.; Song, S.S.; Wang, Z.; Wang, W.Q.; Guan, E.H. Highly sensitive and selective dopamine biosensor using Au nanoparticles-ZnO nanocone arrays/graphene foam electrode. Mater. Sci. Eng. C 2020, 108, 110490. [Google Scholar] [CrossRef] [PubMed]
- RoyChaudhuri, C.; Chakraborty, B. Development of low noise FET biosensor using graphene and ZnO nanostructures on cost effective substrates for biomolecule detection with enhanced performance. CSI Trans. ICT 2020, 8, 129–136. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Varzakas, T.; Erdem, A.; Nikoleli, G.-P. Portable Biosensing of Food Toxicants and Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Zhu, Y.-C.; Mei, L.-P.; Ruan, Y.-F.; Zhang, N.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Enzyme-Based Biosensors and Their Applications. In Advances in Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–223. [Google Scholar]
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A.; Katz, E.; Katz, E.; Wang, J.; Bocharova, V.; Wang, J.; et al. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J.S. Cholesterol biosensors: A review. Steroids 2019, 143, 6–17. [Google Scholar] [CrossRef]
- Saxena, U.; Das, A.B. Nanomaterials towards fabrication of cholesterol biosensors: Key roles and design approaches. Biosens. Bioelectron. 2016, 75, 196–205. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J. Electroanal. Chem. 2018, 816, 195–204. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Chang, Q.; Fan, X.; Zhang, G.; Zhang, F.; Peng, W.; Li, Y. Fabrication of a novel ZnO–CoO/rGO nanocomposite for nonenzymatic detection of glucose and hydrogen peroxide. Ceram. Int. 2018, 44, 5250–5256. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Li, Y.; Be’er, A.; Oren, Y.; Tour, J.M.; Arnusch, C.J. Laser-Induced Graphene Layers and Electrodes Prevents Microbial Fouling and Exerts Antimicrobial Action. Acs Appl. Mater. Interfaces 2017, 9, 18238–18247. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Fernandes, A.J.S.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Piezoresistive Sensors Synthesized Directly on Cork Insoles for Gait Analysis. Adv. Mater. Technol. 2020, 5, 2000630. [Google Scholar] [CrossRef]
- Zhu, C.; Tao, L.-Q.; Wang, Y.; Zheng, K.; Yu, J.; Li, X.; Chen, X.; Huang, Y. Graphene oxide humidity sensor with laser-induced graphene porous electrodes. Sens. Actuators B Chem. 2020, 325, 128790. [Google Scholar] [CrossRef]
- Yagati, A.K.; Behrent, A.; Beck, S.; Rink, S.; Goepferich, A.M.; Min, J.; Lee, M.-H.; Baeumner, A.J. Laser-induced graphene interdigitated electrodes for label-free or nanolabel-enhanced highly sensitive capacitive aptamer-based biosensors. Biosens. Bioelectron. 2020, 164, 112272. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Marques, A.C.; Santos, L.; Carvalho, A.F.; Costa, F.M.; Martins, R.; Sales, M.G.F.; Fortunato, E. Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosens. Bioelectron. 2019, 124–125, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Strain Sensors Produced by Ultraviolet Irradiation of Polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Santos, N.F.; Pereira, S.O.; Moreira, A.B.; Girão, A.V.; Carvalho, A.F.; Fernandes, A.J.S.; Costa, F.M. IR and UV Laser-induced Graphene: Application as dopamine electrochemical sensors. Adv. Mater. Technol. 2021. [Google Scholar] [CrossRef]
- Kulyk, B.; Silva, B.F.R.; Carvalho, A.F.; Silvestre, S.; Fernandes, A.J.S.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene from Paper for Mechanical Sensing. Acs Appl. Mater. Interfaces 2021, 13, 10210–10221. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2018, 1803621. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Zanoni, J.; Gaspar, G.; Fernandes, A.J.S.; Carvalho, A.F.; Santos, N.F.; Monteiro, T.; Costa, F.M. ZnO decorated laser-induced graphene produced by direct laser scribing. Nanoscale Adv. 2019, 1, 3252–3268. [Google Scholar] [CrossRef]
- Ye, R.; Chyan, Y.; Zhang, J.; Li, Y.; Han, X.; Kittrell, C.; Tour, J.M. Laser-Induced Graphene Formation on Wood. Adv. Mater. 2017, 29, 1702211. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Lamberti, A.; Fontana, M.; Giordani, S. Toxicity assessment of laser-induced graphene by zebrafish during development. J. Phys. Mater. 2020, 3, 034008. [Google Scholar] [CrossRef]
- Ye, R.; Peng, Z.; Wang, T.; Xu, Y.; Zhang, J.; Li, Y.; Nilewski, L.G.; Lin, J.; Tour, J.M. In Situ Formation of Metal Oxide Nanocrystals Embedded in Laser-Induced Graphene. Acs Nano 2015, 9, 9244–9251. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Liu, P.; Ji, G.; Gao, L.; Zhao, J. Versatile Strategy to Design Flexible Planar-Integrated Microsupercapacitors Based on Co3O4 -Decorated Laser-Induced Graphene. Acs Appl. Energy Mater. 2020, 3, 10676–10684. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Sodzel, D.; Khranovskyy, V.; Beni, V.; Turner, A.P.F.; Viter, R.; Eriksson, M.O.; Holtz, P.-O.; Janot, J.-M.; Bechelany, M.; Balme, S.; et al. Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles. Microchim. Acta 2015, 182, 1819–1826. [Google Scholar] [CrossRef]
- Viter, R.; Tereshchenko, A.; Smyntyna, V.; Ogorodniichuk, J.; Starodub, N.; Yakimova, R.; Khranovskyy, V.; Ramanavicius, A. Toward development of optical biosensors based on photoluminescence of TiO2 nanoparticles for the detection of Salmonella. Sens. Actuators B Chem. 2017, 252, 95–102. [Google Scholar] [CrossRef]
- Mai, H.H.; Tran, D.H.; Janssens, E. Non-enzymatic fluorescent glucose sensor using vertically aligned ZnO nanotubes grown by a one-step, seedless hydrothermal method. Microchim. Acta 2019, 186, 245. [Google Scholar] [CrossRef]
- Sarangi, S.N.; Nozaki, S.; Sahu, S.N. ZnO Nanorod-Based Non-Enzymatic Optical Glucose Biosensor. J. Biomed. Nanotechnol. 2015, 11, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-Y.; Huang, J.-S.; Lin, C.-F. Improving the property of ZnO nanorods using hydrogen peroxide solution. J. Cryst. Growth 2008, 310, 2806–2809. [Google Scholar] [CrossRef]
- Roberts, J.G.; Hamilton, K.L.; Sombers, L.A. Comparison of electrode materials for the detection of rapid hydrogen peroxide fluctuations using background-subtracted fast scan cyclic voltammetry. Analyst 2011, 136, 3550. [Google Scholar] [CrossRef] [PubMed]
- Sanford, A.L.; Morton, S.W.; Whitehouse, K.L.; Oara, H.M.; Lugo-Morales, L.Z.; Roberts, J.G.; Sombers, L.A. Voltammetric Detection of Hydrogen Peroxide at Carbon Fiber Microelectrodes. Anal. Chem. 2010, 82, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Harada, S.; Sato, T.; Nakajima, T.; Horino, Y.; Morita, K. Carbonization of polyimide film “Kapton”. Carbon N. Y. 1989, 27, 253–257. [Google Scholar] [CrossRef]
- Inagaki, M.; Ohta, N.; Hishiyama, Y. Aromatic polyimides as carbon precursors. Carbon N. Y. 2013, 61, 1–21. [Google Scholar] [CrossRef]
- Lamberti, A.; Perrucci, F.; Caprioli, M.; Serrapede, M.; Fontana, M.; Bianco, S.; Ferrero, S.; Tresso, E. New insights on laser-induced graphene electrodes for flexible supercapacitors: Tunable morphology and physical properties. Nanotechnology 2017, 28, 174002. [Google Scholar] [CrossRef]
- Luo, S.; Hoang, P.T.; Liu, T. Direct laser writing for creating porous graphitic structures and their use for flexible and highly sensitive sensor and sensor arrays. Carbon N. Y. 2016, 96, 522–531. [Google Scholar] [CrossRef]
- Rodrigues, J.; Fernandes, A.J.S.; Monteiro, T.; Costa, F.M. A review on the laser-assisted flow deposition method: Growth of ZnO micro and nanostructures. CrystEngComm 2019, 21, 1071–1090. [Google Scholar] [CrossRef]
- Rodrigues, J.; Fernandes, A.J.S.; Mata, D.; Holz, T.; Carvalho, R.G.; Fath Allah, R.; Ben, T.; Gonzalez, D.; Silva, R.F.; da Cunha, A.F.; et al. ZnO micro/nanocrystals grown by laser assisted flow deposition. In SPIE OPTO; Teherani, F.H., Look, D.C., Rogers, D.J., Eds.; International Society for Optics and Photonics: Bellingham, DC, USA, 2014; p. 89871F. [Google Scholar]
- Rodrigues, J.; Peres, M.; Soares, M.R.N.; Fernandes, A.J.S.; Ferreira, N.; Ferro, M.; Neves, A.J.; Monteiro, T.; Costa, F.M. ZnO Nano/Microstructures Grown by Laser Assisted Flow Deposition. J. Nano Res. 2012, 18–19, 129–137. [Google Scholar] [CrossRef]
- Rodrigues, J.; Cerqueira, A.F.R.; Sousa, M.G.; Santos, N.F.; Pimentel, A.; Fortunato, E.; da Cunha, A.F.; Monteiro, T.; Costa, F.M. Exploring the potential of laser assisted flow deposition grown ZnO for photovoltaic applications. Mater. Chem. Phys. 2016, 177, 322–329. [Google Scholar] [CrossRef]
- Rodrigues, J.; Pimentel, A.; Fortunato, E.; Monteiro, T.; Costa, F.M. Photocatalytic Activity of Laser-Processed ZnO Micro/Nanocrystals. Phys. Status Solidi 2018, 215, 1800155. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Hallam, P.M.; Banks, C.E. Quantifying the electron transfer sites of graphene. Electrochem. Commun. 2011, 13, 8–11. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Calleja, J.M.; Cardona, M. Resonant Raman scattering in ZnO. Phys. Rev. B 1977, 16, 3753–3761. [Google Scholar] [CrossRef]
- Rodrigues, J.; Holz, T.; Fath Allah, R.; Gonzalez, D.; Ben, T.; Correira, M.R.; Monteiro, T.; Costa, F.M. Effect of the N2 and H2 plasma treatments on band edge emission of ZnO microrods. Sci. Rep. 2015, 5, 10783. [Google Scholar] [CrossRef]
- Dingle, R. Luminescent Transitions Associated With Divalent Copper Impurities and the Green Emission from Semiconducting Zinc Oxide. Phys. Rev. Lett. 1969, 23, 579–581. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 3527623957. [Google Scholar]
- Jagadish, C.; Pearton, S. Zinc Oxide Bulk, Thin Films and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780080447223. [Google Scholar]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18, 095702. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Liu, X.; Leung, Y.H. Zinc oxide films and nanomaterials for photovoltaic applications. Phys. Status Solidi Rapid Res. Lett. 2014, 8, 123–132. [Google Scholar] [CrossRef]
- Djurisić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, H.; Wang, Y.; Ni, Z.; Yan, Y.; Shen, Z.X.; Loh, K.P.; Tang, D.Y. Atomic-Layer Graphene as a Saturable Absorber for Ultrafast Pulsed Lasers. Adv. Funct. Mater. 2009, 19, 3077–3083. [Google Scholar] [CrossRef]
- Baibarac, M.; Baltog, I.; Matea, A.; Lefrant, S. Raman scattering and photoluminescence studies of ZnO nanowhiskers assembled as flowers in the presence of fullerene. J. Cryst. Growth 2015, 419, 158–164. [Google Scholar] [CrossRef]
- Wöll, C. The chemistry and physics of zinc oxide surfaces. Prog. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- Hövel, S.; Kolczewski, C.; Wühn, M.; Albers, J.; Weiss, K.; Staemmler, V.; Wöll, C. Pyridine adsorption on the polar ZnO(0001) surface: Zn termination versus O termination. J. Chem. Phys. 2000, 112, 3909–3916. [Google Scholar] [CrossRef]
- Hofmann, O.T.; Deinert, J.-C.; Xu, Y.; Rinke, P.; Stähler, J.; Wolf, M.; Scheffler, M. Large work function reduction by adsorption of a molecule with a negative electron affinity: Pyridine on ZnO(101¯0). J. Chem. Phys. 2013, 139, 174701. [Google Scholar] [CrossRef]
- Turkina, O.; Nabok, D.; Gulans, A.; Cocchi, C.; Draxl, C. Electronic and Optical Excitations at the Pyridine/ZnO(101¯0) Hybrid Interface. Adv. Theory Simul. 2019, 2, 1800108. [Google Scholar] [CrossRef]
- Wang, L.; Giles, N.C. Temperature dependence of the free-exciton transition energy in zinc oxide by photoluminescence excitation spectroscopy. J. Appl. Phys. 2003, 94, 973. [Google Scholar] [CrossRef]
- Rai, R.C. Analysis of the Urbach tails in absorption spectra of undoped ZnO thin films. J. Appl. Phys. 2013, 113, 153508. [Google Scholar] [CrossRef]
- Postica, V.; Gröttrup, J.; Adelung, R.; Lupan, O.; Mishra, A.K.; de Leeuw, N.H.; Ababii, N.; Carreira, J.F.C.; Rodrigues, J.; Sedrine, N.B.; et al. Multifunctional Materials: A Case Study of the Effects of Metal Doping on ZnO Tetrapods with Bismuth and Tin Oxides. Adv. Funct. Mater. 2017, 27, 1604676. [Google Scholar] [CrossRef]
- Boemare, C.; Monteiro, T.; Soares, M.J.; Guilherme, J.G.; Alves, E. Photoluminescence studies in ZnO samples. Phys. B Condens. Matter 2001, 308–310, 985–988. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. Rsc Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry—An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [PubMed]
- Velický, M.; Bissett, M.A.; Toth, P.S.; Patten, H.V.; Worrall, S.D.; Rodgers, A.N.J.; Hill, E.W.; Kinloch, I.A.; Novoselov, K.S.; Georgiou, T.; et al. Electron transfer kinetics on natural crystals of MoS2 and graphite. Phys. Chem. Chem. Phys. 2015, 17, 17844–17853. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge- and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 2248. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, F.; Wang, L.; Mo, C.; Pu, Y.; Fang, W. Influence of hydrogen peroxide solution on the properties of ZnO thin films. J. Cryst. Growth 2004, 268, 71–75. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Viter, R.; Iatsunskyi, I. Metal Oxide Nanostructures in Sensing. In Nanomaterials Design for Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–91. [Google Scholar]
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C.; Rocha-Santos, T.A. Review of analytical figures of merit of sensors and biosensors in clinical applications. Trac Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Shiang, Y.-C.; Huang, C.-C.; Chang, H.-T. Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose. Chem. Commun. 2009, 3437. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Deng, J.; Zhang, Y.; Li, H.; Yao, S. Label-free Si quantum dots as photoluminescence probes for glucose detection. Chem. Commun. 2013, 49, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Zhu, G.; Dai, Y.; Shen, Y.; Yang, J.; Liu, J. Fabrication of Pt-ZnO composite nanotube modified electrodes for the detection of H2O2. J. Electroanal. Chem. 2018, 817, 176–183. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lai, Y.-H.; Balamurugan, A.; Vittal, R.; Lin, C.-W.; Ho, K.-C. Electrode modified with a composite film of ZnO nanorods and Ag nanoparticles as a sensor for hydrogen peroxide. Talanta 2010, 82, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Chen, S.-M.; Sarawathi, R. A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sens. Actuators B Chem. 2012, 166–167, 372–377. [Google Scholar] [CrossRef]
- Hussain, M.; Sun, H.; Karim, S.; Nisar, A.; Khan, M.; ul Haq, A.; Iqbal, M.; Ahmad, M. Noble metal nanoparticle-functionalized ZnO nanoflowers for photocatalytic degradation of RhB dye and electrochemical sensing of hydrogen peroxide. J. Nanoparticle Res. 2016, 18, 95. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Zhang, K.; Shang, Y.; Zhao, R.; Liu, L.; Du, Q.; Guo, T.; Du, J.; Li, J. Size-Controllable Strategy of ZnO Micro/Nanorods for Electrochemical Detection of H2O2. J. Electrochem. Soc. 2021, 168, 027507. [Google Scholar] [CrossRef]
- Xu, H.; Wei, Z.; Verpoort, F.; Hu, J.; Zhuiykov, S. Nanoscale Au-ZnO Heterostructure Developed by Atomic Layer Deposition Towards Amperometric H2O2 Detection. Nanoscale Res. Lett. 2020, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Alsareii, S.A. A Highly Efficient Nonenzymatic Hydrogen Peroxide Electrochemical Sensor Using Mesoporous Carbon Doped ZnO Nanocomposite. J. Electrochem. Soc. 2021, 168, 027512. [Google Scholar] [CrossRef]
- Salih, E.; Mekawy, M.; Hassan, R.Y.A.; El-Sherbiny, I.M. Synthesis, characterization and electrochemical-sensor applications of zinc oxide/graphene oxide nanocomposite. J. Nanostructure Chem. 2016, 6, 137–144. [Google Scholar] [CrossRef]
- Evans, S.A.G.; Elliott, J.M.; Andrews, L.M.; Bartlett, P.N.; Doyle, P.J.; Denuault, G. Detection of Hydrogen Peroxide at Mesoporous Platinum Microelectrodes. Anal. Chem. 2002, 74, 1322–1326. [Google Scholar] [CrossRef] [PubMed]

| Sample | FTO | ZnO | ZnO-LIG1 | ZnO-LIG5 |
|---|---|---|---|---|
| k0 (cm s−1) | - | (3.0 ± 0.4) × 10−3 | (3.9 ± 0.5) × 10−3 | (4.3 ± 0.4) × 10−3 |
| Aeff (cm2) | - | 0.109 ± 0.002 | 0.132 ± 0.001 | 0.206 ± 0.003 |
| Rs (Ω) | 31.6 ± 0.3 | 74.6 ± 1.8 | 86.2 ± 2.7 | 73.6 ± 1.8 |
| RET (Ω) | 136 ± 1 | 29.5 ± 2.1 | 25.7 ± 2.9 | 9.64 ± 2.10 |
| Signal | PL | |||
|---|---|---|---|---|
| Sample | Linear range (mM) | Sensitivity [(IBL/IUV) mM−1] | LOD (mM) | Reference |
| ZnO TPs | 1.8–32.7 | 0.0079 | 0.8 | This work |
| ZnO-LIG5 | 1.8–32.7 | 0.026 | 0.45 | This work |
| ZnO NPs | 0.05–100 | --- | 0.05 (a) | [60] |
| ZnO NRs | 1–30 | --- | --- | [63] |
| Signal | CV | |||
| Sample | Linear range (mM) | Sensitivity (µA mM−1 cm−2) | LOD (mM) | Reference |
| ZnO TPs | 0.8–14.6 | 308.8 | 0.11 | This work |
| ZnO-LIG5 | 0.8–14.6 | 276.8 | 0.19 | This work |
| ZnO/GO composite | 1–15 | --- | 0.8 | [120] |
| ZnO nano/microrods based GCE | 0.1–1 | 10.8 (b) | 0.00028 | [117] |
| Mesoporous carbon doped ZnO modified GCE | 0.2–3.1 0.05–0.981 (b) | 5.52 46.48 (b) | 0.0526 0.00625 (c) | [119] |
| Pt-ZnO/GCE | 0.02–50 | --- | 1.5 | [113] |
| Mesoporous platinum microelectrodes (d) | 0.02–40 | 2800 | 0.0045 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanoni, J.; Moura, J.P.; Santos, N.F.; Carvalho, A.F.; Fernandes, A.J.S.; Monteiro, T.; Costa, F.M.; Pereira, S.O.; Rodrigues, J. Dual Transduction of H2O2 Detection Using ZnO/Laser-Induced Graphene Composites. Chemosensors 2021, 9, 102. https://doi.org/10.3390/chemosensors9050102
Zanoni J, Moura JP, Santos NF, Carvalho AF, Fernandes AJS, Monteiro T, Costa FM, Pereira SO, Rodrigues J. Dual Transduction of H2O2 Detection Using ZnO/Laser-Induced Graphene Composites. Chemosensors. 2021; 9(5):102. https://doi.org/10.3390/chemosensors9050102
Chicago/Turabian StyleZanoni, Julia, Jorge P. Moura, Nuno F. Santos, Alexandre F. Carvalho, António J. S. Fernandes, Teresa Monteiro, Florinda M. Costa, Sónia O. Pereira, and Joana Rodrigues. 2021. "Dual Transduction of H2O2 Detection Using ZnO/Laser-Induced Graphene Composites" Chemosensors 9, no. 5: 102. https://doi.org/10.3390/chemosensors9050102
APA StyleZanoni, J., Moura, J. P., Santos, N. F., Carvalho, A. F., Fernandes, A. J. S., Monteiro, T., Costa, F. M., Pereira, S. O., & Rodrigues, J. (2021). Dual Transduction of H2O2 Detection Using ZnO/Laser-Induced Graphene Composites. Chemosensors, 9(5), 102. https://doi.org/10.3390/chemosensors9050102









