Carbon Dots Fluorescence-Based Colorimetric Sensor for Sensitive Detection of Aluminum Ions with a Smartphone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization and Instrumentation
2.3. Preparation of the CDs
2.4. Determination of Al3+
2.5. Analysis of Spiked Water Sample
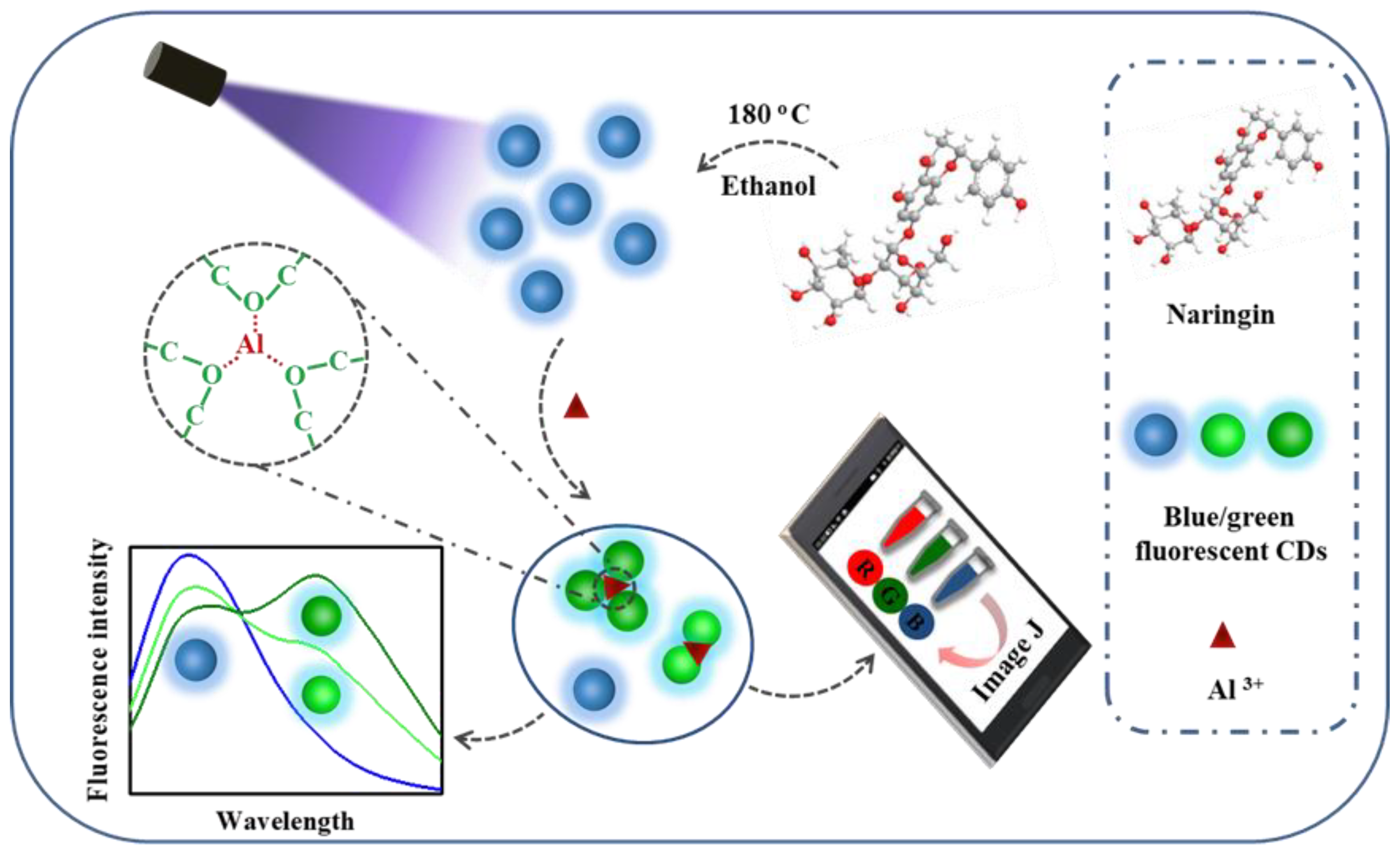
3. Results and Discussion
3.1. Characterization of the CDs
3.2. Detection Mechanism
3.3. Effect of the pH on the Detection of Al3+
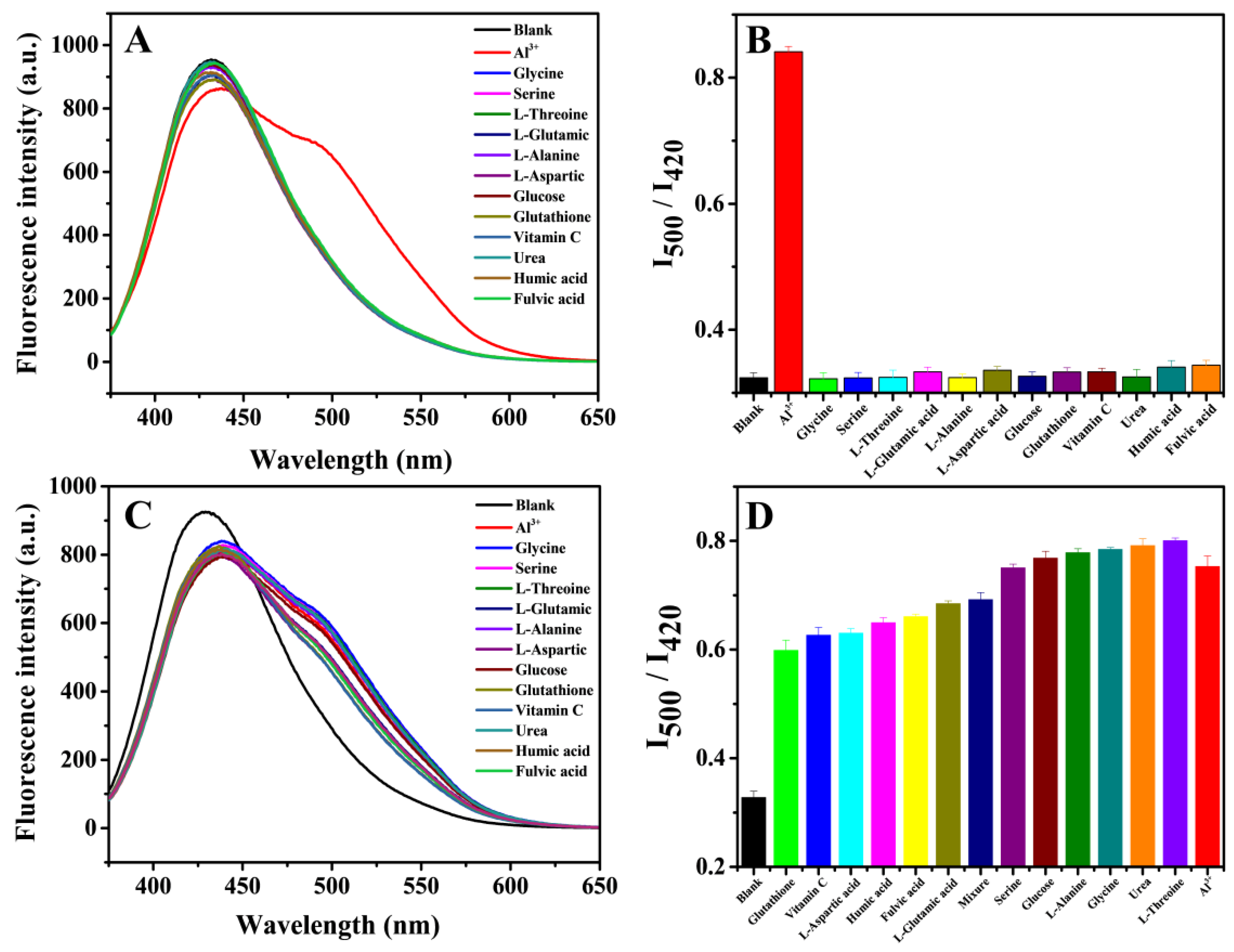
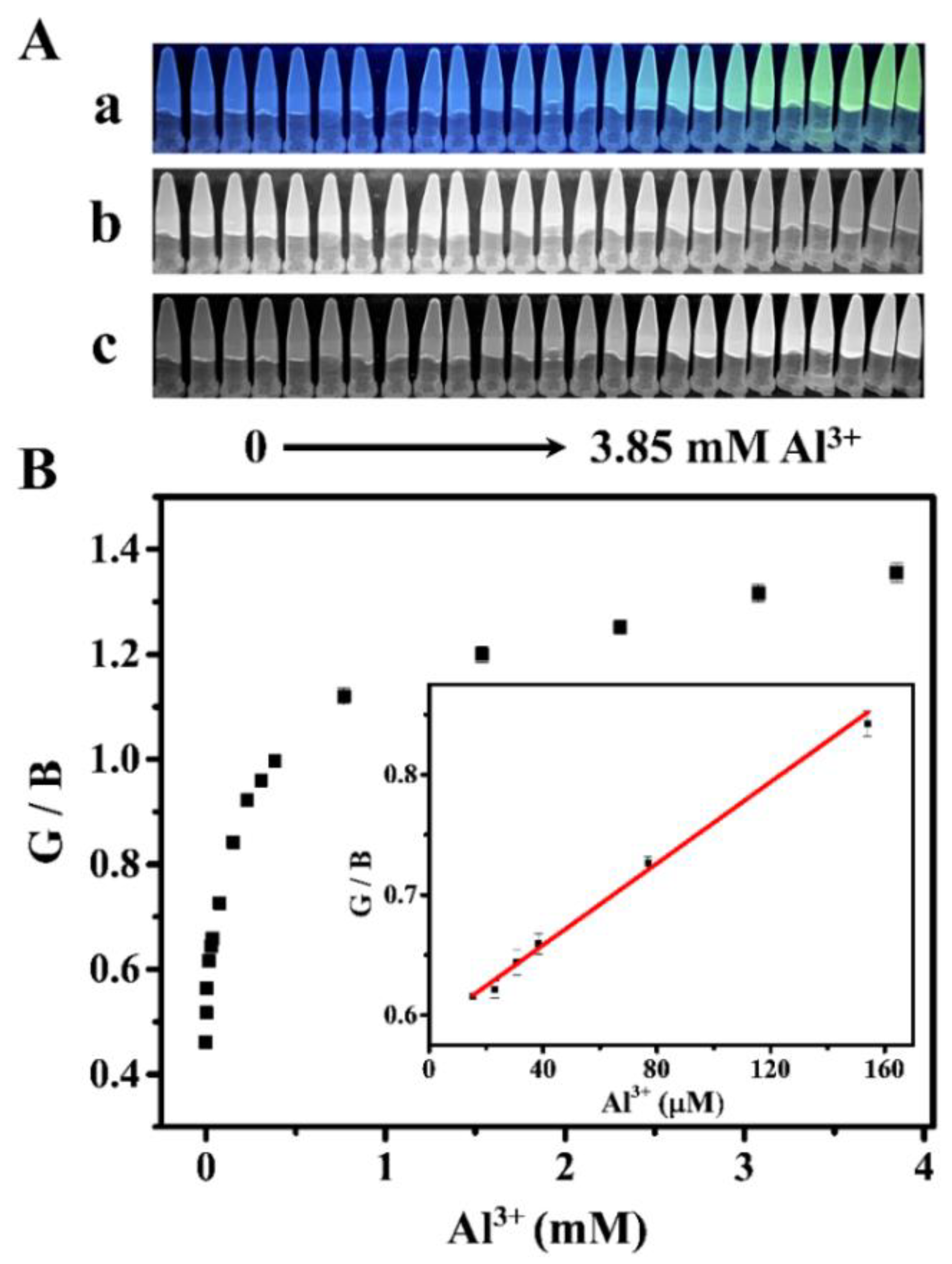
3.4. Fluorescence Detection of Al3+
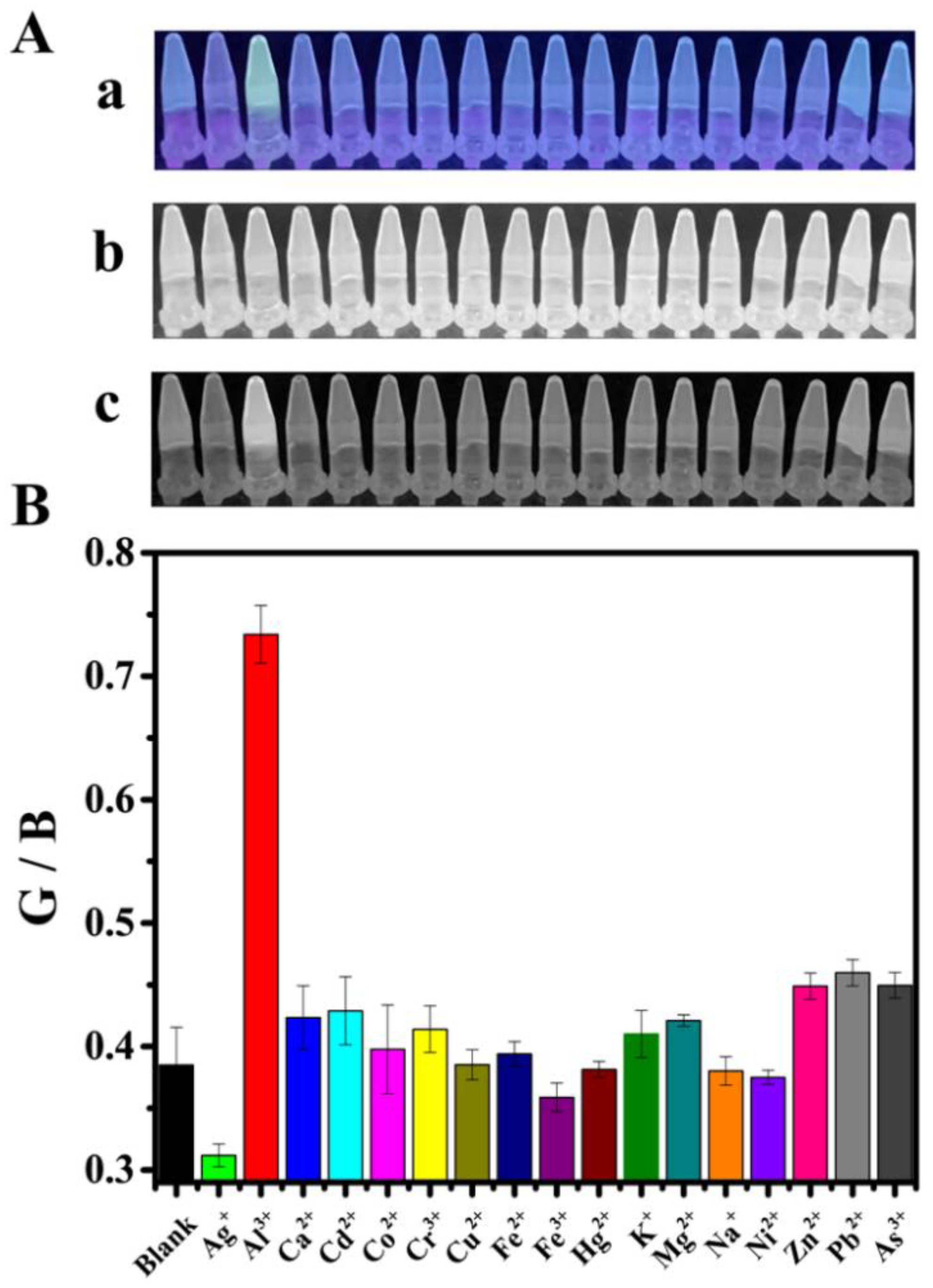
3.5. RGB Detection of Al3+
3.6. Analysis of Spiked Water Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Perl, D.P.; Gajdusek, D.C.; Garruto, R.M.; Yanagihara, R.T.; Gibbs, C.J. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science 1982, 217, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.P.; Brody, A.R. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980, 208, 297–299. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 301.
- Torawane, P.; Tayade, K.; Bothta, S.; Sahoo, S.K.; Singh, N.; Borse, A.; Kuwar, A. A highly selective and sensitive fluorescent turn-on chemosensor for Al3+ based on C=N isomerisation mechanism with nanomolar detection. Sens. Actuators B Chem. 2016, 222, 562–566. [Google Scholar] [CrossRef]
- Purkait, R.; Sinha, C. Solvent-tuned discriminant sensing of Al3+, Mg2+ and HF2- byvanilinyl-picolinyl hydrazide Schiffbase. New J. Chem. 2019, 43, 9815–9823. [Google Scholar] [CrossRef]
- Song, Y.J.; Wei, W.L.; Qu, X.G. Colorimetric biosensing using smart materials. Adv. Mater. 2011, 23, 4215–4236. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, D.; Zhao, Q.; Lu, X.Y.; Shi, X.B. A critical factor for quantifying proteins in unmodified gold nanoparticles-based aptasensing: The effect of pH. Chemosensors 2020, 8, 98. [Google Scholar] [CrossRef]
- Joshi, P.; Painuli, R.; Kumar, D. Label-free colorimetric nanosensor for the selective on-site detection of aqueous Al3+. ACS Sustain. Chem. Eng. 2017, 5, 4552–4562. [Google Scholar] [CrossRef]
- Xue, D.S.; Wang, H.Y.; Zhang, Y.B. Specific and sensitive colorimetric detection of Al3+ using 5-mercaptomethyltetrazole capped gold nanoparticles in aqueous solution. Talanta 2014, 119, 306–311. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lee, I.L.; Sung, Y.M.; Wu, S.P. Colorimetric detection of Al3+ ions using triazole–ether functionalized gold nanoparticles. Talanta 2013, 117, 70–74. [Google Scholar] [CrossRef]
- Chen, W.W.; Jia, Y.X.; Feng, Y.; Zheng, W.S.; Wang, Z.; Jiang, X.Y. Colorimetric detection of Al(III) in vermicelli samples based on ionic liquid group coated gold nanoparticles. RSC Adv. 2015, 5, 62260–62264. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, G.J.; Kim, Y.S.; Lee, S.Y.; Lee, H.J.; Noh, I.; Kim, C. A water-soluble carboxylic-functionalized chemosensor for detecting Al3+ in aqueous media and living cells: Experimental and theoreticalstudies. Biosens. Bioelectron. 2015, 69, 226–229. [Google Scholar] [CrossRef]
- Qin, J.C.; Yang, Z.Y. Ratiometric fluorescent probefor Al3+ based on coumarin derivative in aqueous media. Anal. Methods 2015, 7, 2036–2040. [Google Scholar] [CrossRef]
- Yao, J.L.; Zhang, K.; Zhu, H.J.; Ma, F.; Sun, M.T.; Yu, H.; Sun, J.; Wang, S.H. Efficient ratiometric fluorescence probe based on dual-emission quantum dots hybrid for on-site determination of copper ions. Anal. Chem. 2013, 85, 6461–6468. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, H. Ratiometric fluorescence molecularly imprinted sensor basedon dual-emission quantum dots hybrid for determination of tetracycline. Anal. Bioanal. Chem. 2019, 411, 5809–5816. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.O.; Krull, U. Camera-based ratiometric fluorescence transduction of nucleic acid hybridization with reagentless signal amplification on a paper-based platform using immobilized quantum dots as donors. Anal. Chem. 2014, 86, 10331–10339. [Google Scholar] [CrossRef]
- Khan, I.M.; Niazi, S.; Yu, Y.; Mohsin, A.; Mushtag, B.S.; Iqbal, M.W.; Rehman, A.; Alhtar, W.; Wang, Z.P. Aptamer induced multicolored AuNCs-WS2 “Turn on” FRET nano platform for dual-color simultaneous detection of AflatoxinB1 and Zearalenone. Anal. Chem. 2019, 91, 14085–14092. [Google Scholar] [CrossRef]
- Liu, H.J.; Jia, L.; Wang, Y.X.; Wang, M.Y.; Gao, Z.D.; Ren, X.Q. Ratiometric fluorescent sensor for visual determination of copperions and alkaline phosphatase based on carbon quantum dotsand gold nanoclusters. Anal. Bioanal. Chem. 2019, 411, 2531–2543. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Shi, X.B.; Wen, C.; Fu, Z.D.; Deng, F.M.; Zheng, S.; Liu, Q.Y. Photo properties and applications of single quantum dots. Prog. Chem. 2014, 26, 1781–1792. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hu, A.G. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.B.; Wei, W.; Fu, Z.D.; Gao, W.L.; Zhang, C.Y.; Zhao, Q.; Deng, F.M.; Lu, X.Y. Review on carbon dots in food safety applications. Talanta 2019, 194, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Yang, L.; Chu, S.Y.; Liu, B.H.; Zhang, Q.K.; Zou, L.M.; Yu, S.M.; Jiang, C.L. Semi-quantitative visual detection of lead ions with smartphone via colorimetric paper-based analytical device. Anal. Chem. 2019, 91, 9292–9299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Y.; Xu, N.Z.; Zhang, M.Q.; Wu, G.Z.; Yao, C. A colorimetric and ratiometric fluorescent chemosensor based on furan-pyrene for selective and sensitive sensing Al3+. Chin. Chem. Lett. 2016, 27, 1673–1678. [Google Scholar] [CrossRef]
- Soufeena, P.P.; Aravindakshan, K.K. Antipyrine derived schiff base: A colorimetric sensor for Fe(III) and “Turn-On” fluorescent sensor for Al(III). J. Lumin. 2019, 205, 400–405. [Google Scholar] [CrossRef]
- Peng, B.; Xu, J.M.; Fan, M.M.; Guo, Y.; Ma, Y.J.; Zhou, M.; Fang, Y.J. Smartphone colorimetric determination of hydrogen peroxidein real samples based on B, N, and S co-doped carbon dots probe. Anal. Bioanal. Chem. 2020, 412, 861–870. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.L.; Wang, H.J.; Ji, W.Y.; Zhang, Z.Y.; Zhang, Y.Y.; Yang, Z.C.; Cao, Z.S.; Zhang, S.F.; Chang, J. Intelligent detection platform for simultaneous detection of multiple MiRNAs based on Smartphone. ACS Sens. 2019, 4, 1873–1880. [Google Scholar] [CrossRef]
- Zeng, X.L.; Hu, J.; Zhang, M.; Wang, F.L.; Wu, L.; Hou, X.D. Visual detection of fluoride anions using mixed lanthanide metal-organic frameworks with a smartphone. Anal. Chem. 2020, 92, 2097–2102. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, C.; Yu, X.L.; Li, J.; Wang, Z.Y.; Zhang, Z.P.; Liu, B.H. Microwave synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J. Mater. Chem. C 2018, 6, 9636–9641. [Google Scholar] [CrossRef]
- Hou, L.; Qin, Y.X.; Li, J.Y.; Qin, S.Y.; Huang, Y.L.; Lin, T.R.; Guo, L.Q.; Ye, F.G.; Zhao, S.L. A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens. Bioelectron. 2019, 143, 111605. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.L. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Rad, M.S.; Zucca, P.; Pezzani, R.; Martins, N.; Rad, J.S. The therapeutic potential of naringenin: A review of clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Khan, H.; Aschner, M.; Hasan, M.M.; Hasan, S.S. Therapeutic potential of naringin in neurological disorders. Food Chem. Toxicol. 2019, 132, 110646. [Google Scholar] [CrossRef] [PubMed]
- Hartogh, D.D.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Z.; Sharma, S.; Kaur, A. Antitoxic effects of naringin: A flavonoid with diverse biological activities. World J. Pharm. Res. 2018, 7, 484–489. [Google Scholar] [CrossRef]
- Ye, R.Q.; Xiang, C.S.; Lin, J.; Peng, Z.W.; Huang, K.W.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.C.; Ruan, G.D.; et al. Coal as an abundant source of grapheme quantum dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef]
- Wang, W.S.; Damm, C.; Walter, J.; Nacken, T.J.; Peukert, W. Photobleaching and stabilization of carbon nanodots produced by solvothermal synthesis. Phys. Chem. Chem. Phys. 2016, 18, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Chang, Q.; Xue, C.R.; Yang, J.L.; Hu, S.L. Full-colour carbon dots from energy-efficient synthesis to concentration-dependent photoluminescence properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef] [PubMed]
- Han, C.P.; Wang, R.; Wang, K.Y.; Xu, H.T.; Sui, M.R.; Li, J.J.; Xu, K. Highly fluorescent carbon dots as selective and sensitive “on-off-on”probes for iron(III) ion and apoferritin detection and imaging in living cells. Biosens. Bioelectron. 2016, 83, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, X.P.; Li, G.; Zhao, C.; Liao, X.J. Green Synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, C.Z.; Song, J.H.; Li, H.; Du, D.; Lin, Y.H. Drug-derived bright and color-tunable N-doped carbon dotsfor cell imaging and sensitive detection of Fe3+ in living cells. ACS Appl. Mater. Interfaces 2017, 9, 7399–7405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrion, C.C.; Cardenas, S.; Simonet, B.M.; Valcarcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 35, 5214–5226. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.Z.; Zhou, S.F.; Shimotsuma, Y.; Miura, K.; Qiu, J.R. Effect of UV irradiation on photoluminescence of carbon dots. Opt. Soc. Am. 2014, 4, 213–219. [Google Scholar] [CrossRef]
- Li, X.Y.; Yan, L.H.; Si, J.H.; Xu, H.H.; Xu, Y.M. Tuning the photoluminescence property of carbon dots by ultraviolet light irradiation. RSC Adv. 2019, 9, 12732–12736. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Wang, L.B.; Su, R.X.; Huang, R.L.; Qi, W.; He, Z.M. A carbon dot-based off-on fluorescent probe for highly selective and sensitive detection of phytic acid. Biosens. Bioelectron. 2015, 70, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.J.; Chir, J.L.; Ting, W.; Wu, A.T. A selective colorimetric and ratiometric fluorescent chemosensor for detection of Al3+ ion. J. Lumin. 2015, 158, 371–375. [Google Scholar] [CrossRef]
- Wang, Y.B.; Chang, Q.; Hu, S.L. Carbon dots with concentration-tunable multicoloredphotoluminescence for simultaneous detection of Fe3+ and Cu2+ ions. Sens. Actuators B Chem. 2017, 253, 928–933. [Google Scholar] [CrossRef]
- Li, Y.P.; Liu, X.M.; Zhang, Y.H.; Chang, Z. A fluorescent and colorimetric sensor for Al3+ based on a dibenzo-18-crown-6 derivative. Inorg. Chem. Commun. 2013, 33, 6–9. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zhang, W.H.; Qiu, H.; Tsang, D.C.W.; Morel, J.L.; Qiu, R.L. Effect of coexisting Al(III) ions on Pb(II) sorption on biochars: Role of pH buffer and competition. Chemosphere 2016, 161, 438–445. [Google Scholar] [CrossRef]
- Yan, F.Y.; Shi, D.C.; Zheng, T.C.; Yun, K.Y.; Zhou, X.G.; Chen, L. Carbon dots as nanosensor for sensitive and selective detection ofHg2+ and L-cysteine by means of fluorescence “Off–On” switching. Sens. Actuators B Chem. 2016, 224, 926–935. [Google Scholar] [CrossRef]



| Samples | Fluorescence Analysis | RGB Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Addition (μM) | Found (μM) | Recovery (%) | RSD (%) | Addition (μM) | Found (μM) | Recovery (%) | RSD (%) | |
| Tap water | 0.39 | 0.4 | 101.27 | 2.05 | 15.39 | 14.47 | 94.02 | 4.47 |
| 3.85 | 3.69 | 95.91 | 5.64 | 30.77 | 28.68 | 93.21 | 3.56 | |
| 15.39 | 15.09 | 98.02 | 2.54 | 38.47 | 37.02 | 96.23 | 4.11 | |
| 30.77 | 29.9 | 97.31 | 3.9 | 76.92 | 78.58 | 102.16 | 6.74 | |
| Lake water | 0.39 | 0.37 | 96.31 | 3.02 | 15.39 | 14.23 | 92.46 | 5.3 |
| 3.85 | 3.61 | 93.77 | 2.72 | 30.77 | 30.13 | 97.92 | 5.39 | |
| 15.39 | 16.02 | 104.1 | 5.57 | 38.46 | 36.07 | 93.79 | 7.09 | |
| 30.77 | 28.95 | 94.09 | 3.89 | 76.92 | 74.32 | 96.62 | 4.58 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Huang, J.; Gao, W.; Lu, X.; Shi, X. Carbon Dots Fluorescence-Based Colorimetric Sensor for Sensitive Detection of Aluminum Ions with a Smartphone. Chemosensors 2021, 9, 25. https://doi.org/10.3390/chemosensors9020025
Wei W, Huang J, Gao W, Lu X, Shi X. Carbon Dots Fluorescence-Based Colorimetric Sensor for Sensitive Detection of Aluminum Ions with a Smartphone. Chemosensors. 2021; 9(2):25. https://doi.org/10.3390/chemosensors9020025
Chicago/Turabian StyleWei, Wei, Juan Huang, Wenli Gao, Xiangyang Lu, and Xingbo Shi. 2021. "Carbon Dots Fluorescence-Based Colorimetric Sensor for Sensitive Detection of Aluminum Ions with a Smartphone" Chemosensors 9, no. 2: 25. https://doi.org/10.3390/chemosensors9020025
APA StyleWei, W., Huang, J., Gao, W., Lu, X., & Shi, X. (2021). Carbon Dots Fluorescence-Based Colorimetric Sensor for Sensitive Detection of Aluminum Ions with a Smartphone. Chemosensors, 9(2), 25. https://doi.org/10.3390/chemosensors9020025





