Red-Shifted Environmental Fluorophores and Their Use for the Detection of Gram-Negative Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
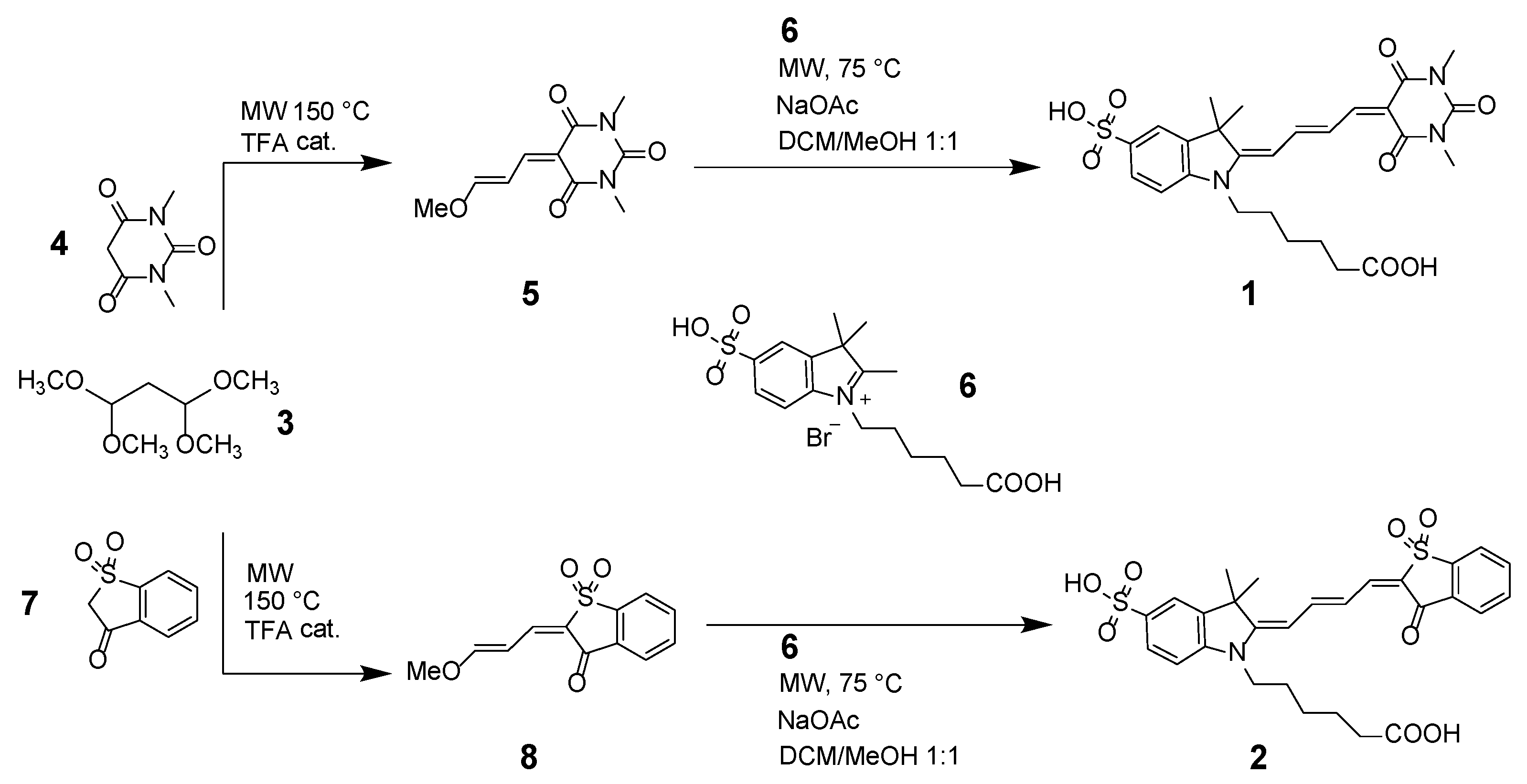
2.2. Synthesis of Compounds 1 and 2
2.3. Photophysical Studies and Characterization of 1 and 2
2.4. Fluorescence Lifetimes
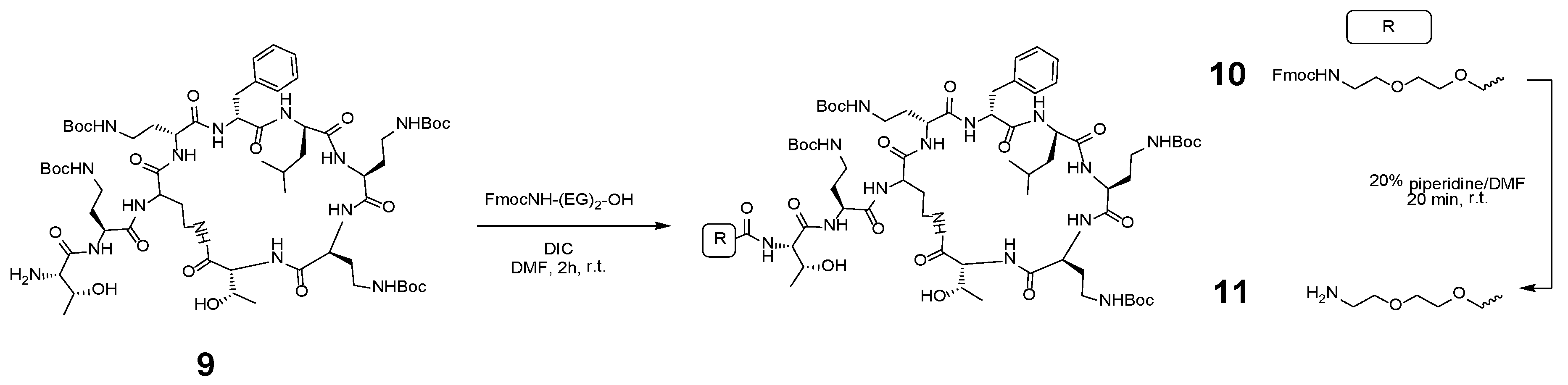
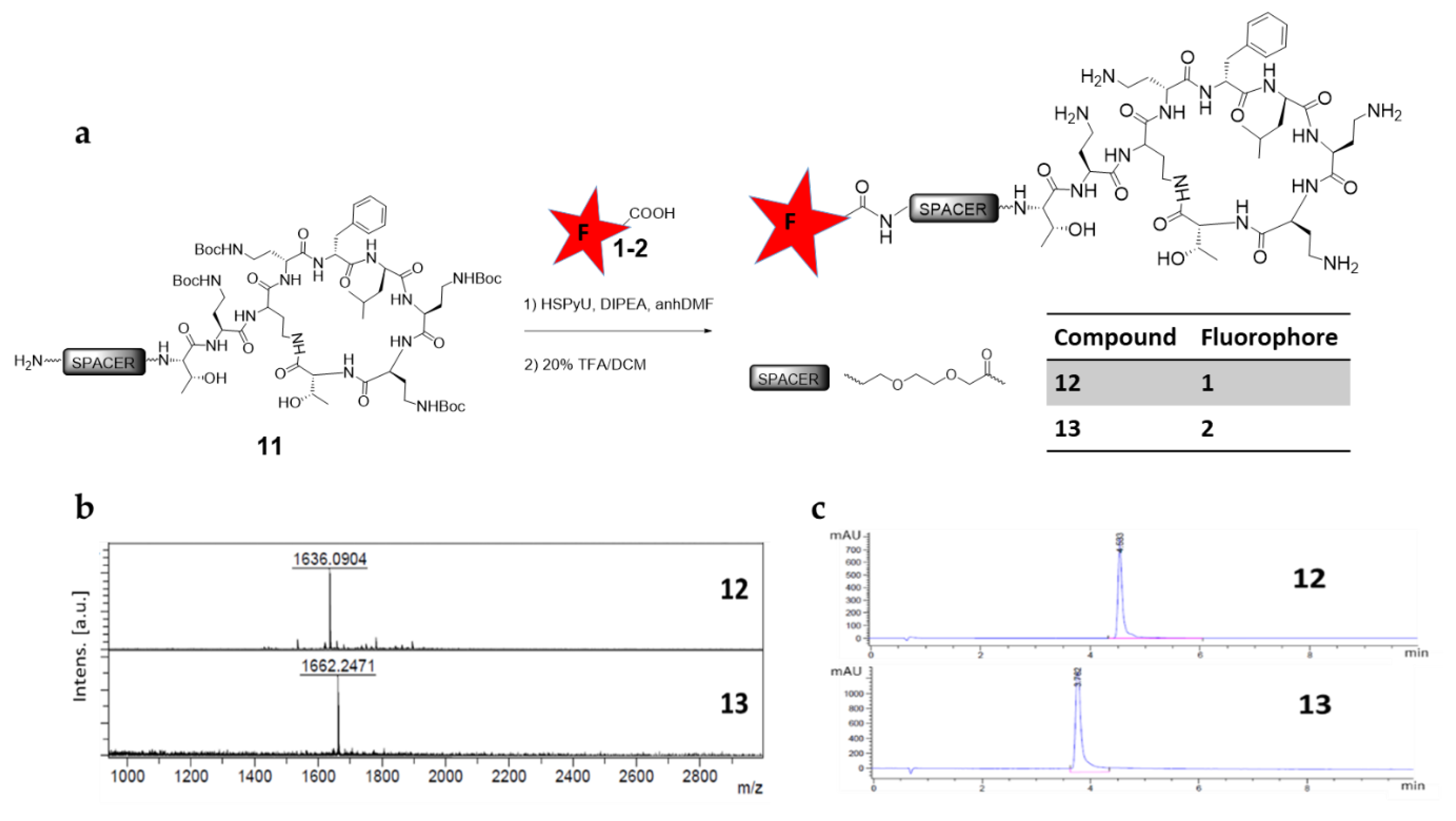
2.5. Synthetic Route to H-(EG)2-Polymyxin(Boc)4 (11)
2.6. General Procedure for Labelling H-(EG)2-Polymyxin(Boc)4 (11) with Fluorophore-COOH
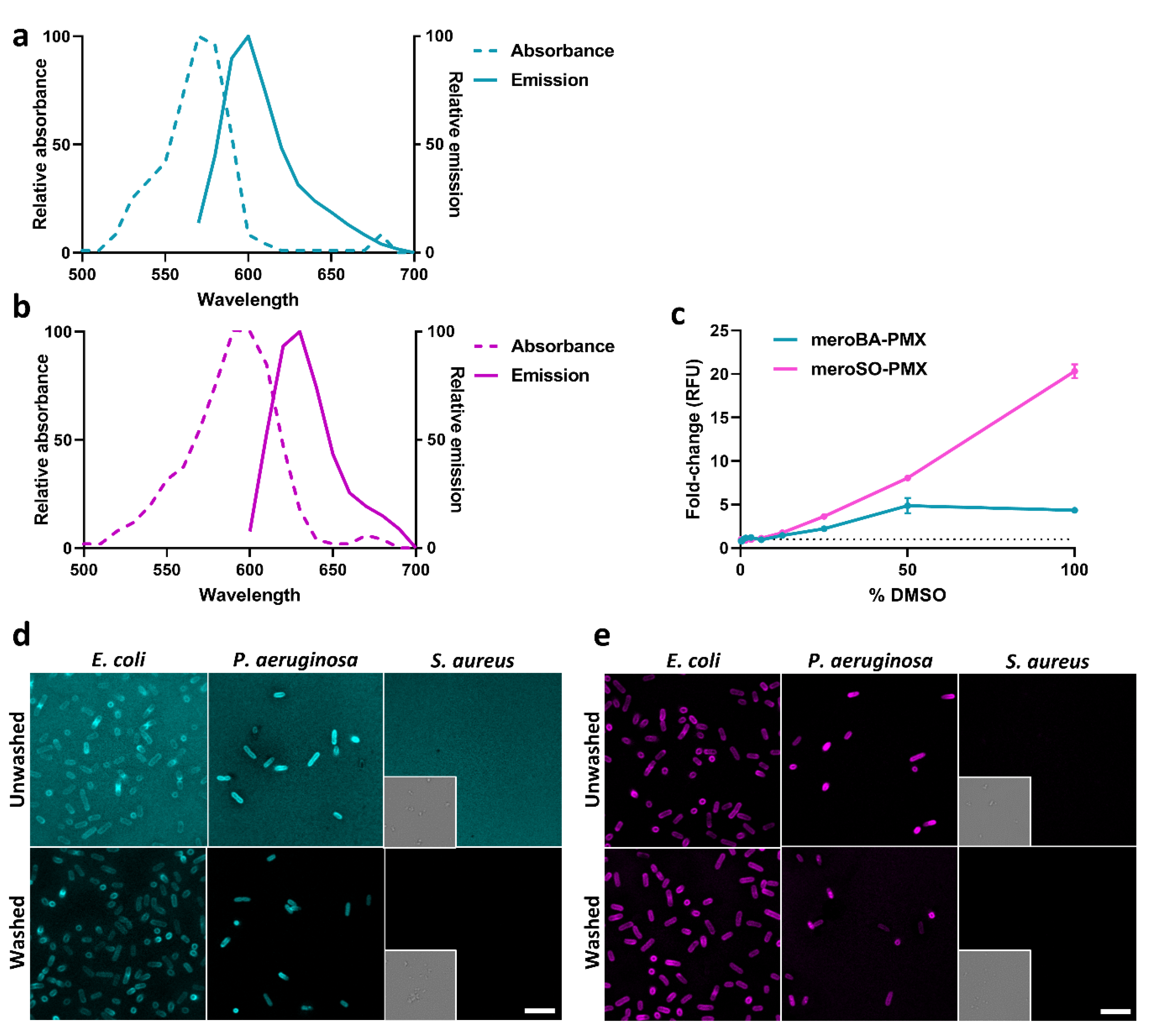
2.7. Spectral Characterization of Red PMX SmartProbes (MeroBA-PMX and MeroSO-PMX)
2.8. Bacteria Imaging with MeroBA-PMX and MeroSO-PMX
3. Results and Discussion
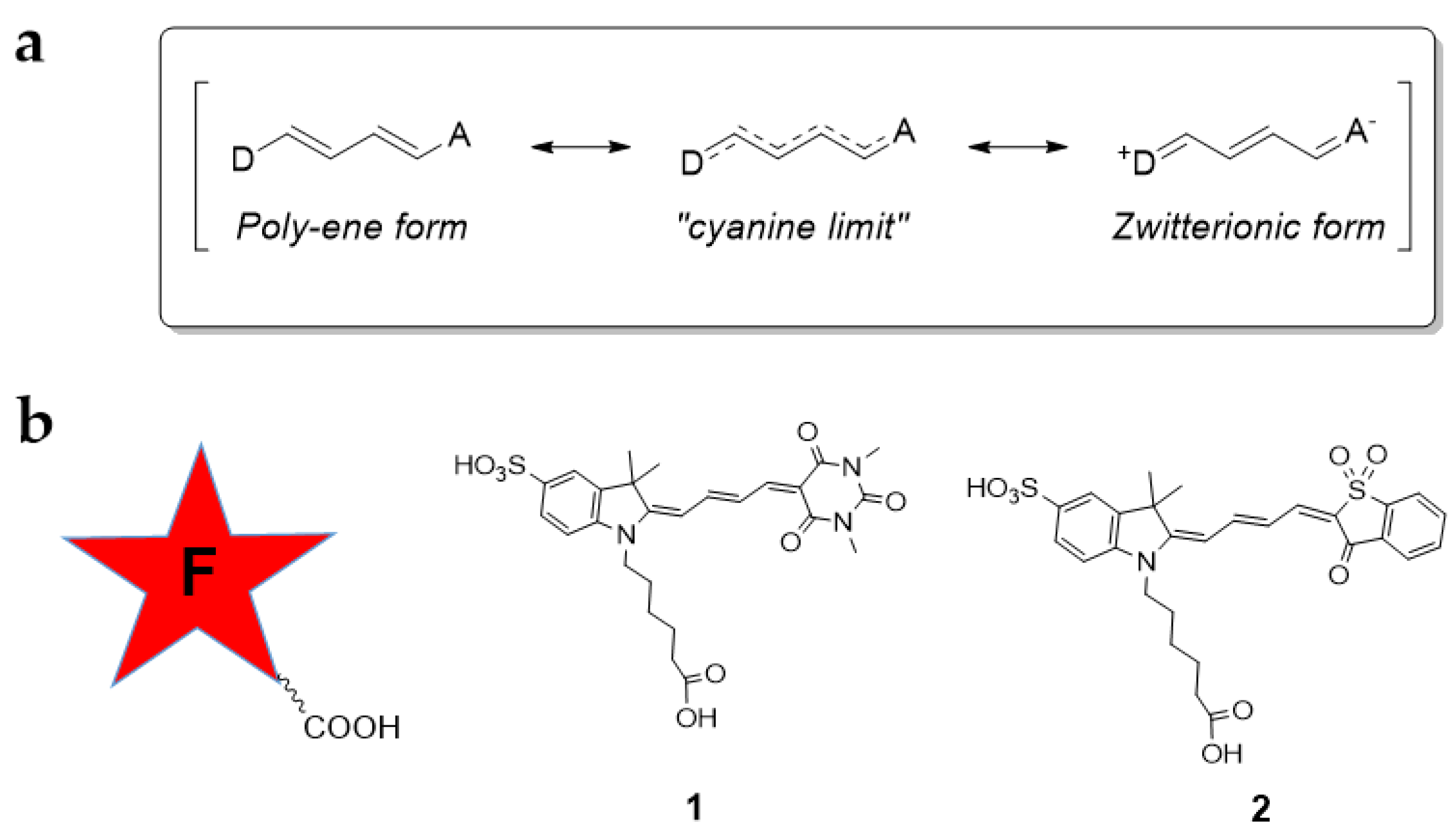
3.1. Design and Synthesis of Red Conjugatable Merocyanine Fluorophores
3.2. Optical Properties of Merocyanine-COOH Fluorophores 1 and 2
3.3. Conjugation of Polymyxin with Red Fluorophores
3.4. Labelling of Bacteria with Polymyxin-Red Fluorophores
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations.The Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 23 March 2020).
- Burggraaf, J.; Kamerling, I.M.C.; Gordon, P.B.; Schrier, L.; de Kam, M.L.; Kales, A.J.; Bendiksen, R.; Indrevoll, B.; Bjerke, R.M.; Moestue, S.A.; et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat. Med. 2015, 21, 955–961. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Sellmyer, M.A.; Gowrishankar, G.; Ruiz-Bedoya, C.A.; Tucker, E.W.; Palestro, C.J.; Hammoud, D.A.; Jain, S.K. Molecular imaging of bacterial infections: Overcoming the barriers to clinical translation. Sci. Trans. Med. 2019, 11, eaax8251. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, W.; Qiao, Q.; Li, X.; Xu, Z. Fluorescent antibiotics for real-time tracking of pathogenic bacteria. J. Pharm. Anal. 2020, 10, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.R.L.; Butler, M.S.; Phetsang, W.; Cooper, M.A.; Blaskovich, M.A.T. Fluorescent Antibiotics: New Research Tools to Fight Antibiotic Resistance. Trends Biotechnol. 2018, 36, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.; Bradley, M.; Dhaliwal, K. Optical imaging of bacterial infections. Clin. Transl. Imaging 2016, 4, 163–174. [Google Scholar] [CrossRef]
- Deris, Z.Z.; Swarbrick, J.D.; Roberts, K.D.; Azad, M.A.K.; Akter, J.; Horne, A.S.; Nation, R.L.; Rogers, K.L.; Thompson, P.E.; Velkov, T.; et al. Probing the Penetration of Antimicrobial Polymyxin Lipopeptides into Gram-Negative Bacteria. Bioconjugate Chem. 2014, 25, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Roberts, K.D.; Thompson, P.E.; Nation, R.L.; Velkov, T.; Li, J. Design and Evaluation of Novel Polymyxin Fluorescent Probes. Sensors 2017, 17, 2598. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, X. Antibiotics-based fluorescent probes for selective labeling of Gram-negative and Gram-positive bacteria in living microbiotas. Sci. China Chem. 2018, 61, 792–796. [Google Scholar] [CrossRef]
- Akram, A.R.; Chankeshwara, S.V.; Scholefield, E.; Aslam, T.; McDonald, N.; Megia-Fernandez, A.; Marshall, A.; Mills, B.; Avlonitis, N.; Craven, T.H.; et al. In situ identification of Gram-negative bacteria in human lungs using a topical fluorescent peptide targeting lipid A. Sci. Transl. Med. 2018, 10, eaal0033. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.R.; Avlonitis, N.; Scholefield, E.; Vendrell, M.; McDonald, N.; Aslam, T.; Craven, T.H.; Gray, C.; Collie, D.S.; Fisher, A.J.; et al. Enhanced avidity from a multivalent fluorescent antimicrobial peptide enables pathogen detection in a human lung model. Sci. Rep. 2019, 9, 8422. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Mely, Y. Chapter Two—Fluorescent Environment-Sensitive Dyes as Reporters of Biomolecular Interactions. In Progress in Molecular Biology and Translational Science; Morris, M.C., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 113, pp. 35–58. [Google Scholar]
- Kulinich, A.V.; Ishchenko, A.A. Merocyanine dyes: Synthesis, structure, properties and applications. Russian Chem. Rev. 2009, 78, 141–164. [Google Scholar] [CrossRef]
- Toutchkine, A.; Kraynov, V.; Hahn, K. Solvent-Sensitive Dyes to Report Protein Conformational Changes in Living Cells. J. Am. Chem. Soc. 2003, 125, 4132–4145. [Google Scholar] [CrossRef] [PubMed]
- Blanchard-Desce, M.; Wortmann, R.; Lebus, S.; Lehn, J.-M.; Krämer, P. Intramolecular charge transfer in elongated donor-acceptor conjugated polyenes. Chem. Phy. Lett. 1995, 243, 526–532. [Google Scholar] [CrossRef]
- Würthner, F.; Wortmann, R.; Matschiner, R.; Lukaszuk, K.; Meerholz, K.; DeNardin, Y.; Bittner, R.; Bräuchle, C.; Sens, R. Merocyanine Dyes in the Cyanine Limit: A New Class of Chromophores for Photorefractive Materials. Angew. Chem. Int. Ed. 1997, 36, 2765–2768. [Google Scholar] [CrossRef]
- MacNevin, C.J.; Gremyachinskiy, D.; Hsu, C.-W.; Li, L.; Rougie, M.; Davis, T.T.; Hahn, K.M. Environment-Sensing Merocyanine Dyes for Live Cell Imaging Applications. Bioconjugate Chem. 2013, 24, 215–223. [Google Scholar] [CrossRef]
- Mills, B.; Megia-Fernandez, A.; Norberg, D.; Duncan, S.; Marshall, A.; Akram, A.R.; Quinn, T.; Young, I.; Bruce, A.M.; Scholefield, E.; et al. Molecular detection of Gram-positive bacteria in the human lung through an optical fiber-based endoscope. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1–8. [Google Scholar] [CrossRef]
- Lopalco, M.; Koini, E.N.; Cho, J.K.; Bradley, M. Catch and release microwave mediated synthesis of cyanine dyes. Org. Biomol. Chem. 2009, 7, 856–859. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Rurack, K. Determination of the photoluminescence quantum yield of dilute dye solutions (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 2005–2026. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Erdogan, A.T.; Walker, R.; Finlayson, N.; Krstajić, N.; Williams, G.; Girkin, J.; Henderson, R. A CMOS SPAD Line Sensor with Per-Pixel Histogramming TDC for Time-Resolved Multispectral Imaging. IEEE J. Solid-State Circuits 2019, 54, 1705–1719. [Google Scholar] [CrossRef]
- O’Dowd, H.; Kim, B.; Margolis, P.; Wang, W.; Wu, C.; Lopez, S.L.; Blais, J. Preparation of tetra-Boc-protected polymyxin B nonapeptide. Tetrahedron Lett. 2007, 48, 2003–2005. [Google Scholar] [CrossRef]
- Würthner, F.; Yao, S.; Debaerdemaeker, T.; Wortmann, R. Dimerization of Merocyanine Dyes. Structural and Energetic Characterization of Dipolar Dye Aggregates and Implications for Nonlinear Optical Materials. J. Am. Chem. Soc. 2002, 124, 9431–9447. [Google Scholar] [CrossRef] [PubMed]
- Rösch, U.; Yao, S.; Wortmann, R.; Würthner, F. Fluorescent H-Aggregates of Merocyanine Dyes. Angew. Chem. Int. Ed. 2006, 45, 7026–7030. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Derevyanko, N.A.; Ishchenko, A.A. Synthesis, structure, and solvatochromism of merocyanine dyes based on barbituric acid. Russ. J. Gen. Chem. 2006, 76, 1441–1457. [Google Scholar] [CrossRef]
- Onganer, Y.; Yin, M.; Bessire, D.R.; Quitevis, E.L. Dynamical solvation effects on the cis-trans isomerization reaction: Photoisomerization of merocyanine 540 in polar solvents. J. Phys. Chem. 1993, 97, 2344–2354. [Google Scholar] [CrossRef]
- Mandal, D.; Pal, S.K.; Sukul, D.; Bhattacharyya, K. Photophysical Processes of Merocyanine 540 in Solutions and in Organized Media. J. Phys. Chem. A 1999, 103, 8156–8159. [Google Scholar] [CrossRef][Green Version]
- Cser, A.; Nagy, K.; Biczók, L. Fluorescence lifetime of Nile Red as a probe for the hydrogen bonding strength with its microenvironment. Chem. Phys. Lett. 2002, 360, 473–478. [Google Scholar] [CrossRef]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [PubMed]
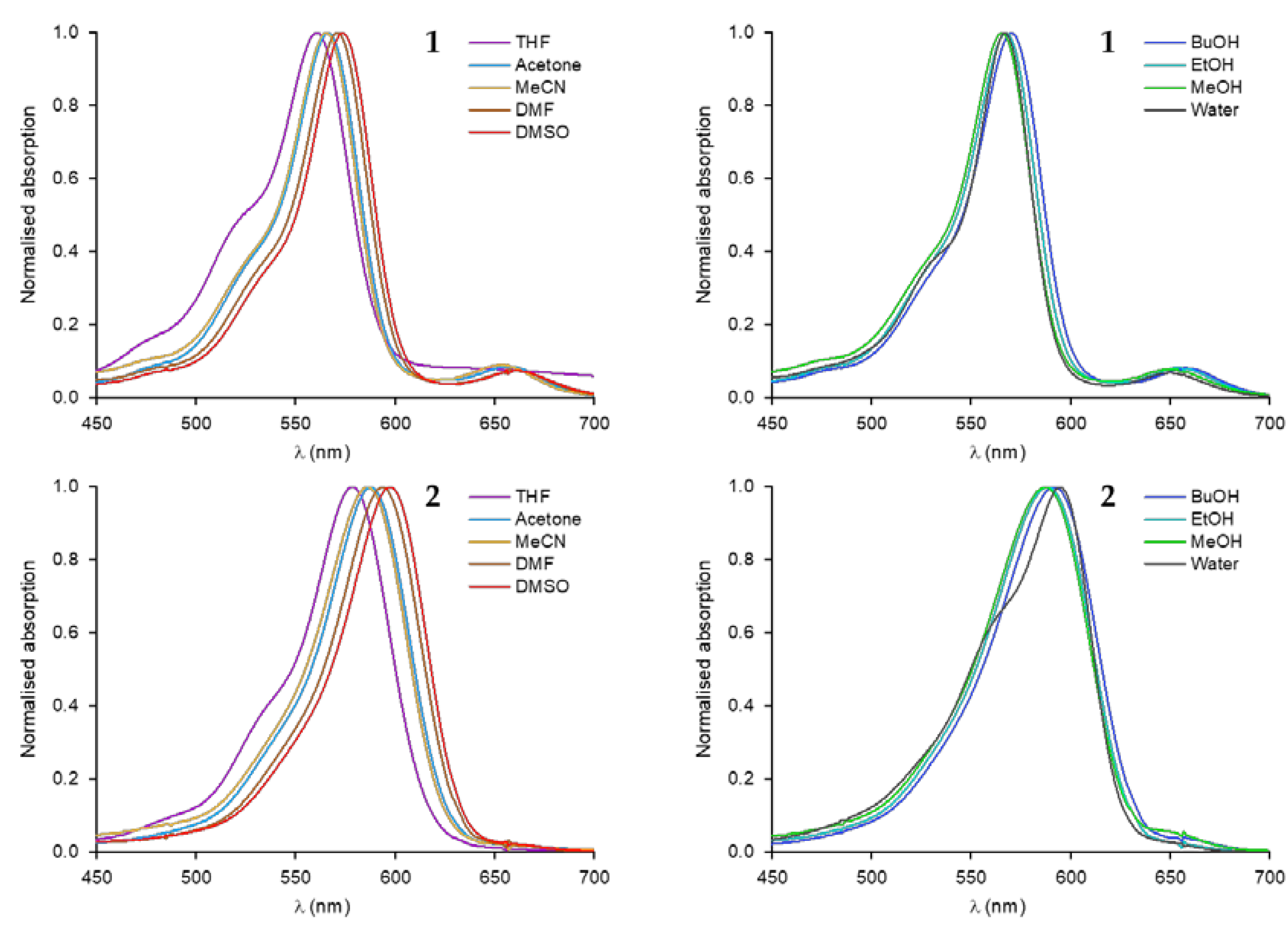

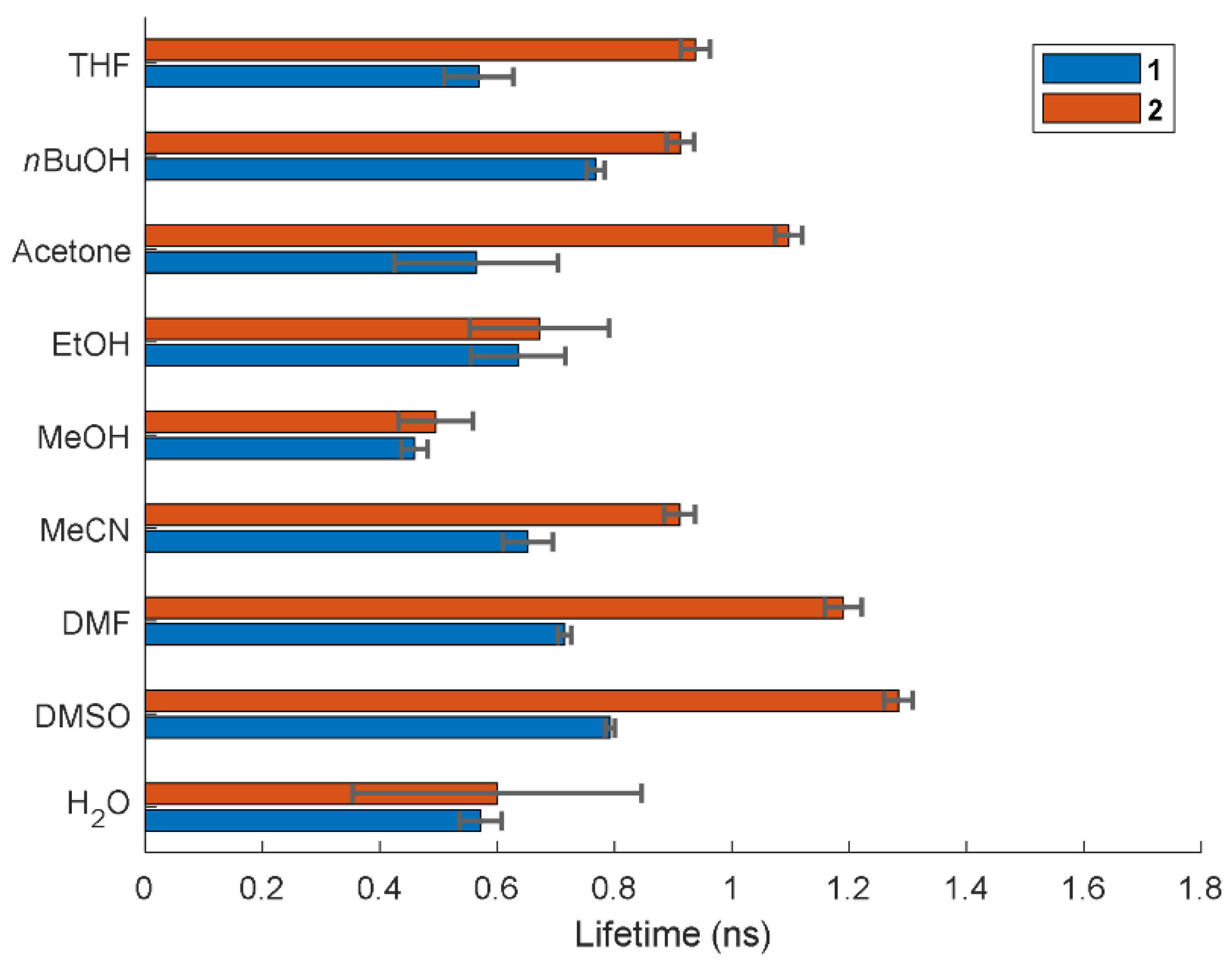
| Compound | Solvent | λabsmax (nm) | εmax (M−1 cm−1) | λemmax (nm) | Stokes Shift (cm−1) | Φf a | εmaxΦf (M−1 cm−1) | τ (ns) b |
|---|---|---|---|---|---|---|---|---|
| 1 | THF | 561 | 2.8 × 104 | 592 | 933 | 0.02 | 0.69 × 103 | 0.57 |
| nBuOH | 570 | 8.3 × 104 | 596 | 765 | 0.23 | 19 × 103 | 0.77 | |
| Acetone | 566 | 7.2 × 104 | 594 | 833 | 0.13 | 9.1 × 103 | 0.56 | |
| EtOH | 568 | 7.8 × 104 | 594 | 771 | 0.11 | 10 × 103 | 0.64 | |
| MeOH | 566 | 7.2 × 104 | 591 | 774 | 0.10 | 7.0 × 103 | 0.46 | |
| MeCN | 566 | 5.4 × 104 | 592 | 776 | 0.11 | 6.0 × 103 | 0.65 | |
| DMF | 570 | 9.3 × 104 | 597 | 793 | 0.16 | 1.5 × 103 | 0.71 | |
| DMSO | 574 | 9.1 × 104 | 600 | 755 | 0.25 | 23 × 103 | 0.79 | |
| H2O | 567 | 8.7 × 104 | 589 | 659 | 0.02 | 1.9 × 103 | 0.57 | |
| 2 | THF | 579 | 1.2 × 104 | 618 | 1090 | 0.23 | 0.29 × 103 | 0.94 |
| nBuOH | 592 | 6.6 × 104 | 629 | 994 | 0.26 | 17 × 103 | 0.91 | |
| Acetone | 587 | 9.4 × 104 | 625 | 1036 | 0.34 | 33 × 103 | 1.10 | |
| EtOH | 589 | 4.2 × 104 | 627 | 1029 | 0.16 | 6.7 × 103 | 0.67 | |
| MeOH | 587 | 5.6 × 104 | 625 | 1036 | 0.09 | 4.8 × 103 | 0.50 | |
| MeCN | 587 | 4.2 × 104 | 621 | 933 | 0.22 | 9.4 × 103 | 0.91 | |
| DMF | 594 | 7.1 × 104 | 628 | 911 | 0.42 | 30 × 103 | 1.19 | |
| DMSO | 598 | 7.5 × 104 | 629 | 824 | 0.38 | 29 × 103 | 1.28 | |
| H2O | 595 | 7.0 × 104 | 621 | 704 | 0.02 | 1.4 × 103 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megia-Fernandez, A.; Klausen, M.; Mills, B.; Brown, G.E.; McEwan, H.; Finlayson, N.; Dhaliwal, K.; Bradley, M. Red-Shifted Environmental Fluorophores and Their Use for the Detection of Gram-Negative Bacteria. Chemosensors 2021, 9, 117. https://doi.org/10.3390/chemosensors9060117
Megia-Fernandez A, Klausen M, Mills B, Brown GE, McEwan H, Finlayson N, Dhaliwal K, Bradley M. Red-Shifted Environmental Fluorophores and Their Use for the Detection of Gram-Negative Bacteria. Chemosensors. 2021; 9(6):117. https://doi.org/10.3390/chemosensors9060117
Chicago/Turabian StyleMegia-Fernandez, Alicia, Maxime Klausen, Bethany Mills, Gillian E. Brown, Heather McEwan, Neil Finlayson, Kevin Dhaliwal, and Mark Bradley. 2021. "Red-Shifted Environmental Fluorophores and Their Use for the Detection of Gram-Negative Bacteria" Chemosensors 9, no. 6: 117. https://doi.org/10.3390/chemosensors9060117
APA StyleMegia-Fernandez, A., Klausen, M., Mills, B., Brown, G. E., McEwan, H., Finlayson, N., Dhaliwal, K., & Bradley, M. (2021). Red-Shifted Environmental Fluorophores and Their Use for the Detection of Gram-Negative Bacteria. Chemosensors, 9(6), 117. https://doi.org/10.3390/chemosensors9060117








