Development of an ImmunoFET for Analysis of Tumour Necrosis Factor-α in Artificial Saliva: Application for Heart Failure Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fabrication of the ISFET Devices
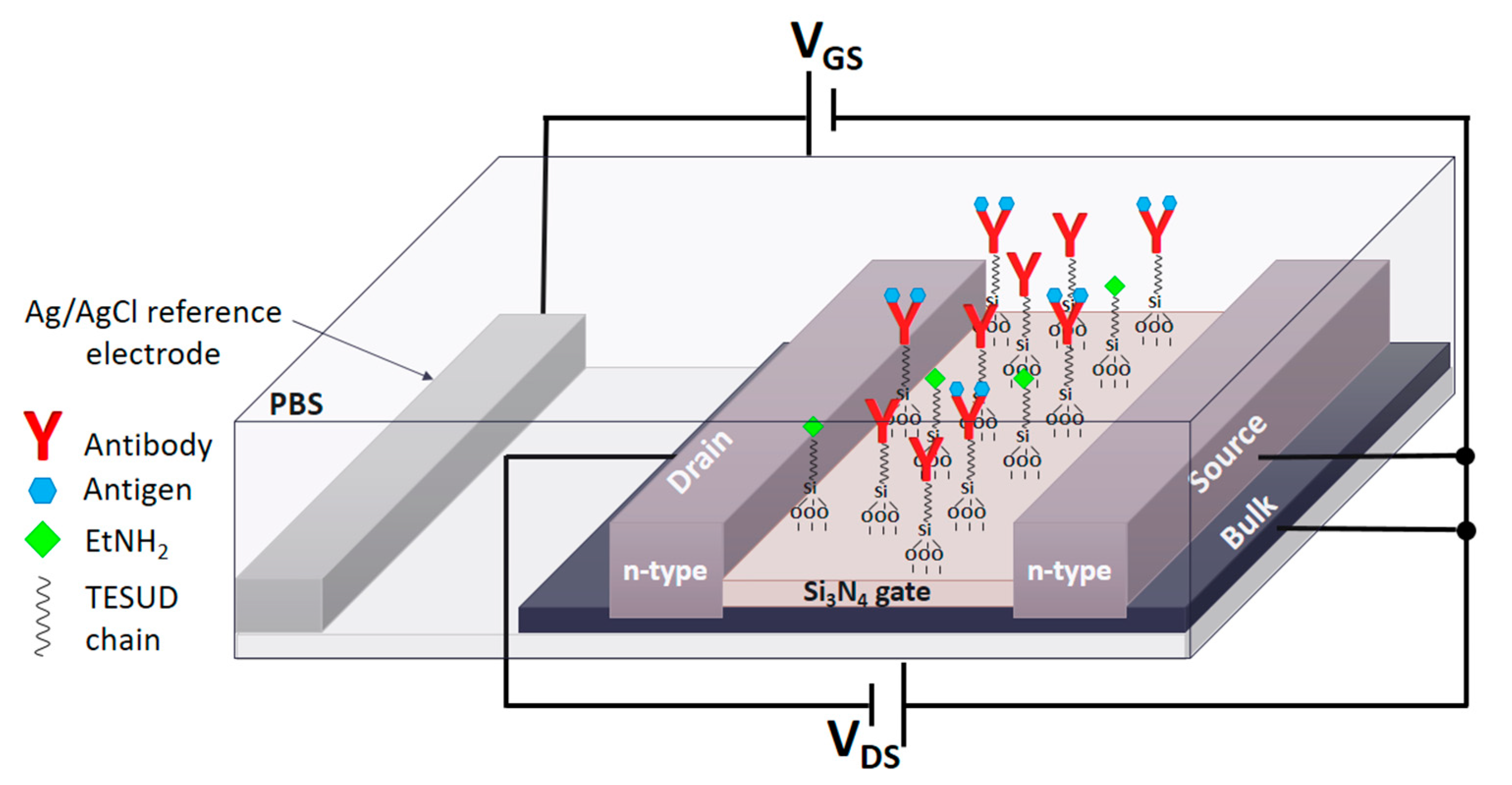
2.3. Functionalization of ISFET Surface with Antibodies
2.4. Preparation of Artificial Saliva (AS)
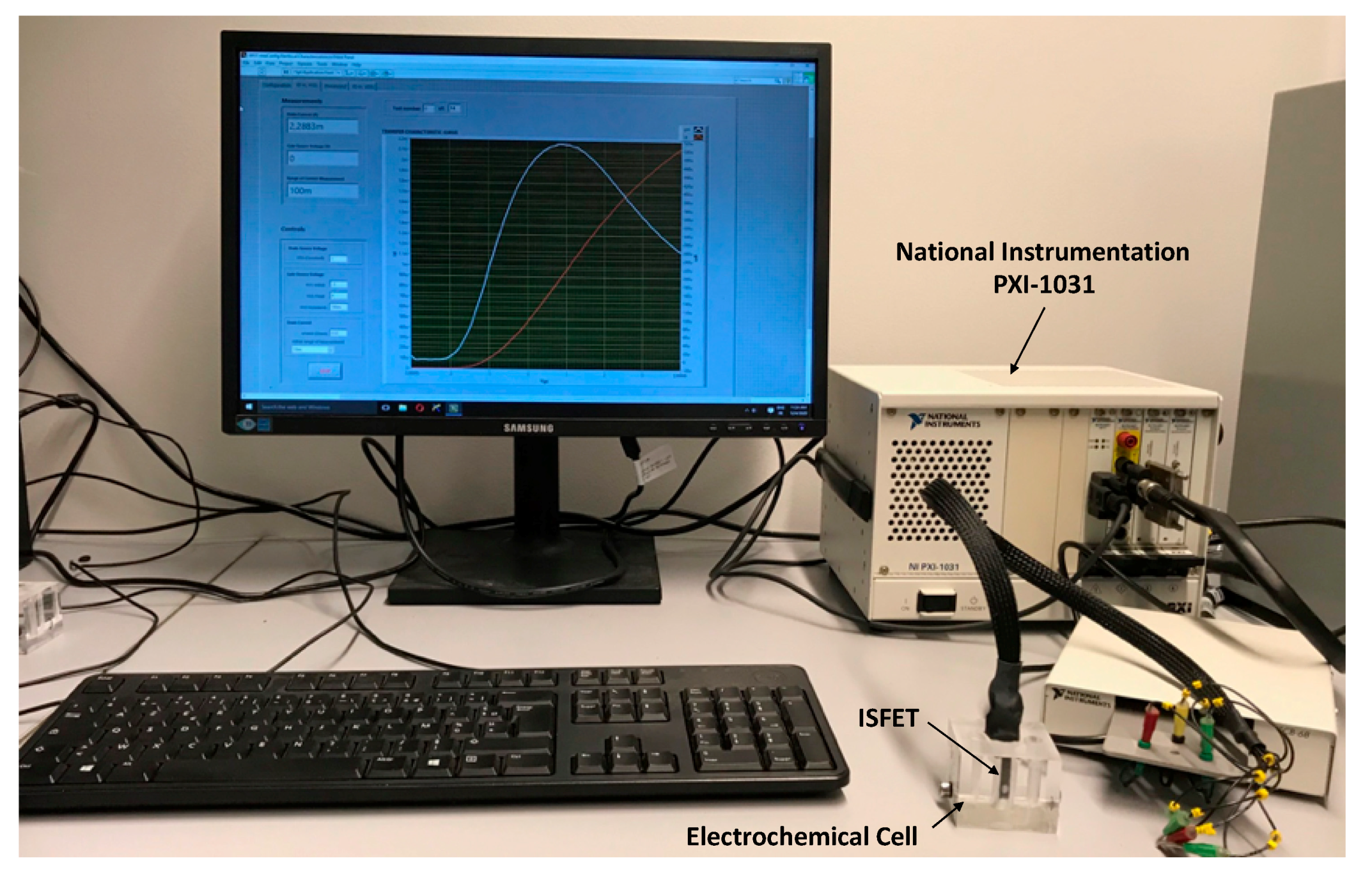
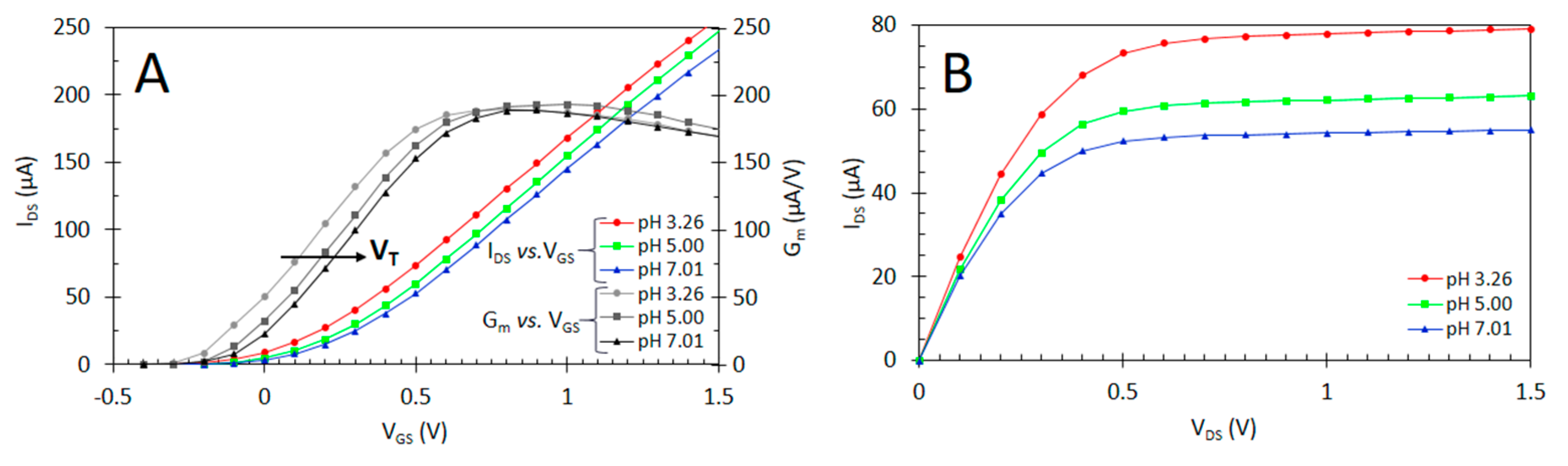
2.5. Electrochemical Characterisation of ISFET
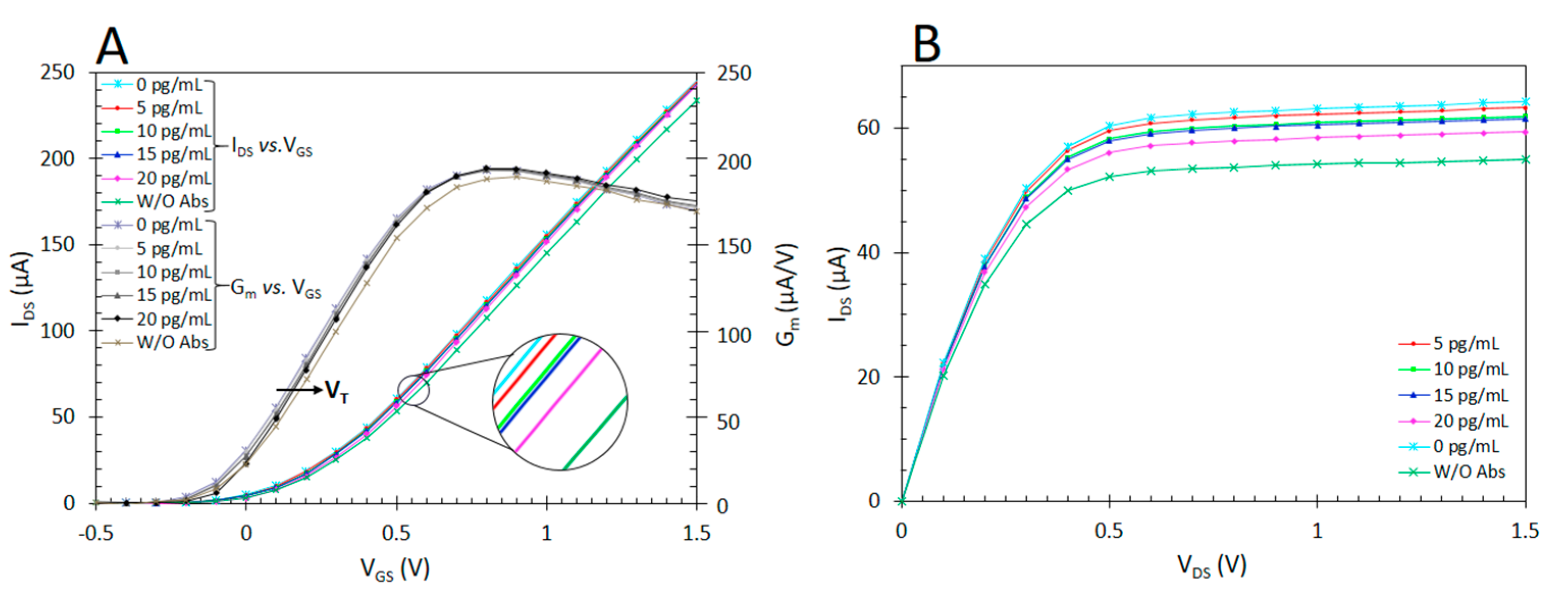
2.6. Detection and Characterization of Antigens (Ag) Using ImmunoFET
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessup, M.; Brozena, S. Heart Failure. N. Engl. J. Med. 2003, 348, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Guha, K.; McDonagh, T. Heart Failure Epidemiology: European Perspective. Available online: https://www.ingentaconnect.com/content/ben/ccr/2013/00000009/00000002/art00005 (accessed on 28 October 2019).
- Schiff, G.D.; Fung, S.; Speroff, T.; McNutt, R.A. Decompensated Heart Failure: Symptoms, Patterns of Onset, and Contributing Factors. Am. J. Med. 2003, 114, 625–630. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in Heart Failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated Circulating Levels of Tumor Necrosis Factor in Severe Chronic Heart Failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef]
- van Kimmenade, R.R.J.; Januzzi, J.L. Emerging Biomarkers in Heart Failure. Clin. Chem. 2012, 58, 127–138. [Google Scholar] [CrossRef]
- Wild, D. The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques; Elsevier: Newnes, Australia, 2013; ISBN 978-0-08-097038-7. [Google Scholar]
- De Moraes, A.C.M.; Kubota, L.T. Recent Trends in Field-Effect Transistors-Based Immunosensors. Chemosensors 2016, 4, 20. [Google Scholar] [CrossRef]
- Barhoumi, L.; Baraket, A.; Bellagambi, F.G.; Karanasiou, G.S.; Ali, M.B.; Fotiadis, D.I.; Bausells, J.; Zine, N.; Sigaud, M.; Errachid, A. A Novel Chronoamperometric Immunosensor for Rapid Detection of TNF-α in Human Saliva. Sens. Actuators B Chem. 2018, 266, 477–484. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in Biosensors: Principle, Architecture and Applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Male, K.B.; Glennon, J.D. Biosensor Technology: Technology Push versus Market Pull. Biotechnol. Adv. 2008, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Piezoelectric Biosensor for the Determination of Tumor Necrosis Factor Alpha. Talanta 2018, 178, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Wang, W.; Zhang, Q.; Liu, D.; Li, X.; Jiang, H. High Sensitivity Determination of TNF-α for Early Diagnosis of Neonatal Infections with a Novel and Reusable Electrochemical Sensor. Sensors 2017, 17, 992. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, L.; Bellagambi, F.G.; Vivaldi, F.M.; Baraket, A.; Clément, Y.; Zine, N.; Ben Ali, M.; Elaissari, A.; Errachid, A. Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva Using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode. Sensors 2019, 19, 692. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Baraket, A.; Longo, A.; Vatteroni, M.; Zine, N.; Bausells, J.; Fuoco, R.; Di Francesco, F.; Salvo, P.; Karanasiou, G.S.; et al. Electrochemical Biosensor Platform for TNF-α Cytokines Detection in Both Artificial and Human Saliva: Heart Failure. Sens. Actuators B Chem. 2017, 251, 1026–1033. [Google Scholar] [CrossRef]
- Bahri, M.; Baraket, A.; Zine, N.; Ben Ali, M.; Bausells, J.; Errachid, A. Capacitance Electrochemical Biosensor Based on Silicon Nitride Transducer for TNF-α Cytokine Detection in Artificial Human Saliva: Heart Failure (HF). Talanta 2020, 209, 120501. [Google Scholar] [CrossRef]
- Kaisti, M. Detection Principles of Biological and Chemical FET Sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianguo, G.; Jianrong, C. Ion Sensitive Field Effect Transducer-Based Biosensors. Biotechnol. Adv. 2003, 21, 527–534. [Google Scholar] [CrossRef]
- Chen, H.; Choo, T.K.; Huang, J.; Wang, Y.; Liu, Y.; Platt, M.; Palaniappan, A.; Liedberg, B.; Tok, A.I.Y. Label-Free Electronic Detection of Interleukin-6 Using Horizontally Aligned Carbon Nanotubes. Mater. Des. 2016, 90, 852–857. [Google Scholar] [CrossRef]
- Selvanayagam, Z.E.; Neuzil, P.; Gopalakrishnakone, P.; Sridhar, U.; Singh, M.; Ho, L.C. An ISFET-Based Immunosensor for the Detection of β-Bungarotoxin. Biosens. Bioelectron. 2002, 17, 821–826. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Xu, L.; Ning, Y.; Xie, S.; Zhang, G.-J. Silicon Nanowire Biosensor for Highly Sensitive and Multiplexed Detection of Oral Squamous Cell Carcinoma Biomarkers in Saliva. Anal. Sci. 2015, 31, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Muangsuwan, W.; Promptmas, C.; Jeamsaksiri, W.; Bunjongpru, W.; Srisuwan, A.; Hruanun, C.; Poyai, A.; Wongchitrat, P.; Yasawong, M. Development of an ImmunoFET Biosensor for the Detection of Biotinylated PCR Product. Heliyon 2016, 2, e00188. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-Chip Platforms for Detection of Cardiovascular Disease and Cancer Biomarkers. Sensors 2017, 17, 2934. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-Chip Technology for Chronic Disease Diagnosis. NPJ Digit. Med. 2018, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Ho, V.; Miller, C.S.; Myers, S.; Ondrey, F. NF-ΚB Dependent Cytokine Levels in Saliva of Patients with Oral Preneoplastic Lesions and Oral Squamous Cell Carcinoma. Cancer Detect. Prev. 2005, 29, 42–45. [Google Scholar] [CrossRef]
- Pezelj-Ribaric, S.; Prso, I.B.; Abram, M.; Glazar, I.; Brumini, G.; Simunovic-Soskic, M. Salivary Levels of Tumor Necrosis Factor-α in Oral Lichen Planus. Mediat. Inflamm. 2004, 13, 131–133. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Degano, I.; Ghimenti, S.; Lomonaco, T.; Dini, V.; Romanelli, M.; Mastorci, F.; Gemignani, A.; Salvo, P.; Fuoco, R.; et al. Determination of Salivary α-Amylase and Cortisol in Psoriatic Subjects Undergoing the Trier Social Stress Test. Microchem. J. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva Sampling: Methods and Devices. An Overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Biagini, D.; Lomonaco, T.; Ghimenti, S.; Fusi, J.; Cerri, E.; De Angelis, F.; Bellagambi, F.G.; Oger, C.; Galano, J.M.; Bramanti, E.; et al. Saliva as a Non-Invasive Tool for Monitoring Oxidative Stress in Swimmers Athletes Performing a VO2max Cycle Ergometer Test. Talanta 2020, 216, 120979. [Google Scholar] [CrossRef]
- Ghimenti, S.; Lomonaco, T.; Bellagambi, F.G.; Biagini, D.; Salvo, P.; Trivella, M.G.; Scali, M.C.; Barletta, V.; Marzilli, M.; Di Francesco, F.; et al. Salivary Lactate and 8-Isoprostaglandin F 2α as Potential Non-Invasive Biomarkers for Monitoring Heart Failure: A Pilot Study. Sci. Rep. 2020, 10, 7441. [Google Scholar] [CrossRef]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The Effect of Sampling Procedures on the Urate and Lactate Concentration in Oral Fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of Cytokines and Inflammation in Heart Function during Health and Disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Januzzi, J.L. Biomarkers and Diagnostics in Heart Failure. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Januzzi, J.L. The Potential Role of Natriuretic Peptides and Other Biomarkers in Heart Failure Diagnosis, Prognosis and Management. Expert Rev. Cardiovasc. Ther. 2015, 13, 1017–1030. [Google Scholar] [CrossRef]
- Magnussen, C.; Blankenberg, S. Biomarkers for Heart Failure: Small Molecules with High Clinical Relevance. J. Intern. Med. 2018, 283, 530–543. [Google Scholar] [CrossRef]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in Routine Heart Failure Clinical Care. Card Fail. Rev. 2019, 5, 50–56. [Google Scholar] [CrossRef]
- Tamariz, L.; Hare, J.M. Inflammatory Cytokines in Heart Failure: Roles in Aetiology and Utility as Biomarkers. Eur. Heart J. 2010, 31, 768–770. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Yaakoubi, N.; Bausells, J.; Errachid, A. A Flexible Electrochemical Micro Lab-on-Chip: Application to the Detection of Interleukin-10. Microchim. Acta 2016, 183, 2155–2162. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Yaakoubi, N.; Trivella, M.G.; Zabala, M.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. Diazonium Modified Gold Microelectrodes onto Polyimide Substrates for Impedimetric Cytokine Detection with an Integrated Ag/AgCl Reference Electrode. Sens. Actuators B Chem. 2013, 189, 165–172. [Google Scholar] [CrossRef]
- Caballero, D.; Martinez, E.; Bausells, J.; Errachid, A.; Samitier, J. Impedimetric Immunosensor for Human Serum Albumin Detection on a Direct Aldehyde-Functionalized Silicon Nitride Surface. Anal. Chim. Acta 2012, 720, 43–48. [Google Scholar] [CrossRef]
- Caballero, D.; Samitier, J.; Bausells, J.; Errachid, A. Direct Patterning of Anti-Human Serum Albumin Antibodies on Aldehyde-Terminated Silicon Nitride Surfaces for HSA Protein Detection. Small 2009, 5, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Ben Halima, H.; Zine, N.; Gallardo-González, J.; Aissari, A.E.; Sigaud, M.; Alcacer, A.; Bausells, J.; Errachid, A. A Novel Cortisol Biosensor Based on the Capacitive Structure of Hafnium Oxide: Application for Heart Failure Monitoring. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1067–1070. [Google Scholar] [CrossRef]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A Novel Biosensor Based on Hafnium Oxide: Application for Early Stage Detection of Human Interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Castellarnau, M.; Zine, N.; Bausells, J.; Madrid, C.; Juárez, A.; Samitier, J.; Errachid, A. ISFET-Based Biosensor to Monitor Sugar Metabolism in Bacteria. Mater. Sci. Eng. C 2008, 28, 680–685. [Google Scholar] [CrossRef]
- Castellarnau, M.; Zine, N.; Bausells, J.; Madrid, C.; Juárez, A.; Samitier, J.; Errachid, A. Integrated Microanalytical System Based on Electrochemical Detection and Cell Positioning. Mater. Sci. Eng. C 2006, 26, 405–410. [Google Scholar] [CrossRef]
- Harame, D.L.; Bousse, L.J.; Shott, J.D.; Meindl, J.D. Ion-Sensing Devices with Silicon Nitride and Borosilicate Glass Insulators. IEEE Trans. Electron. Devices 1987, 34, 1700–1707. [Google Scholar] [CrossRef]
- Castellarnau, M.; Zine, N.; Bausells, J.; Madrid, C.; Juárez, A.; Samitier, J.; Errachid, A. Integrated Cell Positioning and Cell-Based ISFET Biosensors. Sens. Actuators B Chem. 2007, 120, 615–620. [Google Scholar] [CrossRef]
- Errachid, A.; Ivorra, A.; Aguiló, J.; Villa, R.; Zine, N.; Bausells, J. New Technology for Multi-Sensor Silicon Needles for Biomedical Applications. Sens. Actuators B Chem. 2001, 78, 279–284. [Google Scholar] [CrossRef]
- Stoop, R.; Wipf, M.; Müller, S.; Bedner, K.; Wright, I.A.; Martin, C.; Constable, E.; Fu, W.; Tarasov, A.; Calame, M.; et al. Competing surface reactions limiting the performance of ion-sensitive field-effect transistors. Sens. Actuators B Chem. 2015, 220, 500–507. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Yan, L.; Whitesides, G.M. Preparation of mixed self-assembled monolayers (SAMs) that resist adsorption of proteins using the reaction of amines with a SAM that presents interchain carboxylic anhydride groups. Langmuir 2000, 16, 6927–6936. [Google Scholar] [CrossRef]
- Pulikkathodi, A.K.; Sarangadharan, I.; Hsu, C.-P.; Chen, Y.-H.; Hung, L.-Y.; Lee, G.-Y.; Chyi, J.-I.; Lee, G.-B.; Wang, Y.-L. Enumeration of Circulating Tumor Cells and Investigation of Cellular Responses Using Aptamer-Immobilized AlGaN/GaN High Electron Mobility Transistor Sensor Array. Sens. Actuators B Chem. 2018, 257, 96–104. [Google Scholar] [CrossRef]
- Longo, A.; Baraket, A.; Vatteroni, M.; Zine, N.; Baussells, J.; Roger, F.; Di Francesco, F.; Karanasiou, G.S.; Fotiadis, D.I.; Menciassi, A.; et al. Highly Sensitive Electrochemical BioMEMS for TNF-α Detection in Humansaliva: Heart Failure. Procedia Eng. 2016, 168, 97–100. [Google Scholar] [CrossRef]

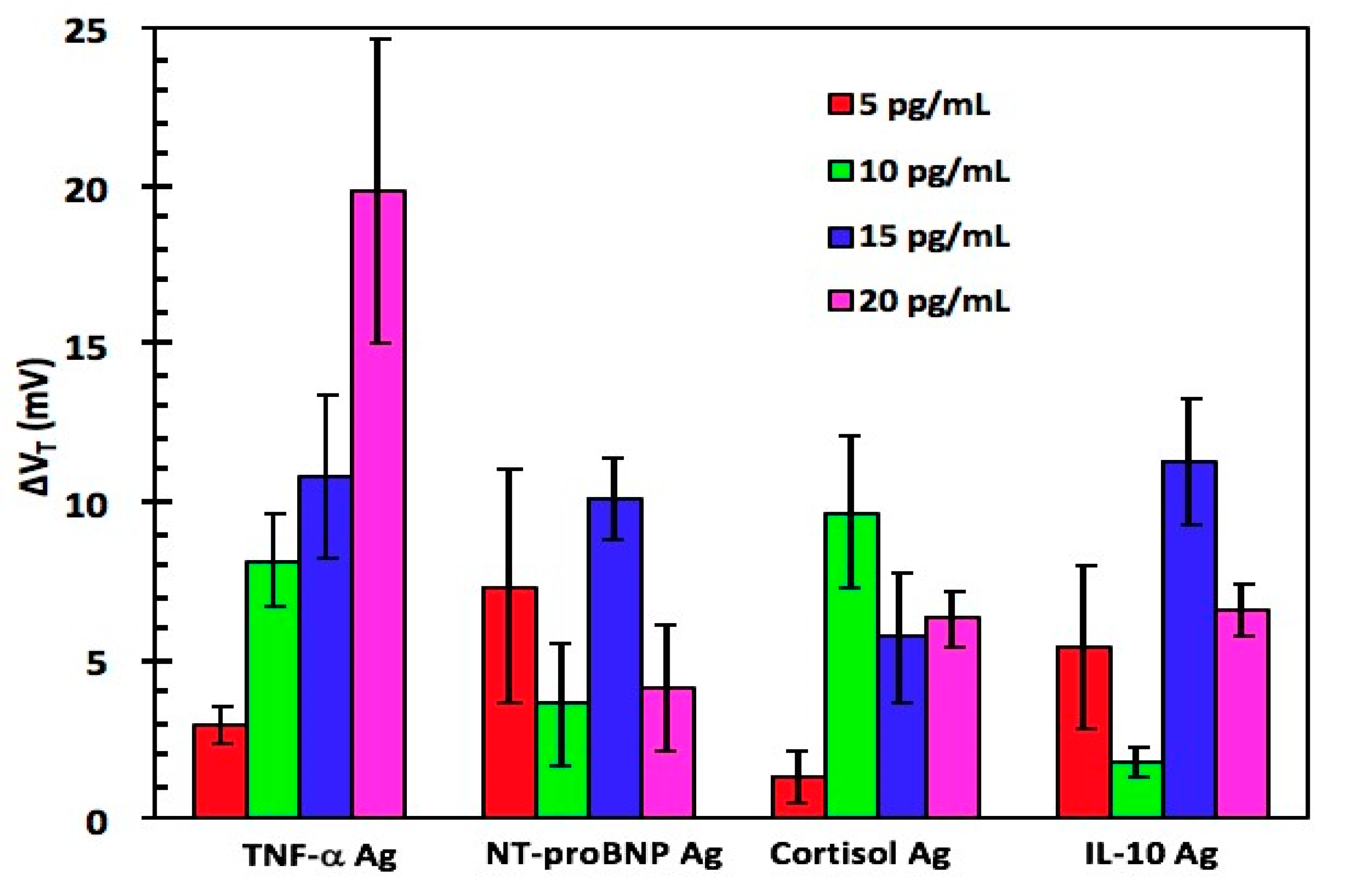
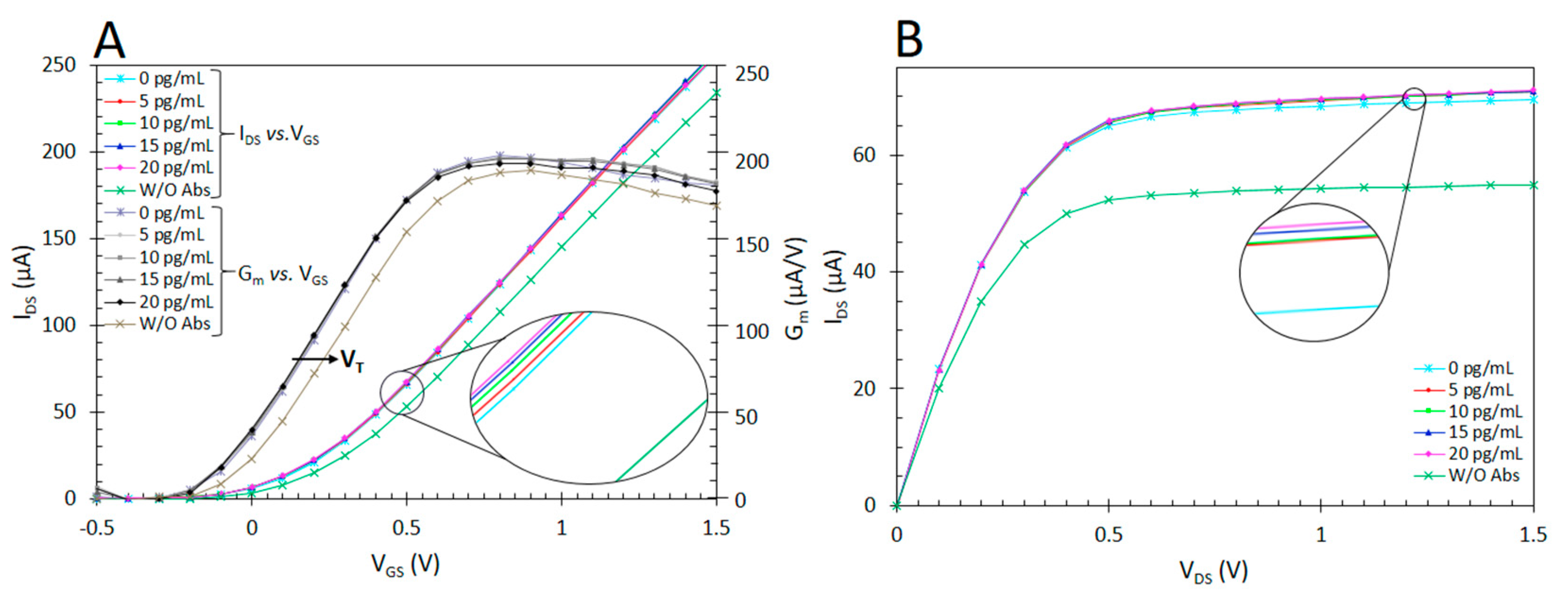
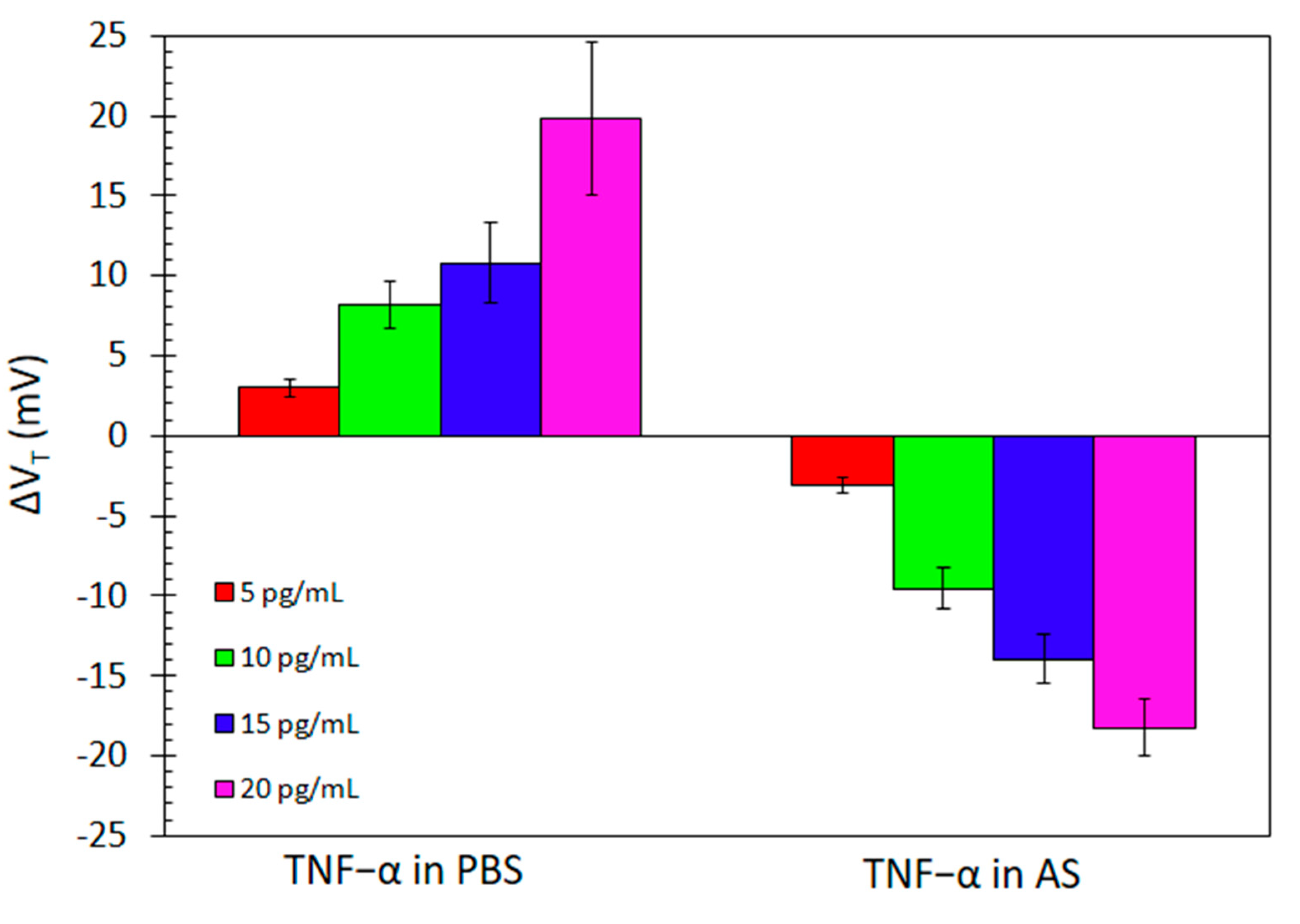
| Technique | Analyte | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Impedimetric | AS | 1–15 pg/mL | 1 pg/mL | [54] |
| Mott shottcky | AS | 1–30 pg/mL | 1 pg/mL | [18] |
| Amperometry | AS | 1–30 pg/mL | 1 pg/mL | [11] |
| Amperometry | AS | 1–15 pg/mL | 0.3 pg/mL | [16] |
| Electrical measurement | AS | 5–20 pg/mL | 5 pg/mL | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vozgirdaite, D.; Ben Halima, H.; Bellagambi, F.G.; Alcacer, A.; Palacio, F.; Jaffrezic-Renault, N.; Zine, N.; Bausells, J.; Elaissari, A.; Errachid, A. Development of an ImmunoFET for Analysis of Tumour Necrosis Factor-α in Artificial Saliva: Application for Heart Failure Monitoring. Chemosensors 2021, 9, 26. https://doi.org/10.3390/chemosensors9020026
Vozgirdaite D, Ben Halima H, Bellagambi FG, Alcacer A, Palacio F, Jaffrezic-Renault N, Zine N, Bausells J, Elaissari A, Errachid A. Development of an ImmunoFET for Analysis of Tumour Necrosis Factor-α in Artificial Saliva: Application for Heart Failure Monitoring. Chemosensors. 2021; 9(2):26. https://doi.org/10.3390/chemosensors9020026
Chicago/Turabian StyleVozgirdaite, Daiva, Hamdi Ben Halima, Francesca G. Bellagambi, Albert Alcacer, Francisio Palacio, Nicole Jaffrezic-Renault, Nadia Zine, Joan Bausells, Abdelhamid Elaissari, and Abdelhamid Errachid. 2021. "Development of an ImmunoFET for Analysis of Tumour Necrosis Factor-α in Artificial Saliva: Application for Heart Failure Monitoring" Chemosensors 9, no. 2: 26. https://doi.org/10.3390/chemosensors9020026
APA StyleVozgirdaite, D., Ben Halima, H., Bellagambi, F. G., Alcacer, A., Palacio, F., Jaffrezic-Renault, N., Zine, N., Bausells, J., Elaissari, A., & Errachid, A. (2021). Development of an ImmunoFET for Analysis of Tumour Necrosis Factor-α in Artificial Saliva: Application for Heart Failure Monitoring. Chemosensors, 9(2), 26. https://doi.org/10.3390/chemosensors9020026












