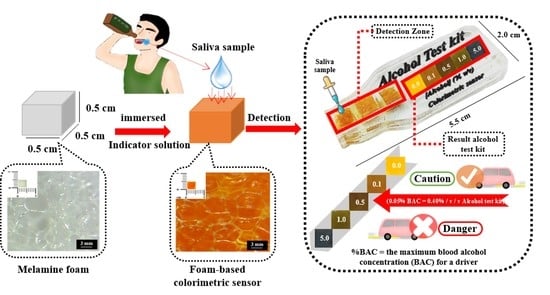
Cost-Effective Foam-Based Colorimetric Sensor for Roadside Testing of Alcohol in Undiluted Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
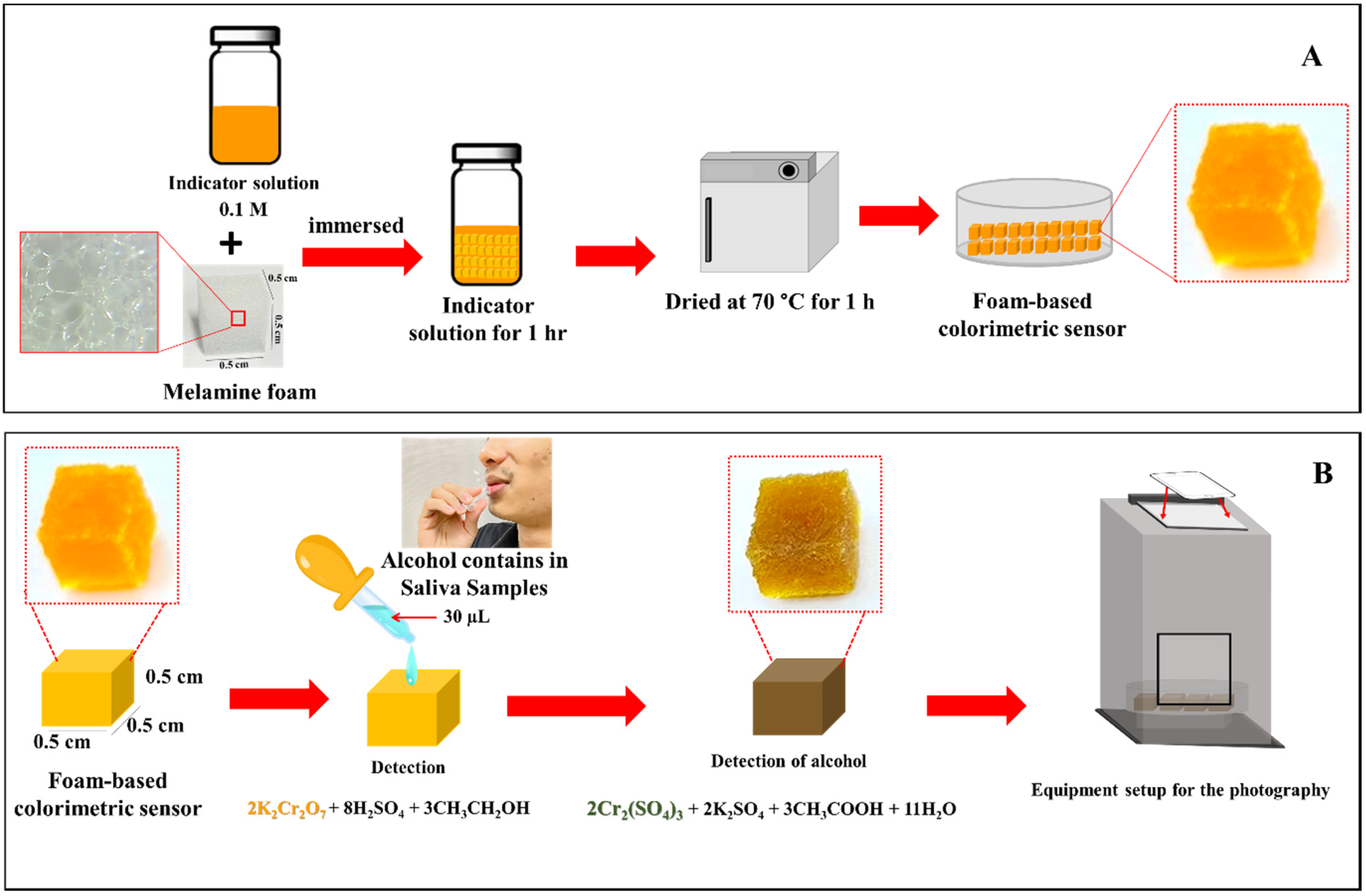
2.2. Preparation of Foam-Based Alcohol Colorimetric Sensor
2.3. RGB Image Processing and Statistical Analysis
2.4. Optimization of the Sensing Conditions of Foam-Based Alcohol Colorimetric Sensor
2.5. Method Validation
2.6. Determination of Alcohol in Saliva Samples
3. Results and Discussion
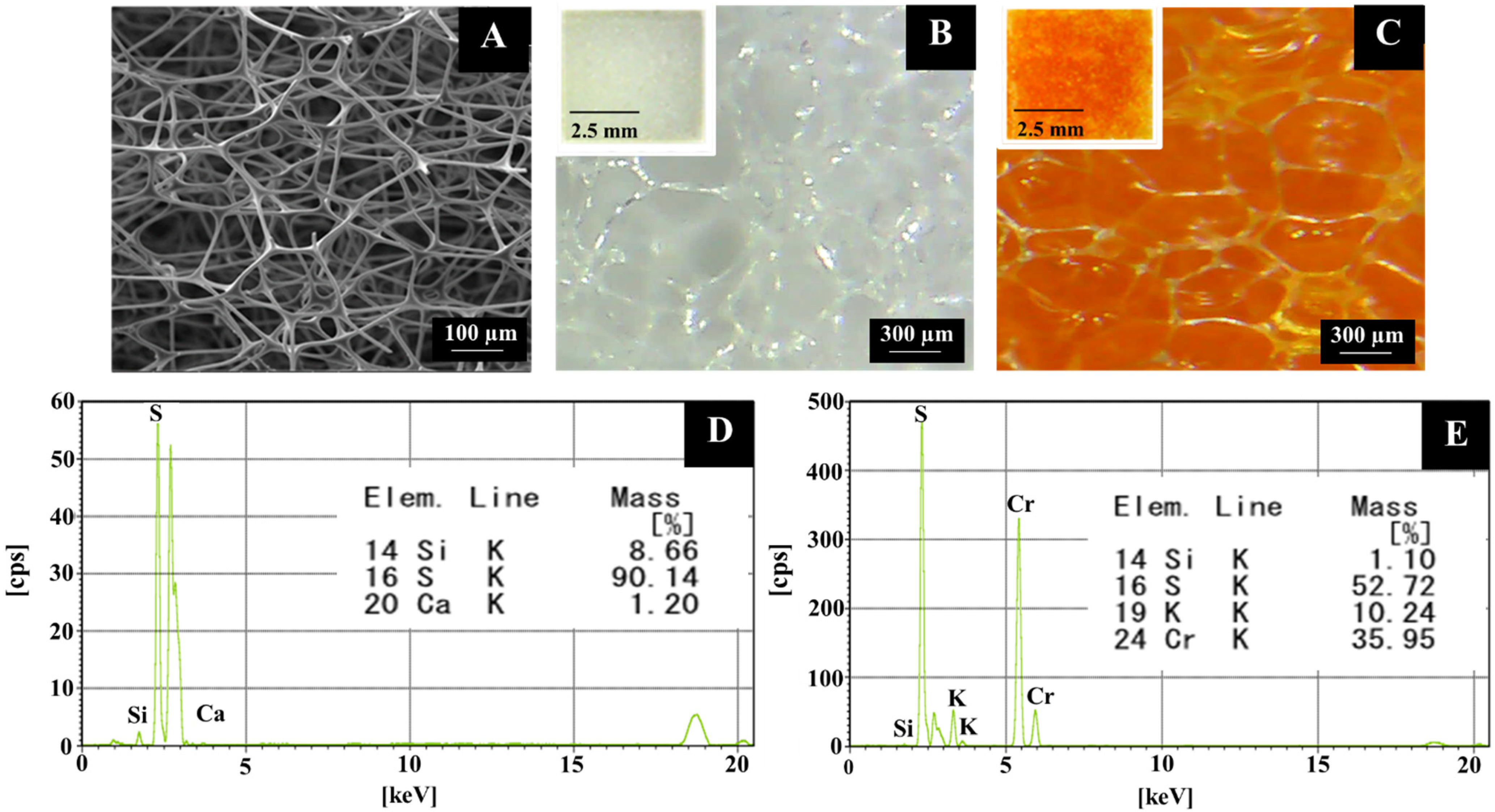
3.1. Design and Characterization of Foam-Based Alcohol Colorimetric Sensor
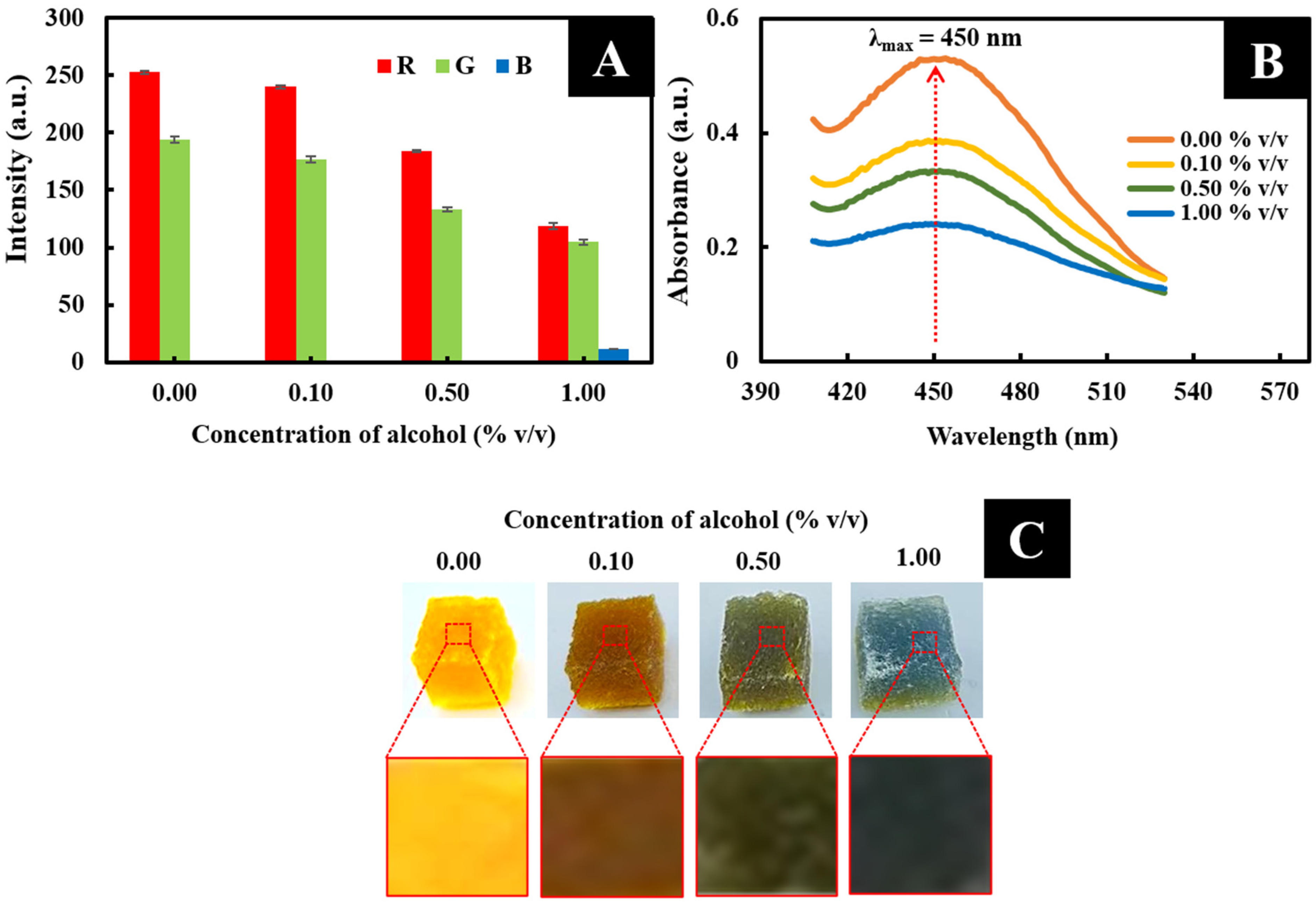
3.2. The Choice of Color Channel (RGB Value) Providing the Analytical Signal
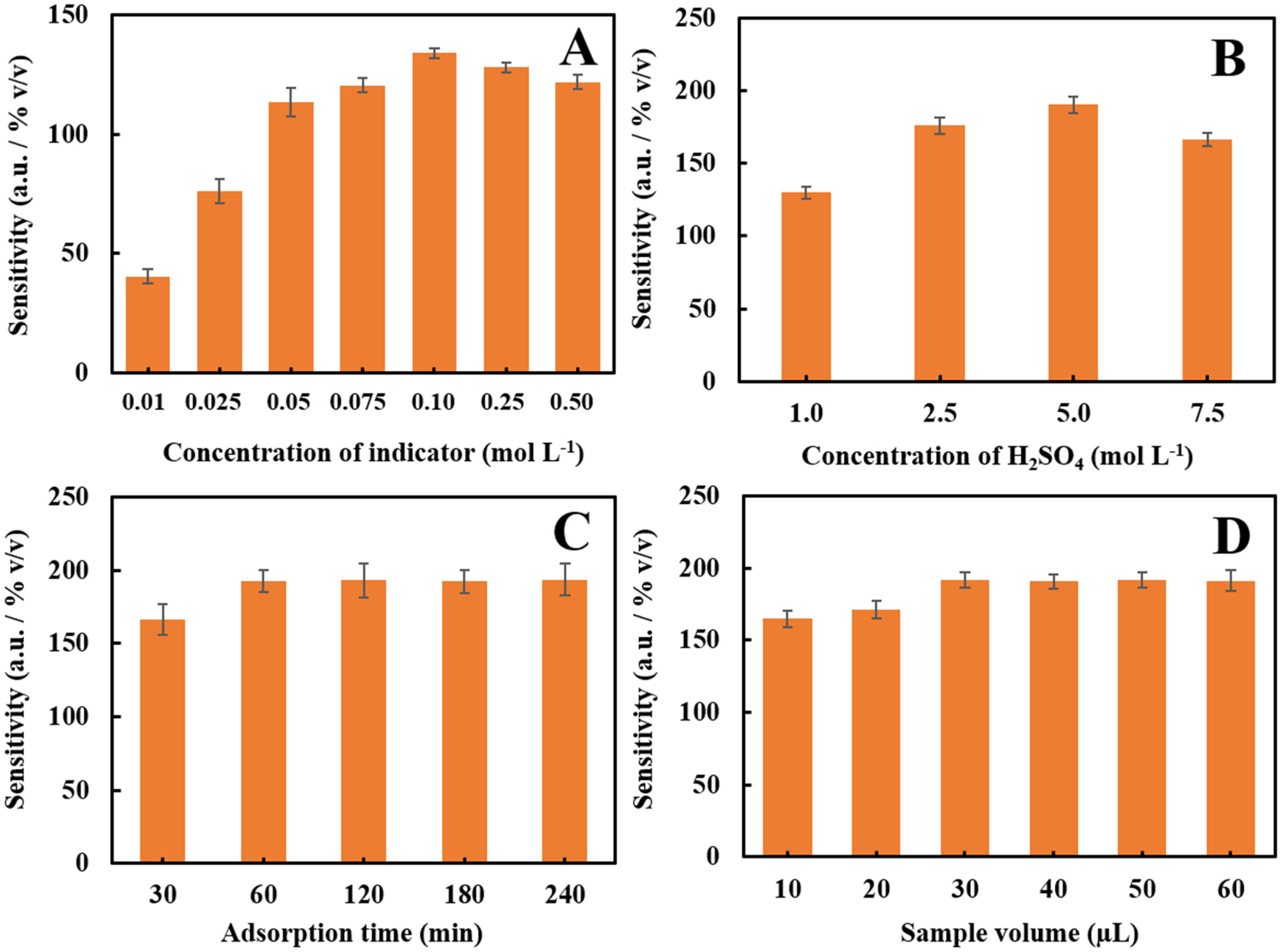
3.3. Optimization
3.3.1. Concentration of the Colorimetric Reagent
3.3.2. Adsorption Time of the Colorimetric Reagent by Foam-Based Sensor
3.3.3. The Loading of Sample Volume
3.4. Analytical Future of the Foam-Based Alcohol Colorimetric Sensor
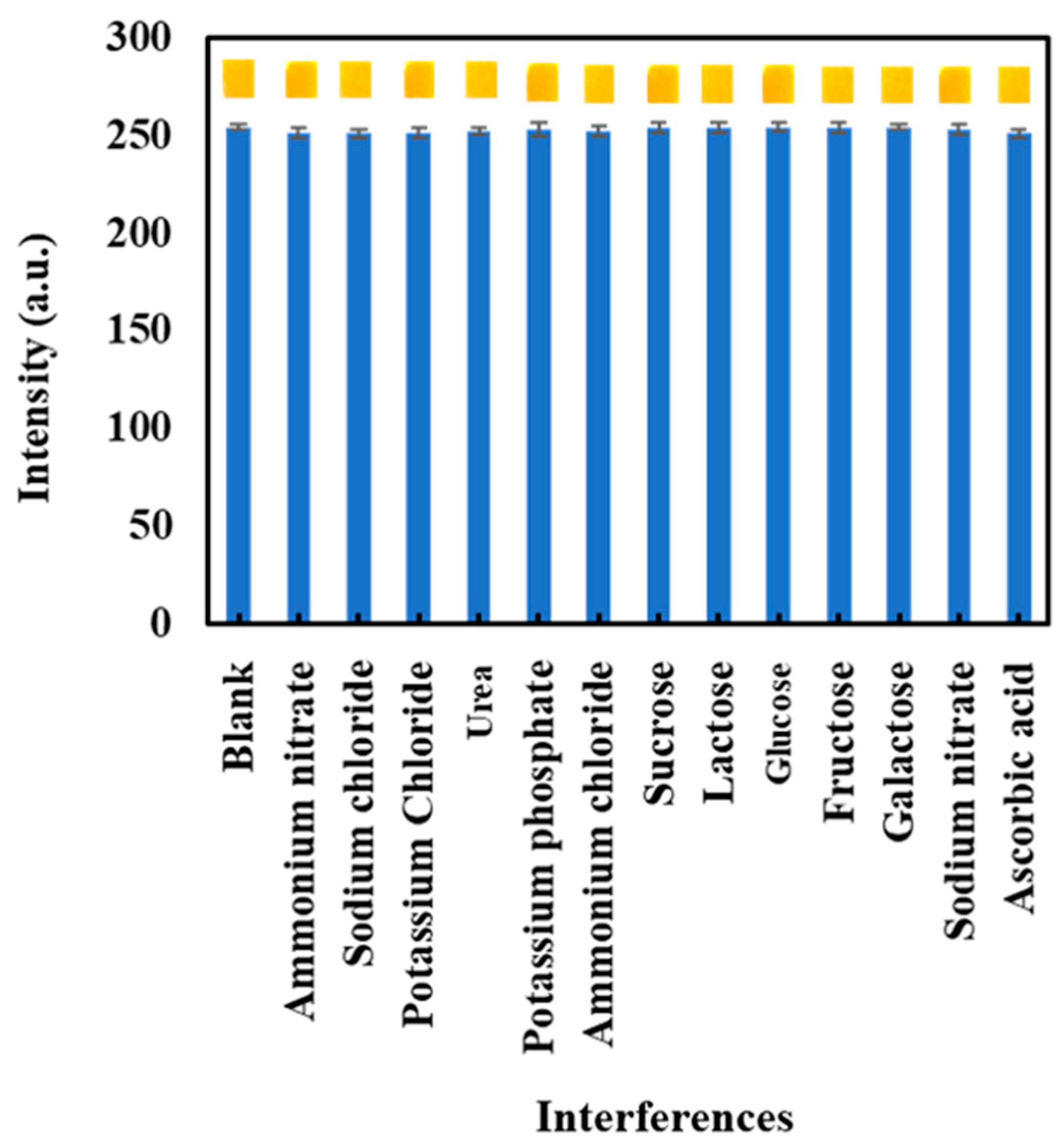
3.5. Interference Study
3.6. Analysis of Alcohol in Saliva Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biancolillo, A.; Bucci, R.; Magrì, A.L.; Magrì, A.D.; Marini, F. Data-fusion for multiplatform characterization of an Italian craft beer aimed at its authentication. Anal. Chim. Acta 2014, 820, 23–31. [Google Scholar] [CrossRef]
- Paasma, R.; Hovda, K.E.; Jacobsen, D. Methanol poisoning and long term sequelae—A six years follow-up after a large methanol outbreak. BMC Clin. Pharmacol. 2009, 9, 5. [Google Scholar] [CrossRef]
- Paine, A.; Dayan, A. Defining a tolerable concentration of methanol in alcoholic drinks. Hum. Exp. Toxicol. 2001, 20, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Thepchuay, Y.; Sonsa-Ard, T.; Ratanawimarnwong, N.; Auparakkitanon, S.; Sitanurak, J.; Nacapricha, D. Based colorimetric biosensor of blood alcohol with in-situ headspace separation of ethanol from whole blood. Anal. Chim. Acta 2020, 1103, 115–121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Road Safety 2018: Summary; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Gubaa, W.; Zuba, D. Saliva as an alternative specimen for alcohol determination in the human body. Pol. J. Pharmacol. 2002, 54, 161–165. [Google Scholar]
- Rao, T.M.; Reddy, D.S.P.; Ramani, P.; Premkumar, P.; Anuja, N.; Sherlin, H.J. Detection of alcohol in saliva for blood alcohol concentration using alcohol saliva strip test: A forensic aid. J. NTR Univ. Health Sci. 2015, 4, 24. [Google Scholar]
- Amal, A.S.S.; Hussain, S.; Jalaluddin, M. Preparation of artificial saliva formulation. In Proceedings of the ICB Pharma II “Current Breakthrough in Pharmacy Materials and Analyse”, Surakarta, Indonesia, 31 October 2015. [Google Scholar]
- Stupak, M.; Kocourek, V.; Kolouchova, I.; Hajslova, J. Rapid approach for the determination of alcoholic strength and overall quality check of various spirit drinks and wines using GC–MS. Food Control 2017, 80, 307–313. [Google Scholar] [CrossRef]
- Wang, M.-L.; Wang, J.-T.; Choong, Y.-M. A rapid and accurate method for determination of methanol in alcoholic beverage by direct injection capillary gas chromatography. J. Food Compos. Anal. 2004, 17, 187–196. [Google Scholar] [CrossRef]
- Choengchan, N.; Mantim, T.; Wilairat, P.; Dasgupta, P.K.; Motomizu, S.; Nacapricha, D. A membraneless gas diffusion unit: Design and its application to determination of ethanol in liquors by spectrophotometric flow injection. Anal. Chim. Acta 2006, 579, 33–37. [Google Scholar] [CrossRef]
- Dorubeţ, D.; Moldoveanu, S.; Mircea, C.; Butnaru, E.; Astărăstoae, V. Development and validation of a quantitative determination method of blood ethanol by gas chromatography with headspace (GC-HS). Rom. J. Leg. Med. 2009, 4, 303–308. [Google Scholar] [CrossRef][Green Version]
- Kristoffersen, L.; Stormyhr, L.-E.; Smith-Kielland, A. Headspace gas chromatographic determination of ethanol: The use of factorial design to study effects of blood storage and headspace conditions on ethanol stability and acetaldehyde formation in whole blood and plasma. Forensic Sci. Int. 2006, 161, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Proença, P.; Tavares, C.; Castañera, A.; Real, F.C. Interference of anesthetics in blood alcohol analysis by HS-GC-FID: A case report. Forensic Sci. Int. 2016, 265, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.; Catharino, R.R.; Eberlin, M.N. Electrospray ionization mass spectrometry fingerprinting of whisky: Immediate proof of origin and authenticity. Analyst 2005, 130, 890–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia, J.S.; Vaz, B.G.; Corilo, Y.E.; Ramires, C.F.; Saraiva, S.A.; Sanvido, G.B.; Schmidt, E.M.; Maia, D.R.; Cosso, R.G.; Zacca, J.J.; et al. Whisky analysis by electrospray ionization-Fourier transform mass spectrometry. Food Res. Int. 2013, 51, 98–106. [Google Scholar] [CrossRef]
- Dias, A.A.; Cardoso, T.M.; Chagas, C.L.; Oliveira, V.X.; Munoz, R.A.; Henry, C.S.; Santana, M.H.; Paixão, T.R.; Coltro, W.K. Detection of analgesics and sedation drugs in whiskey using electrochemical paper-based analytical devices. Electroanalysis 2018, 30, 2250–2257. [Google Scholar] [CrossRef]
- Heller, M.; Vitali, L.; Oliveira, M.A.L.; Costa, A.C.O.; Micke, G.A. A rapid sample screening method for authenticity control of whiskey using capillary electrophoresis with online preconcentration. J. Agric. Food Chem. 2011, 59, 6882–6888. [Google Scholar] [CrossRef]
- Boyaci, I.H.; Genis, H.E.; Guven, B.; Tamer, U.; Alper, N. A novel method for quantification of ethanol and methanol in distilled alcoholic beverages using Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 1171–1176. [Google Scholar] [CrossRef]
- Nogueira, S.A.; Lemes, A.D.; Chagas, A.C.; Vieira, M.L.; Talhavini, M.; Morais, P.A.; Coltro, W.K. Redox titration on foldable paper-based analytical devices for the visual determination of alcohol content in whiskey samples. Talanta 2019, 194, 363–369. [Google Scholar] [CrossRef]
- Chen, J.C.; Naglak, T.J.; Wang, H.Y. An amperometric alcohol sensor based on chemically permeabilized methylotrophic microorganisms. Biotechnol. Prog. 1992, 8, 161–164. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Gómez-Ojea, R.; Tomás-Huercio, O.; Herráez-Hernández, R.; Campíns-Falcó, P. Colorimetric determination of alcohols in spirit drinks using a reversible solid sensor. Food Control 2018, 94, 7–16. [Google Scholar] [CrossRef]
- Wongniramaikul, W.; Limsakul, W.; Choodum, A. A biodegradable colorimetric film for rapid low-cost field determination of formaldehyde contamination by digital image colorimetry. Food Chem. 2018, 249, 154–161. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Simple silver nanoparticle colorimetric sensing for copper by paper-based devices. Talanta 2012, 99, 552–557. [Google Scholar] [CrossRef]
- Choodum, A.; Keson, J.; Kanatharana, P.; Limsakul, W.; Wongniramaikul, W. Selective pre and post blast trinitrotoluene detection with a novel ethylenediamine entrapped thin polymer film and digital image colorimetry. Sens. Actuators B Chem. 2017, 252, 463–469. [Google Scholar] [CrossRef]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Daeid, N.N. Using the iPhone as a device for a rapid quantitative analysis of trinitrotoluene in soil. Talanta 2013, 115, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Zou, X.-B.; Shi, J.-Y.; Li, Z.-H.; Zhao, J.-W. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Apilux, A.; Siangproh, W.; Praphairaksit, N.; Chailapakul, O. Simple and rapid colorimetric detection of Hg (II) by a paper-based device using silver nanoplates. Talanta 2012, 97, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Thipwimonmas, Y.; Jaidam, J.; Samoson, K.; Khunseeraksa, V.; Phonchai, A.; Thiangchanya, A.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes. Chemosensors 2021, 9, 161. [Google Scholar] [CrossRef]
- Fletcher, P.J.; Van Staden, J.F. Determination of ethanol in distilled liquors using sequential injection analysis with spectrophotometric detection. Anal. Chim. Acta 2003, 499, 123–128. [Google Scholar] [CrossRef]
- Vicente, S.; Zagatto, E.A.; Pinto, P.C.; Saraiva, M.L.M.; Lima, J.L.; Borges, E.P. Exploiting gas diffusion for non-invasive sampling in flow analysis: Determination of ethanol in alcoholic beverages. An. Acad. Bras. Ciênc. 2006, 78, 23–29. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; William, H., Ed.; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Phonchai, A.; Rattana, S.; Thongprajukaew, K. A portable sol-gel urea colorimetric method for the determination of urea in feedstuffs. Food Chem. 2020, 319, 126545. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2016; ISBN 978-0-935584-87-5. [Google Scholar]
- Xu, Z.; Miyazaki, K.; Hori, T. Dopamine-Induced Superhydrophobic Melamine Foam for Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1500255. [Google Scholar] [CrossRef]
- Timmer, W.C. An experiment in forensic chemistry: The Breathalyzer. J. Chem. Educ. 1986, 63, 897. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.; Singh, H. Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva. Food Hydrocoll. 2009, 23, 1270–1278. [Google Scholar] [CrossRef]
- Peng, B.; Chen, G.; Li, K.; Zhou, M.; Zhang, J.; Zhao, S. Dispersive liquid-liquid microextraction coupled with digital image colorimetric analysis for detection of total iron in water and food samples. Food Chem. 2017, 230, 667–672. [Google Scholar] [CrossRef]

| Sample | Foam-Based Alcohol Colorimetric Sensor (n = 6) | %Recovery | Spectrophotometric Method (n = 3) | %Recovery | Alcohol Meter (n = 3) | |||
|---|---|---|---|---|---|---|---|---|
| Found | Added | Found | Added | (% v/v) | %BAC | |||
| Saliva 1 | 1.31 ± 0.05 | 0.5 | 97.2 ± 0.01 | 1.33 ± 0.05 | 0.5 | 100.7 ± 0.02 | 1.10 ± 0.02 | 0.15 ± 0.01 |
| 1.0 | 101.4 ± 0.01 | 1.0 | 102.0 ± 0.01 | |||||
| 5.0 | 101.9 ± 0.08 | 5.0 | 98.9 ± 0.08 | |||||
| Saliva 2 | 1.34 ± 0.02 | 0.5 | 101.4 ± 0.01 | 1.30 ± 0.02 | 0.5 | 99.9 ± 0.02 | 1.17 ± 0.03 | 0.16 ± 0.01 |
| 1.0 | 98.7 ± 0.02 | 1.0 | 99.5 ± 0.03 | |||||
| 5.0 | 99.3 ± 0.07 | 5.0 | 100.8 ± 0.06 | |||||
| Saliva 3 | 0.49 ± 0.03 | 0.5 | 98.3 ± 0.01 | 0.51 ± 0.02 | 0.5 | 98.2 ± 0.01 | 1.03 ± 0.03 | 0.14 ± 0.01 |
| 1 | 100.2 ± 0.02 | 1.0 | 98.3 ± 0.02 | |||||
| 5 | 101.2 ± 0.04 | 5.0 | 99.1 ± 0.04 | |||||
| Saliva 4 | 1.04 ± 0.01 | 0.5 | 101.9 ± 0.01 | 1.01 ± 0.01 | 0.5 | 101.6 ± 0.04 | 1.10 ± 0.02 | 0.15 ± 0.0 |
| 1.0 | 99.8 ± 0.02 | 1.0 | 101.1 ± 0.02 | |||||
| 5.0 | 99.8 ± 0.06 | 5.0 | 100.0 ± 0.03 | |||||
| Saliva 5 | 0.35 ± 0.01 | 0.5 | 99.4 ± 0.01 | 0.37 ± 0.01 | 0.5 | 99.2 ± 0.01 | 1.03 ± 0.02 | 0.14 ± 0.01 |
| 1.0 | 101.4 ± 0.01 | 1.0 | 100.5 ± 0.01 | |||||
| 5.0 | 99.6 ± 0.03 | 5.0 | 100.5 ± 0.03 | |||||
| Saliva 6 | 1.23 ± 0.03 | 0.5 | 99.4 ± 0.02 | 1.26 ± 0.03 | 0.5 | 99.2 ± 0.01 | 1.10 ± 0.01 | 0.15 ± 0.01 |
| 1.0 | 101.4 ± 0.01 | 1.0 | 101.2 ± 0.01 | |||||
| 5.0 | 99.9 ± 0.04 | 5.0 | 100.8 ± 0.03 | |||||
| 0.5 | 100.4 ± 0.03 | 0.5 | 99.5 ± 0.01 | |||||
| Saliva 7 * | N.D. | 1.0 | 99.8 ± 0.04 | N.D. | 1.0 | 100.1 ± 0.02 | N.D. | N.D. |
| 5.0 | 99.3 ± 0.04 | 5.0 | 98.4 ± 0.03 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewnu, K.; Promsuwan, K.; Phonchai, A.; Thiangchanya, A.; Somapa, D.; Somapa, N.; Tayayuth, K.; Limbut, W. Cost-Effective Foam-Based Colorimetric Sensor for Roadside Testing of Alcohol in Undiluted Saliva. Chemosensors 2021, 9, 334. https://doi.org/10.3390/chemosensors9120334
Kaewnu K, Promsuwan K, Phonchai A, Thiangchanya A, Somapa D, Somapa N, Tayayuth K, Limbut W. Cost-Effective Foam-Based Colorimetric Sensor for Roadside Testing of Alcohol in Undiluted Saliva. Chemosensors. 2021; 9(12):334. https://doi.org/10.3390/chemosensors9120334
Chicago/Turabian StyleKaewnu, Krittapas, Kiattisak Promsuwan, Apichai Phonchai, Adul Thiangchanya, Dongsayam Somapa, Namchoke Somapa, Kunanunt Tayayuth, and Warakorn Limbut. 2021. "Cost-Effective Foam-Based Colorimetric Sensor for Roadside Testing of Alcohol in Undiluted Saliva" Chemosensors 9, no. 12: 334. https://doi.org/10.3390/chemosensors9120334
APA StyleKaewnu, K., Promsuwan, K., Phonchai, A., Thiangchanya, A., Somapa, D., Somapa, N., Tayayuth, K., & Limbut, W. (2021). Cost-Effective Foam-Based Colorimetric Sensor for Roadside Testing of Alcohol in Undiluted Saliva. Chemosensors, 9(12), 334. https://doi.org/10.3390/chemosensors9120334