Abstract
4(5)-Methylimidazole (4(5)MEI) is a product of the Maillard reaction between sugars and amino acids, which occurs during the thermal processing of foods. This compound is also found in foods with caramel colorants additives. Due to its prevalence in foods and beverages and its potent carcinogenicity, 4(5)MEI has received federal and state regulatory agency attention. The aim of this review is to present the extraction procedures of 4(5)MEI from food matrices and the analytical methods for its determination. Liquid and gas chromatography coupled with mass spectrometry are the techniques most commonly employed to detect 4(5)MEI in food matrices. However, the analysis of 4(5)MEI is challenging due to the high polarity, water solubility, and the absence of chromophores. To overcome this, specialized sample pretreatment and extraction methods have been developed, such as solid-phase extraction and derivatization procedures, increasing the cost and the preparation time of samples. Other analytical methods for the determination of 4(5)MEI, include capillary electrophoresis, paper spray mass spectrometry, micellar electrokinetic chromatography, high-performance cation exchange chromatography, fluorescence-based immunochromatographic assay, and a fluorescent probe.
1. Introduction
Imidazoles, including 4(5)-methylimidazole (4(5)MEI), are important building blocks in organic synthesis [,] and are used in the production process of pharmaceuticals, agrochemicals, and as curing agents in a variety of epoxy resins [,,]. 4(5)-Methylimidazole is a simple molecule with a molecular weight of 82.1 and solubility in water and ethanol []. When it is in a neutral-to-basic pH aqueous solution, 4(5)MEI exists in a tautomeric equilibrium of 4 and 5 MEI (Figure 1) [,].
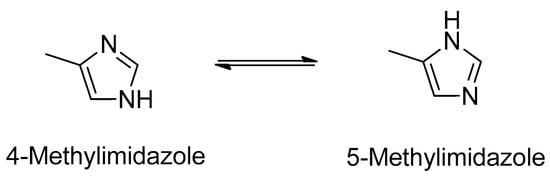
Figure 1.
Tautomeric equilibrium of 4(5)-methylimidazole.
4(5)-Methylimidazole occurs in food matrices as a result of the Maillard reaction that takes place between amino acids and sugars when food is thermally processed [,]. Ammonia caramel colorant additives also constitute a significant source of 4(5)MEI in food products []. In the process of creating ammonia caramel and sulfite ammonia caramel color, the caramelization procedure causes ammonia to interact with reducing sugars, producing 4(5)MEI [,,].
The European Commission Regulation 231/2012 stipulates that the maximum level of 4(5)MEI in ammonia caramel and ammonia sulfate caramel should not exceed 200 and 250 mg/kg, respectively []. The International Agency for Research on Cancer has classified 4(5)MEI as Group 2B, indicating that it is potentially carcinogenic to humans []. Both the National Toxicology Program and the Office of Environmental Health Hazard Assessment recognize the carcinogenicity of 4(5)MEI [,,]. According to the California Environmental Protection Agency, the maximum daily exposure to 4(5)MEI that does not pose a significant level of risk is 29 μg []. These data set the limit of quantification (LOQ) at low levels. Therefore, effective methods are required to accurately monitor 4(5)MEI in food matrices in order to effectively estimate its exposure levels.
A wide range of analytical techniques have been developed for the analysis of 4(5)MEI in foods and beverages. Liquid chromatography (LC) is the main technique used to determine 4(5)MEI [,,,,,,], which is mainly hyphenated with mass spectrometry (MS) in order to obtain lower LOQs [,,,,,,,,,,,,,,,,,,,]. In addition, analytical methods based on gas chromatography-mass spectrometry (GC-MS) have been applied, although a derivatization step with isobutyl chloroformate is required [,,,,,,,,,,,,]. Moreover, 4(5)MEI has been quantified through UV spectrophotometry [], capillary electrophoresis [], paper spray mass spectrometry [], micellar electrokinetic chromatography [], high-performance cation exchange chromatography [], fluorescence-based immunochromatographic assay [], and a fluorescent probe [].
The aim of this review is to provide an overview of the methods that have been employed in the last 15 years for the extraction of 4(5)MEI from food matrices, as well as to present the analytical methods and techniques that have been developed for this purpose. The literature research was performed from March to October 2021, using databases, such as Scopus, Web of Science, and PubMed.
2. Formation of 4(5)-Methylimidazole
Early reports from Radzisewski et al. [] suggested that 4(5)MEI can be synthesized from the reaction of glyoxal and formaldehyde with ammonia, which led to the hypothesis that 4(5)MEI may be formed from the reaction of α-dicarbonyl compounds with ammonia. Several studies indicate that imidazoles can be formed by the Maillard reaction in food matrices. It is known that the Maillard reaction (or nonenzymatic amino-carbonyl browning reaction) takes place between carbonyl and nitrogen-containing compounds []. Amino acids are a potent source of nitrogen-containing compounds in foods via the Strecker degradation []. Sources of carbonyl-containing compounds in foods may be lipids, which upon oxidation produce methylglyoxal, diacetyl, and glyoxal [] or sugars that participate in the non-enzymatic browning reaction, which occur during caramelization [].
Moon and Shibamoto [] studied the Maillard reaction systems of L-rhamnose/NH3, D-glucose/NH3, methylglyoxal/formaldehyde/NH3, and methylglyoxal/NH3. The authors reported that the methylglyoxal/NH3 system provided higher levels of 4(5)MEI compared with the other reaction systems, which indicates that methylglyoxal is a precursor of 4(5)MEI. Moreover, the authors concluded that the formation of 4(5)MEI may occur from the ammonolysis of methylglyoxal.
Further studies in Maillard reaction systems from Jang et al. [] suggested that acidic conditions favor the formation of methylglyoxal and glyoxal. It was found that these compounds may be produced from the degradation of sucrose and glucose. Interestingly, the addition of sodium sulfite, which is utilized in the production of sulfite ammonia caramel color, reduced the levels of 4(5)MEI owing to the formation of a sulfite-aldehyde adduct. A recent research study in Maillard reaction systems from Wu et al. [] confirmed that methylglyoxal is a precursor of 4(5)MEI, and the presence of formaldehyde may increase the concentration of 4(5)MEI.
3. Content of 4(5)-Methylimidazole in Food Matrices
The food industry draws heavily on caramel-colored colorants. Their production is based on the caramelization of sugars and divided into four classes according to their production method [,]. These are:
- Class I: Plain caramel color-E150 a;
- Class II: Sulfite caramel color-E150 b;
- Class III: Ammonia caramel color-E150 c;
- Class IV: Sulfite ammonia caramel color-E150 d.
Among these classes, sulfite ammonia caramel accounts for approx. 70% of the worldwide caramel color production []. 4(5)-Methylimidazole is found in ammonia caramel and sulfite ammonia caramel color due to their preparation method. In particular, E150 c and E150 d are produced by heating sugars with ammonium compounds under specified conditions [,]. Ammonia caramel color is commonly used in various bakery products, soy sauces, soup flavorings, coffee, soups, vinegar, and beers. It represents 60% of the total use of caramel colorants in Europe and 20–25% in the USA. Sulfite ammonia caramel color is used in soft drinks, pet foods, and soups [,,]. Table 1 presents the concentrations of 4(5)MEI in the colorants E150 c and E150 d, as well as in various foods and beverages. Among them, the highest concentration of 4(5)MEI has been measured in sulfite ammonia caramel color. In food and beverages, the highest concentrations of 4(5)MEI were detected in dark beer, soy sauce, and coffee.

Table 1.
Content of 4(5)-methylimidazole in caramel colors and food matrices.
The presence of 4(5)MEI has also been determined at a concentration of 2.7 μg mL−1 in the milk of cows fed with adulterated animal feed. The ammonia-treated feed gives an artificially high nitrogen content that entails a significant amount of proteins, which are a key nutrient in the animal diet [,]. Moreover, the possibility of the formation of 4(5)MEI as a product of the Maillard reaction during the heat treatment of milk has been reported []. Furthermore, 4(5)-methylimidazole can be formed by pyrolysis and it has been detected in cigarette smoke [,].
4. Extraction Methods
Table 2 and Table 3 provide an overview of the extraction methods that have been employed before the analysis of 4(5)MEI with LC and GC, respectively. In addition, the conditions of the chromatographic methods, as well as the LOQs and the method recoveries, are listed with the aim of assisting the researchers in evaluating each method and choosing the appropriate extraction and analysis conditions for the determination of 4(5)MEI. Several studies have reported a simple dilution of samples with water without a subsequent clean-up procedure prior to the LC analysis (Table 2). This practice has been applied in caramel color [,,], beverages [,,,], and vinegar []. Other extraction solvents that have been used are acetonitrile (ACN) [,], methanol (MeOH)/water (50/50 v/v) [], MeOH/formic acid [], ammonium formate in water [], and the mobile phase of the LC method [].
The extract clean-up has been achieved by simple filtration [,,]. Nevertheless, the primary extract clean-up procedure is solid phase extraction (SPE). Cartridges with strong cation exchange sorbents are used, which are functionalized with aromatic sulfonic acid (SCX and MCX) or propyl sulfonic acid (PCX). The SPE method is usually applied after the employment of liquid extraction of 4(5)MEI from food matrices [,,,,,,,,,]. Extracts are acidified before loading onto the SPE cartridges. The cartridges are usually conditioned and equilibrated with MeOH and water with or without acid addition. After the loading of the extract, the cartridges are flushed with acidified water and MeOH. Elution of 4(5)MEI is performed using the ammonia solution in MeOH [,,,,] or ACN/water []. Chen et al. [] used a dispersive micro SPE with PCX sorbent to replace the traditional SPE method, simplifying the clean-up procedure.
The QuEChERS technique has also been employed for the extraction of 4(5)MEI from soft drinks, bakery products [], and soy sauce []. This green extraction method was devised in 2003 and utilizes a solid sorbent that is added directly into the sample solution to absorb the interference components []. Molecularly imprinted polymers (MIPs) have been successfully applied for the extraction of 4(5)MEI. Zhou et al. [] utilized as a template molecule, 4-methylimidazole, in order to prepare a new imprinted polymer for the design and synthesis of alkyl-imidazolium ionic liquid-imprinted polymers. Other research studies have used MIPs for the extraction of 4(5)MEI from beverages [] and soy sauce []. Finally, two studies employed a derivatization reaction with dansyl chloride (Figure 2) prior to the LC analysis, in order to achieve lower detection and quantification limits for the determination of 4(5)MEI in biscuits [] and beverages [].
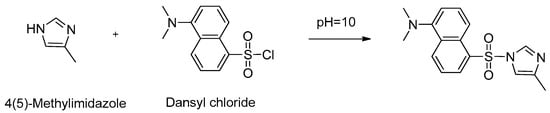
Figure 2.
Derivatization reaction of 4(5)MEI with dansyl chloride.

Table 2.
Liquid chromatography extraction methods and analysis of 4(5)-methylimidazole in food matrices.
Table 2.
Liquid chromatography extraction methods and analysis of 4(5)-methylimidazole in food matrices.
| Matrix | Extraction and Clean-Up | Detector | Column | Mobile Phase | Gradient | Flow Rate (mL min−1) | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Maillard reaction systems, cola soft drink | SCX Disc 15 mg/3 mL | MS/MS | Varian Polaris RP (100 mm × 4.6 mm, 3 μm) | A: ACN/15 mmol ammonia B: water/15 mmol ammonia | 0–3 min, 98% B; 10–15 min, 60% B; 17–20 min, 98% B | 0.4 | - | 102.5 ± 3.61 | [] |
| Caramel color, coffee, dark beer | Water extraction and 1 SPE with MCX 3 cc/60 mg, SCX 500 mg/3 mL, SCX Disc 15 mg/3 mL | 6 DAD: 215 nm 7 MS in 8 SIM mode | MetaChem Polaris C-18A (150 mm × 4.6 mm, 3.5 μm) | A: ACN B: 5 mmol L−1 ammonia in Milli-Q water | 0 min, 98% B; 10 min, 80% B; 15 min, 80% B; 20 min, 98% B | 0.4 | 48.3 ng mL−1 0.4 ng mL−1 | ≥98 | [] |
| Coffee | 2 SFE Water extraction and SPE with SCX Disc 15 mg/3 mL | DAD: 215 nm MS in SIM mode | Atlantis HILIC (150 mm × 2.1 mm, 3 μm) | A: MeOH B: 0.01 mol L−1 ammonium formate | Isocratic 20% B | 0.2 | 48.3 ng mL−1 0.4 ng mL−1 | 98.1 | [] |
| Caramel color | Dilution 10 times in water | DAD: 217 nm | XBridge Shield RP18 (150 mm × 4.6 mm, 3.5 μm) Hypercarb PGC (100 mm × 4.6 mm, 5 μm) | A: MeOH B: 10 mM ammonium formate pH 11 A: ACN B: 10 mM ammonium hydroxide pH 11 | 0–10 min, 100% B 11–20 min, 0 B; 21–30 min, 100% B; 0–20 min, 95% B; 21–27 min, 0% B; 28–30 min, 95% B | 0.7 0.7 | - | - | [] |
| Water, soil | 3 MIP–SPE, 4 NIP–SPE | UV-VIS: 220 nm | - | A: MeOH B: water | Isocratic 20% B | 0.5 | - | 96–102% (MIP-SPE), 34–39% for (NIP-SPE) | [] |
| Cigarette additives | Sonication and SPE | 9 PDA: 215 nm | Acquity UPLC BEH Hilic (100 mm × 2.1 mm, 1.7 μm) | A: ACN B: ammonium formate (5 mmol L−1) | Isocratic 20% B | 0.5 | - | 100.06–101.63 | [] |
| Beverages | SPE with Oasis MCX cartridge 5 DLLME with dansyl chloride derivatization | PDA: 254 nm | Waters Atlantis T3 column (250 mm × 4.6 mm, 5 μm) | A: Water B: ACN | 0–1 min, 40% B; 1–10 min, 60% B; 10–20 min, 80% B; 20–21 min, 40% B; 21–30 min, 40% B | 1.0 | 12.7 ng mL−1 | 91 | [] |
| Cola beverages | Addition of 10 MMIP and shaken for 15 min Separation and wash of MMIP with ACN/formic acid (9:1, v/v) by sonication | PDA: 233 nm | Sino-Chrom ODS-AP column (230 mm × 5 mm, 4.6 μm) | A: MeOH B: KH2PO4 (0.05 mol L−1) | Isocratic 90% B | 1.0 | 0.13 mmol L−1 | 90.19–104.29 | [] |
| Soy sauce, caramel color, Worcestershire sauce, carbonated soft drinks, canned coffee, dark beer | Dilution with water SPE C18 12 cc (2 g) | MS/MS 11 TOFMS | Scherzo SM-C18 (150 mm × 3 mm, 3 μm) | A: 10 mmol/L ammonium formate-water/acetonitrile (90:10) B: 150 mmol/L ammonium formate-water/ACN (30:70) | 0 min, 0% B; 8 min, 20% B; 10 min, 100% B; 21 min, 100% B; 21.1 min, 0% B; 36 min, 0% B | 0.3 | 0.7 ng mL−1 | 95.6–104.5 | [] |
| Cola, tea, beer, coffee beverages, bread, biscuit, instant coffee | QuEChERS extraction | MS/MS | Agilent Polaris C18-A (150 mm × 4.6 mm, 3.5 μm) | A: 5 mM ammonia in water B: ACN | 0 min, 5% B; 10 min, 40% B; 10.1 min, 95% B; 12 min, 95% B | 0.5 | 5–20 μg L−1 20–100 mg kg−1 | 91–113 | [] |
| Sauces, meat | Dillution with water, ultrasonication, centrifuge 8000 r/min, and SPE clean-up with PCX cartridge | MS/MS | Waters XBridge BEH Shield RP18 (150 mm × 3 mm, 3.5 μm) | A: 10 mmol L−1 ammonia in water B: MeOH | 0–10 min, 5% B; 11–15 min, 5% to 100% B; 15–20 min, 100% B | 0.3 | - | - | [] |
| Fermented soy sauce | QuEChERS-isotope dilution | MS/MS | Waters ACQUITY UPLC BEH HILIC (50 mm × 2.1 mm, 1.7 μm) | A: 0.1% formic acid in water B: ACN | 0–0.8 min, 5% B; 0.8–2.1 min, 5–80% B; 2.1–2.6 min, 80–95% B; 2.6–3.6 min, 95% B; 3.6–4.1 min, 95–5% B; 4.1–6.0 min, 5% B | 0.4 | 0.9 μg Kg−1 | 91.2–112.5 | [] |
| Beverages | 1,2-Dichloroethane, ACN and 13 C6-4-MeI were added in diluted samples, centrifuge at 5000× g, SPE clean-up | MS/MS | xBridge Shield RP C18 (250 mm × 4.6 mm, 5 μm) | A: 5 mmol L−1 100% ammonium acetate/0.1% formic acid B: MeOH | 0–5 min, 5% B; 5–6 min, 5%–100% B; for 6–15 min, 100% B; 15–16 min, 100%–5% B; 16–25 min, 5% B | 0.8 | 0.3 μg L−1 | 102.60–113.22 | [] |
| Various food matrices | Ultrasonication, addition of water and 0.1 M HCl, SPE clean-up with Oasis MCX 6 cc/150 mg | MS/MS | CORTECS HILIC (2.1 mm × 100 mm, 1.6 μm), Waters HSS T3 (150 mm × 2.1 mm, 1.8 µm) | A: 10 mM ammonium formate in 95/5 (v/v) ACN/water/0.1% formic acid B: 30 mM ammonium formate in 85/15 (v/v) ACN/water/0.1% formic acid | 0–1 min, 0% B; 1–5 min, 0–100% B; 5–6 min, 100% B; 6–6.1 min, 0% B; 6.1–10 min, 0% B | 0.25 | 5 mg kg−1 | 94–114 | [] |
| Various food matrices | Dilution with water, addition of PCX sorbent for micro SPE | 12 HRMS | Waters BEH HILIC (100 mm × 2.1 mm, 1.7 μm) | A: ACN B: 5 mM ammonium acetate in water | Isocratic 10% B | 0.2 | 1 mg kg−1 | 82.6–115.8 | [] |
| Soft drinks, sauces, vinegars | Dilution 1:5 using 5 mM ammonium formate in water, vortex, and centrifuge at 14.000 rpm | MS/MS | Discovery C18 (250 mm × 4.6 mm, 5 μm) | A: 5 mM ammonium formate in water B: MeOH | 0.1 min, 95% B; 10.0 min, 1% B; 10.1 min, 95% B for 7 min | 0.6 | 4.0 ng mL−1 | 84.2 ± 14.8 | [] |
| Various food matrices | Addition of MeOH/2% formic acid, homogenization at 1500 rpm, and centrifuge at 4000× g, SPE clean-up with Oasis MCX 6 cc/500 mg | MS/MS | Hypercarb Porous Graphitic Carbon (100 mm × 2.1 mm, 3 μm) | A: water/20 mM ammonium formate/0.1% formic acid B: MeOH/20 mM ammonium formate/0.1% formic acid | 0–1 min, 0% B; 1–2.25 min, 80% B; hold 0.75 min, 80% B; 0.1 min, 0% B in 0.1 min, and hold at 0% B for 0 min | 0.5 | 10 mg kg−1 | 102–108 | [] |
| Caramel colors, vinegar, and beverages | Dilution with water and addition of 0.02 mol/L hydrochloric acid | MS/MS | Polaris 3 C18-A (100 mm × 2.1 mm) | A: water/0.05% ammonia B: ACN | 0 min, 5% B; 3.0 min, 5% B; 5.0 min, 40% B; 5.1 min, 5% B; 6.5 min, 5% B | 0.3 | 4.5 µg L−1 | 70–110 | [] |
| Carbonated beverages | Dilution 10 times in water and addition of d6-4-MEI | MS/MS | XDB-C8 (150 mm × 4.6 mm, 5 μm) | A: 0.05% formic acid in water B: 0.05% formic acid in MeOH | 0–0.5 min, 20% B; 10 min, 100% B; 12 min, 100% B; 12–17 min, 20% B | 0.4 | 9.6 ng mL–1 | - | [] |
| Liquorice | Addition of MeOH/water (50/50 v/v), sonication for 15 min, removal of proteins, filtration | MS/MS | Poroshell 120 EC-C18 (50 mm × 4.6 mm, 2.7 μm) | A: 0.1% formic acid/MeOH (99.5/0.5 v/v) B: 0.05% ammonia solution/MeOH (90/10 v/v) | Isocratic 50% B | 0.45 | 0.07 mg Kg−1 | 99.4 | [] |
| Carbonated beverages | Dilution with water | MS/MS | KINETEX PFP (50 mm × 4.6 mm, 2.6 μm) | A: 0.1% formic acid in deionized water B: 0.1% formic acid in MeOH | 0–0.2 min, 5% B; 0.2–1.3 min, 80% B; 1.3–1.8 min, 80% B; 1.8–4 min, 5% B | 0.6 | 2 ng mL–1 | 94.7 ± 1.5 | [] |
| Beverages, sauces | 500 µL of sample + 20 µL of 0.1 M of HCl until 3 mL final volume with water SPE Strata™X-C 100 mg | MS/MS | Thermo Hypercarb (100 mm × 2.1 mm, 5 μm) | A: water B: MeOH | 0 min, 5% B; 11 min, 95% B; 13 min, 95% B; 15 min, 5% B | 0.3 | 5 ng mL–1 | 75.4–92.5 | [] |
| Caramel colors, cola | Dilution 1:20 with a mixture of mobile phase A:B (7:3, V:V), filtration with a membrane filter 0.2 μm | MS/MS | Gemini (Phenomenex) RP 18 (200 mm × 2 mm, 3 μm) | A: 5 mM NH4HCO3 in high-purity water (pH 9) B: 5 mM NH4HCO3 in MeOH (pH 9) | 0–8 min, 30% B; 8–13 min, 80% B; 13–17 min, 80% B; 17–20 min, 30% B; 20–35 min, 30% B | 0.2 | 80 μg L−1 | 98.4 | [] |
| Caramel color, sauces, curry, mixed drinks, vinegar, seasonings | Dilution with ACN, centrifuge at 5000 rpm, filtration with 0.2 μm syringe filter | MS/MS | Luna C18 (100 mm × 2 mm, 3 μm) | A: 5 mM NH4HCO3 (pH 9) B: 5 mM NH4HCO3 50 mL + 950 mL MeOH (pH 9) | Isocratic 30% B | 0.2 | 5 μg Kg−1 | 81.9 ± 0.9–111.0 ± 0.2 | [] |
| Soft drinks | Dilution 10 times in water and filtration with 13 PTFE, 0.22 μm | MS/MS | Eclipse Plus C8 (150 mm × 4.6 mm, 5 μm) | A: 0.05% formic acid in water/ammonium hydroxide (25%) pH 4 B: ACN | B = 0% at first, increased to B = 100% by 7 min | 0.4 | - | - | [] |
| Beverages | Dilution with water | MS/MS | Acclaim C30 reversed-phase (150 mm × 2.1 mm, 3 μm) | A: MeOH/5% ammonium hydroxide (0.7% in DI water) B: water | Isocratic 85% B | 0.3 | 1 ng mL−1 | 96.0–99.5 | [] |
| Caramel model systems | Dilution with water | MS/MS | Varian Polaris RP (100 mm × 4.6 mm, 3 μm) | A: water (15 mmol ammonium hydroxide) B: ACN (15 mmol ammonium hydroxide) | 0–3 min, 2% B; 10–13 min, 40% B; 15–25 min, 2% B; | 0.4 | - | 101.2 ± 1.8 | [] |
| Biscuits | Addition of MeOH, centrifuge, evaporation Addition of n-hexane, centrifuge, and filtration with a 0.2 μm PES membrane | MS/MS | Phenomenex kinetex C18 (100 mm × 3.0 mm, 2.6 μm) | A: 0.1% formic acid in water B: MeOH | 1 min, 5% B; 2 min, 10% B; 3.5 min, 80% B; 5 min, 80% B; 5.5 min, 5% B; 7 min, 5% B | 0.3 | 5 ng g−1 | 93–108 | [] |
| Soy sauce | MMIP | PDA: 233 nm | Sino-Chrom ODS-AP column (230 mm × 5 mm, 4.6 μm) | A: MeOH B: KH2PO4 (0.05 mol L−1) | Isocratic 88% B | 1.0 | 5.64 μg L−1 | 97.33–104.57 | [] |
| Biscuits | SPE with Oasis MCX cartridge DLLME with dansyl chloride derivatization | 14 QqQ-MS Ion trap-MS | Agilent Polaris C18-A (150 mm × 4.6 mm, 3.5 μm) | A: ACN B: 5 mM ammonium hydroxide in water A: water B:ACN | 0 min, 95% B; 10 min, 60% B; 10.1 min, 95% B; 12 min, 59% B; Isocratic 90% B; 15 min | 0.5 0.5 | 0.5 ng mL−1 0.2 ng mL−1 | 103–108 87–102 | [] |
1 SPE: Solid phase extraction; 2 SFE: Supercritical fluid extraction; 3 MIP-SPE: Molecularly imprinted polymer-solid phase extraction; 4 NIP-SPE: Non-imprinted polymer-solid phase extraction; 5 DLLME: Dispersive liquid–liquid microextraction; 6 DAD: Diode array; 7 MS: Mass spectrometry; 8 SIM: Single ion monitoring; 9 PDA: Photodiode array; 10 MMIP: Magnetic molecularly imprinted polymer; 11 TOFMS: Time of flight mass spectrometry; 12 HRMS: High resolution mass spectrometry; 13 PTFE: Polytetrafluoroethylene; 14 QqQ-MS: Triple quadrupole-mass spectrometry.

Table 3.
Gas chromatography methods extraction and analysis of 4(5)-methylimidazole in food matrices.
Table 3.
Gas chromatography methods extraction and analysis of 4(5)-methylimidazole in food matrices.
| Matrix | Extraction and Clean-Up | Detector | Column | Conditions | Oven Temperature Program | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Caramel colors | Addition of 2-methylimidazole, 3 N sodium hydroxide, and Celite 545. Extraction with hot dichloromethane, evaporation, and addition of acetic anhydride | MS | HP5MS (30 m × 0.25 mm, 0.25 μm) | Split ratio of 30:1 at 240 °C | 75 °C for 10 min, then 320 °C | - | - | [] |
| Soft drinks | 1 SPME fibers (100 μm 2 PDMS, 65 μm 3 PDMS-DVB, 85 μm PA, 85 μm 4 CAR-PDMS) | MS | HP-InnoWax (60 m × 0.25 mm, 0.25 μm) | Splitless mode at 270 °C | 70 °C for 1 min, ramped to 250 °C at 15 °C min−1 | 6.0 μg L−1 | - | [] |
| Ammonia caramel color | Ion-pair extraction with 5 BEHPA, derivatization with isobutyl chloroformate | MS | DB-5 MS (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 250 °C | 70 °C for 1 min, then 20 °C min−1 to 280 °C | 1 mg Kg−1 | 95–102 | [] |
| Coffee | Ion-pair extraction with BEHPA, derivatization with isobutyl or ethyl chloroformate | MS 6 FID | DB-5MS and DB-1701 (30 m × 0.25 mm, 0.25 μm) SP-Sil-8CB (25 m × 0.25 mm, 0.12 μm) | Splitless mode at 250 °C | 70 °C for 1 min, then two-step gradient to 280 °C after 16 min (10-min hold) | 0.04 mg kg−1 | 86.5–102.8 | [] |
| Soft drinks and dark beer | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (50:30:20) | MS | DB-5MS (15 m × 0.25 mm, 0.25 μm) | Splitless mode at 270 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 1.83 min. Total run time 9.5 min | 2.2 μg L−1 | 90–101 | [] |
| Caramel model systems | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (50:30:20), and isobutyl chloroformate | MS | DB-5 (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 270 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 1.83 min | - | 84–111 | [] |
| Yellow rice wine and soy sauce | Sample mixed with d5-ethyl carbamate, homogenized and transferred to an alkaline diatomite SPE column, eluted with n-hexane and ethyl acetate | MS | DB-Innowax (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 200 °C | 50 °C for 1 min, increased to 180 °C at 8 °C min, increased to 230 °C at 15 °C min−1, held for 2 min, increased to 240 °C, held for 5 min | 15 μg Kg−1 | 83.3–92.8 | [] |
| Brown colored foods and beverages | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (50:30:20), and isobutyl chloroformate | MS | DB-5MS (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 270 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 1.83 min | 36.87 ng g−1 | 87–117 | [] |
| Red ginseng products | 7 DLLME with in situ derivatization | MS | DB-Wax (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 200 °C | Ramped from 100 to 190 °C at 10 °C min−1, ramped from 190 to 220 °C at 15 °C min−1, maintained for 25 min at 220 °C | 5.79 μg L−1 | 89.86–109.09 | [] |
| Biscuits | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (50:30:20), and isobutyl chloroformate | MS | HP-5MS (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 275 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 2 min | 53 ng mL−1 | - | [] |
| Coffee | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (50:30:20), and isobutyl chloroformate | MS | HP-5MS (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 260 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 2 min | 49.02 ng mL−1 | 103.86–111.08 | [] |
| Balsamic vinegars, sauces | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (50:30:20), and isobutyl chloroformate | MS | DB-5 (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 270 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 1.83 min. Total run time 9.5 min | - | 78–96 | [] |
| Coffee, coffee substitutes | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine, and isobutyl chloroformate | MS | DB-5 MS (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 270 °C | 80 °C for 1 min, ramped to 280 °C at 30 °C min−1, held for 1.83 min. Total run time 9.5 min | 10 μg Kg−1 | 97.3–98.2 | [] |
| Cola, dark beer | Direct derivatization with ACN-isobutanol-pyridine (50:30:20) and isobutyl chloroformate | MS/MS | DB-17 (30 m × 0.25 mm, 0.25 μm) | Splitless mode at 250 °C | 80 °C for 1 min, increase to 250 °C at 20 °C min−1, held for 2 min | 5.5 μg L−1 | 91–107 | [] |
| Ammonia caramel | Ion-pair extraction with BEHPA, derivatization with ACN-isobutanol-pyridine (5:3:2), and isobutyl chloroformate | MS/MS | RTX-5MS (10 m × 0.18 mm, 0.1 μm) | Splitless mode at 280 °C | 70 °C for 1 min, with temperature rise at a rate of 20 °C min−1, to 280 °C | 37.8 μg Kg−1 | 101 | [] |
1 SPME: Solid phase microextraction; 2 PDMS: Polydimethylsiloxane; 3 PDMS-DVB: Polydimethylsiloxane-divinylbenzene; PA: 4 CAR-PDMS: Carboxen-polydimethylsiloxane; 5 BEHPA: Βis-2-ethylhexylphosphate; 6 FID: Flame ionization detector; 7 DLLME: Dispersive liquid–liquid microextraction.
Good to excellent recovery rates have been obtained from the previously described extraction methods. The best recoveries have been reported from Klejdus et al. [] and Lojkova et al. [] (>98%) who both used the water extraction and SPE clean-up, Lee et al. [] (101.2 ± 1.8%) who applied a simple water dilution, Raters et al. [] (99.4%) with MeOH/water extraction and subsequent filtration, Mottier et al. [] (102–108%) with MeOH extraction and subsequent SPE, and Wu et al. [] (103–108%) with dansyl chloride derivatization.
In the case of the employment of gas chromatography (GC) for the analysis of 4(5)MEI, the most common extraction technique is the use of ion-pair extraction with bis-2-ethylhexylphosphate (BEHPA) and subsequent derivatization with isobutyl chloroformate (Figure 3). In the ion-pair extraction procedure with BEHPA, the sample is extracted using a solvent, such as MeOH, which is evaporated to dryness and dissolved in phosphate buffer pH 6.0. A portion of the solution is mixed with 0.1 M BEHPA in chloroform. After centrifugation, a portion of the chloroform phase is mixed with HCl 0.1 M. ACN-isobutanol-pyridine (50:30:20, v/v/v) is then mixed with a portion of the aqueous hydrochloric phase and subsequently, isobutyl chloroformate, 1.0 M NaHCO3−/CO3−2, and chloroform are added. After vortexing, the 4(5)MEI is extracted in the organic layer []. Good to excellent recoveries have been reported with the previously described extraction and derivatization methods from Wieczorek et al. [] (101%) and Hyong et al. [] (103.86–111.08%).

Figure 3.
Derivatization reaction of 4(5)MEI with isobutyl chloroformate.
Solid phase microextraction (SPME) in combination with the GC-MS analysis have also been used by Lim and Shin [] for the extraction of 4(5)MEI from beverages. The researchers tested four fibers, polydimethylsiloxane (PDMS), polydimethylsiloxane-divinylbenzene (PDMS-DVB), polyamide (PA), carboxen-polydimethylsiloxane (CAR-PDMS). As a result, PDMS-DVB was evaluated as the most suitable.
5. Analytical Methods
5.1. Liquid Chromatography
The analysis of 4(5)MEI is most often conducted using LC-based methods. To detect 4(5)MEI, a number of researchers have used high-performance liquid chromatography (HPLC) with the UV absorbance set at 215 [,,], 217 [], 220 [], 233 [,], and 254 nm []. However, 4(5)MEI does not have a strong chromophore group, and impurities are absorbed at 210 nm. Therefore, most of the LC methods use mass spectrometry (MS) for detection (Table 2). Reversed-phase [,,,,,,,,,,,,,,] and hydrophilic interaction chromatography (HILIC) columns [,,,,] have been commonly used. The high polarity and solubility of 4(5)MEI in water has a negative impact on the separation from other co-extracted substances. This can be overcome by the use of an alkaline mobile phase with the addition of ammonium hydroxide, ammonium formate or ammonium acetate. Several researchers have also employed the Hypercarb column, with a porous graphitic carbon stationary phase, which is specifically applied for the retention of very polar analytes [,,].
The lowest LOQ from the employment of HPLC with UV detection was determined at 5.64 μg L−1 []. However, lower LOQs have been acquired with MS detection. The lowest LOQ has been observed in a study by Wu et al. [] with the use of an ion trap-LC-MS, which was 0.2 ng mL−1. Similar LOQs have been obtained by the use of LC-MS/MS (0.3 μg L−1) [], LC-MS in SIM mode (0.4 ng mL−1) [,], and LC-QqQ-MS (0.5 ng mL−1) [].
5.2. Gas Chromatography
The most frequently employed detector in GC is MS (Table 3), with an exception of Casal et al. [] who reported the use of FID detector for the analysis of 4(5)MEI in coffee. However, the authors reported that the use of FID proved unsuitable and used the MS detector instead. The GC injector is usually operated in splitless mode at temperatures ranging from 200 to 280 °C. The capillary columns used for the chromatographic separation of 4(5)MEI are non-polar with a stationary phase of diphenyl dimethyl polysiloxane. The lowest LOQs determined with the GC-MS technique were from Cunha et al. [] (2.2 μg L−1), Choi and Jung [] (5.5 μg L−1), and Lee et al. [] (5.79 μg L−1).
5.3. Spectroscopic Techniques
The first attempt for the spectroscopic determination of 4(5)MEI was made by Gutiérrez et al. [] using fluorescence spectroscopy. The method was based on the oxidation of 1,1,3-tricyano-2-amino-1-propene, which is favored by 4(5)MEI, in the presence of hydrogen peroxide and copper. The fluorescent reaction system presented λex = 345 and λem = 415 nm and the detection levels of 4(5)MEI were at 10−5 M.
A UV-Vis spectroscopic determination of 4(5)MEI in soy sauce has been reported by Li et al. []. The extraction of 4(5)MEI from soy sauce was conducted using dichloromethane, followed by a diazotization reaction to form a red color compound, which absorbs at 440 nm. The recoveries obtained from this method were 97.25–99.44%.
Another UV-Vis spectroscopic method, with a detection wavelength at 315 nm, has been developed by Altunay and Gürkan []. The researchers reported the use of ionic liquids (C6mim PF6 and Cyphos IL 101) for the application of vortex assisted-ionic liquid-based dispersive liquid-liquid microextraction (VA-IL-DLLM) of 4(5)MEI from a variety of food matrices. The 4(5)MEI is extracted from aqueous extracts to the ionic liquid phase after the formation of ion-pair anionic chelate complex AgIm(ImH)(H2N-C(CH2OH)2(CH2O)-, at pH 7.0. The validation study performed on honey and tea quality control samples provided low LOQs (4.24 and 4.73 μg L−1) and the recovery ranged from 91.5–96.3%.
5.4. Other Techniques
Paper spray mass spectrometry has been used for the analysis of 4(5)MEI in caramel and beverage samples []. The solution of the sample was applied to a cellulose chromatography paper followed by the application of a high voltage (+2.5–3.5 kV). The researchers evaluated three different types of mass spectrometers for different purposes (linear ion trap, triple quadrupole, and orbitrap). The triple quadrupole mass spectrometer provided a detection limit of 5 pg μL−1, while LOQ was 20 pg μL−1. The analytical results from the linear ion trap MS were similar to those obtained from the triple quadrupole, while the orbitrap mass spectrometer provided a detection limit of 100 ng mL−1.
To determine the 4(5)MEI in caramel color, Petruci et al. [] developed a capillary electrophoresis method. An uncoated fused-silica capillary of 75 μm i.d. was utilized with a length of 40 cm. The running electrolyte consisted of 160 mmol L−1 phosphate plus 30% acetonitrile at pH 2.5, which was adjusted with triethylamine. The method provided very good recoveries (89.1 ± 2.0–106.9 ± 1.8%), and a LOQ at 0.54 mg L−1.
Xu et al. [] used high-performance cation exchange chromatography with pulsed integrated amperometric electrochemical detection (HPCEC-PAD) for the analysis of 4(5)MEI in caramel color and beverages. Samples were SPE pretreated and filtered through a clarification kit of 0.45-μm pore size. Separation was achieved on a CS12A cation exchange column (250 × 4 mm) and a CS12A guard column (50 × 4 mm) utilizing the isocratic elution of 10 mmol/L methane sulfonic acid for 50 min. The recoveries obtained were 93.7–98.3%, while the LOQ was lower compared to the capillary electrophoresis method (0.045 mg L−1).
An amino trap column coupled with pulsed amperometric detection (AMTC-PAD) has been employed by Xu et al. [] for the determination of 4(5)MEI in beverages. Samples were pretreated with SPE using SCX Disc 15 mg/3 mL cartridges and filtered through a 0.45-μm pore size clarification kit. An amino trap column (4 × 50 mm) was used with an isocratic elution of 100 mmol L−1 NaOH for 60 min. The LOQ obtained was comparable with the previously described HPCEC-PAD method (0.045 mg L−1) and the recovery ranged from 95.1–103.4%.
An indirect competitive enzyme-linked immunoassay (ic-ELISA) was developed by Wu et al. [] based on the monoclonal antibody for the detection of 4(5)MEI in caramels. Ovalbumin or bovine serum albumin and the 4(5)MEI hapten were used for the preparation of artificial antigens. The cross-reactivity of 4(5)MEI structural analogues was less than 5.62%. The linear detection range was 0.64–20.48 mg L−1 and the recoveries were between 88.69% and 114.09%.
Shih et al. [] developed a novel method employing cation-selective exhaustive injection and sweeping micellar electrokinetic chromatography (CSEI-sweeping-MEKC). The samples analyzed were soft drinks and alcoholic beverages, which were diluted with 0.05 mM H3PO4 and injected for direct analysis in the online CSEI-sweeping-MEKC. The researchers also prepared an SPME adsorbent by incorporation of activated carbon into a monolithic column with poly(butyl methacrylate-co-ethylene dimethacrylate). This adsorbent was used to extract 4(5)MEI from the samples with subsequent analysis by CSEI-sweeping-MEKC. The LOQs were 20 and 125.9 μg L−1 for the CSEI-MEKC method and the SPME CSEI-MEKC method, respectively, and the recoveries ranged from 94.0 to 94.8%.
A fluorescence-based immunochromatographic assay has been developed by Wu et al. [] for the quantitative detection of 4(5)MEI in caramel color. The authors used as a probe conjugates of fluorescent microspheres and the 4(5)MEI monoclonal antibody. The method recovery ranged from 82.85 to 102.31% and the LOQ was 0.6 mg L−1.
A THz technique was utilized by Shin et al. [] for the detection of 4(5)MEI. The researchers fabricated THz metamaterial with a metal array, using an electric-field-coupled inductor-capacitor resonator structure, and a finite-difference time-domain simulation. The THz spectrum of 4(5)MEI was measured in the frequency range of 0.2–2 THz, utilizing the THz transmission mode spectroscopy at concentrations of 0–20 mg L−1. This technique was able to detect the presence of 4(5)MEI at a few mg L−1. Nevertheless, the researchers concluded that the sensitivity of the metamaterial must be improved by the enhancement of the interactions among the over-layered materials or using a higher sharp resonant peak.
Manshaei et al. [] developed a fluorescent probe based on carbon dots, which were prepared by ultrasound in a metallic deep eutectic solvent. This method was implemented for the detection of 4(5)MEI in soft drinks. The synthesis of carbon dots-chelated metals was performed in situ in the presence of 4(5)MEI, which enables a kinetically fluorescence emission, thereby avoiding the multi-step analysis. The LOQ of the method was 0.6 ng mL−1 and the recoveries were in the range of 89–108%. This fluorescent probe along with the previously described method of triple quadrupole paper spray mass spectrometry provided the most promising results compared to the other methods. Therefore, it can be considered more effective for the analysis of 4(5)MEI.
6. Conclusions
The presence of 4(5)MEI in food matrices is ascribed to the use of ammonia caramel colorants in foods and beverages, and to its production through the Maillard reaction where methylglyoxal, a Maillard reaction by-product, is considered an important precursor of 4(5)MEI. Dark beer, soy sauce, and coffee are the food matrices with the highest concentration of 4(5)MEI, while very high amounts of 4(5)MEI have been detected in sulfite ammonia caramel color. In order to address this issue, the reaction conditions during the preparation of the caramel color could be adjusted and the levels of 4(5)MEI should be continuously monitored with an appropriate method of detection. Τhe absence of chromophores and the high polarity and water solubility of 4(5)MEI pose limitations to the applicability of liquid and gas chromatography analysis. These chromatographic techniques are usually combined with MS detection in order to achieve lower LOQs. Although a variety of alternative analytical methods have been developed to address these issues, novel determination methods of 4(5)MEI with simplified procedures need to be developed to facilitate the in situ monitoring of this compound during ammonia caramel color preparation and food production, which will assist the audit authorities with more consistent investigations.
Author Contributions
Conceptualization, P.-K.R. and E.A.; writing—original draft preparation P.-K.R.; writing—review and editing, P.-K.R., M.X., E.A., C.S.P., and P.A.T.; critical revising, M.X., E.A., C.S.P., and P.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is co-funded 50% by the Hellenic Ministry of Rural Development and Food and 50% by the EU, under the Commission Delegated Regulation (EU) 2015/1366 of 11 May 2015 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council with regard to aid in the apiculture sector.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 4(5)MEI | 4(5)-Methylimidazole |
| LC | Liquid Chromatography |
| GC | Gas Chromatography |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| HRMS | High Resolution Mass Spectrometry |
| FID | Flame Ionization Detector |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| SPE | Solid Phase Extraction |
| SFE | Supercritical Fluid Extraction |
| HPLC | High Performance Liquid Chromatography |
| HILIC | Hydrophilic Interaction Chromatography |
| MS | Mass Spectrometry |
| QqQ-MS | Triple Quadrupole-Mass Spectrometry |
| LOQ | Limit of Quantification |
| DAD | Diode Array |
| SIM | Single Ion Monitoring |
| TOFMS | Time of Flight Mass Spectrometry |
| PTFE | Polytetrafluoroethylene |
| SPME | Solid Phase Microextraction |
| PDA | Photodiode-Array Detection |
| MIP-SPE | Molecularly Imprinted Polymer-Solid Phase Extraction |
| NIP-SPE | Non-Imprinted Polymer- Solid Phase Extraction |
| MMIP | Magnetic Molecularly Imprinted Polymer |
| DLLME | Dispersive Liquid–Liquid Microextraction |
| ic-ELISA | Indirect Competitive Enzyme-Linked Immunoassay |
| HPCEC-PAD | High-Performance Cation Exchange Chromatography-PulsedIntegrated Amperometric Electrochemical Detection |
| AMTC-PAD | Amino Trap Column- Pulsed Amperometric Detection |
| BEHPA | Bis-2-ethylhexylphosphate |
| PDMS | Polydimethylsiloxane |
| PDMS-DVB | Polydimethylsiloxane-Divinylbenzene |
| CAR-PDMS | Carboxen-Polydimethylsiloxane |
| VA-IL-DLLM | Vortex Assisted-Ionic Liquid-Based Dispersive Liquid-Liquid Microextraction |
| CSEI-sweeping-MEKC | Cation-Selective Exhaustive Injection and Sweeping Micellar Electrokinetic Chromatography |
References
- Grimmett, M.R. 4.08-Imidazoles and Their Benzo Derivatives: (iii) Synthesis and Applications. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: New York, NY, USA, 1984; pp. 457–498. ISBN 978-0-08-096519-2. [Google Scholar]
- Xi, N.; Huang, Q.; Liu, L. Imidazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 143–364. ISBN 978-0-08-044992-0. [Google Scholar]
- Lei, D.; Ma, W.; Wang, L.; Zhang, D. Preparation of 2-Ethyl-4-Methylimidazole Derivatives as Latent Curing Agents and Their Application in Curing Epoxy Resin. J. Appl. Polym. Sci. 2015, 132, 42563. [Google Scholar] [CrossRef]
- Mehri, F.; Salimi, A.; Jamali, Z.; Kahrizi, F.; Faizi, M. Exposure to 4-Methylimidazole as a Food Pollutant Induces Neurobehavioral Toxicity in Mother and Developmental Impairments in the Offspring. Toxin Rev. 2020, 1–6. [Google Scholar] [CrossRef]
- Jonsson, T.; Emteborg, M.; Irgum, K. Heterocyclic Compounds as Catalysts in the Peroxyoxalate Chemiluminescence Reaction of Bis(2,4,6-Trichlorophenyl)Oxalate. Anal. Chim. Acta 1998, 361, 205–215. [Google Scholar] [CrossRef]
- Humans, I.W.G. 4-METHYLIMIDAZOLE. Available online: https://www.ncbi.nlm.nih.gov/books/NBK373183/ (accessed on 17 November 2021).
- Campos, R.B.; Menezes, L.R.A.; Barison, A.; Tantillo, D.J.; Orth, E.S. The Importance of Methyl Positioning and Tautomeric Equilibria for Imidazole Nucleophilicity. Chem. A Eur. J. 2016, 22, 15521–15528. [Google Scholar] [CrossRef]
- Li, G.-S.; Ruiz-López, M.F.; Maigret, B. Ab Initio Study of 4(5)-Methylimidazole in Aqueous Solution. J. Phys. Chem. A 1997, 101, 7885–7892. [Google Scholar] [CrossRef]
- Hengel, M.; Shibamoto, T. Carcinogenic 4(5)-Methylimidazole Found in Beverages, Sauces, and Caramel Colors: Chemical Properties, Analysis, and Biological Activities. J. Agric. Food Chem. 2013, 61, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-K.; Shibamoto, T. Formation of Carcinogenic 4(5)-Methylimidazole in Maillard Reaction Systems. J. Agric. Food Chem. 2011, 59, 615–618. [Google Scholar] [CrossRef]
- Vollmuth, T.A. Caramel Color Safety—An Update. Food Chem. Toxicol. 2018, 111, 578–596. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 231/2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32012R0231 (accessed on 17 November 2021).
- Chan, PC. NTP technical report on the toxicity studies of 2- and 4-Methylimidazole (CAS No. 693-98-1 and 822-36-6) administered in feed to F344/N rats and B6C3F1 mice. Toxic Rep. Ser. 2004, 67, 1–12. [Google Scholar]
- Program, NT. Toxicology and Carcinogenesis Studies of 4-Methylimidazole (Cas No. 822-36-6) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2007, 535, 1–274. [Google Scholar]
- Monserrat, L. Notice of Amendment of Text Title 27, California Code of Regulations Amendment of Section 25705 Specific Regulatory Levels: No Significant Risk Levels 4-Methylimidazole (4-MEI). Available online: https://oehha.ca.gov/proposition-65/crnr/notice-amendment-text-title-27-california-code-regulations-amendment-section (accessed on 26 September 2021).
- Morita, T.; Uneyama, C. Genotoxicity Assessment of 4-Methylimidazole: Regulatory Perspectives. Genes Environ. 2016, 38, 20. [Google Scholar] [CrossRef]
- Klejdus, B.; Moravcová, J.; Lojková, L.; Vacek, J.; Kubáň, V. Solid-Phase Extraction of 4(5)-Methylimidazole (4MeI) and 2-Acetyl-4(5)-(1,2,3,4-Tetrahydroxybutyl)-Imidazole (THI) from Foods and Beverages with Subsequent Liquid Chromatographic-Electrospray Mass Spectrometric Quantification. J. Sep. Sci. 2006, 29, 378–384. [Google Scholar] [CrossRef]
- Lojková, L.; Klejdus, B.; Moravcová, J.; Kubáň, V. Supercritical Fluid Extraction (SFE) of 4(5)-Methylimidazole (4-MeI) and 2-Acetyl-4(5)-(1,2,3,4)-Tetrahydroxybutyl-Imidazole (THI) from Ground-Coffee with High-Performance Liquid Chromatographic-Electrospray Mass Spectrometric Quantification (HPLC/ESI-MS). Food Addit. Contam. 2006, 23, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Moretton, C.; Crétier, G.; Nigay, H.; Rocca, J.-L. Quantification of 4-Methylimidazole in Class III and IV Caramel Colors: Validation of a New Method Based on Heart-Cutting Two-Dimensional Liquid Chromatography (LC-LC). J. Agric. Food Chem. 2011, 59, 3544–3550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fan, J.; Gao, X.; Wang, J. A Surface Molecularly Imprinted Polymer for Selective Extraction and Liquid Chromatographic Determination of 4-Methylimidazole in Environmental Samples. Adsorpt. Sci. Technol. 2013, 31, 791–806. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, H.; Wei, Y.; Bie, Z.; Ji, L. Determination of Imidazole, 4-Methylimidazole, and 2-Methylimidazole in Cigarette Additives by Ultra-High Performance Liquid Chromatography. Anal. Lett. 2015, 48, 2708–2714. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Li, H.; Yu, S. Combination of Solid-Phase Extraction with Microextraction Techniques Followed by HPLC for Simultaneous Determination of 2-Methylimidazole and 4-Methylimidazole in Beverages. Food Chem. 2020, 305, 125389. [Google Scholar] [CrossRef]
- Ye, H.; Chen, X.; Feng, Z. Preparations of Magnetic Molecularly Imprinted Polymer for Selective Recognition and Determination of 4-Methylimidazole in Soft Beverage by High Performance Liquid Chromatography. Adsorpt. Sci. Technol. 2017, 35, 37–54. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Masuda, T. Determination of 4(5)-Methylimidazole in Soy Sauce and Other Foods by LC-MS/MS after Solid-Phase Extraction. J. Agric. Food Chem. 2011, 59, 9770–9775. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Li, H.; Yu, S. Determination of 4(5)-Methylimidazole in Foods and Beverages by Modified QuEChERS Extraction and Liquid Chromatography-Tandem Mass Spectrometry Analysis. Food Chem. 2019, 280, 278–285. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Wu, C.; Yu, S. Maillard Reaction in Chinese Household-Prepared Stewed Pork Balls with Brown Sauce: Potentially Risky and Volatile Products. Food Sci. Hum. Wellness 2021, 10, 221–230. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C. Simultaneous Analysis of 2-Methylimidazole, 4-Methylimidazole, and 5-Hydroxymethylfurfural Potentially Formed in Fermented Soy Sauce by “Quick, Easy, Cheap, Effective, Rugged, and Safe” Purification and UHPLC with Tandem Mass Spectrometry. J. Sep. Sci. 2019, 42, 501–508. [Google Scholar] [CrossRef]
- Feng, T.-T.; Wu, J.-H.; Liang, X.; Du, M.; Qin, L.; Xu, X.-B. Isotope Dilution Determination for the Trace Level of 4(5)-Methylimidazole in Beverages Using Dispersive Liquid-Liquid Microextraction Coupled with ESI-HPLC–MS/MS. Food Chem. 2018, 245, 687–691. [Google Scholar] [CrossRef]
- Jacobs, G.; Voorspoels, S.; Vloemans, P.; Fierens, T.; Van Holderbeke, M.; Cornelis, C.; Sioen, I.; De Maeyer, M.; Vinkx, C.; Vanermen, G. Caramel Colour and Process By-Products in Foods and Beverages: Part I—Development of a UPLC-MS/MS Isotope Dilution Method for Determination of 2-Acetyl-4-(1,2,3,4-Tetrahydroxybutyl)Imidazole (THI), 4-Methylimidazole (4-MEI) and 2-Methylimidazol (2-MEI). Food Chem. 2018, 255, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yan, P.; Lv, B.; Zhao, Y.; Wu, Y. Parallel Reaction Monitoring to Improve the Detection Performance of Carcinogenic 4-Methylimidazole in Food by Liquid Chromatography-High Resolution Mass Spectrometry Coupled with Dispersive Micro Solid-Phase Extraction. Food Control 2018, 88, 1–8. [Google Scholar] [CrossRef]
- Tzatzarakis, M.N.; Vakonaki, E.; Moti, S.; Alegakis, A.; Tsitsimpikou, C.; Tsakiris, I.; Goumenou, M.; Nosyrev, A.E.; Rizos, A.K.; Tsatsakis, A.M. Quantification of 4-Methylimidazole in Soft Drinks, Sauces and Vinegars of Greek Market Using Two Liquid Chromatography Techniques. Food Chem. Toxicol. 2017, 107, 565–571. [Google Scholar] [CrossRef]
- Mottier, P.; Mujahid, C.; Tarres, A.; Bessaire, T.; Stadler, R.H. Process-Induced Formation of Imidazoles in Selected Foods. Food Chem. 2017, 228, 381–387. [Google Scholar] [CrossRef]
- Wang, L.; Ren, B.; Liu, Y.; Lu, Y.; Chang, F.; Yang, L. 2-Acetyl-4-Tetrahydroxybutylimidazole and 4-Methylimidazole in Caramel Colours, Vinegar and Beverages in China. Food Addit. Contam. Part B 2015, 8, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, G.; Halldorson, T.; Bestvater, L.; Tomy, G.T. Determination of 4(5)-Methylimidazole in Carbonated Beverages by Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A 2015, 32, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Raters, M.; Elsinghorst, P.W.; Goetze, S.; Dingel, A.; Matissek, R. Determination of 2-Methylimidazole, 4-Methylimidazole, and 2-Acetyl-4-(1,2,3,4-Tetrahydroxybutyl)Imidazole in Licorice Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry Stable-Isotope Dilution Analysis. J. Agric. Food Chem. 2015, 63, 5930–5934. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-H.; Shin, K.-O.; Seo, C.-H.; Lee, S.-H.; Yoo, H.-S.; Yoon, H.-R.; Kim, J.-W.; Lee, Y.-M. Quantification of 4-Methylimidazole in Carbonated Beverages by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Arch. Pharm. Res. 2015, 38, 1363–1368. [Google Scholar] [CrossRef]
- Goscinny, S.; Hanot, V.; Trabelsi, H.; Van Loco, J. Determination of Caramel Colorants’ by-Products in Liquid Foods by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Food Addit. Contam. Part A 2014, 31, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Schlee, C.; Markova, M.; Schrank, J.; Laplagne, F.; Schneider, R.; Lachenmeier, D.W. Determination of 2-Methylimidazole, 4-Methylimidazole and 2-Acetyl-4-(1,2,3,4-Tetrahydroxybutyl)Imidazole in Caramel Colours and Cola Using LC/MS/MS. J. Chromatogr. B 2013, 927, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Kim, S.U.; Shin, Y.; Kim, J.Y.; Lee, S.M.; Kim, J.H. Determination of 4-Methylimidazole and 2-Acetyl-4()-Tetrahydroxybutylimidazole in Caramel Color and Processed Foods by LC-MS/MS. Prev. Nutr. Food Sci. 2013, 18, 263–268. [Google Scholar] [CrossRef][Green Version]
- Lim, H.-H.; Shin, H.-S. Simple Determination of 4-Methylimidazole in Soft Drinks by Headspace SPME GC–MS. Chromatographia 2013, 76, 97–101. [Google Scholar] [CrossRef]
- Wang, J.; Schnute, W.C. Simultaneous Quantitation of 2-Acetyl-4-Tetrahydroxybutylimidazole, 2- and 4-Methylimidazoles, and 5-Hydroxymethylfurfural in Beverages by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.O.; Ferreira, M.A. Gas Chromatographic-Mass Spectrometric Determination of 4-(5) Methylimidazole in Ammonia Caramel Colour Using Ion-Pair Extraction and Derivatization with Isobutylchloroformate. J. Chromatogr. A 1997, 786, 299–308. [Google Scholar] [CrossRef]
- Casal, S.; Fernandes, J.O.; Oliveira, M.B.P.P.; Ferreira, M.A. Gas Chromatographic–Mass Spectrometric Quantification of 4-(5-)Methylimidazole in Roasted Coffee after Ion-Pair Extraction. J. Chromatogr. A 2002, 976, 285–291. [Google Scholar] [CrossRef]
- Cunha, S.C.; Barrado, A.I.; Faria, M.A.; Fernandes, J.O. Assessment of 4-(5-)Methylimidazole in Soft Drinks and Dark Beer. J. Food Compos. Anal. 2011, 24, 609–614. [Google Scholar] [CrossRef]
- Seo, S.; Ka, M.-H.; Lee, K.-G. Reduction of Carcinogenic 4(5)-Methylimidazole in a Caramel Model System: Influence of Food Additives. J. Agric. Food Chem. 2014, 62, 6481–6486. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.; Wang, L.; Zhang, J.; Tan, Y.; Tang, J.; Ma, B.; Pan, X.; Jiang, W. Simultaneous Determination of Ethyl Carbamate and 4-(5-)Methylimidazole in Yellow Rice Wine and Soy Sauce by Gas Chromatography with Mass Spectrometry. J. Sep. Sci. 2014, 37, 2172–2176. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.-G. Analysis and Risk Assessment of 4(5)-Methylimidazole in Brown Colored Foods and Beverages. Food Addit. Contam. Part B 2016, 9, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.M.F.; Shuang, S.; Lai, H.Y.; Cheng, S.C.; Cheng, R.C.W.; Cheung, B.K.B.; Lee, A.W.M. Gas Chromatography-Mass Spectrometric Determination of Total Isothiocyanates in Chinese Medicinal Herbs. Anal. Chim. Acta 2004, 516, 155–163. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Nam, T.G.; Jang, H.W. Dispersive Liquid–Liquid Microextraction with in Situ Derivatization Coupled with Gas Chromatography and Mass Spectrometry for the Determination of 4-Methylimidazole in Red Ginseng Products Containing Caramel Colors. J. Sep. Sci. 2018, 41, 3415–3423. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Jeleń, H.H. Volatile Compounds of Selected Raw and Cooked Brassica Vegetables. Molecules 2019, 24, 391. [Google Scholar] [CrossRef] [PubMed]
- Mousa, R.M.A. Simultaneous Mitigation of 4(5)-Methylimidazole, Acrylamide, and 5-Hydroxymethylfurfural in Ammonia Biscuits by Supplementing with Food Hydrocolloids. Food Sci. Nutr. 2019, 7, 3912–3921. [Google Scholar] [CrossRef]
- Hyong, S.; Chu, M.; Park, H.; Park, J.; Lee, K.-G. Analysis of α-Dicarbonyl Compounds and 4-Methylimidazole in Coffee Made with Various Roasting and Brewing Conditions. LWT 2021, 151, 112231. [Google Scholar] [CrossRef]
- Altunay, N.; Gürkan, R. Ion Pair Vortex Assisted-Ionic Liquid Based Dispersive Liquid-Liquid Microextraction for Selective Separation and Preconcentration of 4-Methylimidazole from Caramel Colour Drinks and Foodstuffs Prior to Its Spectrophotometric Determination. Microchem. J. 2019, 147, 999–1009. [Google Scholar] [CrossRef]
- Petruci, J.F.d.S.; Pereira, E.A.; Cardoso, A.A. Determination of 2-Methylimidazole and 4-Methylimidazole in Caramel Colors by Capillary Electrophoresis. J. Agric. Food Chem. 2013, 61, 2263–2267. [Google Scholar] [CrossRef]
- Li, A.; Wei, P.; Hsu, H.-C.; Cooks, R.G. Direct Analysis of 4-Methylimidazole in Foods Using Paper Spray Mass Spectrometry. Analyst 2013, 138, 4624–4630. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Lirio, S.; Li, C.-K.; Liu, W.-L.; Huang, H.-Y. Determination of Imidazole Derivatives by Micellar Electrokinetic Chromatography Combined with Solid-Phase Microextraction Using Activated Carbon-Polymer Monolith as Adsorbent. J. Chromatogr. A 2016, 1428, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-B.; Liu, D.-B.; Zhao, Y.; Yu, S.-J.; Zhao, Z.-G. Simultaneous Analysis of 2- and 4-Methylimidazole in Caramel Color and Soft Drinks Using IC-PAD with Post-Column Addition of Hydroxide. Food Anal. Methods 2015, 8, 467–473. [Google Scholar] [CrossRef]
- Wu, X.; Huang, M.; Yu, S.; Kong, F. Rapid and Quantitative Detection of 4(5)-Methylimidazole in Caramel Colours: A Novel Fluorescent-Based Immunochromatographic Assay. Food Chem. 2016, 190, 843–847. [Google Scholar] [CrossRef]
- Manshaei, F.; Bagheri, H.; Es-haghi, A. Turn-off Chelation-Enhanced Fluorescence Sensing of Carbon Dot-Metallic Deep Eutectic Solvent by Imidazole-Based Small Molecules. Sens. Actuators B Chem. 2021, 344, 130228. [Google Scholar] [CrossRef]
- Radzisewski, B. Ueber Glyoxalin Und Seine Homologe. Ber. Der Dtsch. Chem. Ges. 1882, 15, 2706–2708. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.-T. Flavour Chemistry of Methylglyoxal and Glyoxal. Chem. Soc. Rev. 2012, 41, 4140–4149. [Google Scholar] [CrossRef]
- Strecker, A. Notiz Über Eine Eigenthümliche Oxydation Durch Alloxan. Justus Liebigs Ann. Der Chem. 1862, 123, 363–365. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Hengel, M.; Pan, C.; Seiber, J.N.; Shibamoto, T. Determination of Toxic α-Dicarbonyl Compounds, Glyoxal, Methylglyoxal, and Diacetyl, Released to the Headspace of Lipid Commodities upon Heat Treatment. J. Agric. Food Chem. 2013, 61, 1067–1071. [Google Scholar] [CrossRef]
- Hollnagel, A.; Kroh, L.W. Formation of α-Dicarbonyl Fragments from Mono- and Disaccharides under Caramelization and Maillard Reaction Conditions. Z Lebensm Unters Forsch 1998, 207, 50–54. [Google Scholar] [CrossRef]
- Jang, H.W.; Jiang, Y.; Hengel, M.; Shibamoto, T. Formation of 4(5)-Methylimidazole and Its Precursors, α-Dicarbonyl Compounds, in Maillard Model Systems. J. Agric. Food Chem. 2013, 61, 6865–6872. [Google Scholar] [CrossRef]
- Wu, X.; Huang, M.; Kong, F.; Yu, S. Short Communication: Study on the Formation of 2-Methylimidazole and 4-Methylimidazole in the Maillard Reaction. J. Dairy Sci. 2015, 98, 8565–8571. [Google Scholar] [CrossRef] [PubMed]
- Licht, B.H.; Shaw, K.; Smith, C.; Mendoza, M.; Orr, J.; Myers, D.V. Characterization of Caramel Colour IV. Food Chem. Toxicol. 1992, 30, 365–373. [Google Scholar] [CrossRef]
- Chappel, C.I.; Howell, J.C. Caramel Colours—A Historical Introduction. Food Chem. Toxicol. 1992, 30, 351–357. [Google Scholar] [CrossRef]
- Houben, G.F.; Penninks, A.H. Immunotoxicity of the Colour Additive Caramel Colour III; A Review on Complicated Issues in the Safety Evaluation of a Food Additive. Toxicology 1994, 91, 289–302. [Google Scholar] [CrossRef]
- Brusick, D.J.; Jagannath, D.R.; Galloway, S.M.; Nestmann, E.R. Genotoxicity Hazard Assessment of Caramel Colours III and IV. Food Chem. Toxicol. 1992, 30, 403–410. [Google Scholar] [CrossRef]
- Ciolino, L.A. Determination and Classification of Added Caramel Color in Adulterated Acerola Juice Formulations. J. Agric. Food Chem. 1998, 46, 1746–1753. [Google Scholar] [CrossRef]
- Allen, J.A.; Brooker, P.C.; Jones, E.; Adams, K.; Richold, M. Absence of Mutagenic Activity in Salmonella and of Clastogenic Activity in Cho Cells of Caramel Colours I, II, III and IV. Food Chem. Toxicol. 1992, 30, 389–395. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Fujiwara, M. Determination of 4 (5)-Methylimidazole in Food by Thin Layer Chromatography. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1981, 22, 189–196_1. [Google Scholar] [CrossRef][Green Version]
- Muller, L.; Sivertsen, T.; Langseth, W. Ammoniated Forage Poisoning: Concentrations of Alkylimidazoles in Ammoniated Forage and in Milk, Plasma and Urine in Sheep and Cow. Acta Vet. Scand. 1998, 39, 511–514. [Google Scholar] [CrossRef]
- Müller, L.; Langseth, W.; Solheim, E.; Sivertsen, T. Ammoniated Forage Poisoning: Isolation and Characterization of Alkyl-Substituted Imidazoles in Ammoniated Forage and in Milk. J. Agric. Food Chem. 1998, 46, 3172–3177. [Google Scholar] [CrossRef]
- Moore-Testa, P.; Saint-Jalm, Y.; Testa, A. Identification and Determination of Imidazole Derivatives in Cigarette Smoke. J. Chromatogr. A 1984, 290, 263–274. [Google Scholar] [CrossRef]
- Klupinski, T.P.; Strozier, E.D.; Friedenberg, D.A.; Brinkman, M.C.; Gordon, S.M.; Clark, P.I. Identification of New and Distinctive Exposures from Little Cigars. Chem. Res. Toxicol. 2016, 29, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Jang, H.; Shibamoto, T. Formation of Carcinogenic 4(5)-Methylimidazole in Caramel Model Systems: A Role of Sulphite. Food Chem. 2013, 136, 1165–1168. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Guo, X.; Li, H.; Yu, S. Simultaneous Detection of 4(5)-Methylimidazole and Acrylamide in Biscuit Products by Isotope-Dilution UPLC-MS/MS. Food Control 2019, 105, 64–70. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Feng, Z.; Lu, Y.; Zhao, Y.; Ye, H. Fast Extraction and Detection of 4-Methylimidazole in Soy Sauce Using Magnetic Molecularly Imprinted Polymer by HPLC. Molecules 2017, 22, 1885. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Li, H.; Yu, S. Enhancement of Liquid Chromatography-Ion Trap Mass Spectrometry Analysis of 4(5)-Methylimidazole in Biscuits through Derivatization with Dansyl Chloride. J. Chromatogr. A 2019, 1596, 1–7. [Google Scholar] [CrossRef]
- Cunha, C.; Senra, L.; Fernandes, J.O.; Cunha, S.C. Gas Chromatography–Mass Spectrometry Analysis of 4-Methylimidazole in Balsamic Vinegars and Processed Sauces. Food Anal. Methods 2014, 7, 1519–1525. [Google Scholar] [CrossRef]
- Cunha, S.C.; Senra, L.; Cruz, R.; Casal, S.; Fernandes, J.O. 4-Methylimidazole in Soluble Coffee and Coffee Substitutes. Food Control 2016, 63, 15–20. [Google Scholar] [CrossRef]
- Choi, S.J.; Jung, M.Y. Simple and Fast Sample Preparation Followed by Gas Chromatography-Tandem Mass Spectrometry (GC-MS/MS) for the Analysis of 2- and 4-Methylimidazole in Cola and Dark Beer. J. Food Sci. 2017, 82, 1044–1052. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Przygoński, K.; Jeleń, H.H. Determination of 4-Methylimidazole in Ammonia Caramel Using Gas Chromatography–Tandem Mass Spectrometry (GC-MS/MS). J. Food Qual. 2018, 2018, 4696074. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Gómez-Hens, A.; Valcárcel, M. Individual and Joint Kinetic Fluorometric Determination of Imidazole and 4-Methylimidazole. Microchem. J. 1986, 34, 332–339. [Google Scholar] [CrossRef]
- Li, J.M.; Wang, Y.J.; Zou, N. Detemination of 4-Methyliminidazol in Soy Sauce by Ultraviolet-Visible Spectrometry. Chin. J. Spectrosc. Lab. (Chin. J.) 2008, 25, 84–87. [Google Scholar]
- Xu, X.-B.; Liu, D.-B.; Yu, S.-J.; Yu, P.; Zhao, Z.-G. Separation and Determination of 4-Methylimidazole, 2-Methylimidazole and 5-Hydroxymethylfurfural in Beverages by Amino Trap Column Coupled with Pulsed Amperometric Detection. Food Chem. 2015, 169, 224–229. [Google Scholar] [CrossRef]
- Wu, X.-L.; Yu, S.-J.; Kang, K.-R. Development of a Monoclonal Antibody-Based Indirect Competitive Immunosorbent Assay for 4(5)-Methylimidazole Detection in Caramels. Food Chem. 2015, 170, 354–359. [Google Scholar] [CrossRef]
- Shin, H.J.; Jang, H.W.; Ok, G. Highly Sensitive Detection of 4-Methylimidazole Using a Terahertz Metamaterial. Sensors 2018, 18, 4304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).