Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation Process
2.2. Characterization Techniques
2.3. Sensing Tests
3. Results and Discussion
3.1. Characterization
3.2. Gas Sensing Studies
3.2.1. Effect of Al on Mg Doped ZnO NPs-Based Sensor
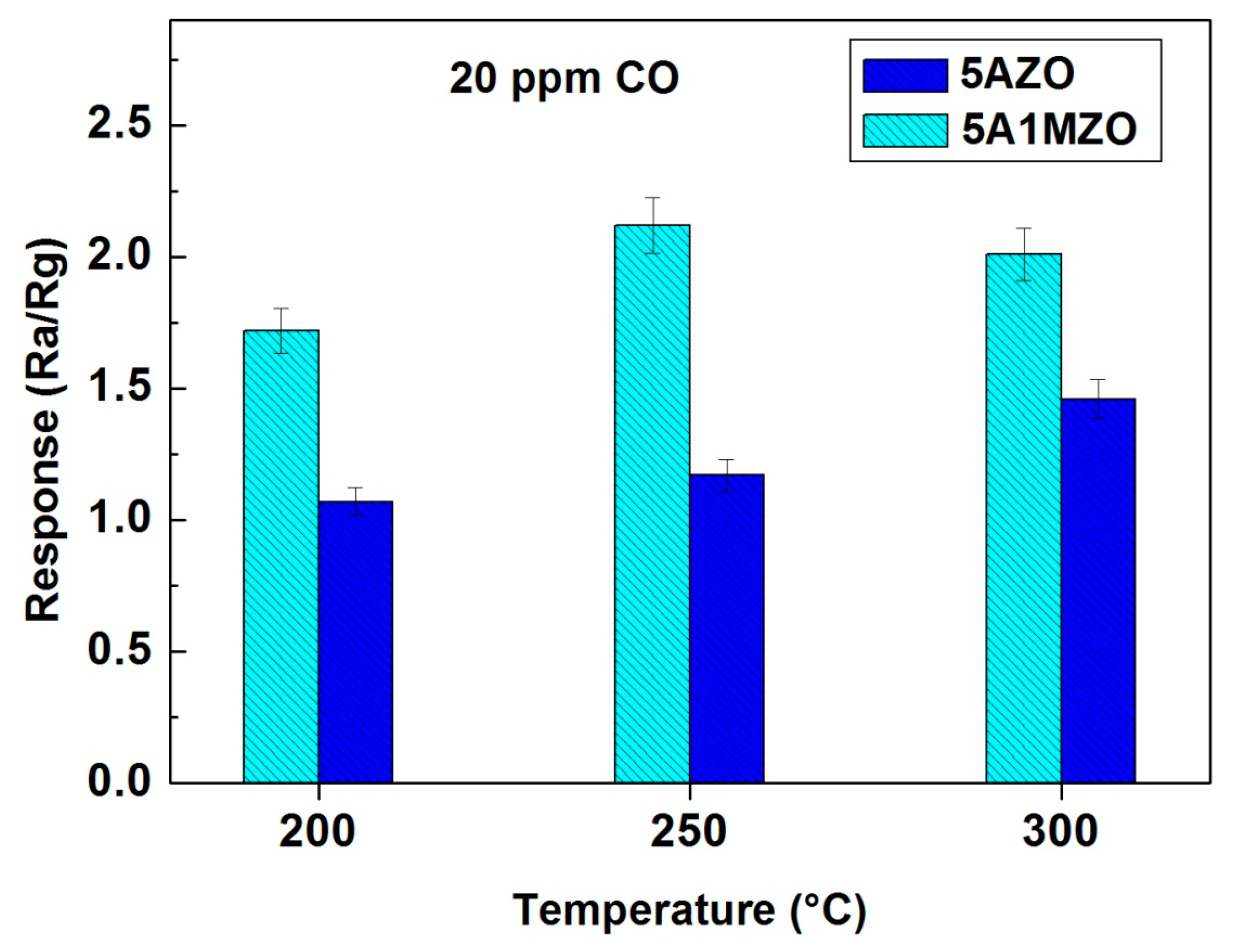
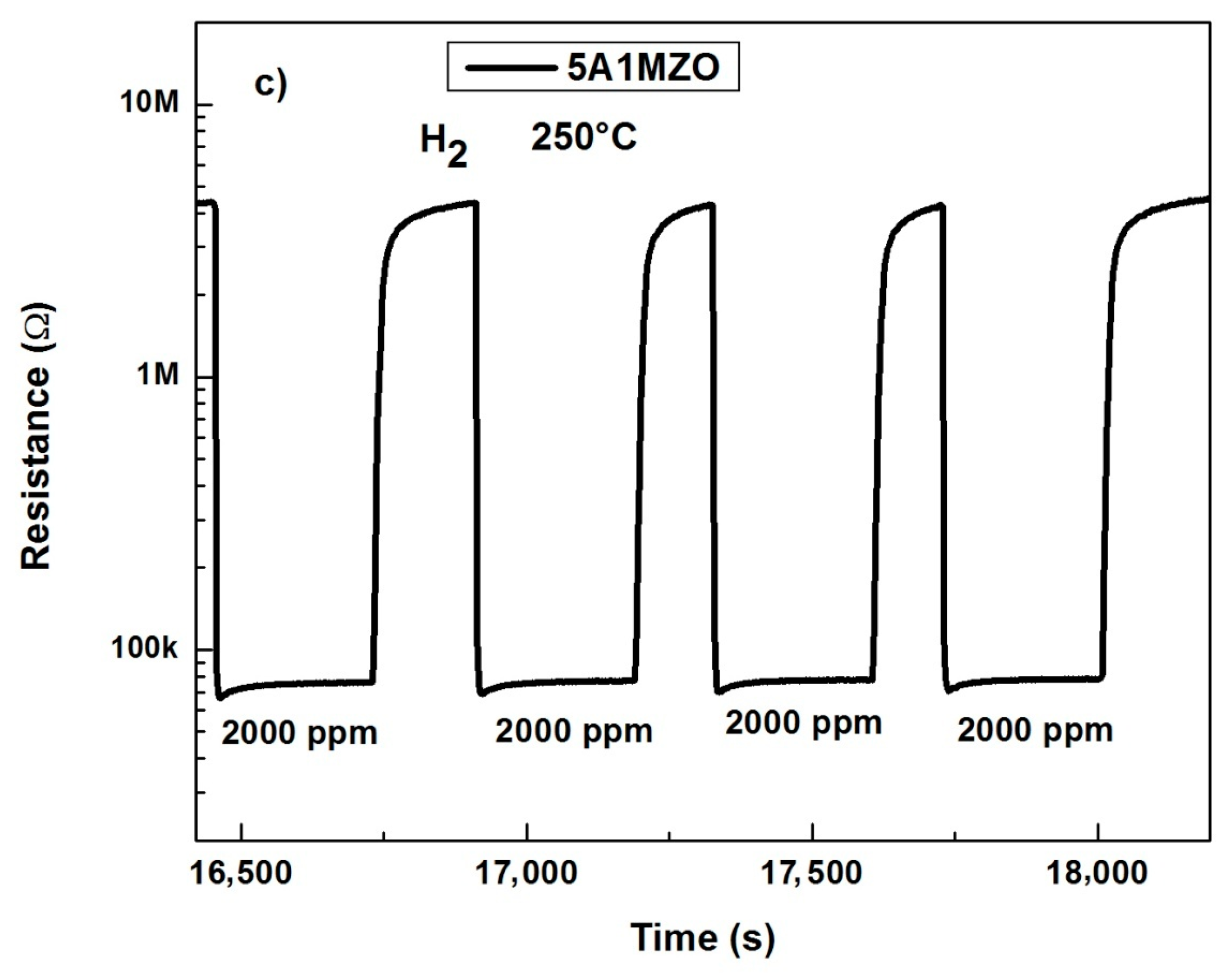
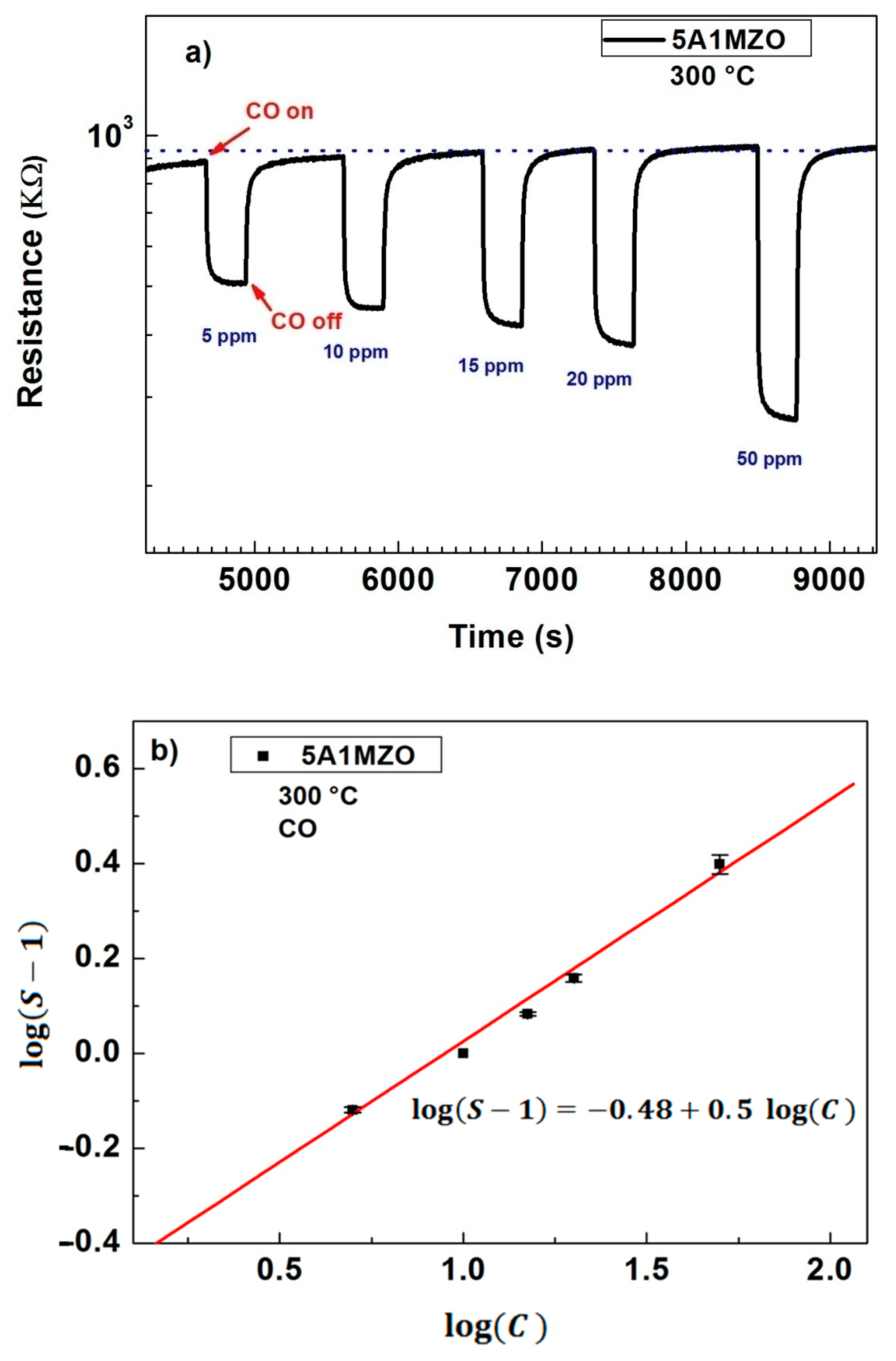
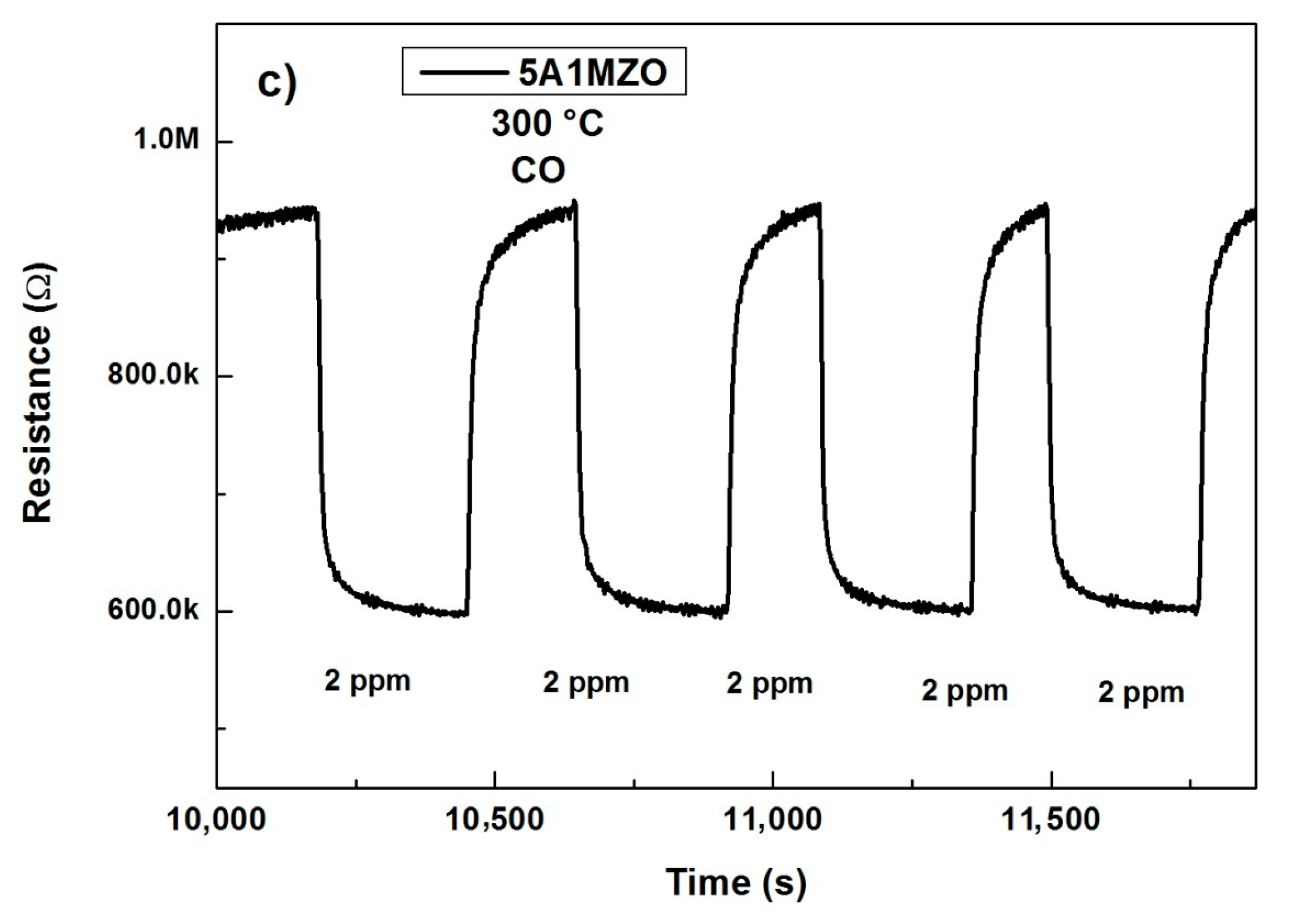
3.2.2. Effect of Mg Doping on Al Doped ZnO NPs-Based Sensor for CO Sensing
3.2.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Manjunath, G.; Nagaraju, P.; Mandal, S. Ultra-sensitive clogging free combustible molecular precursor-based screen-printed ZnO sensors: A detection of ammonia and formaldehyde breath markers. J. Mater. Sci. Mater. Electron. 2021, 32, 5713–5728. [Google Scholar] [CrossRef]
- Rosli, N.; Halim, M.M.; Hashim, M.R. Effect of CBD growth times on the ZnO microrods prepared on macroporous silicon. Appl. Phys. A 2021, 127, 1–12. [Google Scholar] [CrossRef]
- Que, M.; Lin, C.; Sun, J.; Chen, L.; Sun, X.; Sun, Y. Progress in ZnO Nanosensors. Sensors 2021, 21, 5502. [Google Scholar] [CrossRef]
- Nakate, U.; Ahmad, R.; Patil, P.; Bhat, K.S.; Wang, Y.; Mahmoudi, T.; Yu, Y.; Suh, E.-K.; Hahn, Y.-B. High response and low concentration hydrogen gas sensing properties using hollow ZnO particles transformed from polystyrene@ZnO core-shell structures. Int. J. Hydrogen Energy 2019, 44, 15677–15688. [Google Scholar] [CrossRef]
- Dhahri, R.; Leonardi, S.; Hjiri, M.; El Mir, L.; Bonavita, A.; Donato, N.; Iannazzo, D.; Neri, G. Enhanced performance of novel calcium/aluminum co-doped zinc oxide for CO2 sensors. Sens. Actuators B Chem. 2016, 239, 36–44. [Google Scholar] [CrossRef]
- El Ghoul, J.; Omri, K.; El Mir, L.; Barthou, C.; Alaya, S. Sol-gel synthesis and luminescence properties of SiO2/Zn2SiO4 and SiO2/Zn2SiO4:V composite materials. J. Lumin. 2012, 132, 2288. [Google Scholar] [CrossRef]
- Rajput, P.; Vashishtha, P.; Gupta, G.; Kamni. A comparative study on structural and optical properties of ZnO nanoparticles prepared by three different synthesis methods. Mater. Today Proc. 2021, 43, 3856–3861. [Google Scholar] [CrossRef]
- Nguyen, H.T.P.; Nguyen, T.M.T.; Hoang, C.N.; Le, T.K.; Lund, T.; Huynh, T.K.X. Characterization and photocatalytic activity of new photocatalysts based on Ag, F-modified ZnO nanoparticles prepared by thermal shock method. Arab. J. Chem. 2020, 13, 1837–1847. [Google Scholar] [CrossRef]
- Anugrahwidya, R.; Yudasari, N.; Tahir, D. Optical and structural investigation of synthesis ZnO/Ag Nanoparticles prepared by laser ablation in liquid. Mater. Sci. Semicond. Process. 2019, 105, 104712. [Google Scholar] [CrossRef]
- Taha, K.K.; Modwi, A.; Elamin, M.; Arasheed, R.; Al-Fahad, A.J.; Albutairi, I.; Arasheed, H.; Alfaify, M.; Anojaidi, K.; Algethami, F.K.; et al. Impact of Hibiscus extract on the structural and activity of sonochemically fabricated ZnO nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 390, 112263. [Google Scholar] [CrossRef]
- Husin, H.; Chen, H.-M.; Su, W.-N.; Pan, C.-J.; Chuang, W.-T.; Sheu, H.-S.; Hwang, B.-J. Green fabrication of La-doped NaTaO3 via H2O2 assisted sol–gel route for photocatalytic hydrogen production. Appl. Catal. B Environ. 2010, 102, 343–351. [Google Scholar] [CrossRef]
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S. Recent Advances in Perylene Diimide-Based Active Materials in Electrical Mode Gas Sensing. Chemosensors 2021, 9, 30. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-Oxide Based Nanomaterials: Synthesis, Characterization and Their Applications in Electrical and Electrochemical Sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, X.-N.; Cao, J.-l. Rapid detection of low concentration CO using Pt-loaded ZnO nanosheets. J. Hazard. Mater. 2020, 381, 120944. [Google Scholar] [CrossRef]
- Ani, A.; Poornesh, P.; Nagaraja, K.; Kolesnikov, E.; Shchetinin, I.V.; Antony, A.; Kulkarni, S.D.; Sanjeev, G.; Petwal, V.C.; Verma, V.P. Tuning of CO gas sensing performance of spray pyrolyzed ZnO thin films by electron beam irradiation. Mater. Sci. Semicond. Process. 2020, 119, 105249. [Google Scholar] [CrossRef]
- González-Garnica, M.; Galdámez-Martínez, A.; Malagón, F.; Ramos, C.; Santana, G.; Abolhassani, R.; Panda, P.K.; Kaushik, A.; Mishra, Y.K.; Karthik, T.V. One dimensional Au-ZnO hybrid nanostructures based CO2 detection: Growth mechanism and role of the seed layer on sensing performance. Sens. Actuators B Chem. 2021, 337, 129765. [Google Scholar] [CrossRef]
- Nguyen, T.T.O.; Truong, T.H.; Le, A.T.; Dai Nguyen, T. Facile synthesis of different ZnO nanostructures for detecting sub-ppm NO2 gas. Mater. Today Commun. 2020, 22, 100826. [Google Scholar]
- Lou, C.; Wang, K.; Mei, H.; Xie, J.; Zheng, W.; Liu, X.; Zhang, J. ZnO nanoarrays via a thermal decomposition–deposition method for sensitive and selective NO2 detection. Cryst. Eng. Comm. 2021, 23, 3654–3663. [Google Scholar] [CrossRef]
- Kumar, M.; Bhati, V.S.; Ranwa, S.; Singh, J. Pd/ZnO nanorods based sensor for highly selective detection of extremely low concentration hydrogen. Sci. Rep. 2017, 7, 236. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yi, G.; Li, H.; Shi, C.; Sun, G.; Zhang, Z. Hydrothermally synthesized porous ZnO nanosheets for methane sensing at lower temperature. J. Porous Mater. 2020, 27, 1363–1368. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Highly sensitive and ultra-fast responsive ammonia gas sensor based on 2D ZnO nanoflakes. Mater. Sci. Energy Technol. 2020, 3, 91–96. [Google Scholar] [CrossRef]
- Nakarungsee, P.; Srirattanapibul, S.; Issro, C.; Tang, I.-M.; Thongmee, S. High performance Cr doped ZnO by UV for NH3 gas sensor. Sens. Actuators A Phys. 2020, 314, 112230. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Karthick, K. Influence of Mg doping on the morphology and optical properties of ZnO films for enhanced H2 sensing. Microsc. Res. Tech. 2013, 76, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kumar, S.; Agrawal, H.; Moinuddin, M.G.; Kumar, M.; Sharma, S.K.; Awasthi, K. An Efficient Hydrogen Gas Sensor Based on Hierarchical Ag/ZnO Hollow Microstructures. Sens. Actuators B Chem. 2021, 130510. [Google Scholar] [CrossRef]
- Jaballah, S.; Benamara, M.; Dahman, H.; Ly, A.; Lahem, D.; Debliquy, M.; Mir, L.E. Effect of Mg-doping ZnO nanoparticles on detection of low ethanol concentrations. Mater. Chem. Phys. 2020, 255, 123643. [Google Scholar] [CrossRef]
- Jaballah, S.; Benamara, M.; Dahman, H.; Lahem, D.; Debliquy, M.; El Mir, L. Formaldehyde sensing characteristics of calcium-doped zinc oxide nanoparticles-based gas sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 8230–8239. [Google Scholar] [CrossRef]
- Samoei, V.K.; Jayatissa, A.H. Aluminum doped zinc oxide (AZO)-based pressure sensor. Sens. Actuators A Phys. 2020, 303, 111816. [Google Scholar] [CrossRef]
- El Mir, L.; Ayadi, Z.B.; Saadoun, M.; Djessas, K.; von Bardeleben, H.J.; Alaya, S. Preparation and characterization of n-type conductive (Al, Co) co-doped ZnO thin films deposited by sputtering from aerogel nanopowders. Appl. Surf. Sci. 2007, 254, 570–573. [Google Scholar] [CrossRef]
- Omri, K.; Alyamani, A.; El Mir, L. Photoluminescence and cathodoluminescence of Mn doped zinc silicate nanophosphors for green and yellow field emissions displays. Appl. Phys. A Mater. Sci. Process. 2018, 124, 215. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; Mir, L.E.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing properties under UV radiation of Ga-doped ZnO nanopowders. Appl. Surf. Sci. 2015, 355, 1321–1326. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Gopalakrishnan, R. Structural, FTIR and photoluminescence studies of Cu doped ZnO nanopowders by co-precipitation method. Opt. Mater. 2012, 34, 1946–1953. [Google Scholar] [CrossRef]
- Karthik, S.; Siva, P.; Balu, K.S.; Suriyaprabha, R.; Rajendran, V.; Maaza, M. Acalypha indica–mediated green synthesis of ZnO nanostructures under differential thermal treatment: Effect on textile coating, hydrophobicity, UV resistance, and antibacterial activity. Adv. Powder Technol. 2017, 28, 3184–3194. [Google Scholar] [CrossRef]
- Han, B.; Liu, X.; Xing, X.; Chen, N.; Xiao, X.; Liu, S.; Wang, Y. A high response butanol gas sensor based on ZnO hollow spheres. Sens. Actuators B Chem. 2016, 237, 423–430. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Leontie, L.; Galca, A.C.; Enculescu, M.; Carlescu, A.; Guerboub, M.; Mothudi, B.M.; Jorio, A. Graphene Oxide Concentration Effect on the Optoelectronic Properties of ZnO/GO Nanocomposites. Nanomaterials 2020, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Siregar, N.; Panggabean, J. The effect magnesium (Mg) on structural and optical properties of ZnO: Mg thin film by sol-gel spin coating method. J. Phys. Conf. Ser. 2020, 1428, 012026. [Google Scholar] [CrossRef]
- Pradeev Raj, K.; Sadaiyandi, K.; Kennedy, A.; Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.B.; Aziz, F.A.; Rafique, R.F.; Thamiz Selvi, R.; Rathina Bala, R. Influence of Mg Doping on ZnO Nanoparticles for Enhanced Photocatalytic Evaluation and Antibacterial Analysis. Nanoscale Res. Lett. 2018, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Panda, E. Mg addition in undoped and Al-doped ZnO films: Fabricating near UV transparent conductor by bandgap engineering. J. Alloy. Compd. 2019, 788, 1037–1047. [Google Scholar] [CrossRef]
- Rouchdi, M.; Salmani, E.; Fares, B.; Hassanain, N.; Mzerd, A. Synthesis and characteristics of Mg doped ZnO thin films: Experimental and ab-initio study. Results Phys. 2017, 7, 620–627. [Google Scholar] [CrossRef]
- Varudkar, H.; Umadevi, G.; Nagaraju, P.; Dargad, J.; Mote, V. Fabrication of Al-doped ZnO nanoparticles and their application as a semiconductor-based gas sensor for the detection of ammonia. J. Mater. Sci. Mater. Electron. 2020, 31, 12579–12585. [Google Scholar] [CrossRef]
- Caglar, Y.; Caglar, M.; Ilican, S. Microstructural, optical and electrical studies on sol gel derived ZnO and ZnO: Al films. Curr. Appl. Phys. 2012, 12, 963–968. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Al-Asedy, H.J.; Bidin, N.; Al-khafaji, S.A.; Bakhtiar, H. Sol-gel grown aluminum/gallium co-doped ZnO nanostructures: Hydrogen gas sensing attributes. Mater. Sci. Semicond. Process. 2018, 77, 50–57. [Google Scholar] [CrossRef]
- Khojier, K. Preparation and investigation of Al-doped ZnO thin films as a formaldehyde sensor with extremely low detection limit and considering the effect of RH. Mater. Sci. Semicond. Process. 2021, 121, 105283. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Liu, T.; Zeb, S.; Nie, Y.; Zhao, Y.; Qin, C.; Jiang, X. Advanced development of metal oxide nanomaterials for H2 gas sensing applications. Mater. Adv. 2021, 2, 1530–1569. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; Cheng, J.; Zhang, L.; Jia, J.; Li, X. Synthesis and ethanol sensing properties of Al-doped ZnO nanofibers. Curr. Appl. Phys. 2013, 13, 403–407. [Google Scholar] [CrossRef]
- Qin, C.; Wang, B.; Li, P.; Sun, L.; Han, C.; Wu, N.; Wang, Y. Metal-organic framework-derived highly dispersed Pt nanoparticles-functionalized ZnO polyhedrons for ppb-level CO detection. Sens. Actuators B Chem. 2021, 331, 129433. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Li, S.; Yang, P.; Xi, Y.; Cai, C.; Liu, W. Preparation of Al3+-doped ZnO micro–nanospheres for highly sensitive CO gas sensors. Appl. Phys. A 2021, 127, 176. [Google Scholar] [CrossRef]
- Lim, S.K.; Hong, S.H.; Hwang, S.-H.; Choi, W.M.; Kim, S.; Park, H.; Jeong, M.G. Synthesis of Al-doped ZnO nanorods via microemulsion method and their application as a CO gas sensor. J. Mater. Sci. Technol. 2015, 31, 639–644. [Google Scholar] [CrossRef]
- Hadipour, N.L.; Ahmadi Peyghan, A.; Soleymanabadi, H. Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J. Phys. Chem. C 2015, 119, 6398–6404. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Bonavita, A.; Iannazzo, D.; Latino, M.; Donato, N.; Leonardi, S.G.; Neri, G. Gas sensing properties of Al-doped ZnO for UV-activated CO detection. J. Phys. D Appl. Phys. 2016, 49, 135502. [Google Scholar] [CrossRef]
- Amin, M.; Shah, N.A.; Bhatti, A.S.; Malik, M.A. Effects of Mg doping on optical and CO gas sensing properties of sensitive ZnO nanobelts. CrystEngComm 2014, 16, 6080–6088. [Google Scholar] [CrossRef]
- Mohamed, R.; Mamat, M.; Ismail, A.; Malek, M.; Zoolfakar, A.; Khusaimi, Z.; Suriani, A.; Mohamed, A.; Ahmad, M.; Rusop, M. Hierarchically assembled tin-doped zinc oxide nanorods using low-temperature immersion route for low temperature ethanol sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 16292–16305. [Google Scholar]
- Sen, S.; Kundu, S. Reduced graphene oxide (rGO) decorated ZnO-SnO2: A ternary nanocomposite towards improved low concentration VOC sensing performance. J. Alloys Compd. 2021, 881, 160406. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, F.; Han, D.; Wang, Z.; Guo, G. Synthesis, characterization and alcohol-sensing properties of rare earth doped In2O3 hollow spheres. Sens. Actuators B Chem. 2013, 177, 1180–1188. [Google Scholar] [CrossRef]
- Kumaresan, M.; Venkatachalam, M.; Saroja, M.; Gowthaman, P. TiO2 nanofibers decorated with monodispersed WO3 heterostruture sensors for high gas sensing performance towards H2 gas. Inorg. Chem. Commun. 2021, 129, 108663. [Google Scholar] [CrossRef]
- Han, Z.; Ren, J.; Zhou, J.; Zhang, S.; Zhang, Z.; Yang, L.; Yin, C. Multilayer porous Pd-WO3 composite thin films prepared by sol-gel process for hydrogen sensing. Int. J. Hydrog. Energy 2020, 45, 7223–7233. [Google Scholar] [CrossRef]
- Ani, A.; Poornesh, P.; Nagaraja, K.; Hegde, G.; Kolesnikov, E.; Shchetinin, I.V.; Antony, A.; Kulkarni, S.D. Evaluation of spray pyrolysed In: ZnO nanostructures for CO gas sensing at low concentration. J. Mater. Sci. Mater. Electron. 2021, 32, 22599–22616. [Google Scholar] [CrossRef]
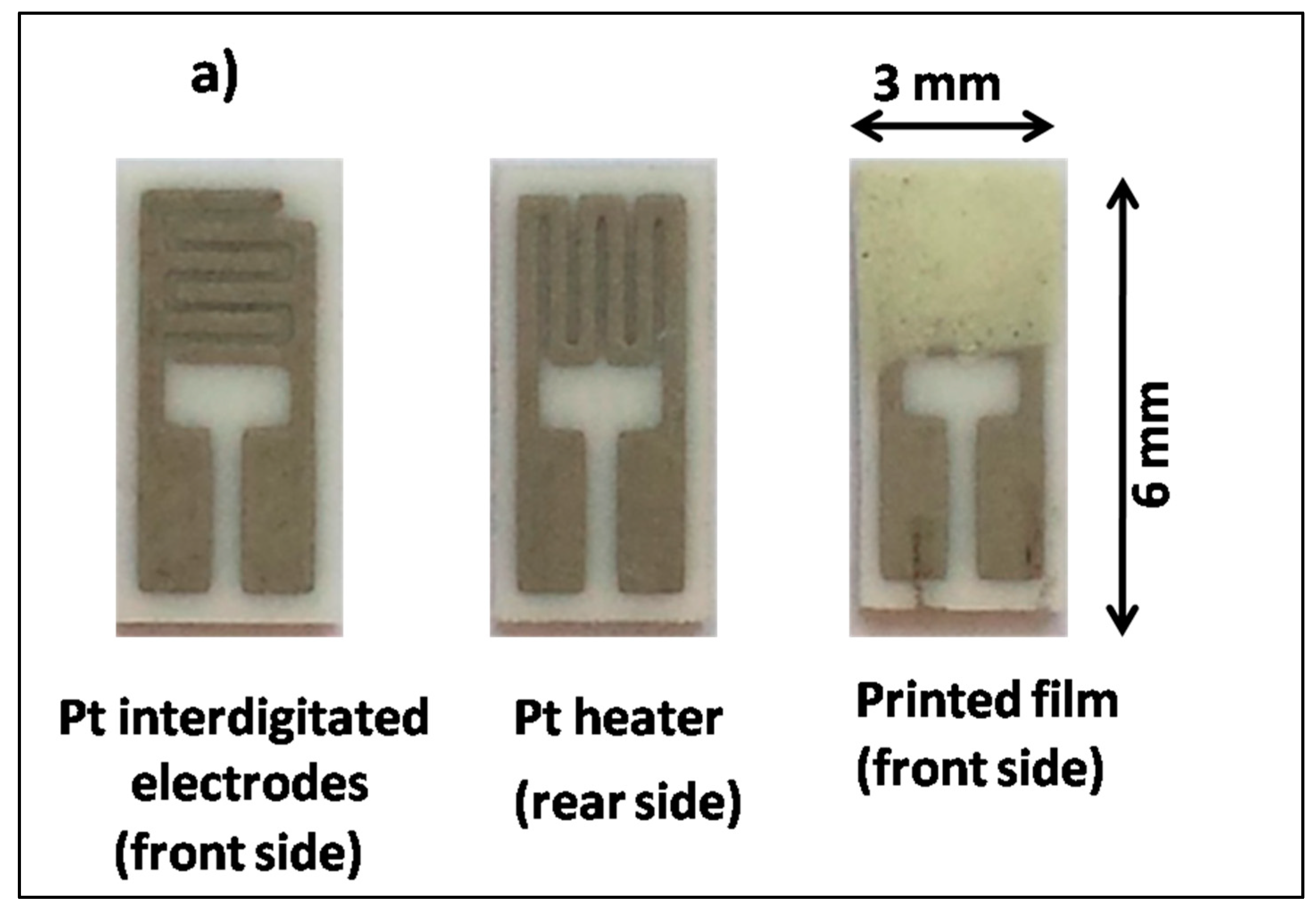
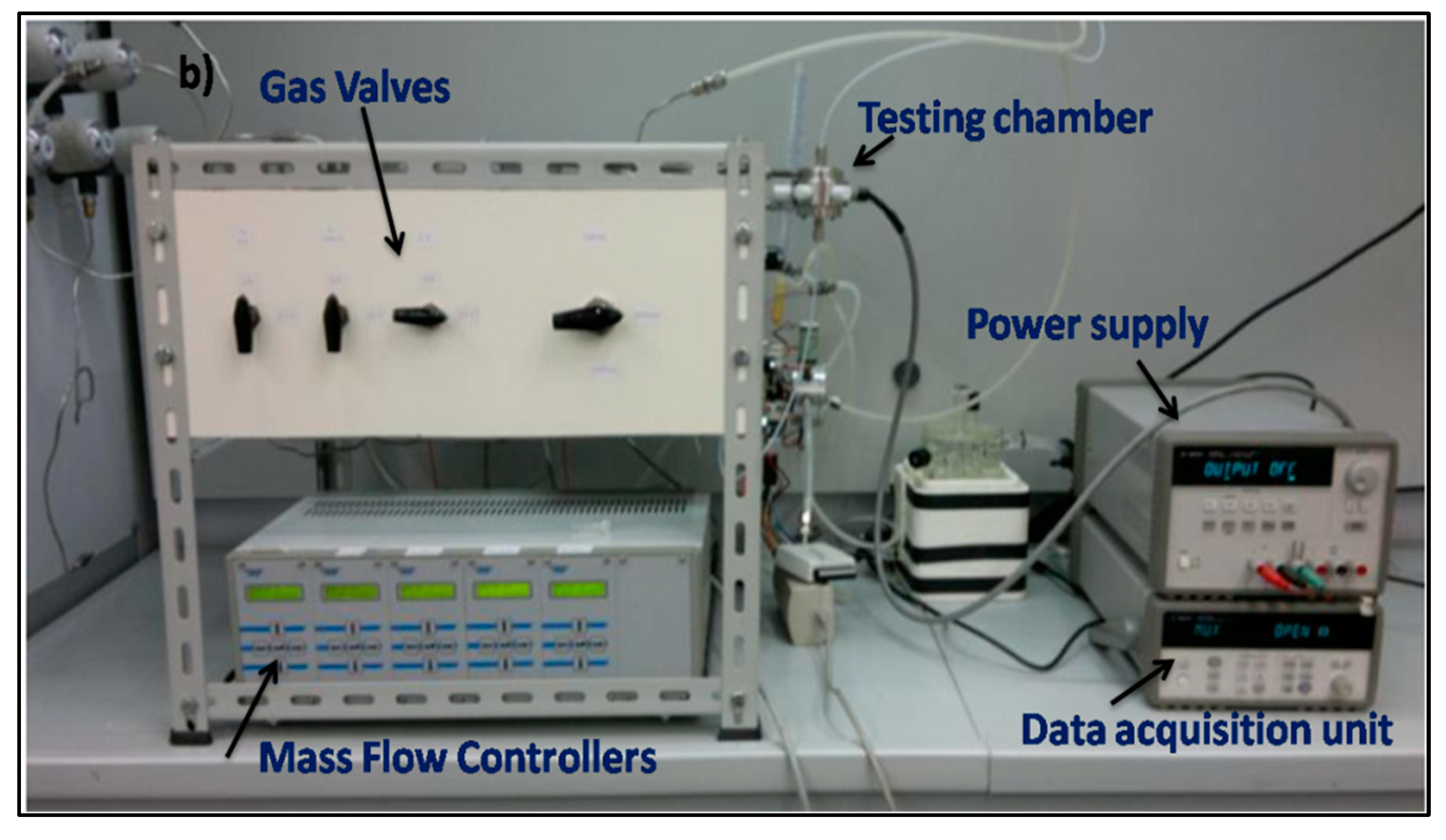
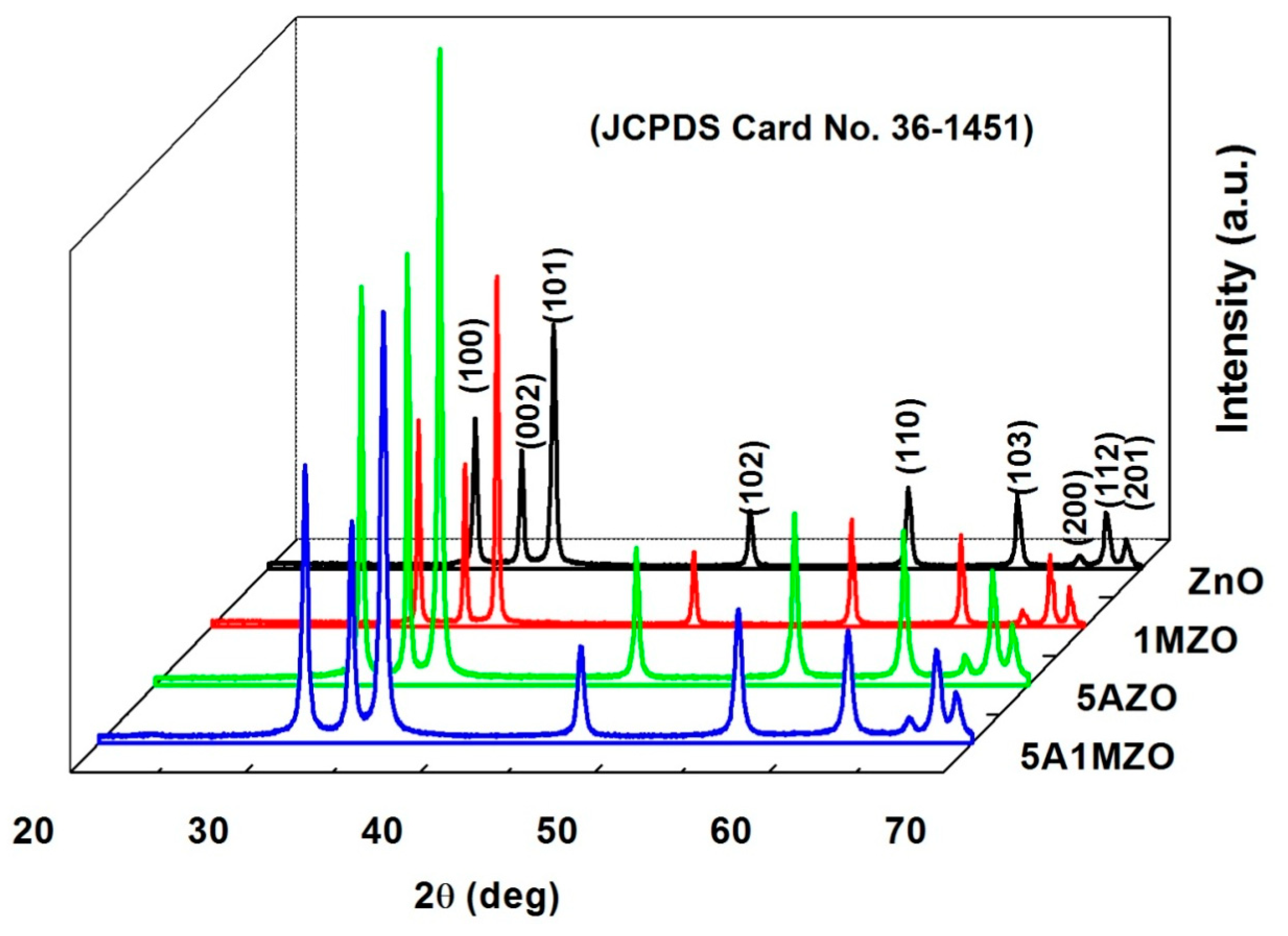
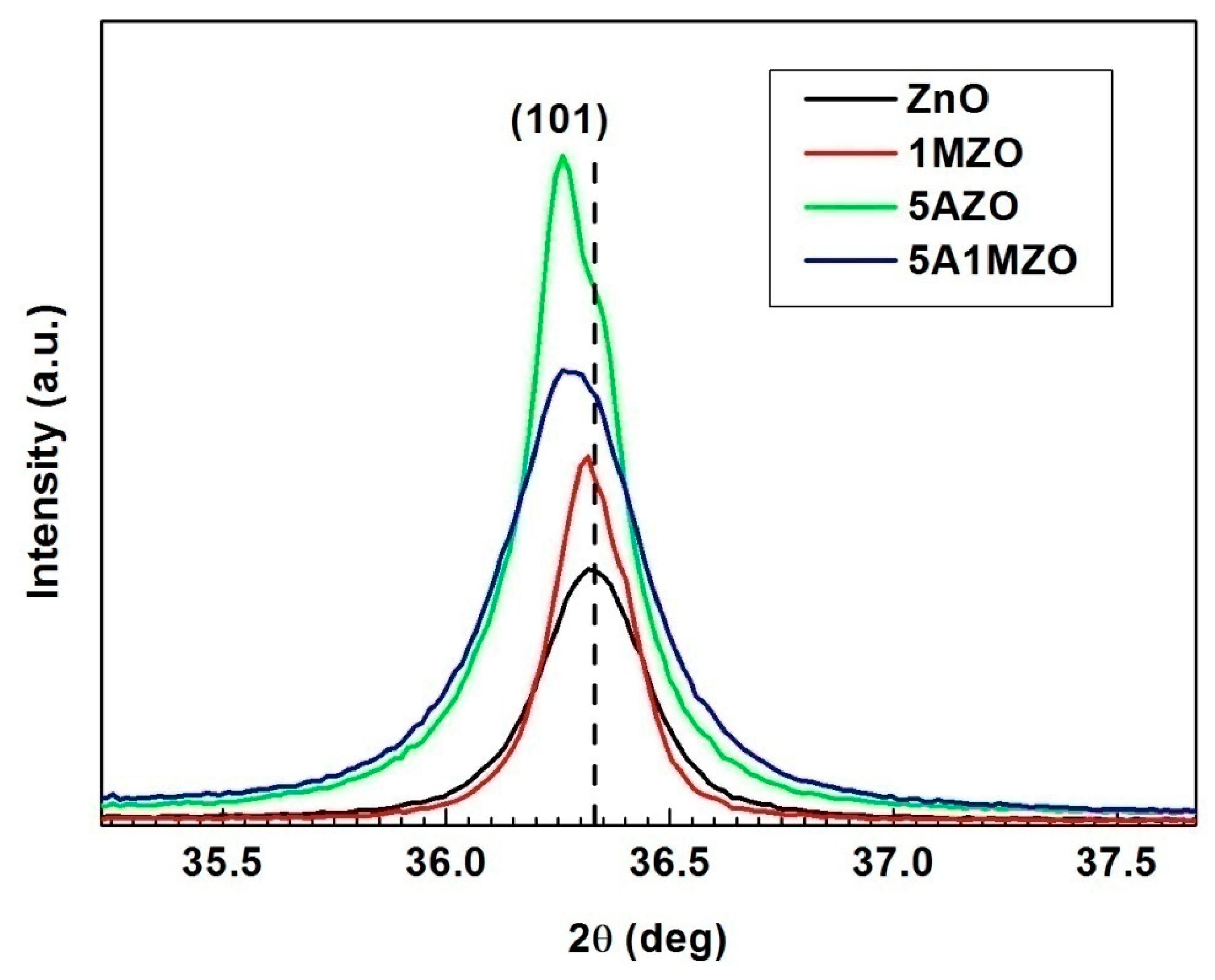


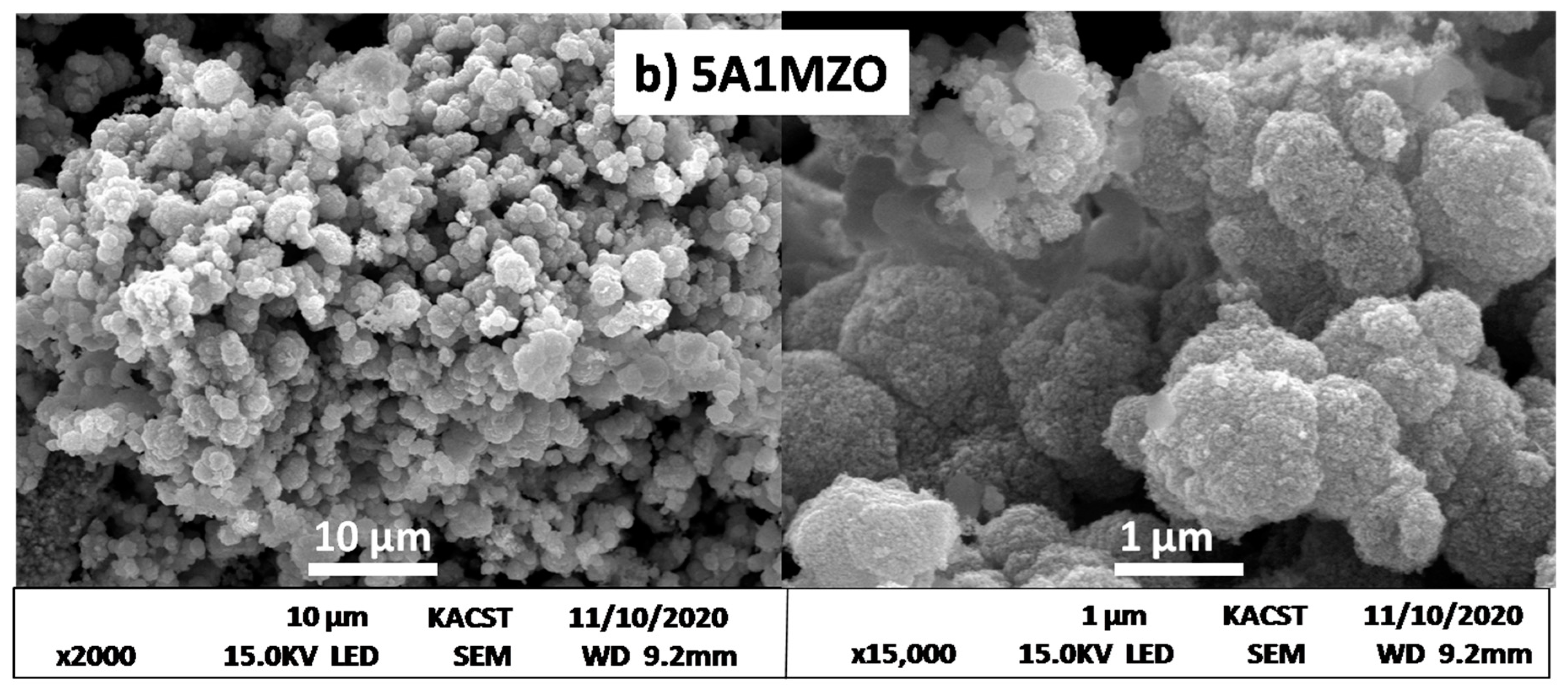
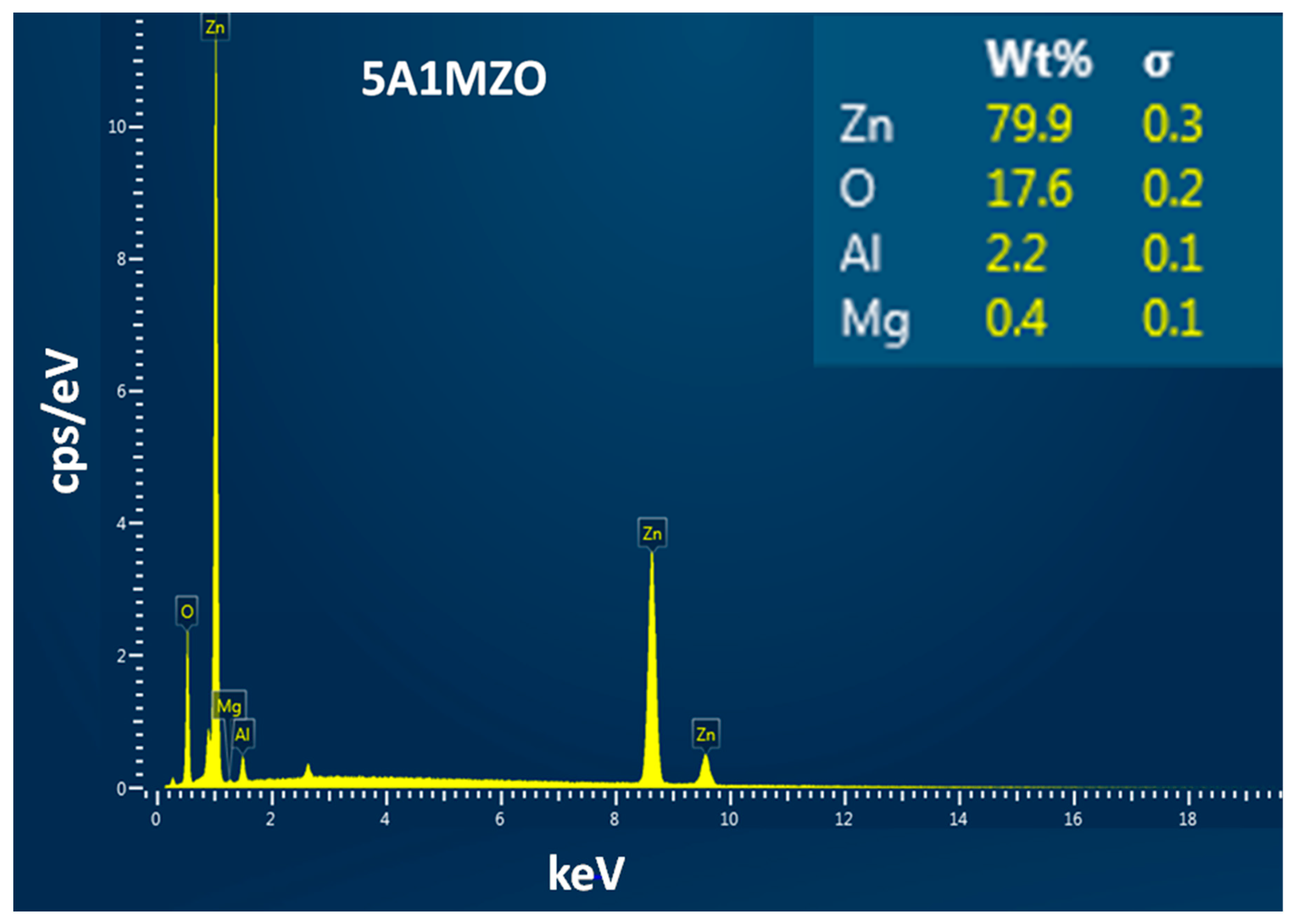

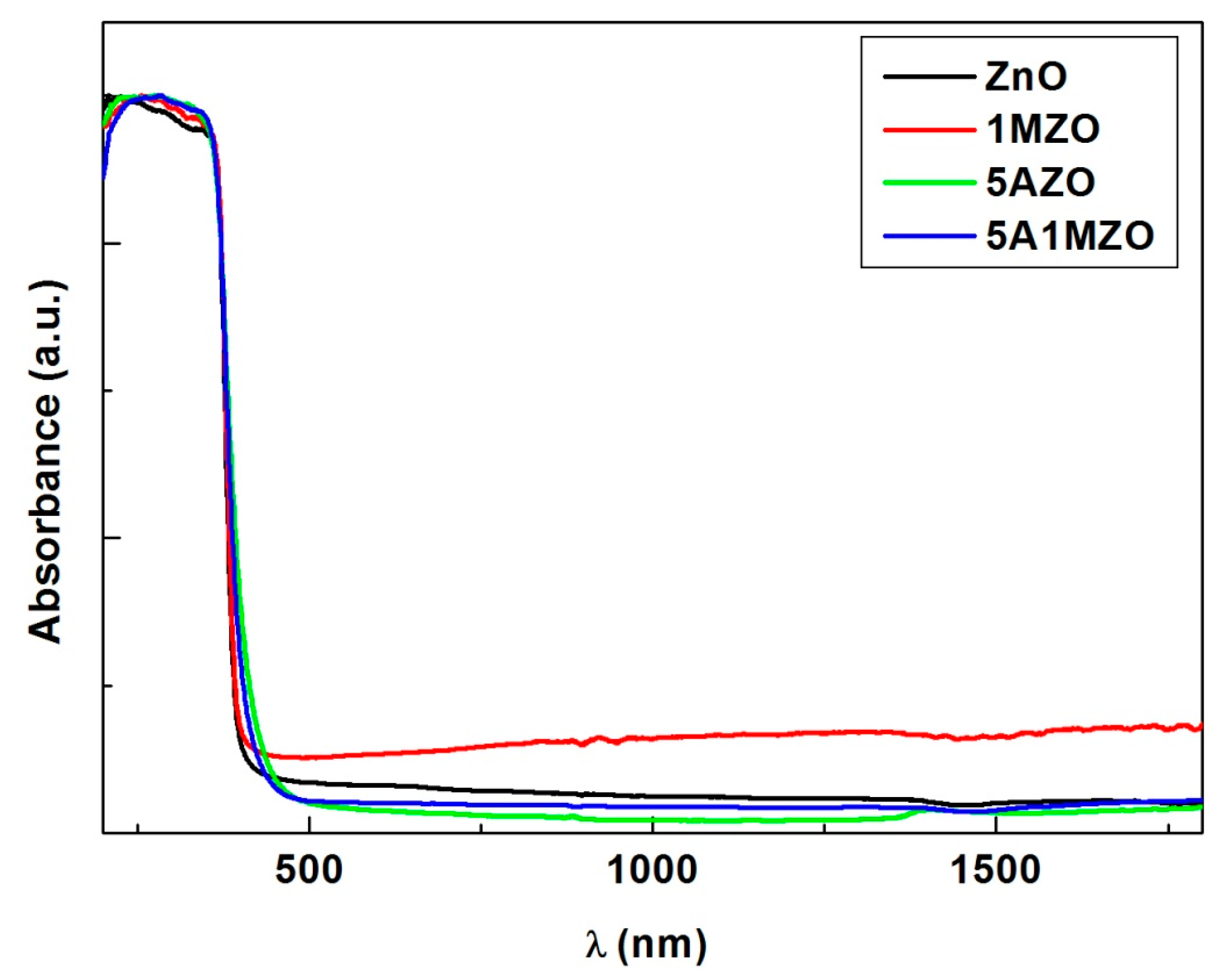
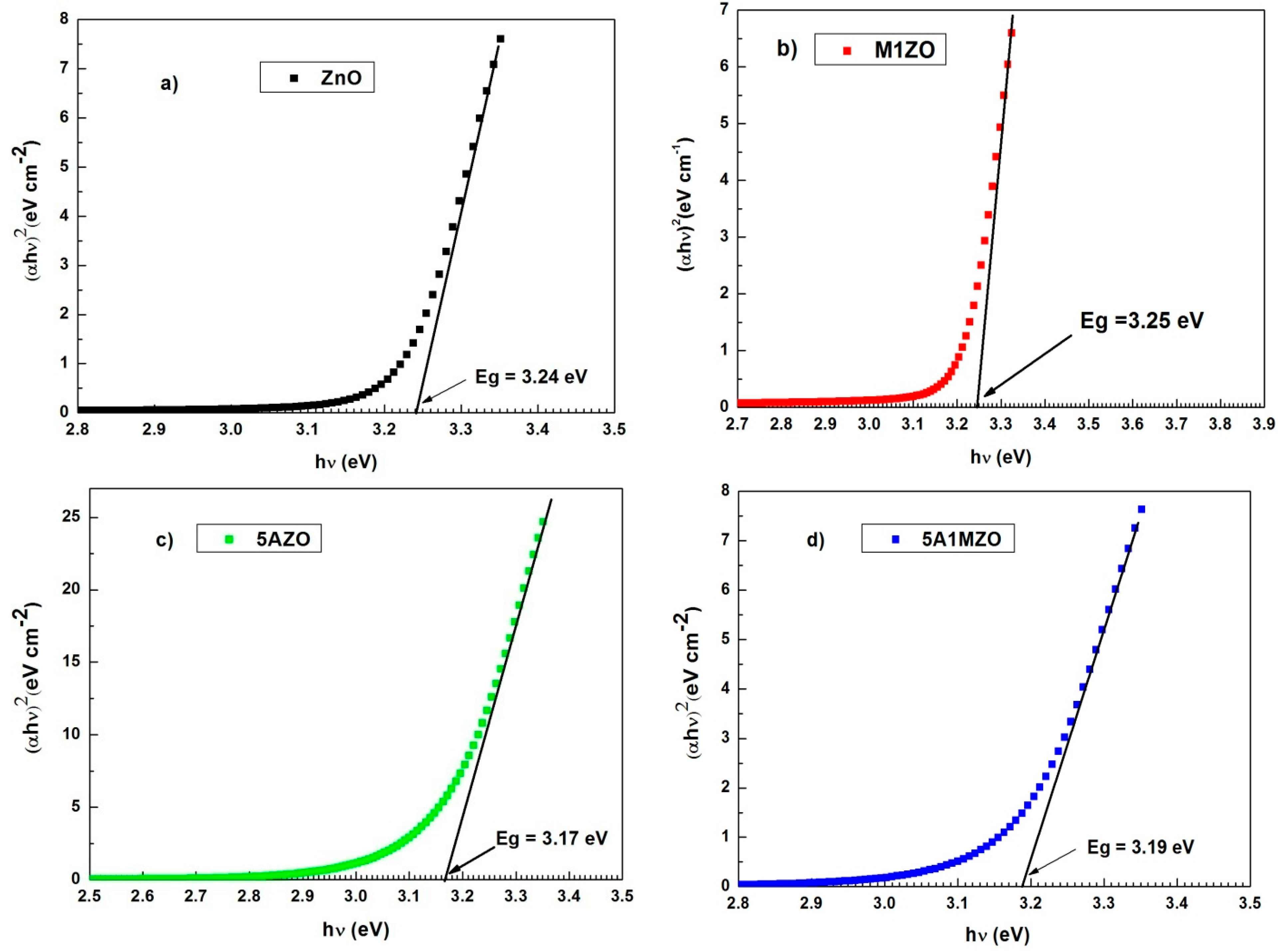



| Samples | Crystallites Size (nm) | Lattice Parameters (Å) | Volume (Å3) |
|---|---|---|---|
| ZnO | 36 | a = 3.246 c = 5.201 | 47.47 |
| 1MZO | 57 | a = 3.249 c = 5.206 | 47.61 |
| 5AZO | 35 | a = 3.250 c = 5.206 | 47.64 |
| 5A1MZO | 27 | a = 3.249 c = 5.203 | 47.58 |
| Gas | Sample | Concentration (ppm) | Response | Temperature (°C) | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|---|
| H2 | WO3/TiO2 nanofibers | 1000 | (Rg-Ra)/Ra = 78.21% | RT | 20 | 23 | [56] |
| Pd-WO3 composite thin films | 2000 | Ra/Rg = 2000 | 250 | 7 | 299 | [57] | |
| 5A1MZO NPs | 2000 | Ra/Rg = 70 | 250 | 3 | 70 | This work | |
| CO | In:ZnO thin films | 5 | (Ra-Rg)/Rg = 4.17 | 300 | 24 | 273 | [58] |
| Pt@ZnOpolyhedrons | 50 | (Ra-Rg)/Ra = 65% | 125 | - | - | [47] | |
| 5A1MZO NPs | 50 | Ra/Rg = 2.01 | 300 | 24 | 35 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaballah, S.; Alaskar, Y.; AlShunaifi, I.; Ghiloufi, I.; Neri, G.; Bouzidi, C.; Dahman, H.; El Mir, L. Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles. Chemosensors 2021, 9, 300. https://doi.org/10.3390/chemosensors9110300
Jaballah S, Alaskar Y, AlShunaifi I, Ghiloufi I, Neri G, Bouzidi C, Dahman H, El Mir L. Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles. Chemosensors. 2021; 9(11):300. https://doi.org/10.3390/chemosensors9110300
Chicago/Turabian StyleJaballah, Soumaya, Yazeed Alaskar, Ibrahim AlShunaifi, Imed Ghiloufi, Giovanni Neri, Chaker Bouzidi, Hassen Dahman, and Lassaad El Mir. 2021. "Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles" Chemosensors 9, no. 11: 300. https://doi.org/10.3390/chemosensors9110300
APA StyleJaballah, S., Alaskar, Y., AlShunaifi, I., Ghiloufi, I., Neri, G., Bouzidi, C., Dahman, H., & El Mir, L. (2021). Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles. Chemosensors, 9(11), 300. https://doi.org/10.3390/chemosensors9110300







