Highly Fluorescent Carbon Dots as a Potential Fluorescence Probe for Selective Sensing of Ferric Ions in Aqueous Solution
Abstract
:1. Introduction
2. Experimental
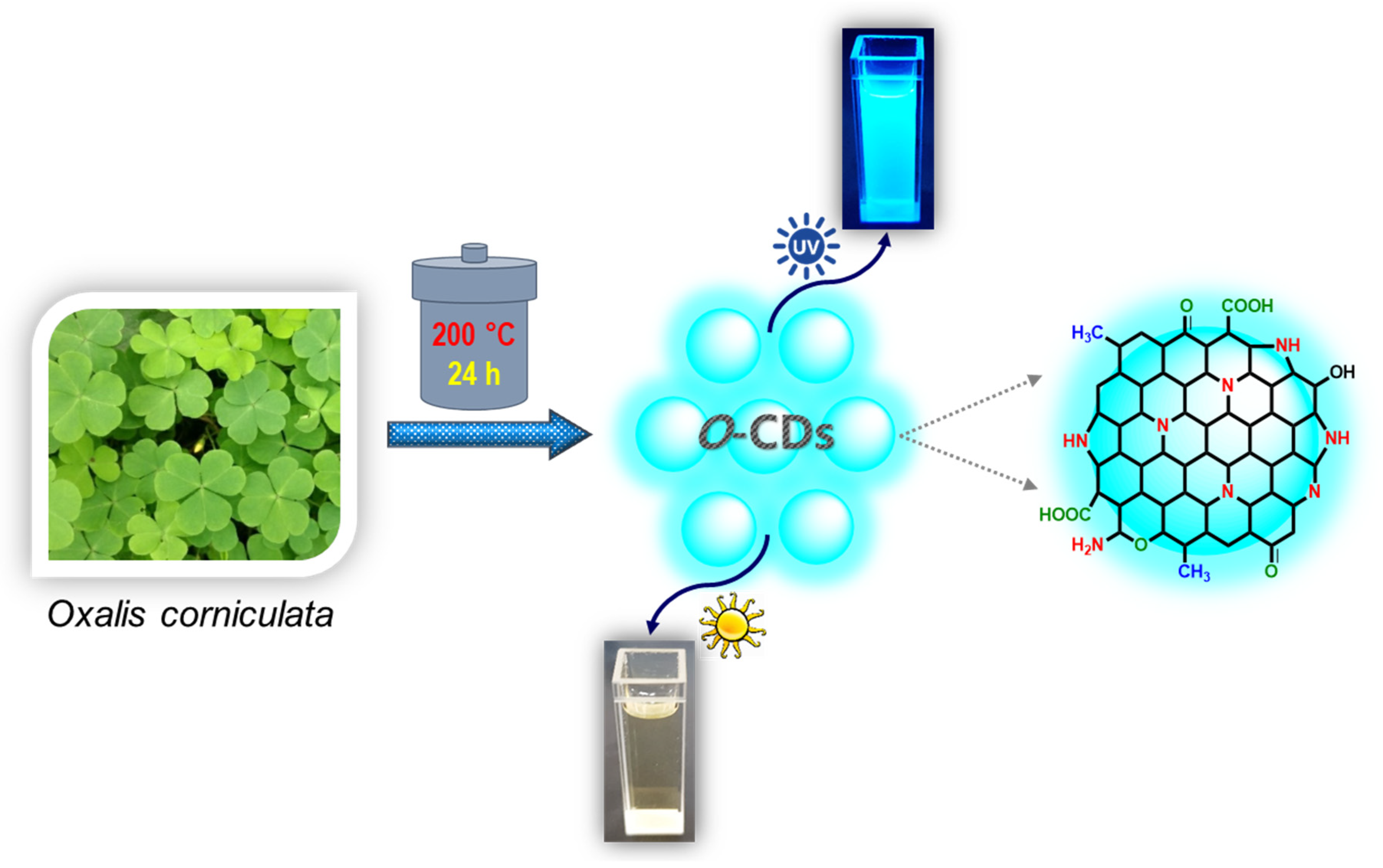
Preparation of O-CDs
3. Results and Discussion
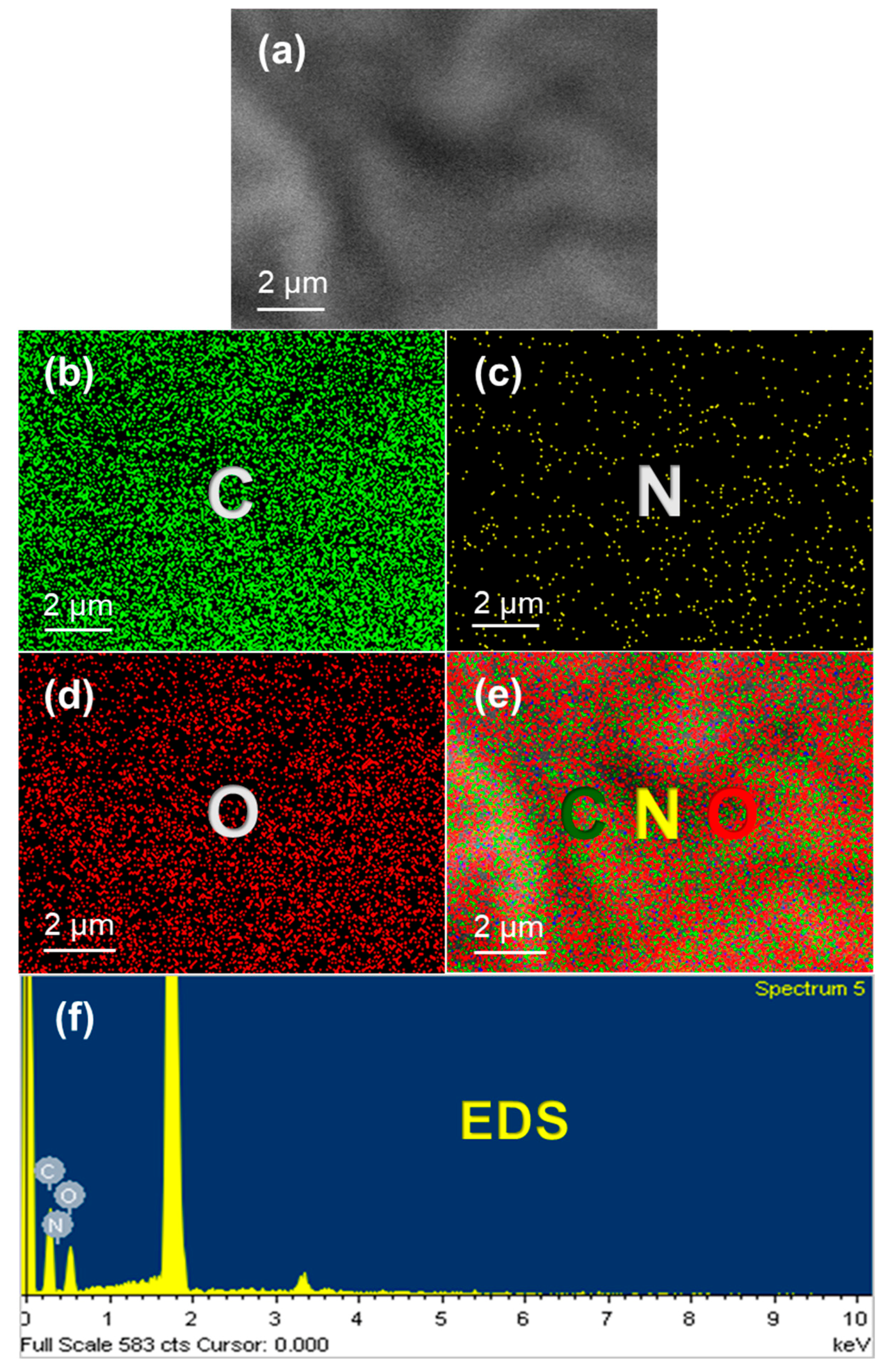
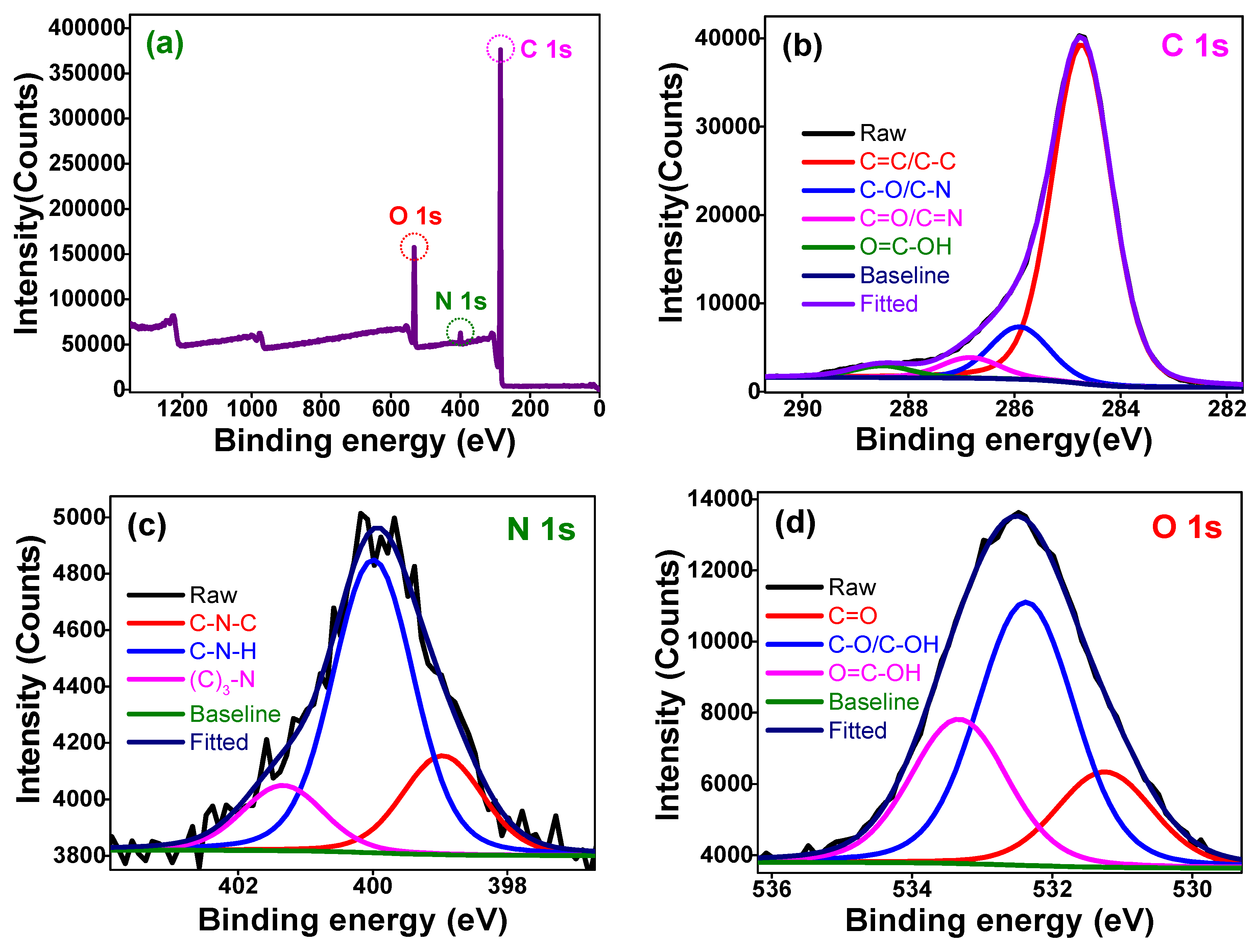
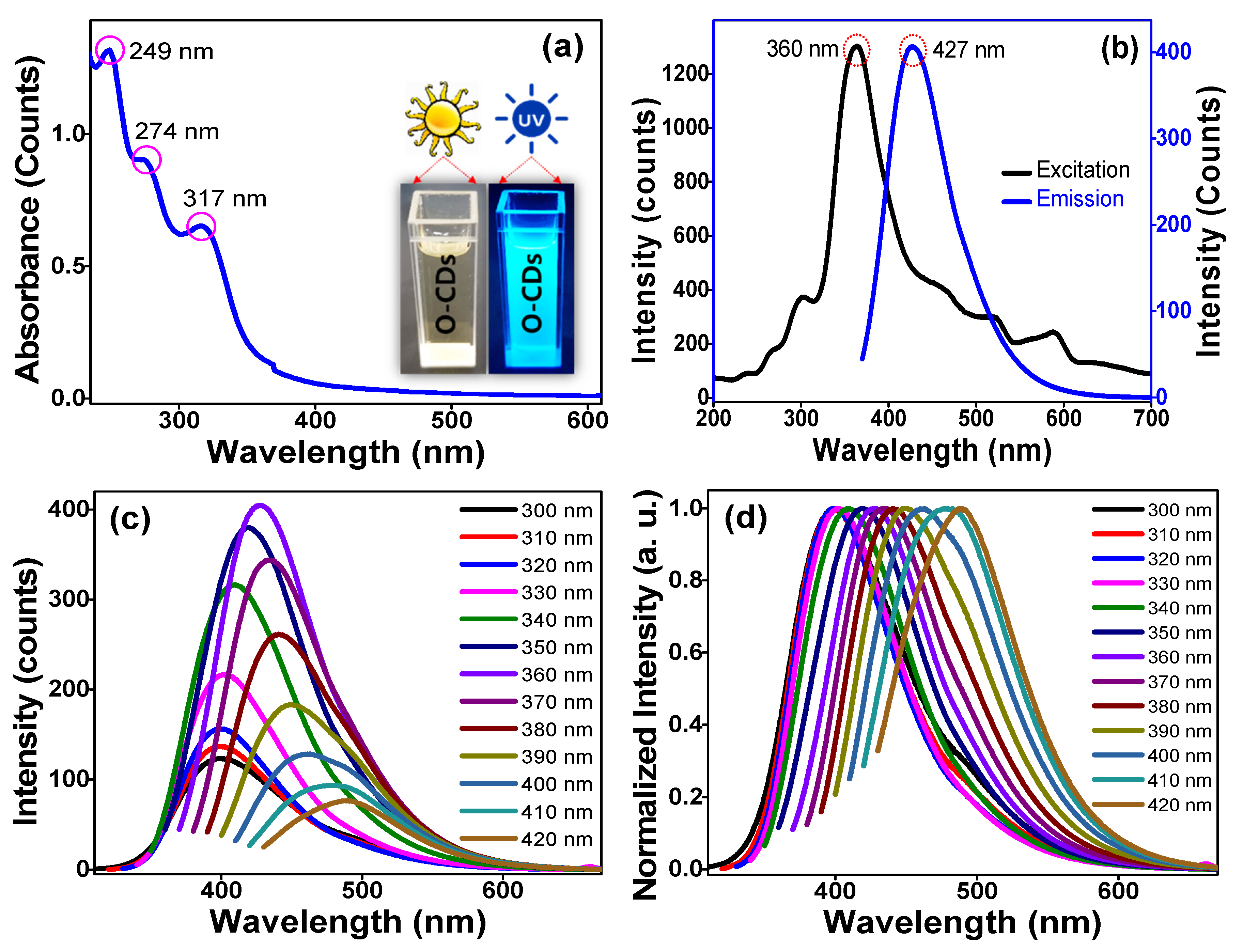
3.1. Structural and Optical Properties of the Hydrophilic O-CDs
3.2. Stability Studies of the Hydrophilic O-CDs
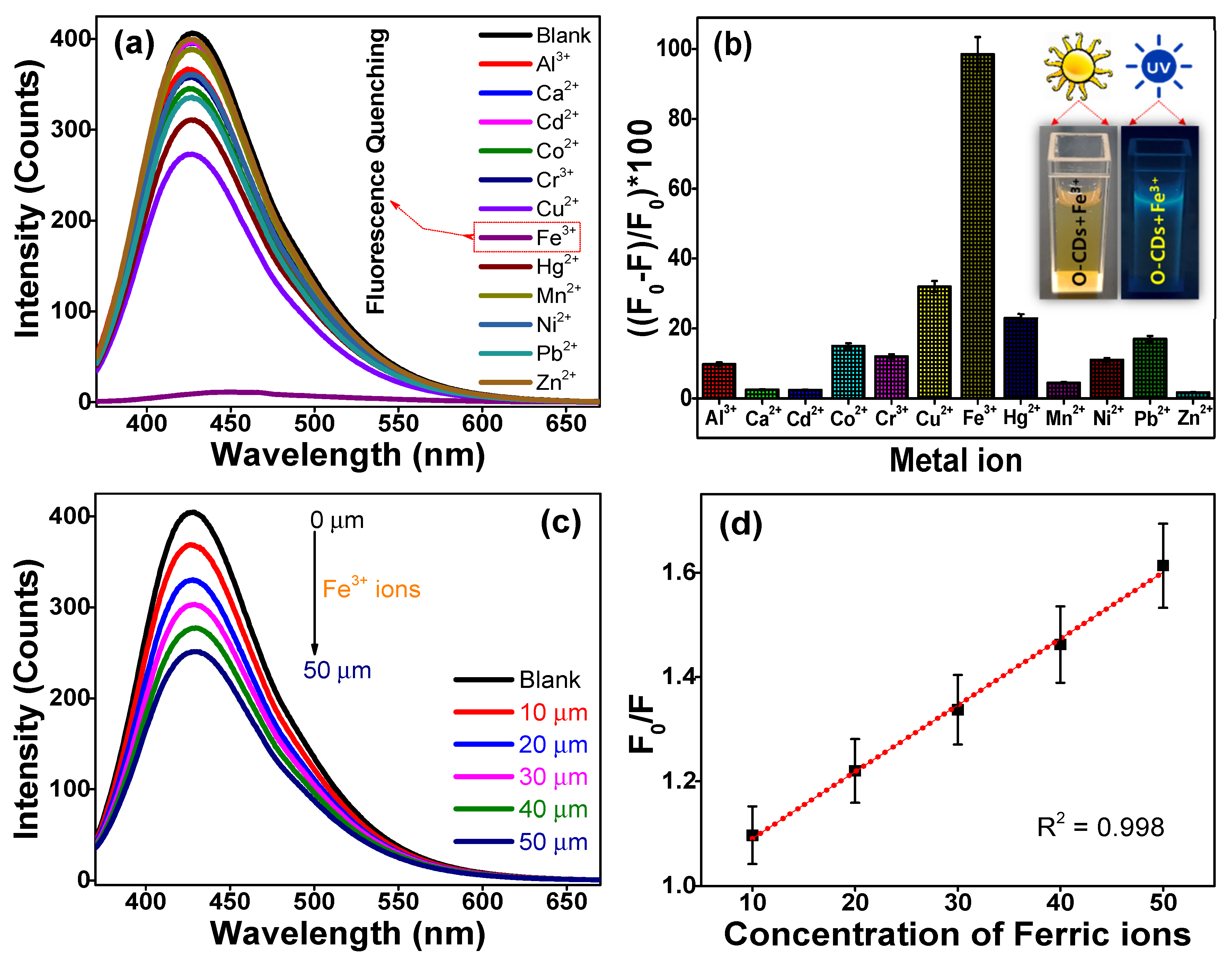
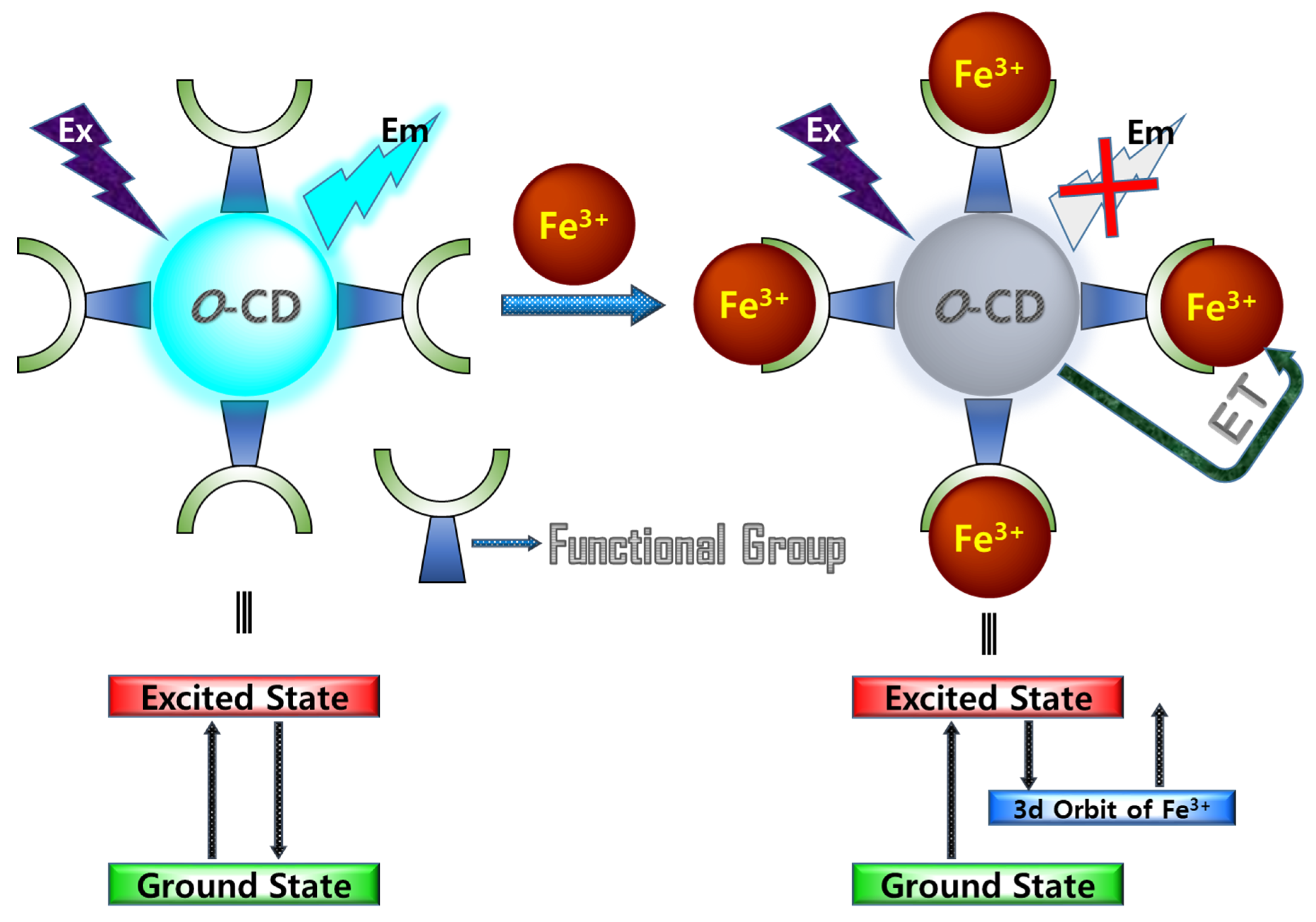
3.3. Fluorometric Sensing of Metal Ions Using Hydrophilic O-CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Zhang, X.; Zhou, Z.; Lu, C. Dual functional biocomposites based on polydopamine modified cellulose nanocrystal for Fe3+-pollutant detecting and autoblocking. ACS Sustain. Chem. Eng. 2016, 4, 5667–5673. [Google Scholar] [CrossRef]
- Li, N.; Araya, S.S.; Kær, S.K. The effect of Fe3+ contamination in feed water on proton exchange membrane electrolyzer performance. Int. J. Hydrogen Energy 2019, 44, 12952–12957. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V. Essential Trace Elements in Human Health: A Physician’s View; Publishing House of Tomsk State University: Tomsk, Russia, 2018. [Google Scholar]
- Saboor, M.; Zehra, A.; Hamali, H.A.; Mobarki, A.A. Revisiting Iron Metabolism, Iron Homeostasis and Iron Deficiency Anemia. Clin. Lab. 2021, 67. [Google Scholar]
- Barić, N.; Cetina, M. Initial bonding of ferric ion (Fe3+) and amyloid beta (Aß) peptide as the precondition for oxidative stress in Alzheimer’s disease. Glycative Stress Res. 2017, 4, 250–265. [Google Scholar]
- Borase, P.N.; Thale, P.B.; Sahoo, S.K.; Shankarling, G.S. An “off–on” colorimetric chemosensor for selective detection of Al3+, Cr3+ and Fe3+: Its application in molecular logic gate. Sens. Actuators B: Chem. 2015, 215, 451–458. [Google Scholar]
- Kaviani, S.; Izadyar, M.; Housaindokht, M.R. DFT investigation on the selective complexation of Fe3+ and Al3+ with hydroxypyridinones used for treatment of the aluminium and iron overload diseases. J. Mol. Graph. Model. 2018, 80, 182–189. [Google Scholar] [PubMed]
- Van Campenhout, A.; Van Campenhout, C.; Lagrou, A.R.; Manuel-y-Keenoy, B. Effects of in vitro glycation on Fe3+ binding and Fe3+ isoforms of transferrin. Clin. Chem. 2004, 50, 1640–1649. [Google Scholar]
- Shahab, U.; Tabrez, S.; Khan, M.S.; Akhter, F.; Khan, M.S.; Saeed, M.; Ahmad, K.; Srivastava, A.; Ahmad, S. Immunogenicity of DNA-advanced glycation end product fashioned through glyoxal and arginine in the presence of Fe3+: Its potential role in prompt recognition of diabetes mellitus auto-antibodies. Chem.-Biol. Interact. 2014, 219, 229–240. [Google Scholar] [PubMed]
- Oh, J.-W.; Kim, T.H.; Yoo, S.W.; Lee, Y.O.; Lee, Y.; Kim, H.; Kim, J.; Kim, J.S. Multisignaling metal sensor: Optical, electrochemical, and electrochemiluminescent responses of cruciform-shaped alkynylpyrene for selective recognition of Fe3+. Sens. Actuators B Chem. 2013, 177, 813–817. [Google Scholar]
- Sayed, A.; Othman, I.M.; Hamam, M.; Gomaa, H.; Gadallah, M.I.; Mostfa, M.; Ali, H.R.H.; Emran, M.Y.; Abdel-Hakim, M.; Mahross, M. A novel fluorescent sensor for fast and highly selective turn-off detection of Fe3+ in water and pharmaceutical samples using synthesized azopyrazole-benzenesulfonamide derivative. J. Mol. Struct. 2021, 1225, 129175. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.; Zhang, A.; Li, B. Catalytic kinetic determination of trace amounts of iron (III) with polarographic detection. Anal. Lett. 1997, 30, 2099–2107. [Google Scholar]
- Bakkaus, E.; Collins, R.N.; Morel, J.-L.; Gouget, B. Anion exchange liquid chromatography–inductively coupled plasma-mass spectrometry detection of the Co2+, Cu2+, Fe3+ and Ni2+ complexes of mugineic and deoxymugineic acid. J. Chromatogr. A 2006, 1129, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.D.; Curtis, R.M.; Wallace, K.J. Low molecular weight fluorescent probes (LMFPs) to detect the group 12 metal triad. Chemosensors 2019, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Fares, N.V.; Medhat, P.M.; El Maraghy, C.M.; Okeil, S.; Ayad, M.F. Influence of Nitrogen-Doped Carbon Dot and Silver Nanoparticle Modified Carbon Paste Electrodes on the Potentiometric Determination of Tobramycin Sulfate: A Comparative Study. Chemosensors 2021, 9, 52. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef]
- Singh, P.; Mittal, L.S.; Vanita, V.; Kumar, K.; Walia, A.; Bhargava, G.; Kumar, S. Self-assembled vesicle and rod-like aggregates of functionalized perylene diimide: Reaction-based near-IR intracellular fluorescent probe for selective detection of palladium. J. Mater. Chem. B 2016, 4, 3750–3759. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S.; Singha, N.; Das, D. Self-Assembly Assisted Tandem Sensing of Pd2+ and CN− by a Perylenediimide-Peptide Conjugate. ChemistrySelect 2017, 2, 10061–10066. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar]
- Wei, X.; Li, H.; Li, Z.; Vuki, M.; Fan, Y.; Zhong, W.; Xu, D. Metal-enhanced fluorescent probes based on silver nanoparticles and its application in IgE detection. Anal. Bioanal. Chem. 2012, 402, 1057–1063. [Google Scholar] [PubMed]
- Rizvi, S.B.; Ghaderi, S.; Keshtgar, M.; Seifalian, A.M. Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev. 2010, 1, 5161. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Lee, Y.R. Hydrothermal conversion of Magnolia liliiflora into nitrogen-doped carbon dots as an effective turn-off fluorescence sensing, multi-colour cell imaging and fluorescent ink. Colloids Surf. B Biointerfaces 2018, 169, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Calì, F.; Cantaro, V.; Fichera, L.; Ruffino, R.; Trusso Sfrazzetto, G.; Li-Destri, G.; Tuccitto, N. Carbon Quantum Dots from Lemon Waste Enable Communication among Biodevices. Chemosensors 2021, 9, 202. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Sonkar, S.K.; Yang, F.; Veca, L.M.; Wang, P.; Tackett, K.N.; Yu, J.-J.; Vasile, E.; Qian, H.; Liu, Y.; et al. Toward Structurally Defined Carbon Dots as Ultracompact Fluorescent Probes. ACS Nano 2014, 8, 4522–4529. [Google Scholar] [CrossRef]
- Lee, H.; Su, Y.-C.; Tang, H.-H.; Lee, Y.-S.; Lee, J.-Y.; Hu, C.-C.; Chiu, T.-C. One-Pot Hydrothermal Synthesis of Carbon Dots as Fluorescent Probes for the Determination of Mercuric and Hypochlorite Ions. Nanomaterials 2021, 11, 1831. [Google Scholar]
- Xie, Y.; Cheng, D.; Liu, X.; Han, A. Green Hydrothermal Synthesis of N-doped Carbon Dots from Biomass Highland Barley for the Detection of Hg(2). Sensors 2019, 19, 3169. [Google Scholar] [CrossRef] [Green Version]
- Pu, Z.-F.; Wen, Q.-L.; Yang, Y.-J.; Cui, X.-M.; Ling, J.; Liu, P.; Cao, Q.-E. Fluorescent carbon quantum dots synthesized using phenylalanine and citric acid for selective detection of Fe3+ ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117944. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Jorns, M.; Pappas, D. A Review of Fluorescent Carbon Dots, Their Synthesis, Physical and Chemical Characteristics, and Applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Lee, Y.R. Leftover Kiwi Fruit Peel-Derived Carbon Dots as a Highly Selective Fluorescent Sensor for Detection of Ferric Ion. Chemosensors 2021, 9, 166. [Google Scholar]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar]
- Li, F.; Yang, D.; Xu, H. Non-Metal-Heteroatom-Doped Carbon Dots: Synthesis and Properties. Chem.–A Eur. J. 2019, 25, 1165–1176. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Shim, J.-J.; Kalimuthu, S.; Ahn, B.-C.; Lee, Y.R. Turn-off fluorescence sensor for the detection of ferric ion in water using green synthesized N-doped carbon dots and its bio-imaging. J. Photochem. Photobiol. B Biol. 2016, 158, 235–242. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Lee, Y.R. Betel-derived nitrogen-doped multicolor carbon dots for environmental and biological applications. J. Mol. Liq. 2019, 296, 111817. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar]
- Kiran, K.; Awathade, K.; Kumar, J.; Kanthesh, B. Protective Effect of Oxalis Corniculate Extract against Toxic Enzymes of Najanaja and Daboiarusselli Venom. Ann. Rom. Soc. Cell Biol. 2021, 25, 7304–7316. [Google Scholar]
- Wei, W.; Huang, J.; Gao, W.; Lu, X.; Shi, X. Carbon Dots Fluorescence-Based Colorimetric Sensor for Sensitive Detection of Aluminum Ions with a Smartphone. Chemosensors 2021, 9, 25. [Google Scholar]
- Hsu, P.-C.; Shih, Z.-Y.; Lee, C.-H.; Chang, H.-T. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Boukaoud, A.; Chiba, Y.; Sebbar, D. A periodic DFT study of IR spectra of amino acids: An approach toward a better understanding of the NH and OH stretching regions. Vib. Spectrosc. 2021, 116, 103280. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Z.; Hu, Y.; Li, J.; Fan, X. Multiple functionalization of multi-walled carbon nanotubes with carboxyl and amino groups. Appl. Surf. Sci. 2013, 276, 476–481. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe(3+) sensing and cell imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Jia, J.; Lin, B.; Gao, Y.; Jiao, Y.; Li, L.; Dong, C.; Shuang, S. Highly luminescent N-doped carbon dots from black soya beans for free radical scavenging, Fe3+ sensing and cellular imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 363–372. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Jana, G.C.; Aktara, M.N.; Das, S.; Nayim, S.; Patra, A.; Bhattacharjee, P.; Bhadra, K.; Hossain, M. Carbon dots derived from lychee waste: Application for Fe(3+) ions sensing in real water and multicolor cell imaging of skin melanoma cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110429. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Cranberry Beans Derived Carbon Dots as a Potential Fluorescence Sensor for Selective Detection of Fe3+ Ions in Aqueous Solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.U.; Wang, D.; Zhang, W.; Tang, Z.; Ma, X.; Ding, X.; Du, S.; Wang, Y. High quantum yield green-emitting carbon dots for Fe (III) detection, biocompatible fluorescent ink and cellular imaging. Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- Yang, G.; Wan, X.; Su, Y.; Zeng, X.; Tang, J. Acidophilic S-doped carbon quantum dots derived from cellulose fibers and their fluorescence sensing performance for metal ions in an extremely strong acid environment. J. Mater. Chem. A 2016, 4, 12841–12849. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, G.; Tan, X.; Qian, X.; Zhang, T.; Gui, J.; Yang, L.; Xie, X. Nitrogen-doped carbon dots with high quantum yield for colorimetric and fluorometric detection of ferric ions and in a fluorescent ink. Microchim. Acta 2019, 186, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Zhao, X.; Deng, Y.; Xia, Y. Facile Synthesis of Nitrogen-Doped Carbon Quantum Dots with Chitosan for Fluorescent Detection of Fe3+. Polymers 2019, 11, 1731. [Google Scholar] [CrossRef] [Green Version]


| No. | Carbon Precursor | Excitation (nm) | QY (%) | LOD/Linear Range (μM) | Reference |
|---|---|---|---|---|---|
| 1 | Onion waste | 380 | 28 | 0.31/ 0–20 | [46] |
| 2 | Rice residue | 365 | 23.48 | 0.74/3.3–32.2 | [47] |
| 3 | Sweet potato | 360 | 8.64 | 0.32/1–100 | [48] |
| 4 | Black soya bean | 360 | 38.7 | 0.096/0.2−300 | [49] |
| 5 | Lychee waste | 365 | 23.5 | 0.023/0–1.6 | [50] |
| 6 | Cranberry beans | 380 | 10.85 | 9.55/30–600 | [51] |
| 7 | Phyllanthus acidus | 350 | 14 | 0.9/2–25 | [18] |
| 8 | Prunus avium | 310 | 13 | 0.96/0–100 | [38] |
| 9 | Chionanthus retusus | 340 | 9 | 70/ 0–25 | [24] |
| 10 | Diammonium hydrogen citrate | 420 | 46.4 | 19/0–300 | [52] |
| 11 | Cellulose fibers | 360 | 32 | 0.96/25–250 | [53] |
| 12 | Kiwi fruit peel | 360 | 19 | 0.85/5–25 | [33] |
| 13 | Oxalis corniculata | 360 | 20 | 0.73/10–50 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchudan, R.; Kishore, S.C.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Alagan, M.; Lee, Y.R. Highly Fluorescent Carbon Dots as a Potential Fluorescence Probe for Selective Sensing of Ferric Ions in Aqueous Solution. Chemosensors 2021, 9, 301. https://doi.org/10.3390/chemosensors9110301
Atchudan R, Kishore SC, Edison TNJI, Perumal S, Vinodh R, Sundramoorthy AK, Babu RS, Alagan M, Lee YR. Highly Fluorescent Carbon Dots as a Potential Fluorescence Probe for Selective Sensing of Ferric Ions in Aqueous Solution. Chemosensors. 2021; 9(11):301. https://doi.org/10.3390/chemosensors9110301
Chicago/Turabian StyleAtchudan, Raji, Somasundaram Chandra Kishore, Thomas Nesakumar Jebakumar Immanuel Edison, Suguna Perumal, Rajangam Vinodh, Ashok K. Sundramoorthy, Rajendran Suresh Babu, Muthulakshmi Alagan, and Yong Rok Lee. 2021. "Highly Fluorescent Carbon Dots as a Potential Fluorescence Probe for Selective Sensing of Ferric Ions in Aqueous Solution" Chemosensors 9, no. 11: 301. https://doi.org/10.3390/chemosensors9110301
APA StyleAtchudan, R., Kishore, S. C., Edison, T. N. J. I., Perumal, S., Vinodh, R., Sundramoorthy, A. K., Babu, R. S., Alagan, M., & Lee, Y. R. (2021). Highly Fluorescent Carbon Dots as a Potential Fluorescence Probe for Selective Sensing of Ferric Ions in Aqueous Solution. Chemosensors, 9(11), 301. https://doi.org/10.3390/chemosensors9110301












