Comparing Surface Plasmon-Optical and Electronic Immuno-Sensing of Affinity Interactions—A Case Study
Abstract
1. Introduction
2. Surface Plasmon Optical Detection
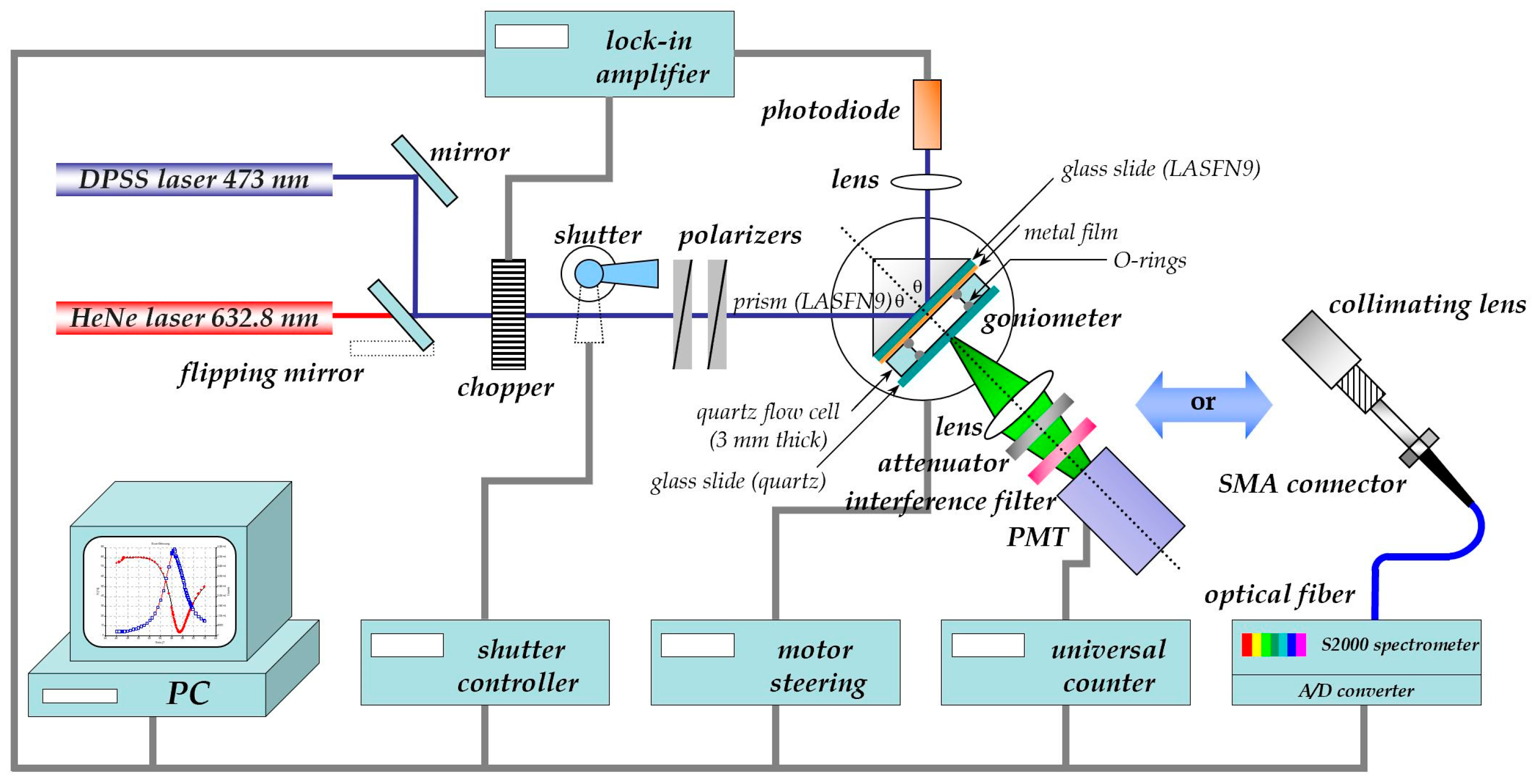
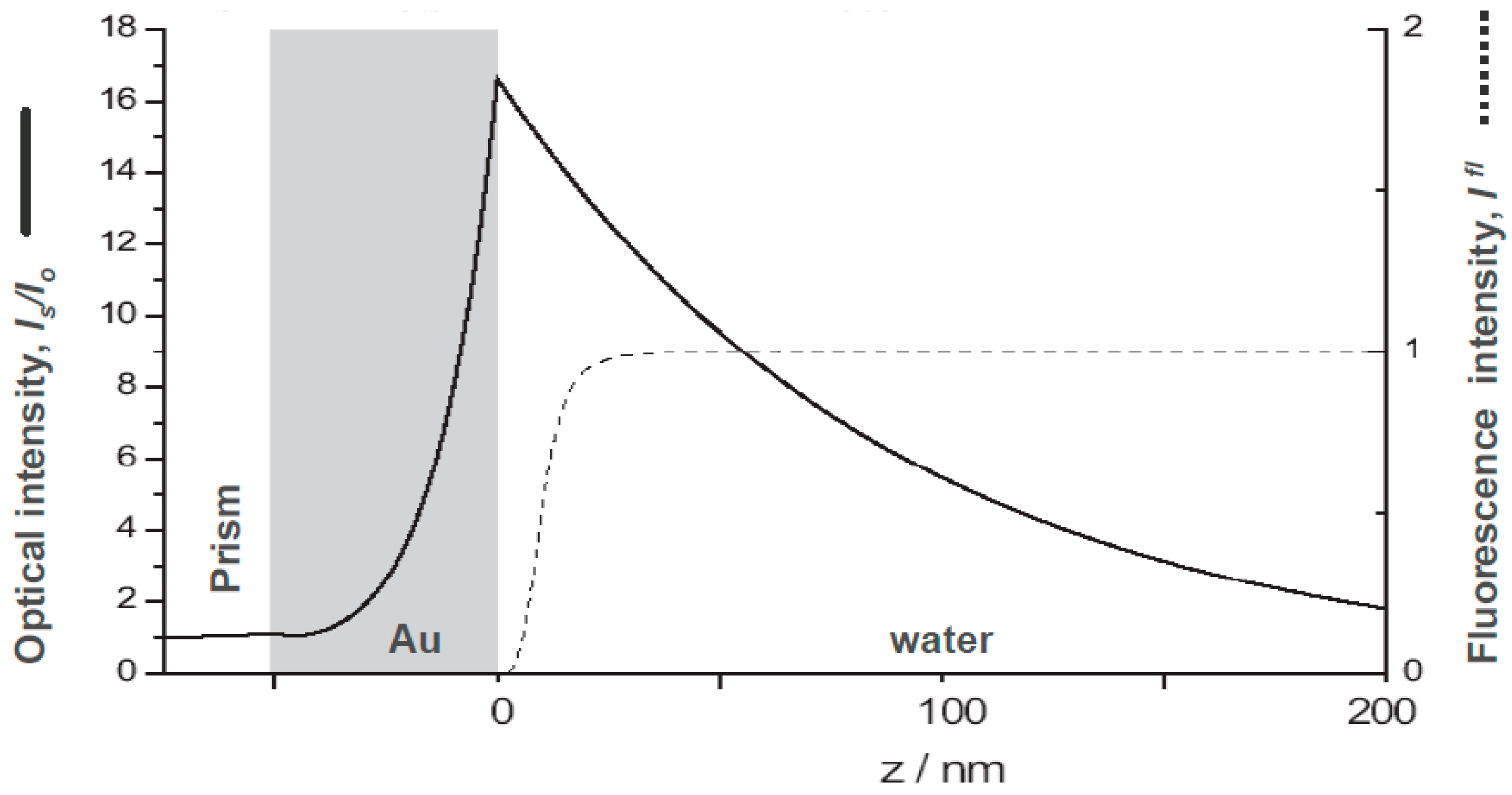
2.1. The Basics of Surface Plasmon Fluorescence Spectroscopy
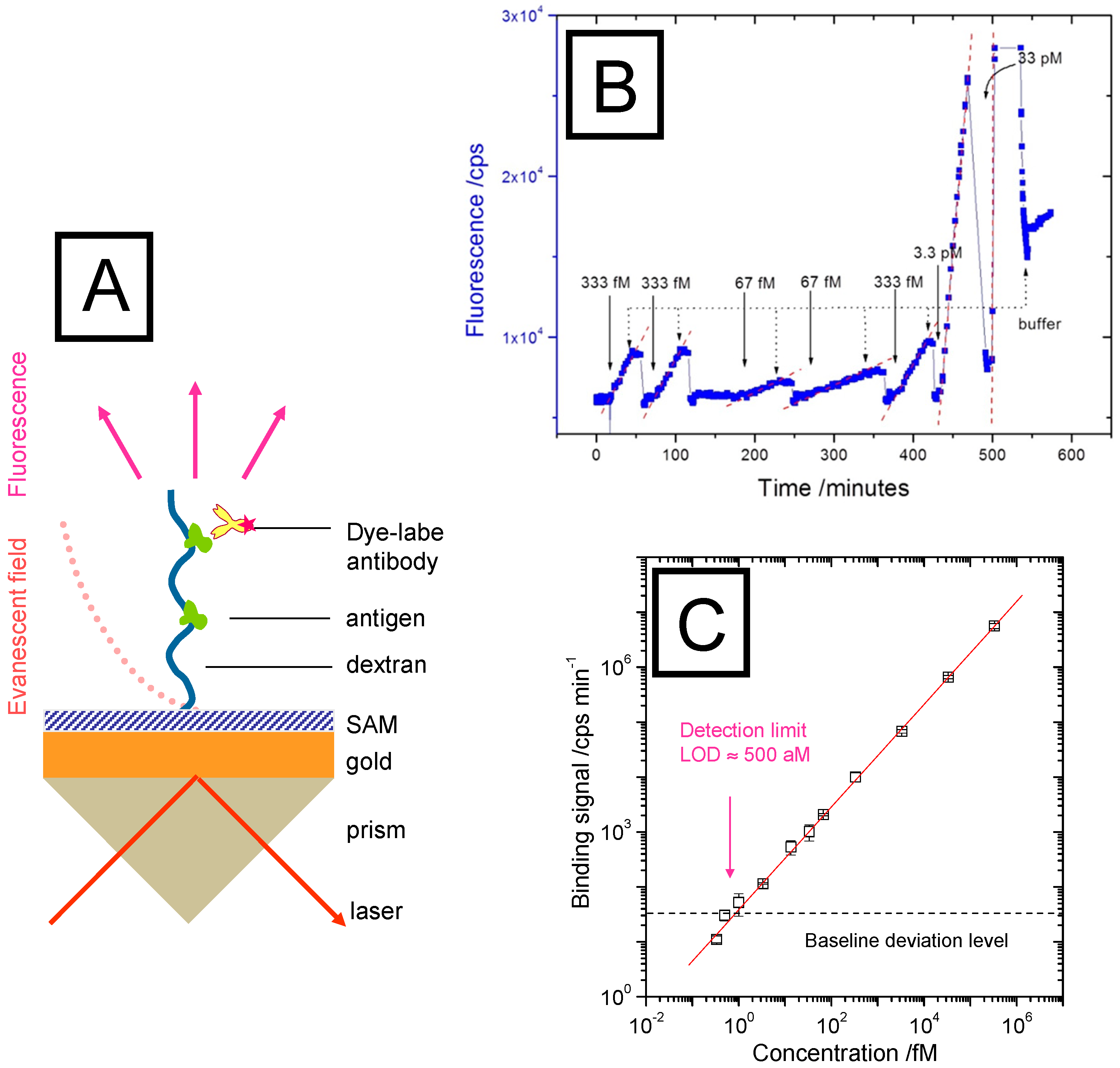
2.2. Sandwich Fluorescence Assays for Human Chorionic Gonadotropin Monitoring
2.3. Extending the Sensitivity by A Polymer Brush Architecture as Binding Matrix
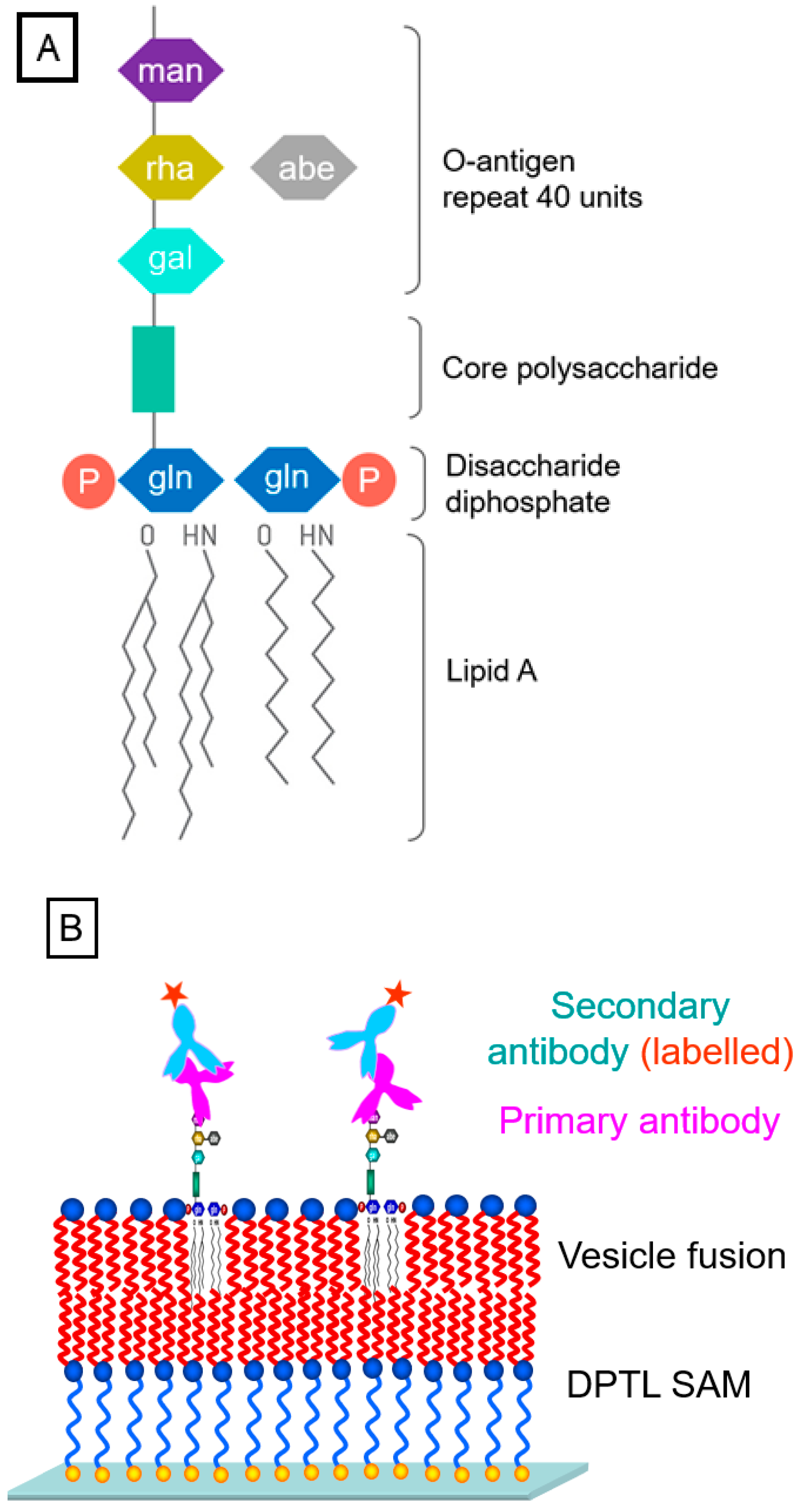
2.4. Immuno-Detection of Lipopolysaccharides in A Tethered Bimolecular Lipid Membrane
3. Electronic Bio-Sensing
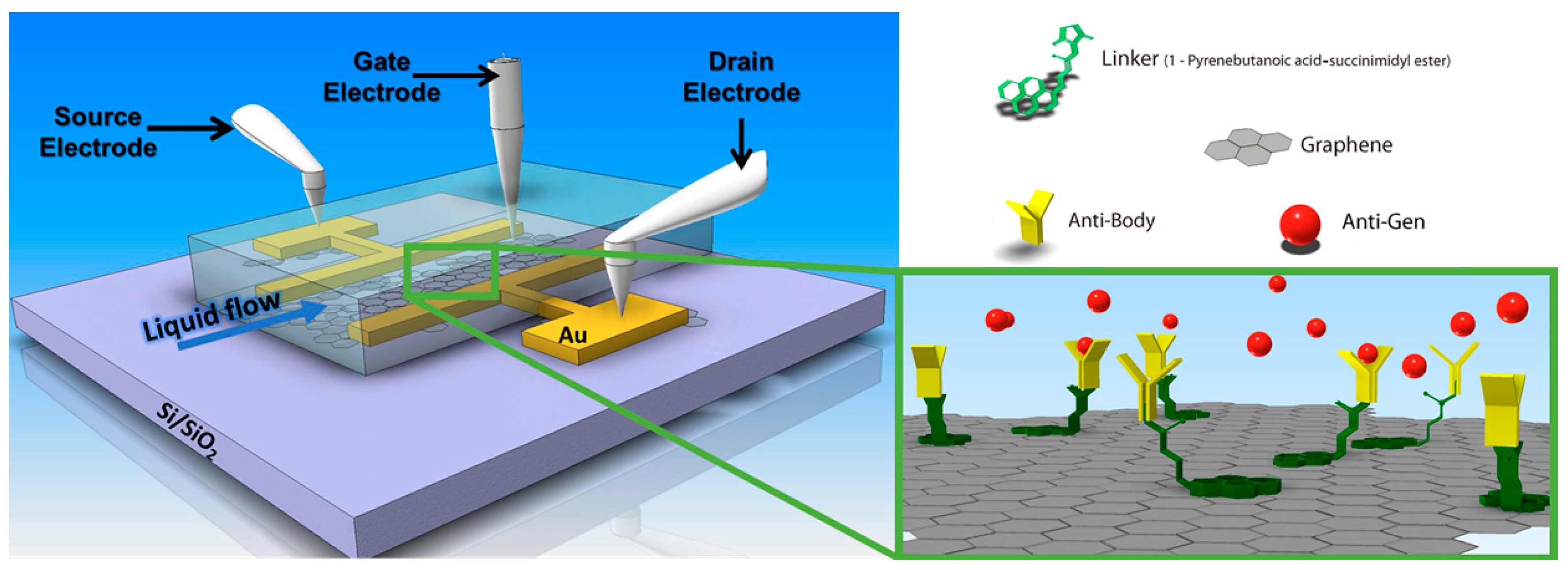
3.1. rGO Based Field Effect Transistors
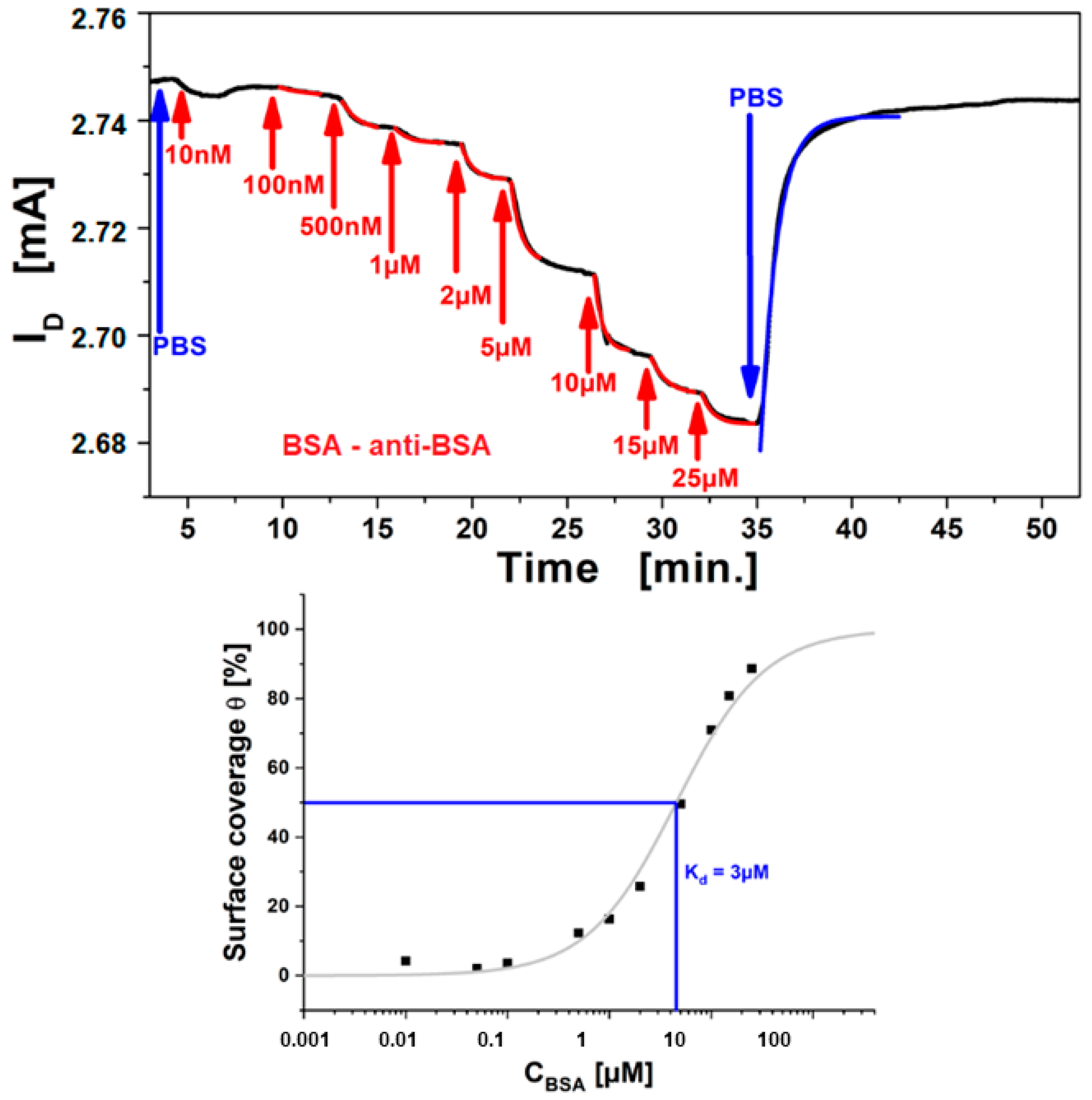
3.2. Immuno-Sensing of Antigens by rGO FETs
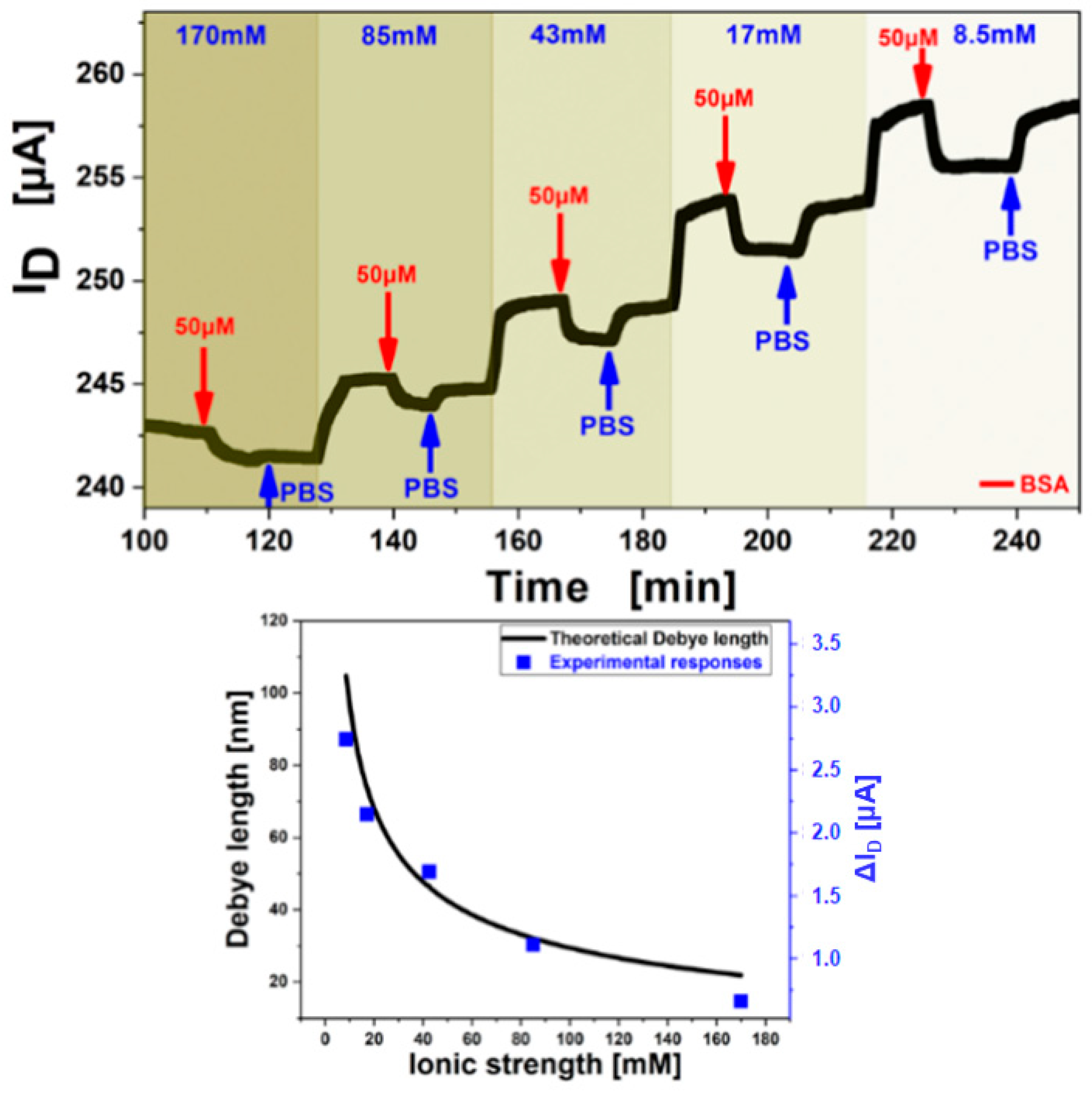
3.3. Debye Length Dependence
3.4. Limit of Detection in Electronic Bio-Sensing
3.5. Small Analyte Detection by Antibodies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glossary, National Agricultural Library. Available online: https://agclass.nal.usda.gov/mtwdk.exe?k=glossary&l=60&w=7776&s=5&t=2 (accessed on 1 November 2020).
- Knoll, W. Interfaces and Thin Films as Seen by Bound Electromagnetic Waves. Ann. Rev. Phys. Chem. 1998, 49, 569–638. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, T.; Knoll, W. Surface-Plasmon Field-Enhanced Fluorescence Spectroscopy. Colloids Surf. A 2000, 171, 115–130. [Google Scholar] [CrossRef]
- Zong, Y.; Xu, F.; Su, X.D.; Knoll, W. Quartz crystal microbalance with integrated surface plasmon grating coupler. Anal. Chem. 2008, 80, 5246–5250. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, W.; El-sayed, M.A. On the Universal Scaling Behavior of the Distance Decay of Plasmon Coupling in Metal Nanoparticle Pairs: A Plasmon Ruler Equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Kuhn, H. Classical Aspects of Energy Transfer in Molecular Systems. J. Chem. Phys. 1970, 53, 101–108. [Google Scholar] [CrossRef]
- Berger, P.; Sturgeon, C.; Bidart, J.M.; Paus, E.; Gerth, R.; Niang, M.; Bristow, A.; Birken, S.; Stenman, U.H. The ISOBM TD-7 workshop on hCG and related molecules—Towards user-oriented standardization of pregnancy and tumor diagnosis: Assignment of epitopes to the three-dimensional structure of diagnostically and commercially relevant monoclonal antibodies directed against human chorionic gonadotropin and derivatives. Tumor Biol. 2002, 23, 1–38. [Google Scholar]
- Fotinou, C.; Beauchamp, J.; Emsley, P.; de Haan, A.; Schielen, W.J.; Bos, E.; Isaacs, N.W. Structure of an Fab fragment against a C-terminal peptide of hCG at 2.0 Angstrom resolution. J. Biol. Chem. 1998, 273, 22515–22518. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.Y.; Cheung, V.Y.T. Time to Revisit the Human Chorionic Gonadotropin Discriminatory Level in the Management of Pregnancy of Unknown Location. J. Ultrasound Med. 2014, 33, 465–471. [Google Scholar] [CrossRef]
- Jena, S.C.; Shrivastava, S.; Saxena, S.; Kumar, N.; Maiti, S.K.; Mishra, B.P.; Singh, R.K. Surface plasmon resonance immunosensor for label-free detection of BIRC5 biomarker in spontaneously occurring canine mammary tumours. Sci. Rep. 2019, 9, 13485. [Google Scholar] [CrossRef]
- Spinke, J.; Liley, M.; Guder, H.J.; Angermaier, L.; Knoll, W. Molecular Recognition at Self—Assembled Monolayers—The Construction of Multicomponent Multilayers. Langmuir 1993, 9, 1821–1825. [Google Scholar] [CrossRef]
- Yu, F.; Persson, B.; Lofas, S.; Knoll, W. Attomolar sensitivity in bioassays based on surface plasmon fluorescence spectroscopy. J. Am. Chem. Soc. 2004, 126, 8902–8903. [Google Scholar] [CrossRef] [PubMed]
- Wilchek, M.; Bayer, E.A. Foreword and introduction to the book (strept)avidin-biotin system. Biomol. Eng. 1999, 16, 1–4. [Google Scholar] [PubMed]
- Spinke, J.; Liley, M.; Schmitt, F.-J.; Guder, H.-J.; Angermaier, L.; Knoll, W. Molecular Recognition at Self-Assembled Monolayers—Optimization of Surface Functionalization. J. Chem. Phys. 1993, 99, 7012–7019. [Google Scholar] [CrossRef]
- Grabbe, E.S. Total Internal-Reflection Fluorescence with Energy-Transfer—A Method for Analyzing IgG Adsorption on Nylon Thin-Films. Langmuir 1993, 9, 1574–1581. [Google Scholar] [CrossRef]
- Liu, J. Systematic Studies of Protein Immobilization by Surface Plasmon Field-Enhanced Fluorescence Spectroscopy. Ph.D. Thesis, University of Mainz, Mainz, Germany, 2005. [Google Scholar]
- Available online: https://www.biacore.com/lifesciences/index.html (accessed on 1 November 2020).
- Robertson, J.L. The lipid bilayer membrane and its protein constituents. J. Gen. Physiol. 2018, 150, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Knoll, W. Tethered Lipid Membranes as Platforms for Biophysical Studies and Advanced Biosensors. In Biomimetic Lipid Membranes: Fundamentals, Applications, and Commercialization; Springer: Cham, Germany, 2019; pp. 183–191. [Google Scholar]
- Jackman, J.A.; Knoll, W.; Cho, N.J. Biotechnology applications of tethered lipid bilayer membranes. Materials 2012, 5, 2637–2657. [Google Scholar] [CrossRef]
- Niu, L.; Wohland, T.; Knoll, W.; Köper, I. Interaction of a synthetic antimicrobial peptide with a model bilayer platform mimicking bacterial membranes. Biointerphases 2017, 12, 04E404. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, H.; Cao, L.; Chen, Z. A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg. Biosensors 2020, 10, 24. [Google Scholar] [CrossRef]
- De Moraes, A.C.M.; Kubota, L.T. Recent Trends in Field-Effect Transistors-Based Immunosensors. Chemosensors 2016, 4, 20. [Google Scholar] [CrossRef]
- MAgliolo, M.; De Tullio, D.; Vikholm-Lundin, I.; Albers, W.M.; Munter, T.; Manoli, K.; Palazzo, G.; Torsi, L. Label-free C-reactive protein electronic detection with an electrolyte-gated organic field-effect transistor-based immunosensor. Anal. Bioanal. Chem. 2016, 408, 3943–3952. [Google Scholar] [CrossRef]
- Khan, H.U.; Jang, J.; Kim, J.-J.; Knoll, W. In Situ Antibody Detection and Charge Discrimination Using Aqueous Stable Pentacene Transistor Biosensors. J. Am. Chem. Soc. 2011, 133, 2170–2176. [Google Scholar] [CrossRef]
- Roberts, M.E.; Mannsfeld, S.C.B.; Queralto, N.; Reese, C.; Locklin, C.; Knoll, W.; Bao, Z.N. Water-stable organic transistors and their application in chemical and biological sensors. Proc. Natl. Acad. Sci. USA 2008, 105, 12134–12139. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Huang, Y.; Chen, Y. Focussing on Energy and Optoelectronic Applications: A Journey for Graphene and Graphene Oxide at Large Scale. Acc. Chem. Res. 2012, 45, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene Field-Effect Transistor and its Application for Electronic Sensing. Small 2014, 10, 4042–40065. [Google Scholar] [CrossRef] [PubMed]
- Larisika, M.; Huang, J.F.; Tok, A.; Knoll, W.; Nowak, C. An improved synthesis route to graphene for molecular sensor applications. Mater. Chem. Phys. 2012, 136, 304–308. [Google Scholar] [CrossRef]
- Rozman, C.; Larisika, M.; Nowak, C.; Knoll, W. Graphene-Based Liquid-Gated Field Effect Transistor for Biosensing: Theory and Experiments. Biosens. Bioelectron. 2015, 70, 21–27. [Google Scholar] [CrossRef]
- Kodali, V.K.; Scrimgeour, J.; Kim, S.; Hankinson, J.H.; Carroll, K.M.; de Heer, W.A.; Berger, C.; Curtis, J.E. Nonperturbative chemical modification of graphene for protein micropatterning. Langmuir 2011, 27, 863–865. [Google Scholar] [CrossRef]
- Larisika, M.; Kotlowski, C.; Steininger, C.; Mastrogiacomo, R.; Pelosi, P.; Schütz, S.; Peteu, S.F.; Kleber, C.; Reiner-Rozman, C.; Nowak, C.; et al. Electronic olfactory sensor based on A. mellifera odorant-binding protein 14 on a reduced graphene oxide field-effect transistor. Angew. Chem. Int. Ed. 2015, 54, 13245–13248. [Google Scholar] [CrossRef]
- Reiner-Rozman, C.; Kotlowski, C.; Knoll, W. Electronic Biosensing with Functionalized rGO FETs. Biosensors 2016, 6, 17. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. II. Liq. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Shreeve, B.J.; Patterson, D.S.P.; Roberts, B.A. Mycotoxins and Their Metabolites in Humans and Animals. Food Cosmet. Toxicol. 1979, 17, 151–152. [Google Scholar] [CrossRef]
- Paniel, N.; Radoi, A.; Marty, J.L. Development of an electrochemical biosensor for the detection of aflatoxin M1 in milk. Sensors 2010, 10, 9439–9448. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 466/2001. Available online: http://ec.europa.eu/food/fs/sfp/fcr/fcr02_en.pdf (accessed on 12 April 2016).
- Wang, Y.; Dostalek, J.; Knoll, W. Long Range Surface Plasmon-enhanced Fluorescence Spectroscopy for the Detection of Aflatoxin M-1 in milk. Biosens. Bioelectron. 2009, 24, 2264–2267. [Google Scholar] [CrossRef]
- Feng, C.L.; Zhong, X.; Steinhart, M.; Majoral, J.P.; Knoll, W. Functional Quantum-Dot/Dendrimer Nanotubes for Sensitive Detection of DNA Hybridization. Small 2008, 4, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Virasamy, S.; Knoll, W.; Liu, K.Y.; Tse, M.S.; Yen, L.W. Fabrication of Gold Nanocrescents by Angle Deposition with Nanosphere Lithography for Localized Surface Plasmon Resonance Applications. J. Nanosci. Nanotechnol. 2008, 8, 3369–3378. [Google Scholar] [CrossRef]
- Wang, Y.; Dostalek, J.; Knoll, W. Magnetic Nanoparticle-Enhanced Biosensor Based on Grating-Coupled Surface Plasmon Resonance. Anal. Chem. 2011, 83, 6202–6207. [Google Scholar] [CrossRef]
- Kotlowski, C.; Larisika, M.; Guerin, P.M.; Kleber, C.; Kröber, T.; Mastrogiacomo, R.; Nowak, C.; Pelosi, P.; Schütz, S.; Schwaighofer, A.; et al. Fine discrimination of volatile compounds by graphene-immobilized odorant-binding proteins. Sens. Act. B Chem. 2018, 256, 564–572. [Google Scholar] [CrossRef]
- Khan, H.U.; Roberts, M.E.; Johnson, O.; Förch, R.; Knoll, W.; Bao, Z. In Situ, Label-Free DNA Detection Using Organic Transistor Sensors. Adv. Mater. 2010, 22, 4452–4456. [Google Scholar] [CrossRef]
- Bruno, J.G. Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics. Molecules 2015, 20, 6866–6887. [Google Scholar] [CrossRef]
- Tiede, C. Affimer proteins are versatile and renewable affinity reagents. eLife 2017, 6, e24903. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.aptabiosciences.com/ (accessed on 1 November 2020).
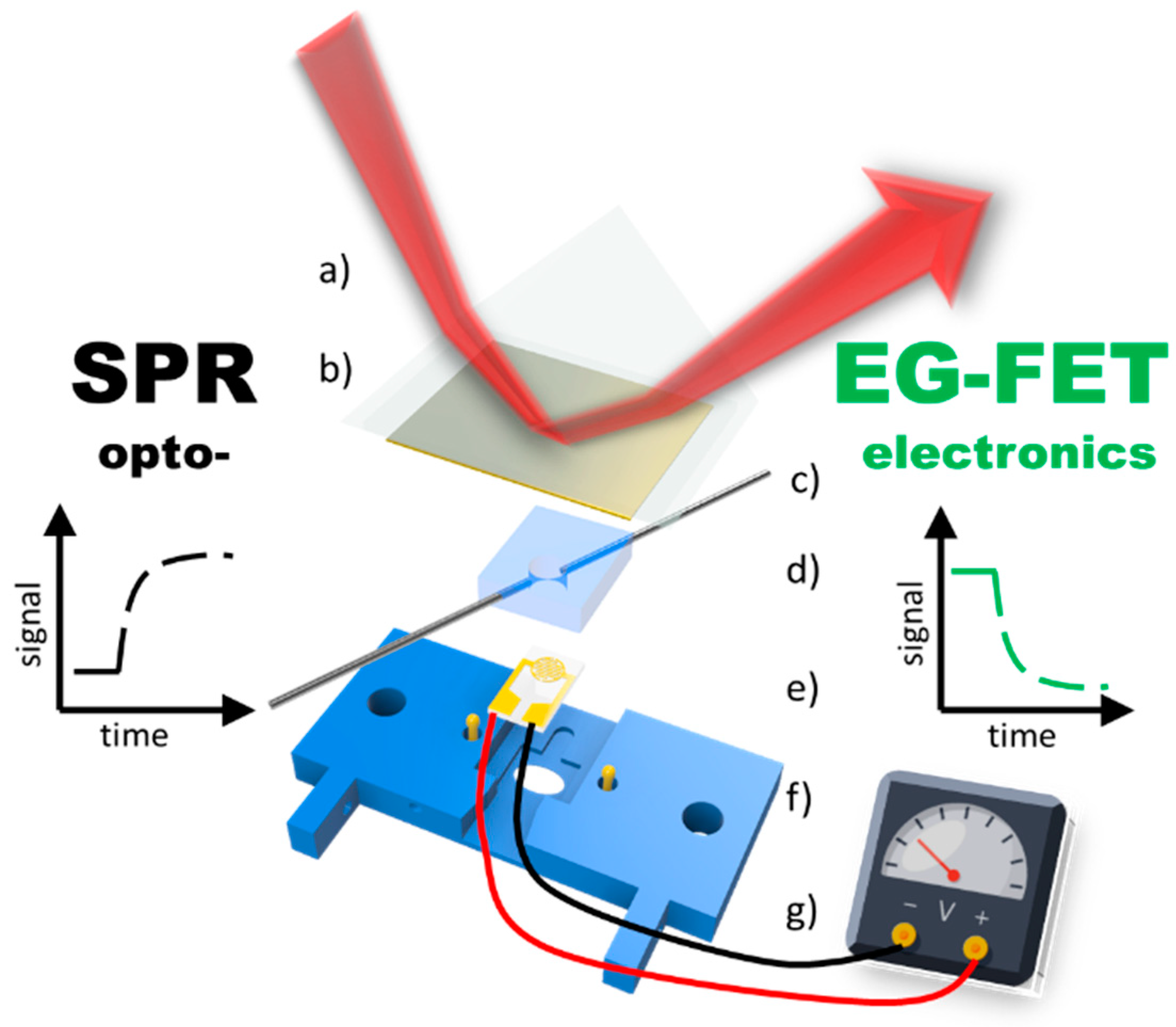
- Aspermair, C.; Ramach, U.; Reiner-Rozman, C.; Fossati, S.; Lechner, B.; Moya, S.E.; Azzaroni, O.; Dostalek, J.; Szunerits, S.; Knoll, W.; et al. Dual Monitoring of Surface Reactions in Real Time by Combined Surface-Plasmon Resonance and Field-Effect Transistor Interrogation. J. Am. Chem. Soc. 2020, 142, 11709–11716. [Google Scholar] [CrossRef] [PubMed]





| 1st week | 10–30 |
| 2nd week | 30–100 |
| 3rd week | 100–1000 |
| 4th week | 1000–10,000 |
| 2nd & 3rd month | 30,000–100,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoll, W.; Liu, J.; Yu, F.; Niu, L.; Reiner-Rozman, C.; Köper, I. Comparing Surface Plasmon-Optical and Electronic Immuno-Sensing of Affinity Interactions—A Case Study. Chemosensors 2021, 9, 11. https://doi.org/10.3390/chemosensors9010011
Knoll W, Liu J, Yu F, Niu L, Reiner-Rozman C, Köper I. Comparing Surface Plasmon-Optical and Electronic Immuno-Sensing of Affinity Interactions—A Case Study. Chemosensors. 2021; 9(1):11. https://doi.org/10.3390/chemosensors9010011
Chicago/Turabian StyleKnoll, Wolfgang, Jing Liu, Fang Yu, Lifang Niu, Ciril Reiner-Rozman, and Ingo Köper. 2021. "Comparing Surface Plasmon-Optical and Electronic Immuno-Sensing of Affinity Interactions—A Case Study" Chemosensors 9, no. 1: 11. https://doi.org/10.3390/chemosensors9010011
APA StyleKnoll, W., Liu, J., Yu, F., Niu, L., Reiner-Rozman, C., & Köper, I. (2021). Comparing Surface Plasmon-Optical and Electronic Immuno-Sensing of Affinity Interactions—A Case Study. Chemosensors, 9(1), 11. https://doi.org/10.3390/chemosensors9010011






