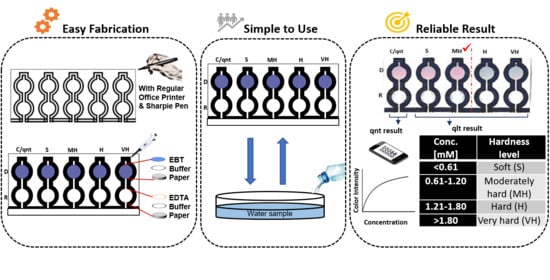
Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device
Abstract
1. Introduction
2. Materials and methods
2.1. Chemicals and Materials
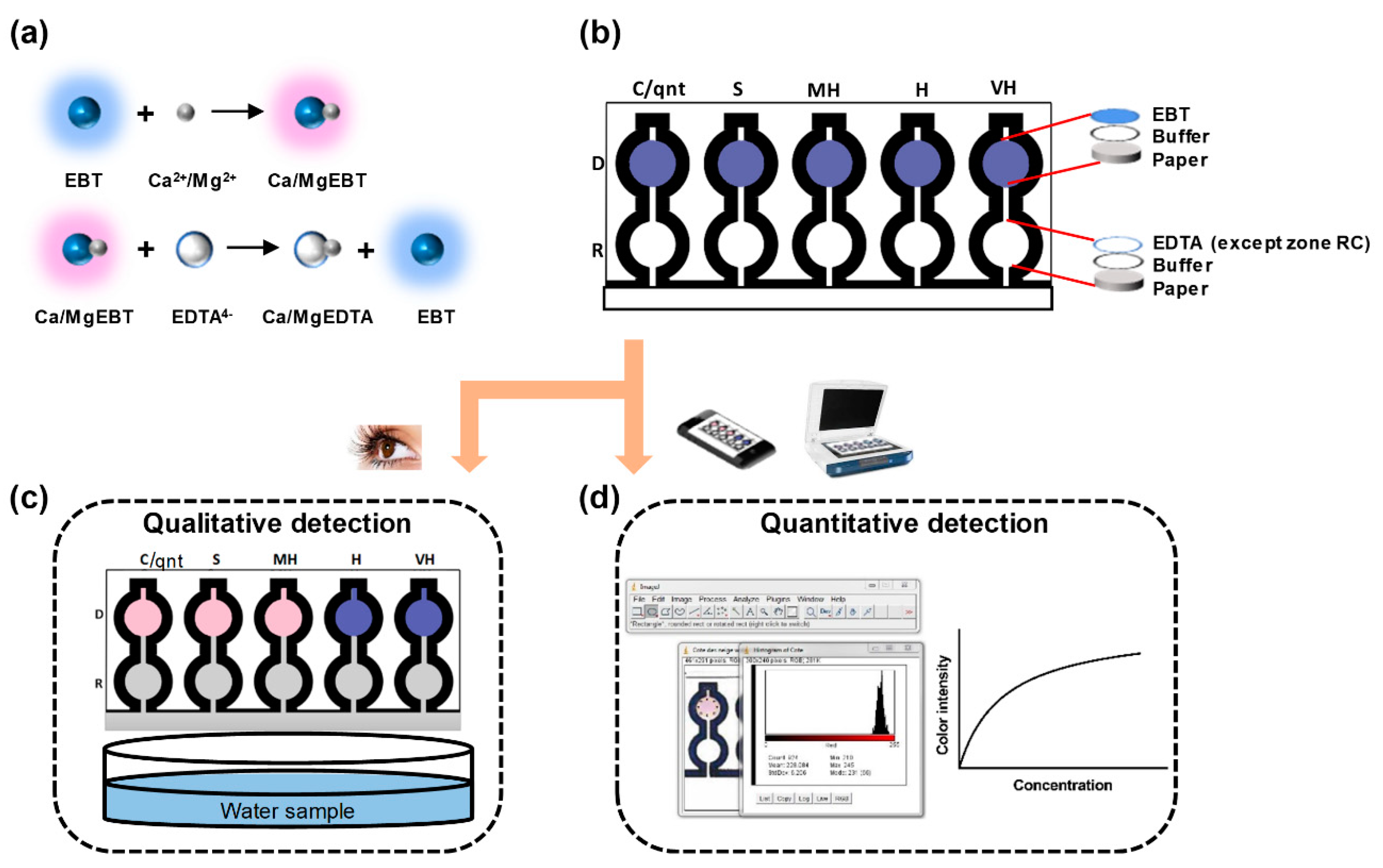
2.2. Principle of Complexometric Chelate Titration
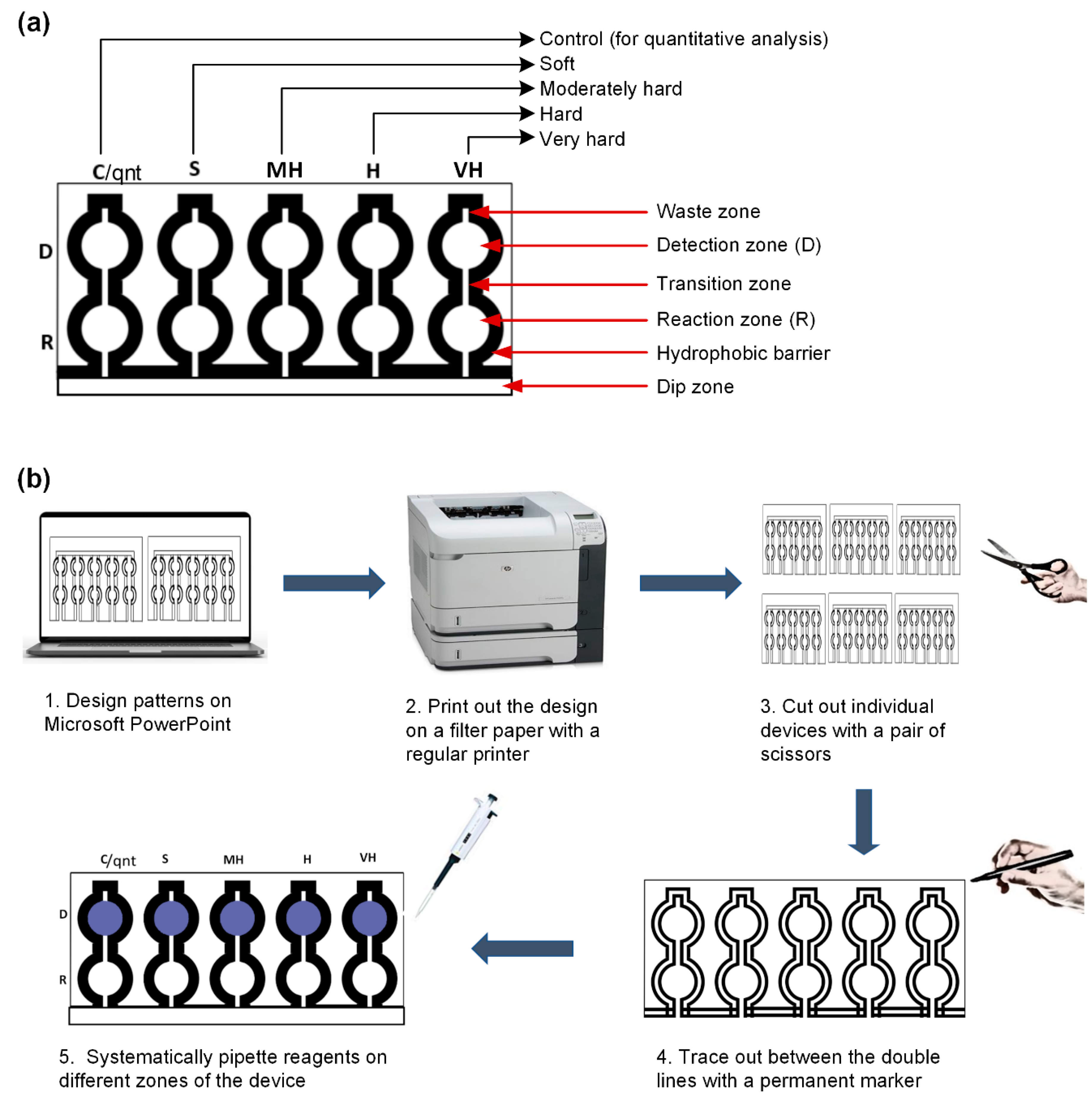
2.3. Microfluidic Paper-Based Analytical Device Fabrication
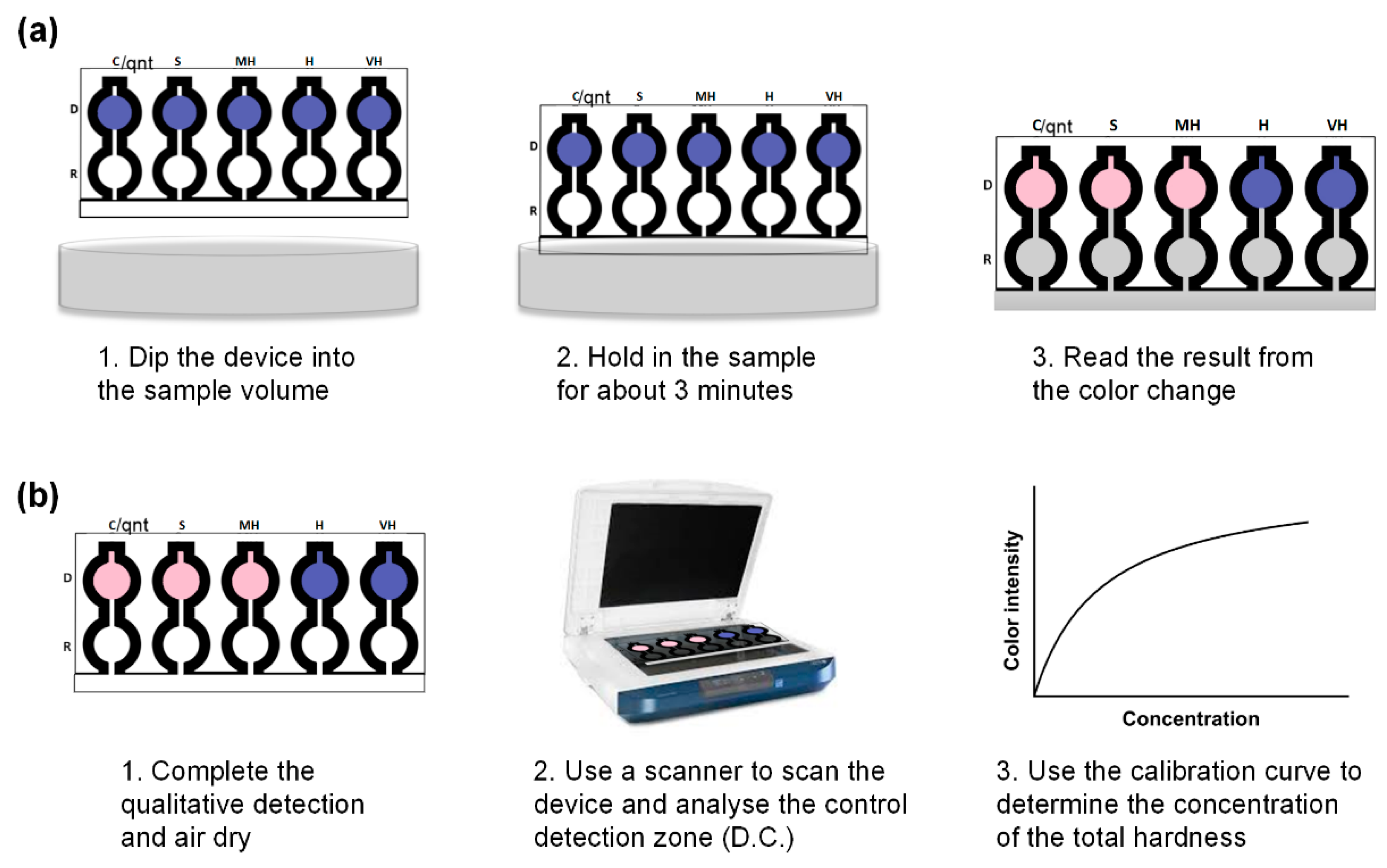
2.4. Systematic Introduction of Reagents on the Fabricated Microfluidic Paper-Based Analytical Device
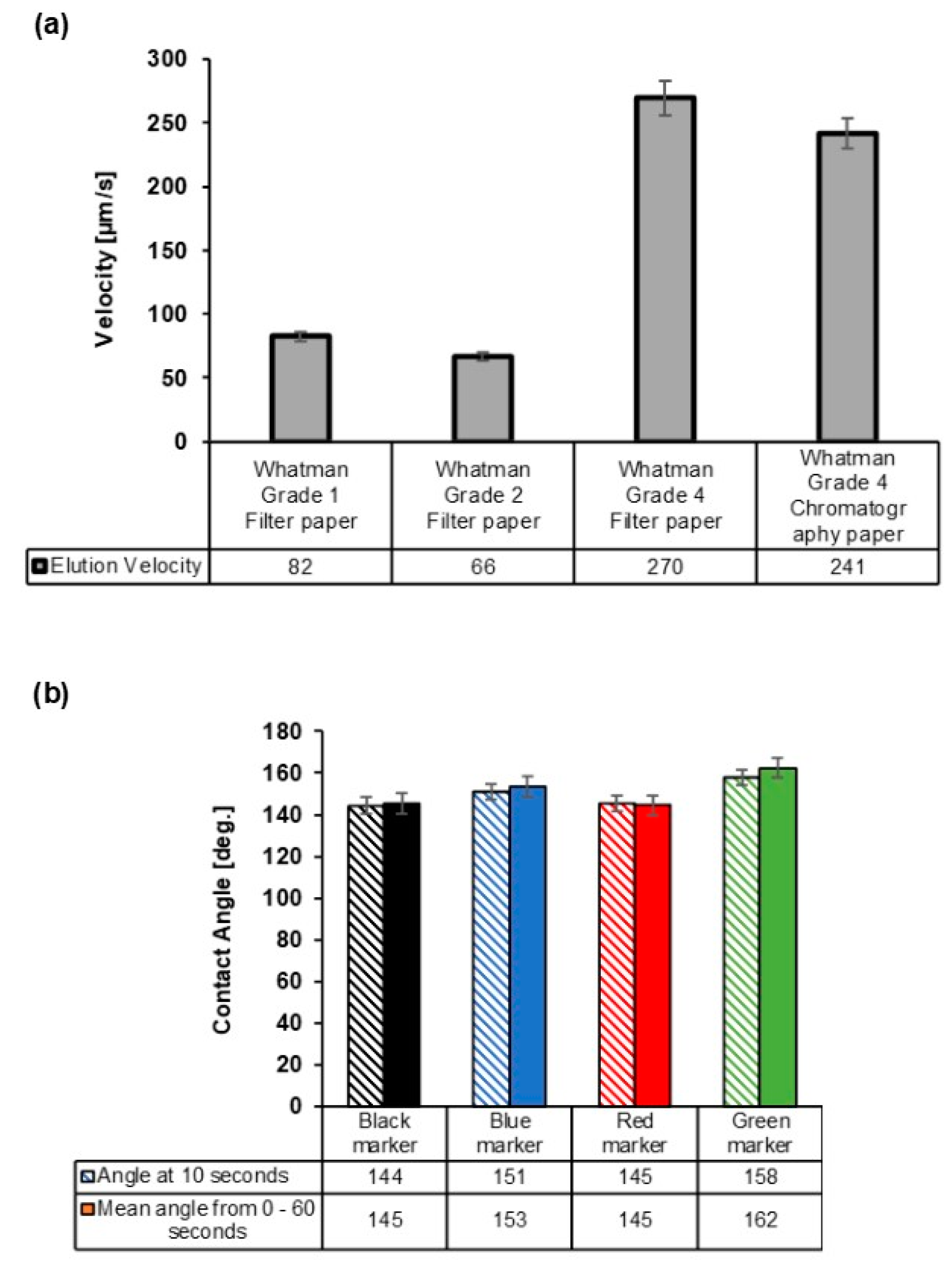
2.5. Elution Velocity Determination
2.6. Contact Angle Determination
2.7. Real-World Samples
2.8. Stability Analysis
2.9. Statistical Analysis
3. Results and Discussion
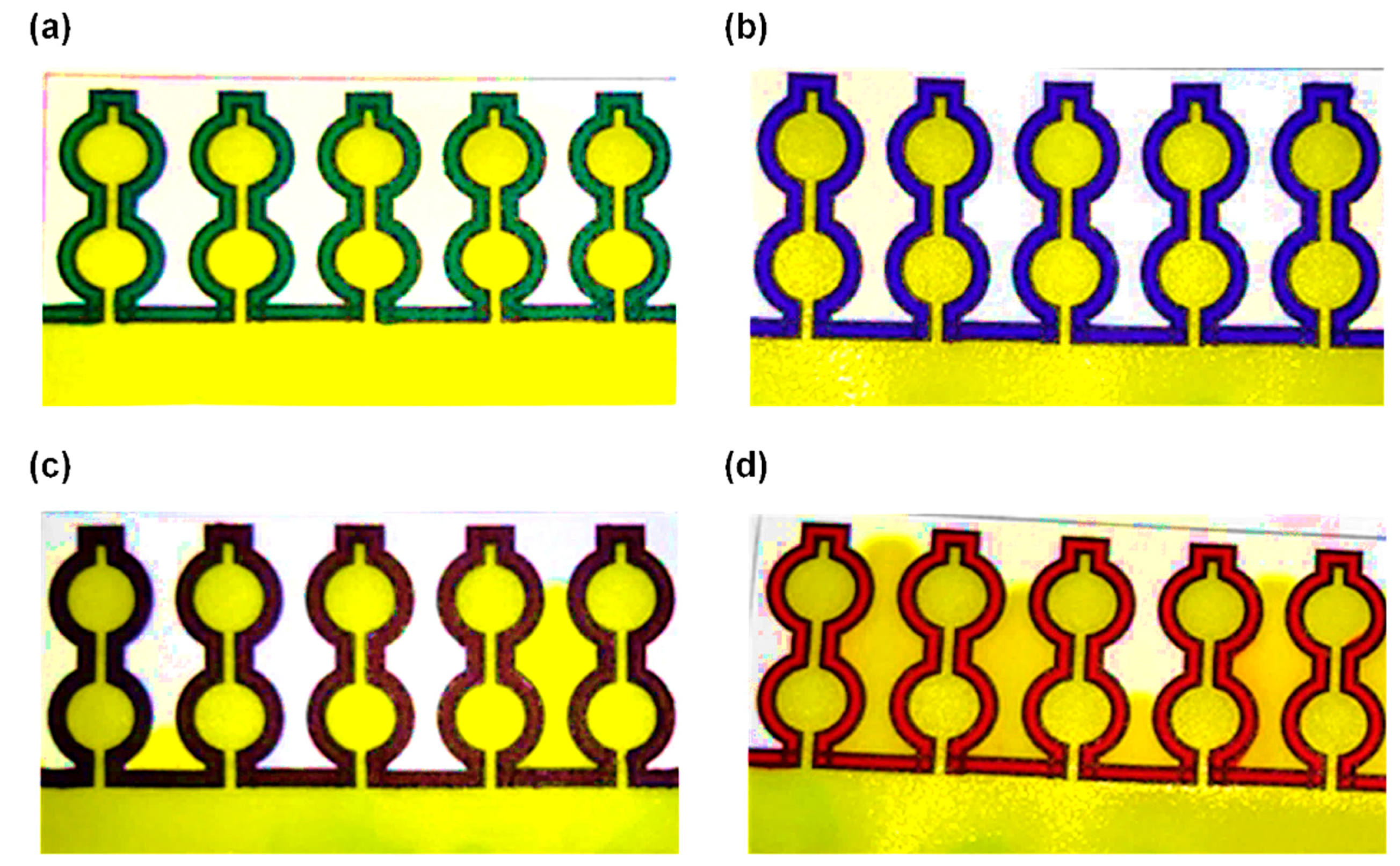
3.1. Selection of the Paper Type and Marker Color for the Creation of Hydrophobic Barrier
3.2. Analysis of Water Sample Using the µPAD
3.2.1. Optimization of the Reagents on the µPAD, Stoichiometric Cancellation, and Expectations
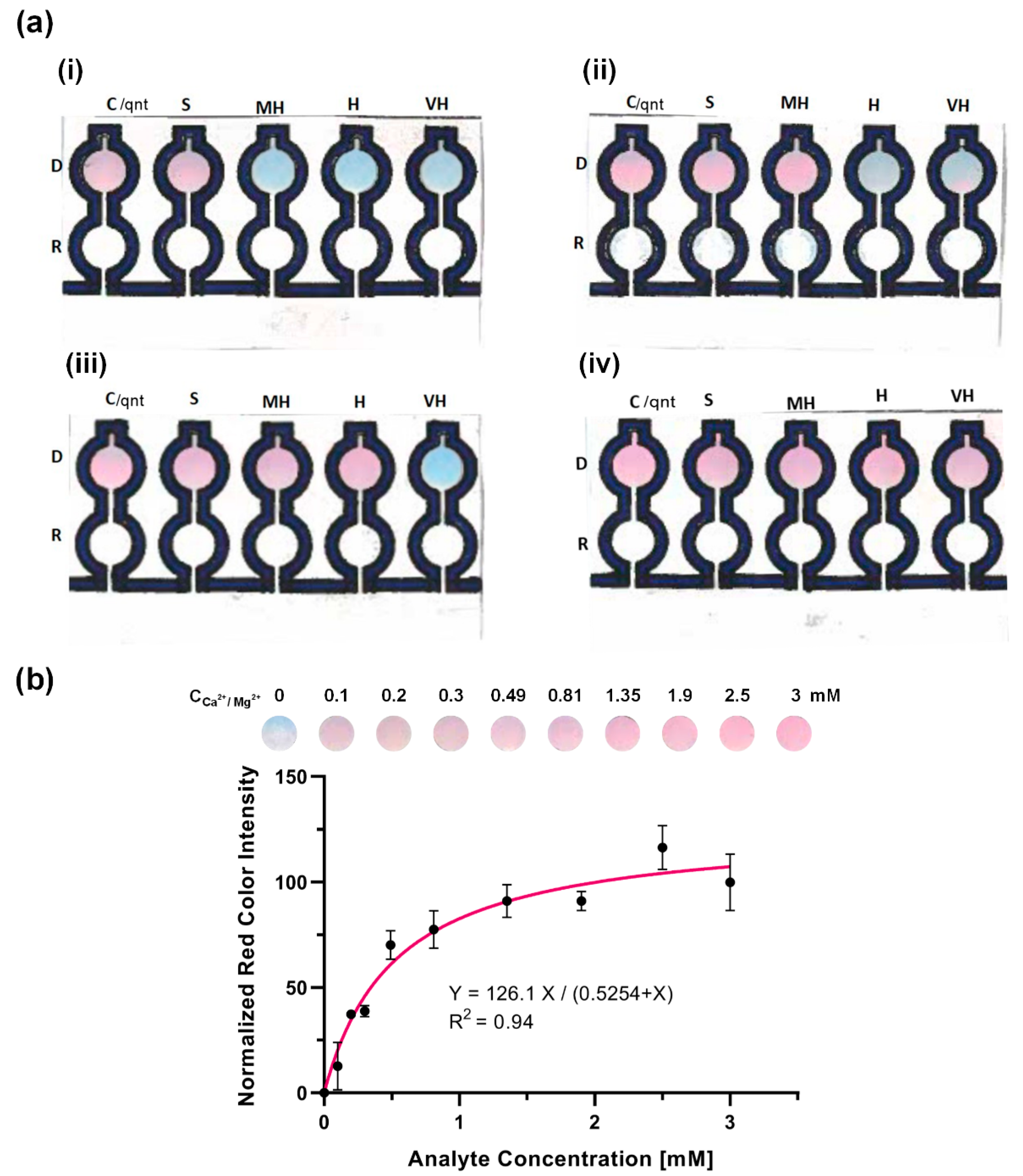
3.2.2. Qualitative Determination of the Total Hardness of Water for Spiked Sample
3.2.3. Quantitative Determination of Total Hardness of Water for Spiked Sample
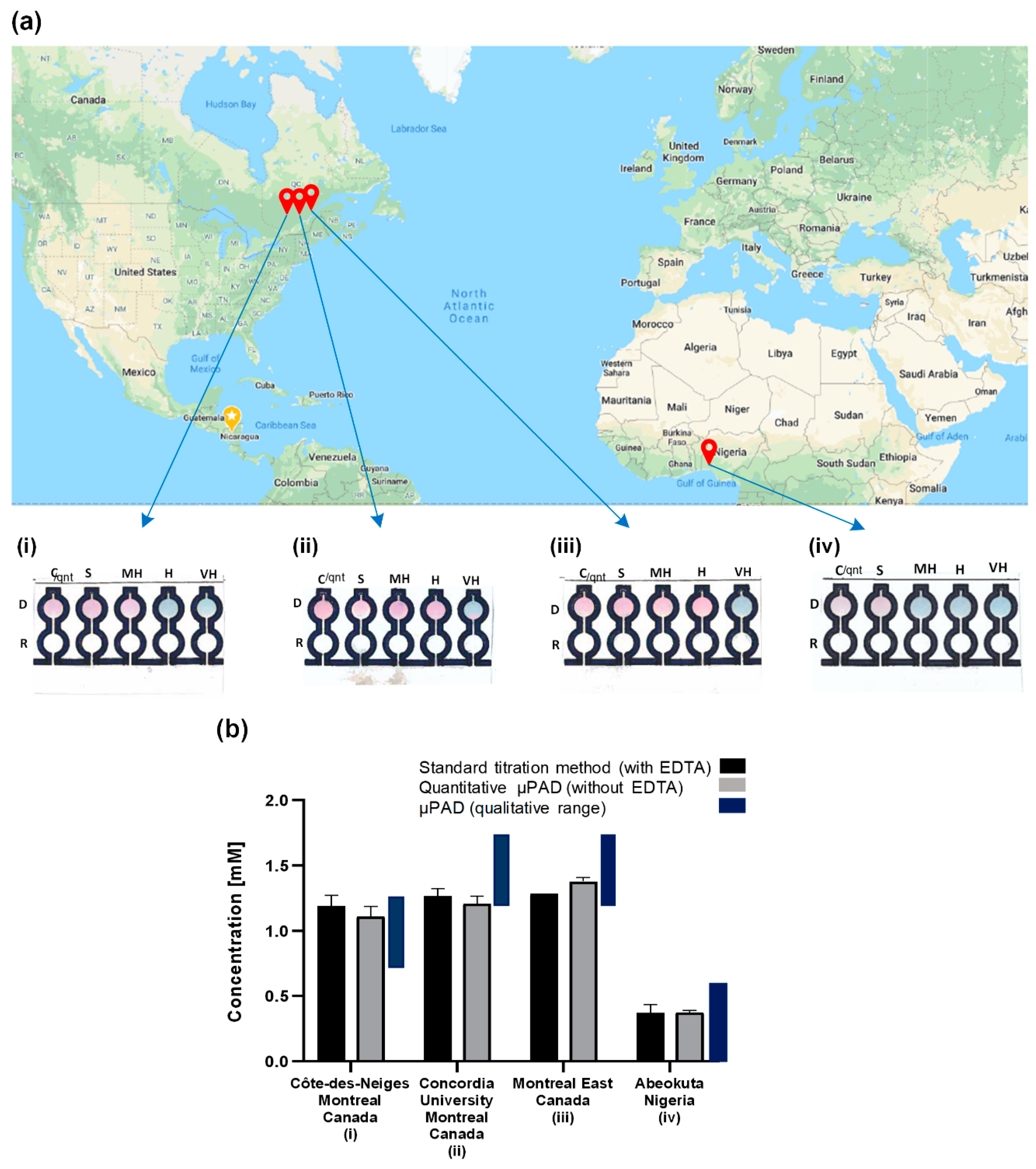
3.2.4. Real-World Sample Analysis
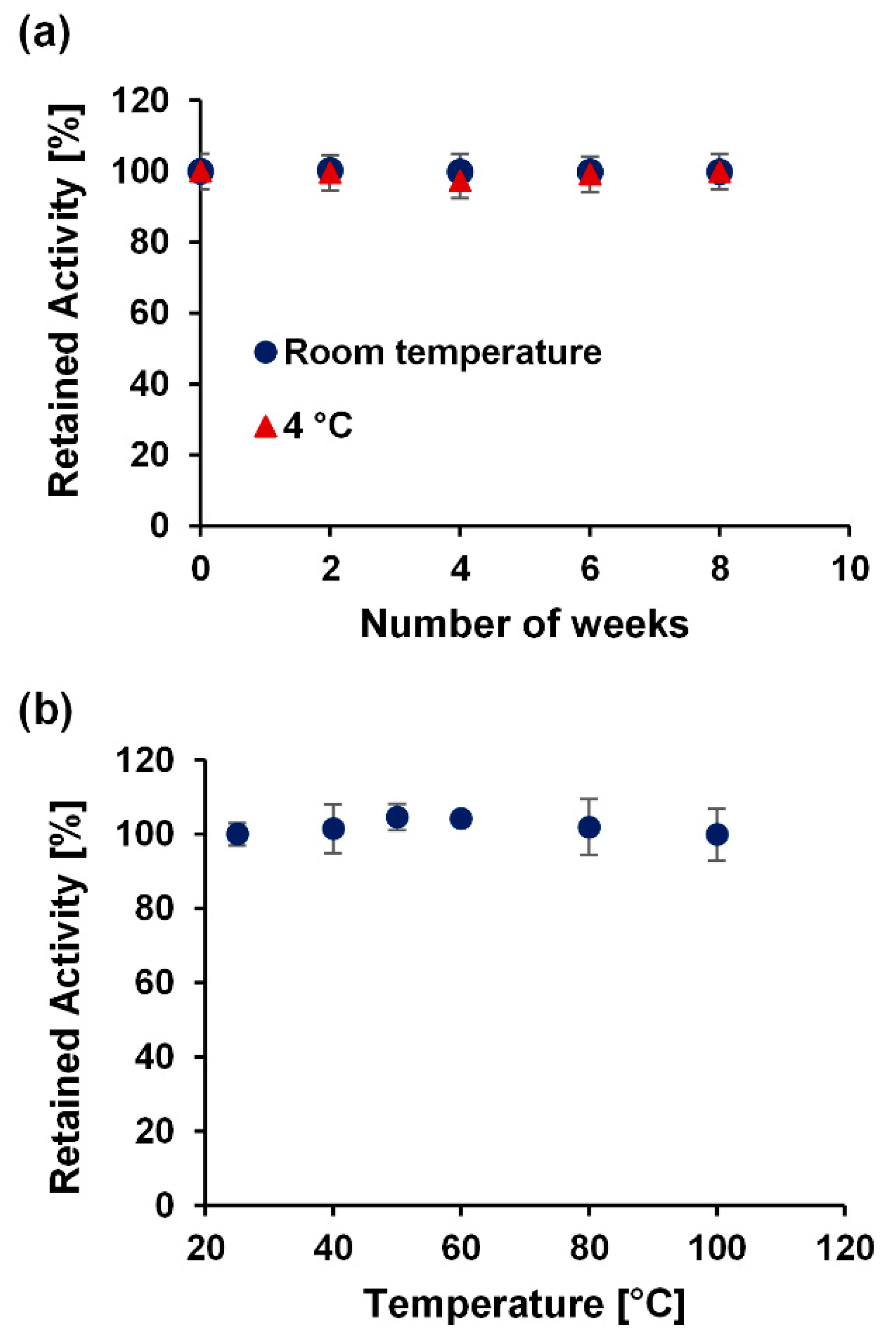
3.3. Stability Test
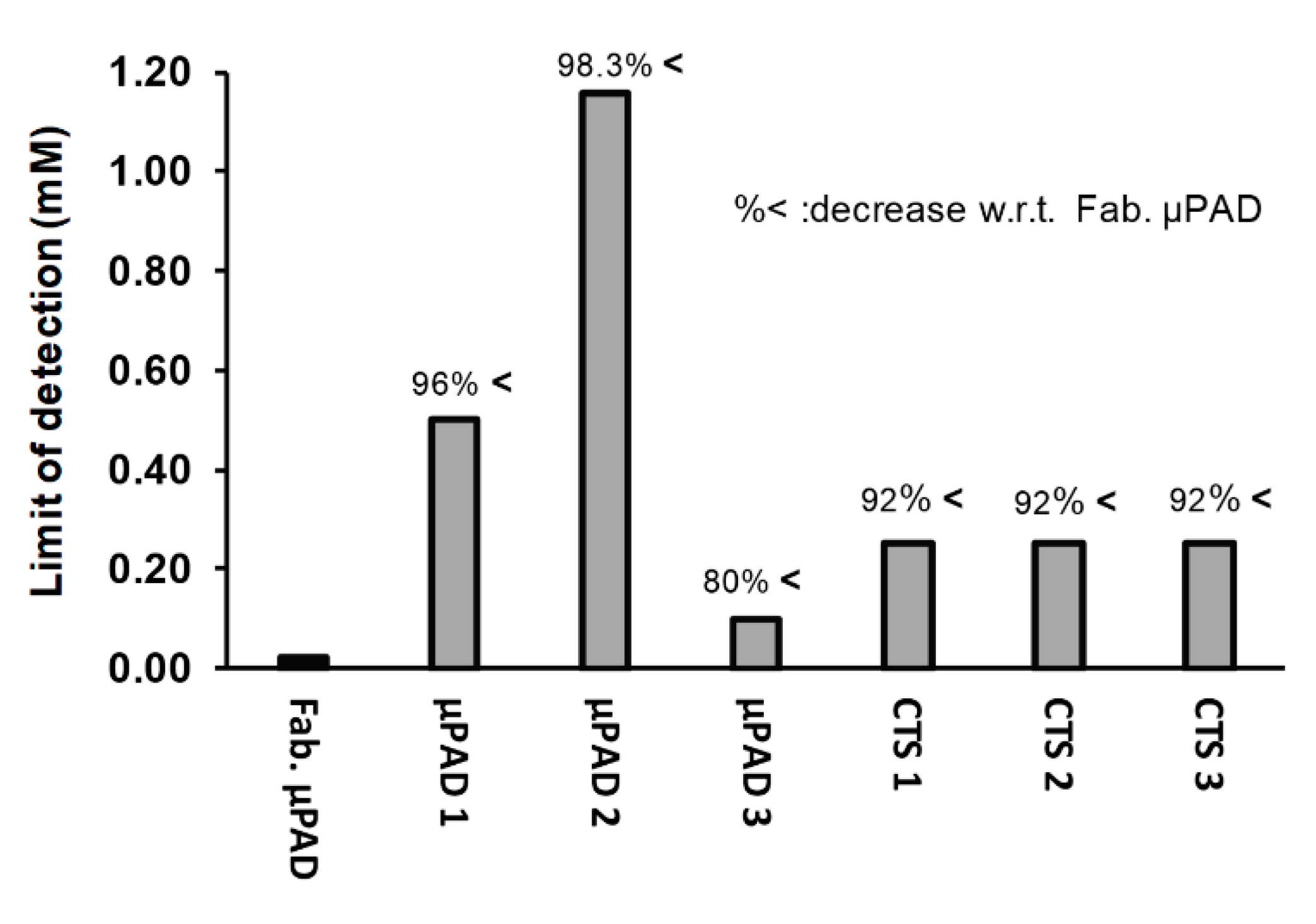
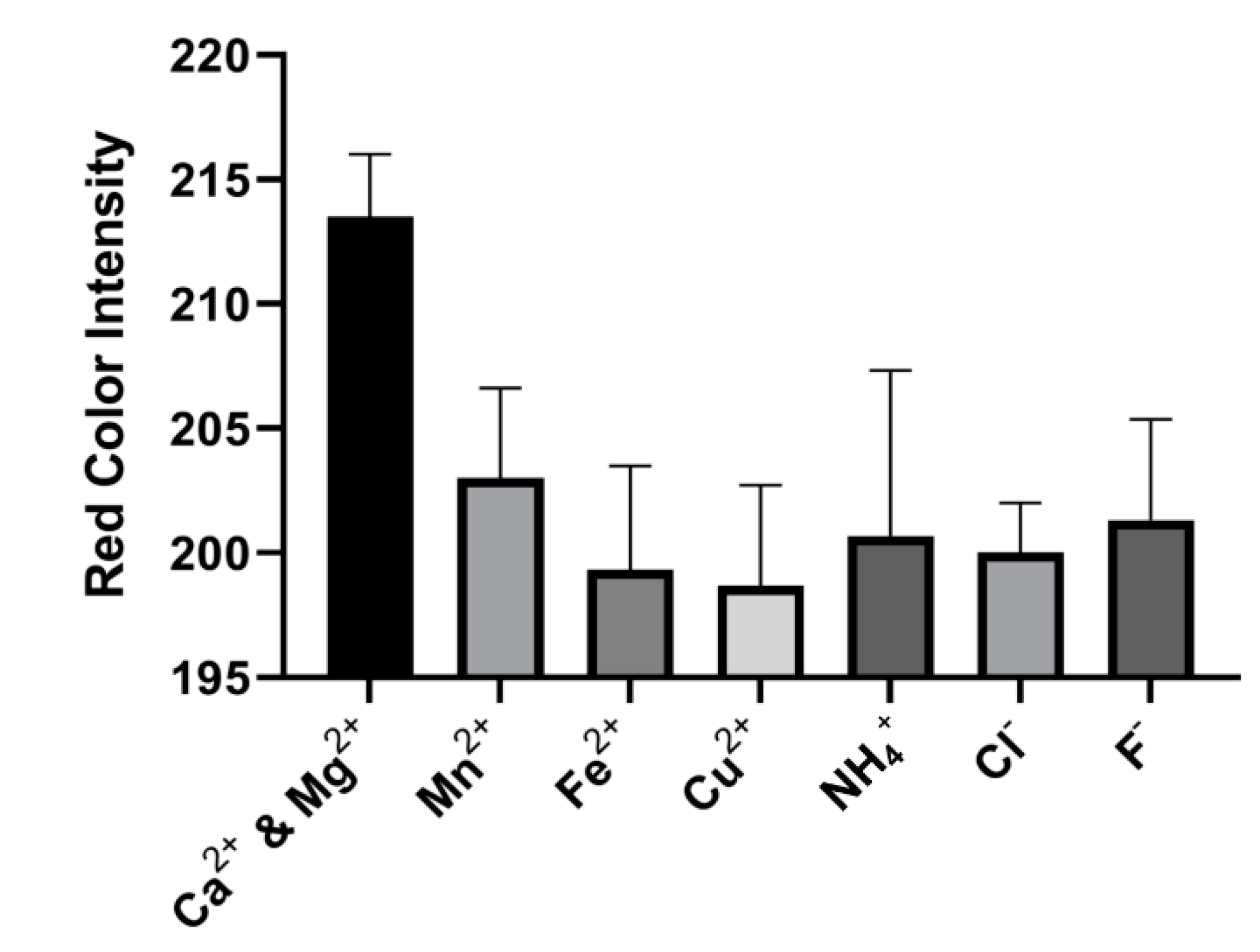
3.4. Interference Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Hardness in Drinking Water: Background Document for Development of WHO Guidelines for Drinking Water Quality; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Abeliotis, K.; Candan, C.; Amberg, C.; Ferri, A.; Osset, M.; Owens, J.; Stamminger, R. Impact of water hardness on consumers’ perception of laundry washing result in five European countries. Int. J. Consum. Stud. 2014, 39, 60–66. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, F.; Zhang, Y.; Li, N.; Chen, Y. The use of graphene oxide membranes for the softening of hard water. Sci. China Ser. E Technol. Sci. 2014, 57, 284–287. [Google Scholar] [CrossRef]
- Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada. Guidelines for Canadian Drinking Water Quality—Summary Table; Health Canada: Ottawa, ON, Canada, 2019.
- Leurs, L.J.; Schouten, L.J.; Mons, M.N.; Goldbohm, R.A.; Brandt, P.A.V.D. Relationship between Tap Water Hardness, Magnesium, and Calcium Concentration and Mortality due to Ischemic Heart Disease or Stroke in the Netherlands. Environ. Health Perspect. 2009, 118, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. Potential Health Impacts of Hard Water. Int. J. Prev. Med. 2013, 4, 866–875. [Google Scholar] [PubMed]
- Cotruvo, J.A.; Costello, R.; Weglicki, W.B. Public Health—Magnesium, Hard Water, and Health. J. Am. Water Work. Assoc. 2017, 109, 62–68. [Google Scholar] [CrossRef]
- Bakker, E. Ion-Selective Electrodes. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-409547-2. [Google Scholar]
- Butcher, D.J. Advances in Inductively Coupled Plasma Optical Emission Spectrometry for Environmental Analysis. Instrum. Sci. Technol. 2010, 38, 458–469. [Google Scholar] [CrossRef]
- Yang, C.-H.; Shi, X.-H.; Yuan, J.-H.; Chang-Hu, Y.; Xiang-Hua, S.; Jian-Hui, Y. Study on the Application of Raman Spectroscopy on Detecting Water Hardness. Water Environ. Res. 2014, 86, 417–420. [Google Scholar] [CrossRef]
- Channon, R.B.; Yang, Y.; Feibelman, K.M.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Development of an Electrochemical Paper-Based Analytical Device for Trace Detection of Virus Particles. Anal. Chem. 2018, 90, 7777–7783. [Google Scholar] [CrossRef]
- De Oliveira, T.R.; Fonseca, W.T.; Setti, G.D.O.; Faria, R.C. Fast and flexible strategy to produce electrochemical paper-based analytical devices using a craft cutter printer to create wax barrier and screen-printed electrodes. Talanta 2019, 195, 480–489. [Google Scholar] [CrossRef]
- Nantaphol, S.; Kava, A.A.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Janus electrochemistry: Simultaneous electrochemical detection at multiple working conditions in a paper-based analytical device. Anal. Chim. Acta 2019, 1056, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.H.; Brennan, J.D.; Filipe, C.D. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi-Anbuhi, S.; Henry, A.; Leung, V.; Sicard, C.; Pennings, K.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Paper-based microfluidics with an erodible polymeric bridge giving controlled release and timed flow shutoff. Lab Chip 2014, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi-Anbuhi, S.; Pennings, K.; Leung, V.; Liu, M.; Carrasquilla, C.; Kannan, B.; Li, Y.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Pullulan Encapsulation of Labile Biomolecules to Give Stable Bioassay Tablets. Angew. Chem. Int. Ed. 2014, 53, 6155–6158. [Google Scholar] [CrossRef] [PubMed]
- Satarpai, T.; Shiowatana, J.; Siripinyanond, A. Paper-based analytical device for sampling, on-site preconcentration and detection of ppb lead in water. Talanta 2016, 154, 504–510. [Google Scholar] [CrossRef]
- Wu, Q.; He, J.; Meng, H.; Wang, Y.; Zhang, Y.; Li, H.; Feng, L. A paper-based microfluidic analytical device combined with home-made SPE column for the colorimetric determination of copper(II) ion. Talanta 2019, 204, 518–524. [Google Scholar] [CrossRef]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of Mercury(II) Ions Using Colorimetric Gold Nanoparticles on Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Aprian, A.; Meileza, N.; Hitsmi, M.; Elvia, R.; Rahmidar, L.; Khaydarov, R.R. Smartphone Coupled with a Paper-Based Colorimetric Device for Sensitive and Portable Mercury Ion Sensing. Chemosensors 2019, 7, 25. [Google Scholar] [CrossRef]
- Marquez, S.; Liu, J.; Morales-Narváez, E. Paper-based analytical devices in environmental applications and their integration with portable technologies. Curr. Opin. Environ. Sci. Health 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Karita, S.; Kaneta, T. Chelate titrations of Ca2+ and Mg2+ using microfluidic paper-based analytical devices. Anal. Chim. Acta 2016, 924, 60–67. [Google Scholar] [CrossRef]
- Shibata, H.; Hiruta, Y.; Citterio, D. Fully inkjet-printed distance-based paper microfluidic devices for colorimetric calcium determination using ion-selective optodes. Analyst 2019, 144, 1178–1186. [Google Scholar] [CrossRef]
- Rattanarat, P.; Dungchai, W.; Cate, D.M.; Siangproh, W.; Volckens, J.; Chailapakul, O.; Henry, C.S. A microfluidic paper-based analytical device for rapid quantification of particulate chromium. Anal. Chim. Acta 2013, 800, 50–55. [Google Scholar] [CrossRef]
- Kudo, H.; Yamada, K.; Watanabe, D.; Suzuki, K.; Citterio, D. Paper-Based Analytical Device for Zinc Ion Quantification in Water Samples with Power-Free Analyte Concentration. Micromachines 2017, 8, 127. [Google Scholar] [CrossRef]
- Yamada, K.; Citterio, D.; Henry, C.S. “Dip-and-read” paper-based analytical devices using distance-based detection with color screening. Lab Chip 2018, 18, 1485–1493. [Google Scholar] [CrossRef]
- Sun, H.; Li, W.; Dong, Z.-Z.; Hu, C.; Leung, C.-H.; Ma, D.-L.; Ren, K. A suspending-droplet mode paper-based microfluidic platform for low-cost, rapid, and convenient detection of lead(II) ions in liquid solution. Biosens. Bioelectron. 2018, 99, 361–367. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Macka, M. A novel highly flexible, simple, rapid and low-cost fabrication tool for paper-based microfluidic devices (μPADs) using technical drawing pens and in-house formulated aqueous inks. Anal. Chim. Acta 2016, 919, 70–77. [Google Scholar] [CrossRef]
- Juang, Y.-J.; Chen, P.-S.; Wang, Y. Rapid fabrication of microfluidic paper-based analytical devices by microembossing. Sens. Actuators B Chem. 2019, 283, 87–92. [Google Scholar] [CrossRef]
- Juang, Y.-J.; Li, W.-S.; Chen, P.-S. Fabrication of microfluidic paper-based analytical devices by filtration-assisted screen printing. J. Taiwan Inst. Chem. Eng. 2017, 80, 71–75. [Google Scholar] [CrossRef]
- Jahanshahi-Anbuhi, S.; Pennings, K.; Leung, V.; Kannan, B.; Brennan, J.D.; Filipe, C.D.M.; Pelton, R. Design Rules for Fluorocarbon-Free Omniphobic Solvent Barriers in Paper-Based Devices. ACS Appl. Mater. Interfaces 2015, 7, 25434–25440. [Google Scholar] [CrossRef] [PubMed]
- Devadhasan, J.P.; Kim, J. A chemically functionalized paper-based microfluidic platform for multiplex heavy metal detection. Sens. Actuators B Chem. 2018, 273, 18–24. [Google Scholar] [CrossRef]
- Chiang, C.-K.; Kurniawan, A.; Kao, C.; Wang, M.-J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2018, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.F.; Garcia, C.D.; Schaumburg, F.; Kler, P.A.; Berli, C.; Hashimoto, M.; Carrilho, E. Patterning and Modeling Three-Dimensional Microfluidic Devices Fabricated on a Single Sheet of Paper. Anal. Chem. 2019, 91, 8298–8303. [Google Scholar] [CrossRef] [PubMed]
- Spicar-Mihalic, P.; Toley, B.; Houghtaling, J.; Liang, T.; Yager, P.; Fu, E. CO2 laser cutting and ablative etching for the fabrication of paper-based devices. J. Micromech. Microeng. 2013, 23, 67003. [Google Scholar] [CrossRef]
- Yu, L.; Shi, Z.Z. Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Li, Y.; Karyappa, R.; Ohno, A.; Toh, Y.-C.; Hashimoto, M.; Yingying, L. Fabrication of integrated microfluidic devices by direct ink writing (DIW) 3D printing. Sens. Actuators B Chem. 2019, 297, 126609. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, K.R.; Thomas, J.L.; Torres, M.N.; Oliveira, S.; Mccord, B.R. Simultaneous colorimetric detection of metallic salts contained in low explosives residue using a microfluidic paper-based analytical device (µPAD). Forensic Chem. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, L.; Doery, J.C.; Shen, W. Three-dimensional microfluidic tape-paper-based sensing device for blood total bilirubin measurement in jaundiced neonates. Lab Chip 2020, 20, 394–404. [Google Scholar] [CrossRef]
- Yehia, A.M.; Farag, M.A.; Tantawy, M.A. A novel trimodal system on a paper-based microfluidic device for on-site detection of the date rape drug “ketamine”. Anal. Chim. Acta 2020, 1104, 95–104. [Google Scholar] [CrossRef]
- Xerox Inc. Available online: https://www.xerox.com/en-us/office/solid-ink (accessed on 17 March 2020).
- Gallibu, C.; Gallibu, C.; Avoundjian, A.; Gomez, F.A. Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays. Micromachines 2016, 7, 6. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Meredith, N.A.; Kelly, S.P.; Henry, C.S. Design considerations for reducing sample loss in microfluidic paper-based analytical devices. Anal. Chim. Acta 2018, 1017, 20–25. [Google Scholar] [CrossRef]
- Masoodi, R.; Pillai, K.M. Darcy’s law-based model for wicking in paper-like swelling porous media. AIChE J. 2010, 56. [Google Scholar] [CrossRef]
- Mott, C.R. Aqueous Permanent Coloring Composition for a Marker. U.S. Patent 5131776A, 21 July 1992. [Google Scholar]
- Sanborn, K.B.; Loftin, R.M. Permanent Aqueous Marker Inks. U.S. Patent 1994002542A1, 3 February 1994. [Google Scholar]
- Van Der Werf, I.D.; Germinario, G.; Palmisano, F.; Sabbatini, L. Characterisation of permanent markers by pyrolysis gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2011, 399, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Shariati-Rad, M.; Heidari, S. Classification and determination of total hardness of water using silver nanoparticles. Talanta 2020, 219, 121297. [Google Scholar] [CrossRef] [PubMed]
- Ostad, M.A.; Hajinia, A.; Heidari, T. A novel direct and cost effective method for fabricating paper-based microfluidic device by commercial eye pencil and its application for determining simultaneous calcium and magnesium. Microchem. J. 2017, 133, 545–550. [Google Scholar] [CrossRef]
- HoneForest. Available online: http://www.honeforest.net/product/water-hardness-test-strips/ (accessed on 7 September 2020).
- Health Metric. Available online: https://www.health-metric.com/products/well-water-test-kit (accessed on 7 September 2020).
- Thomas Scientific. Available online: https://www.thomassci.com/Laboratory-Supplies/Water-Quality-Test-Strips/_/Total-Hardness-Test-Strips?q=Test%20Strips%20Total%20Hardness (accessed on 7 September 2020).
- Liu, W.; Kou, J.; Xing, H.; Li, B. Paper-based chromatographic chemiluminescence chip for the detection of dichlorvos in vegetables. Biosens. Bioelectron. 2014, 52, 76–81. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyewunmi, O.D.; Safiabadi-Tali, S.H.; Jahanshahi-Anbuhi, S. Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device. Chemosensors 2020, 8, 97. https://doi.org/10.3390/chemosensors8040097
Oyewunmi OD, Safiabadi-Tali SH, Jahanshahi-Anbuhi S. Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device. Chemosensors. 2020; 8(4):97. https://doi.org/10.3390/chemosensors8040097
Chicago/Turabian StyleOyewunmi, Oyejide Damilola, Seyed Hamid Safiabadi-Tali, and Sana Jahanshahi-Anbuhi. 2020. "Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device" Chemosensors 8, no. 4: 97. https://doi.org/10.3390/chemosensors8040097
APA StyleOyewunmi, O. D., Safiabadi-Tali, S. H., & Jahanshahi-Anbuhi, S. (2020). Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device. Chemosensors, 8(4), 97. https://doi.org/10.3390/chemosensors8040097