Health Monitoring of Aviation Hydraulic Fluids Using Opto-Chemical Sensor Technologies
Abstract
1. Introduction
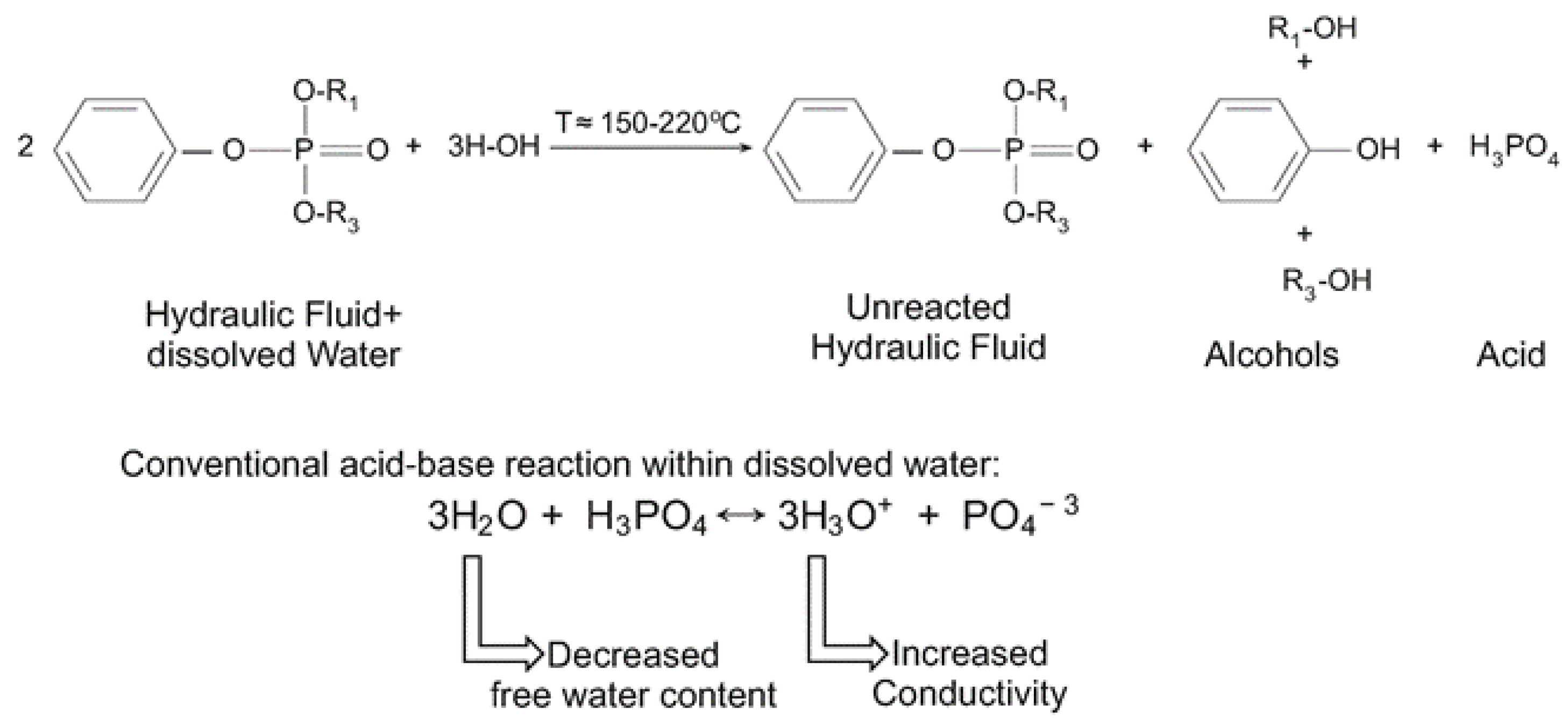
2. Hydraulic Fluids and Fluid Degradation
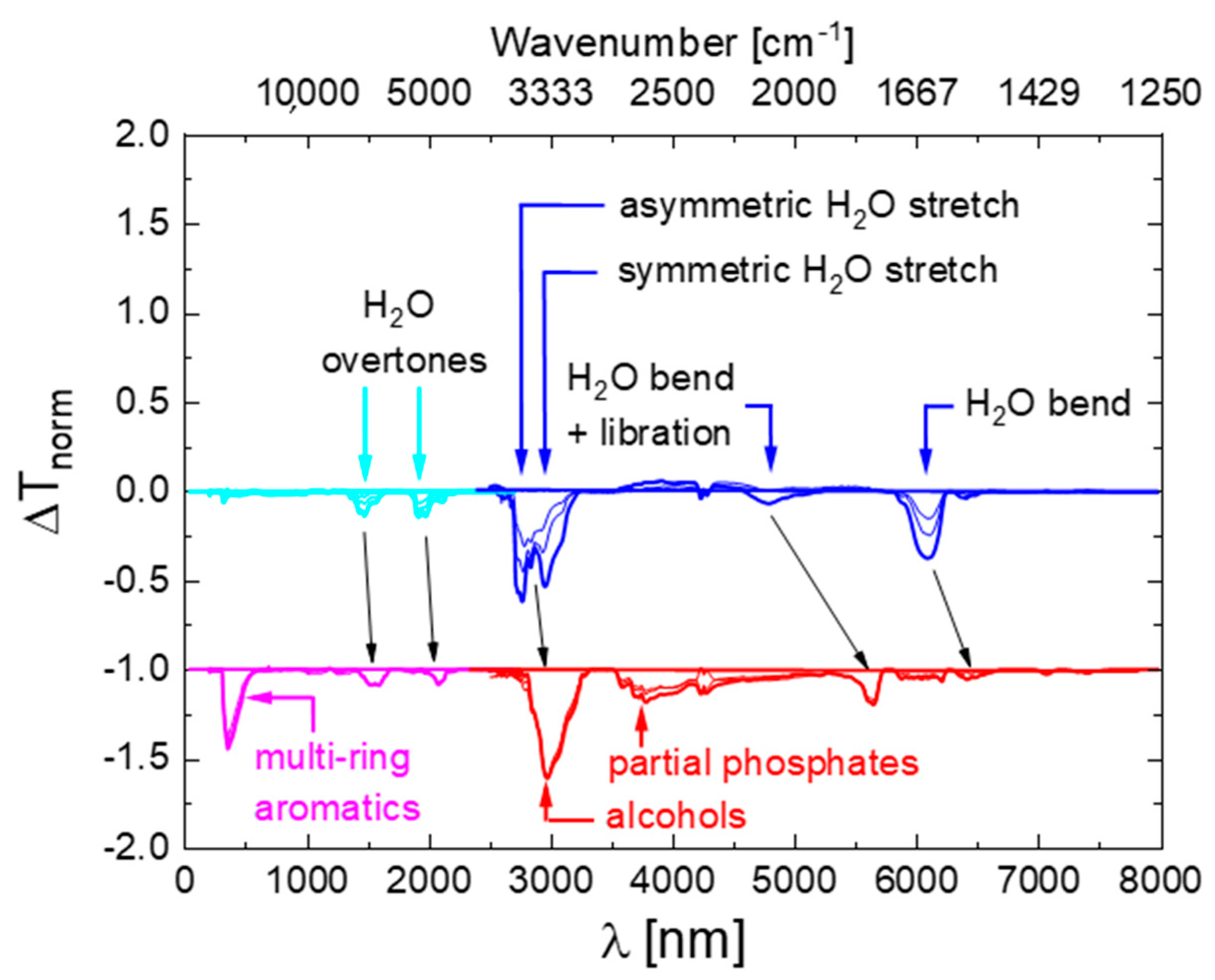
3. Optical Signatures of Hydraulic Fluid Degradation
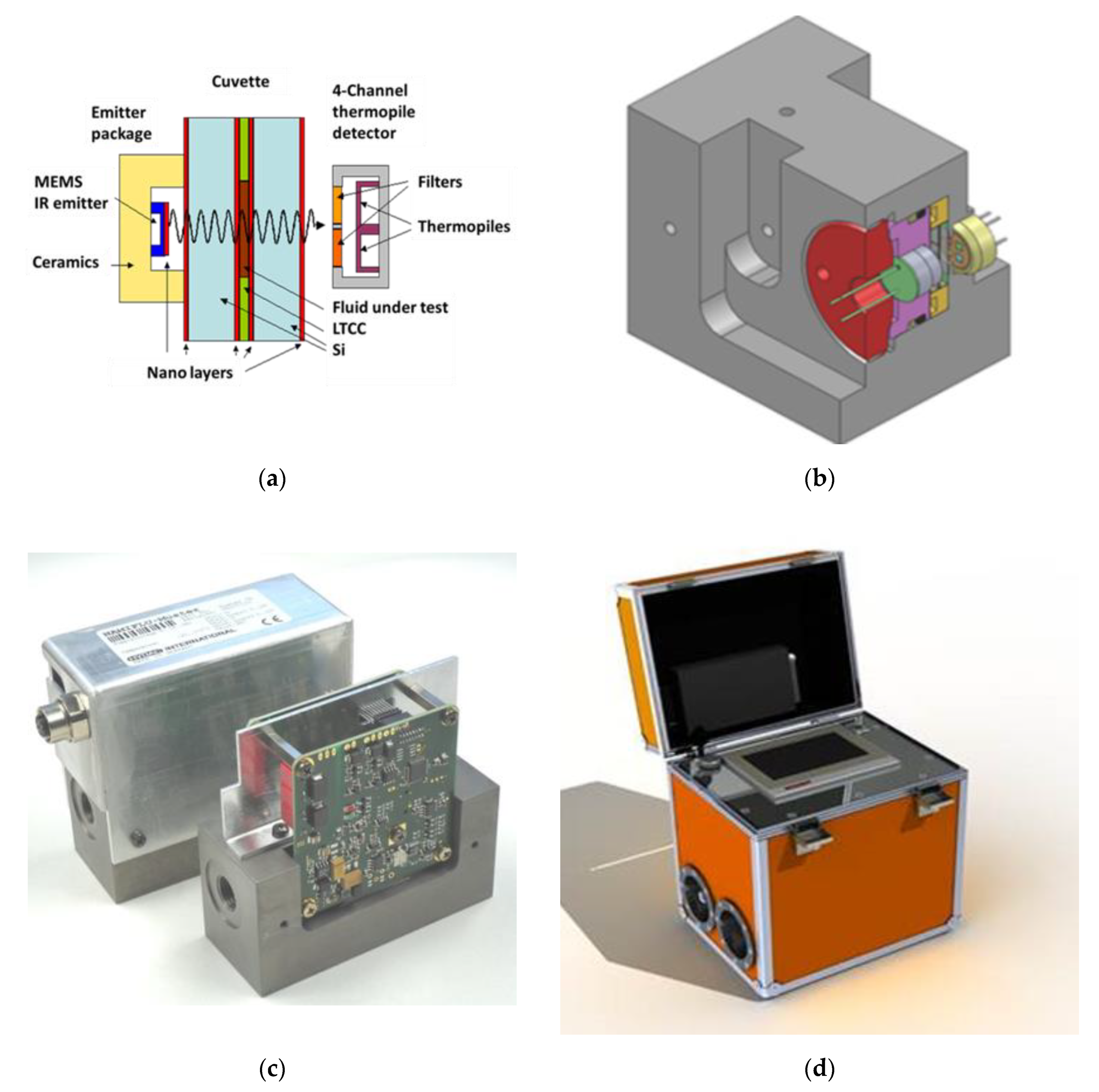
4. MIR Monitoring of Aviation Hydraulic Fluids
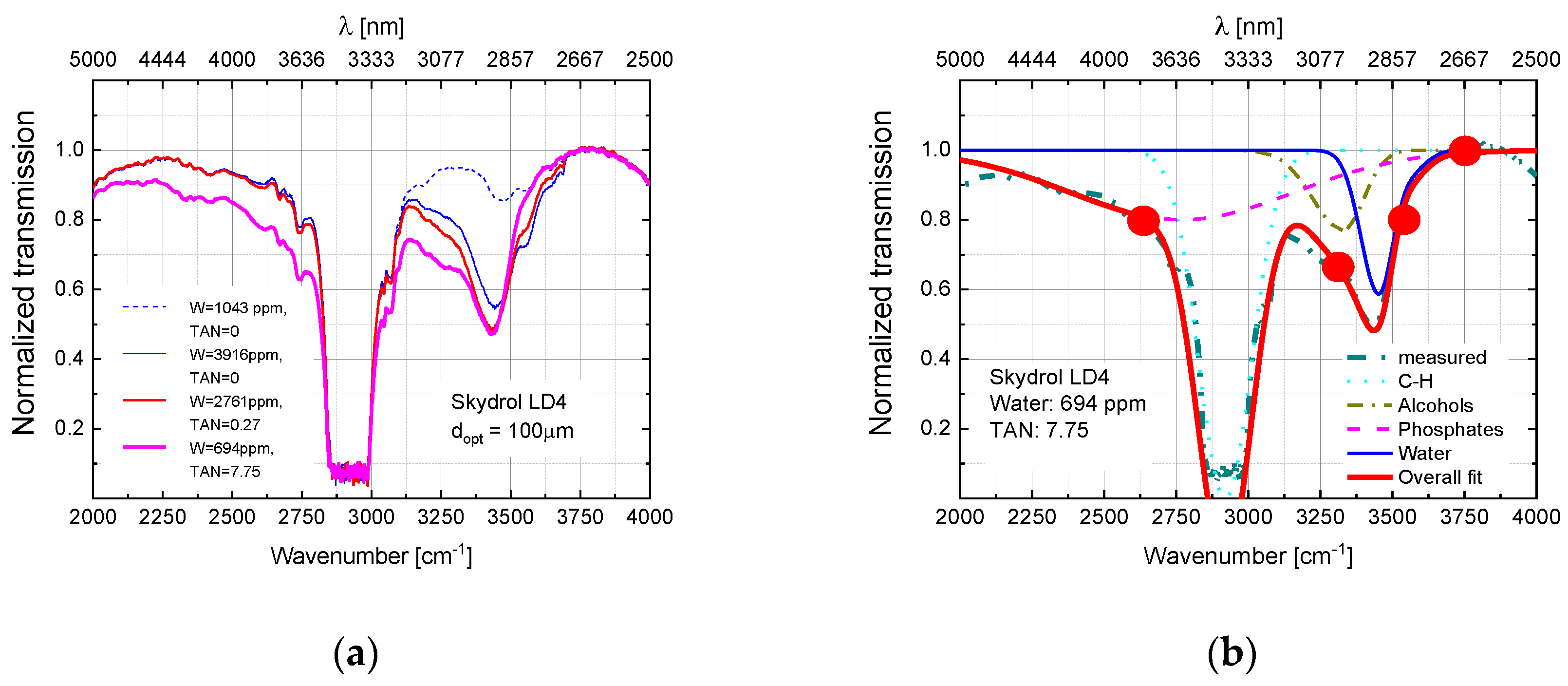
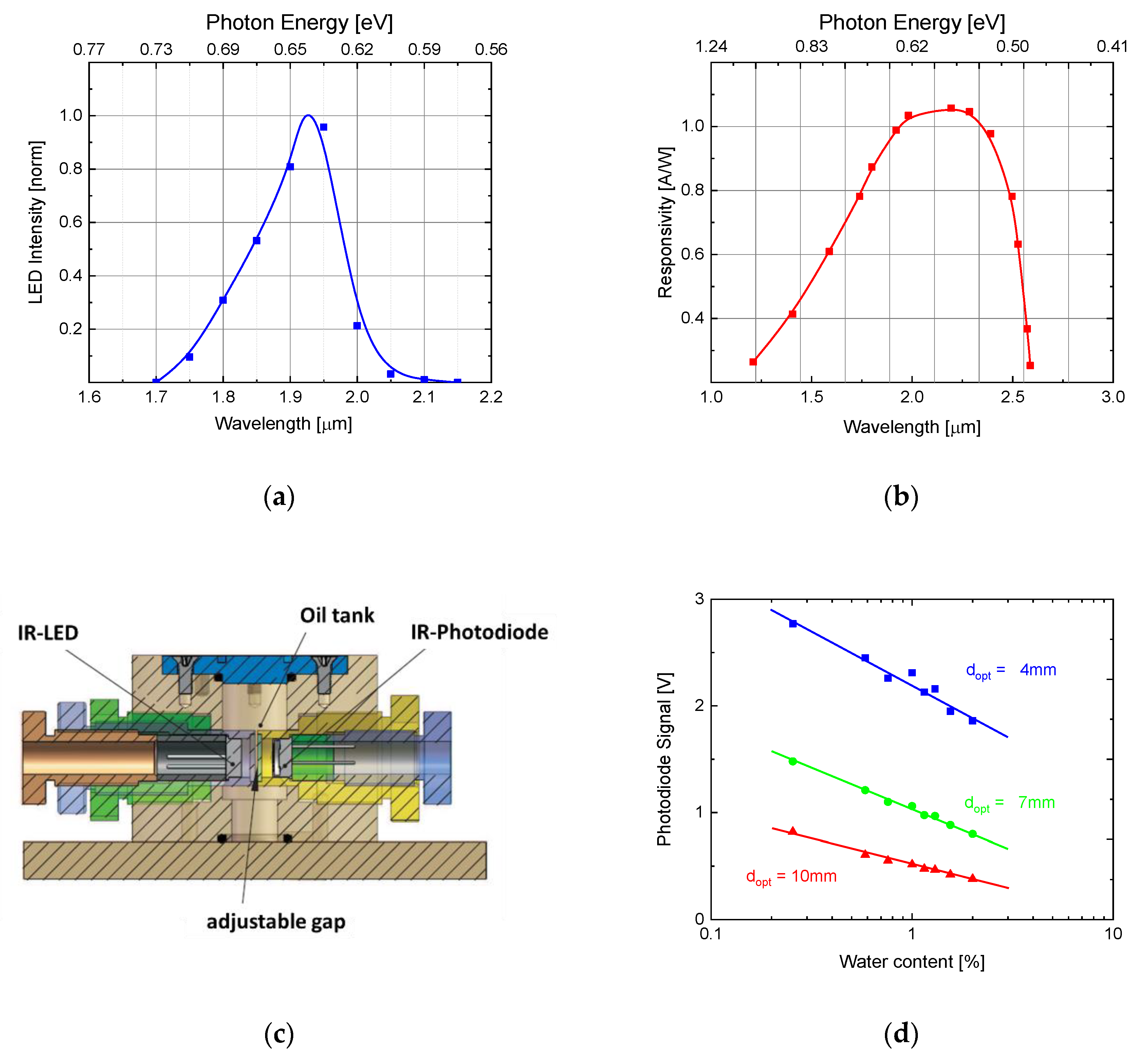
5. Optical Absorption Monitoring of Aviation Hydraulic Fluids in the Visible and Near Infrared Ranges
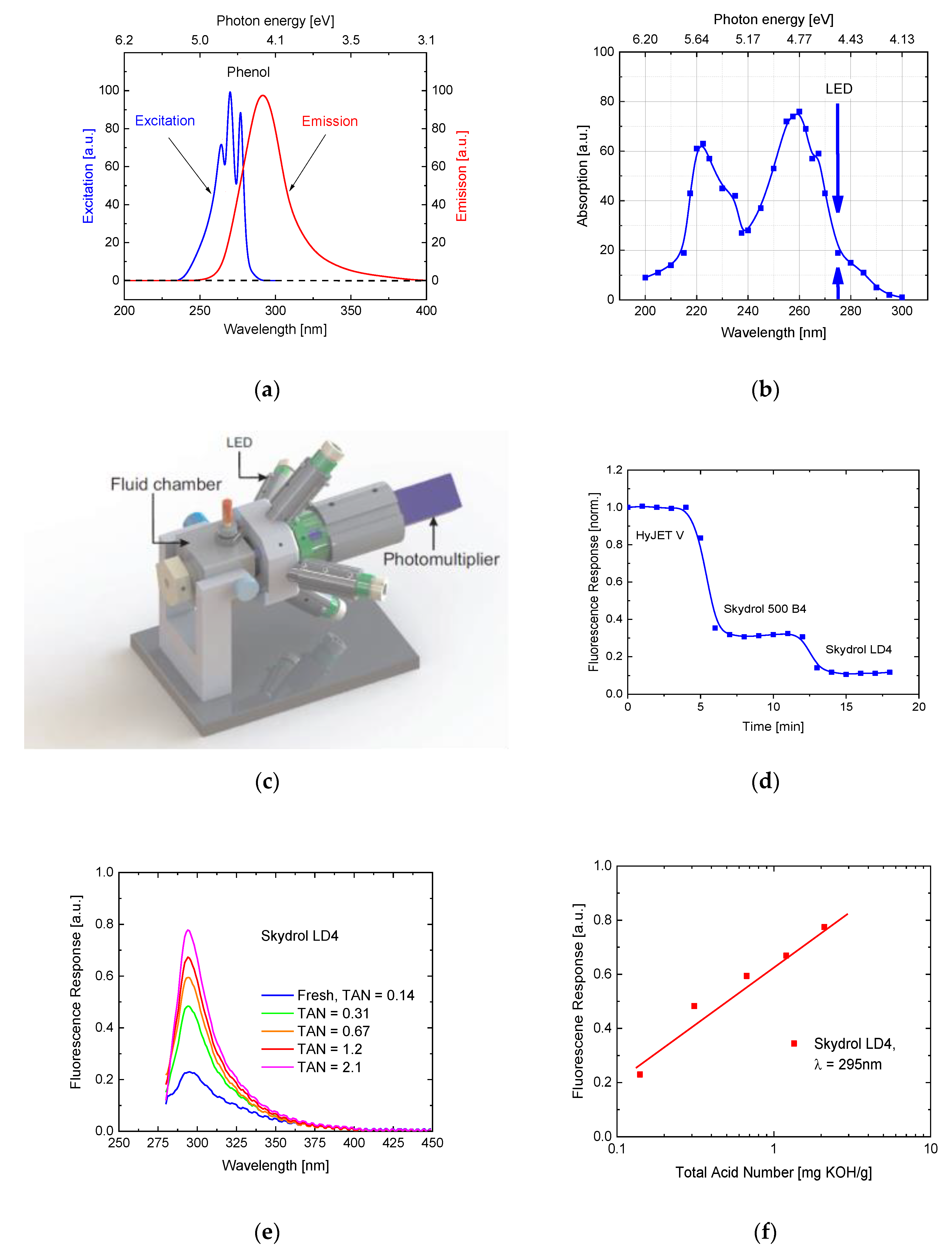
6. Fluorescence Monitoring of Aviation Hydraulic Fluids
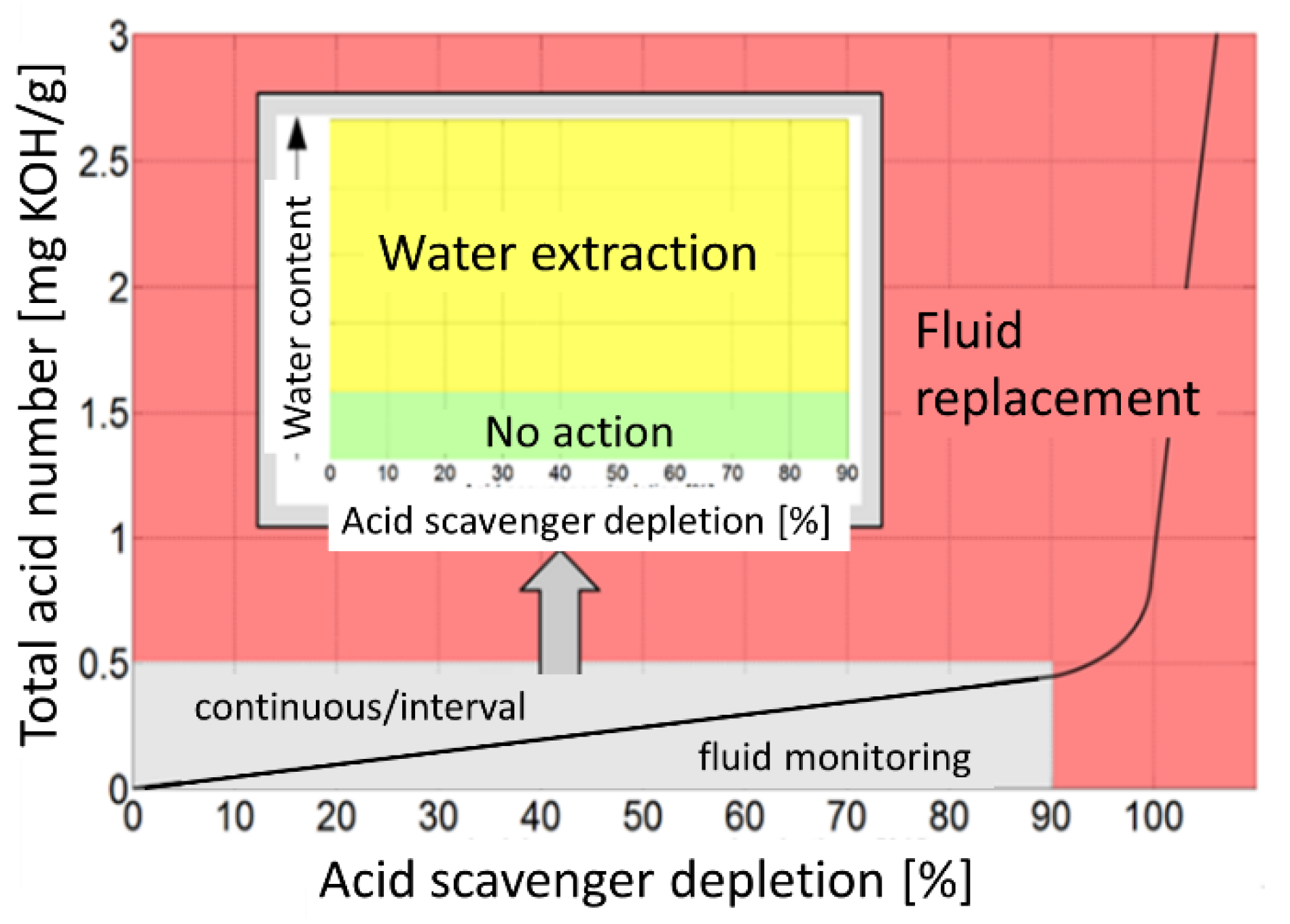
7. Achievements and Possible Ways Forward
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Difference Spectra of Widely Used Aviation Hydraulic Fluids
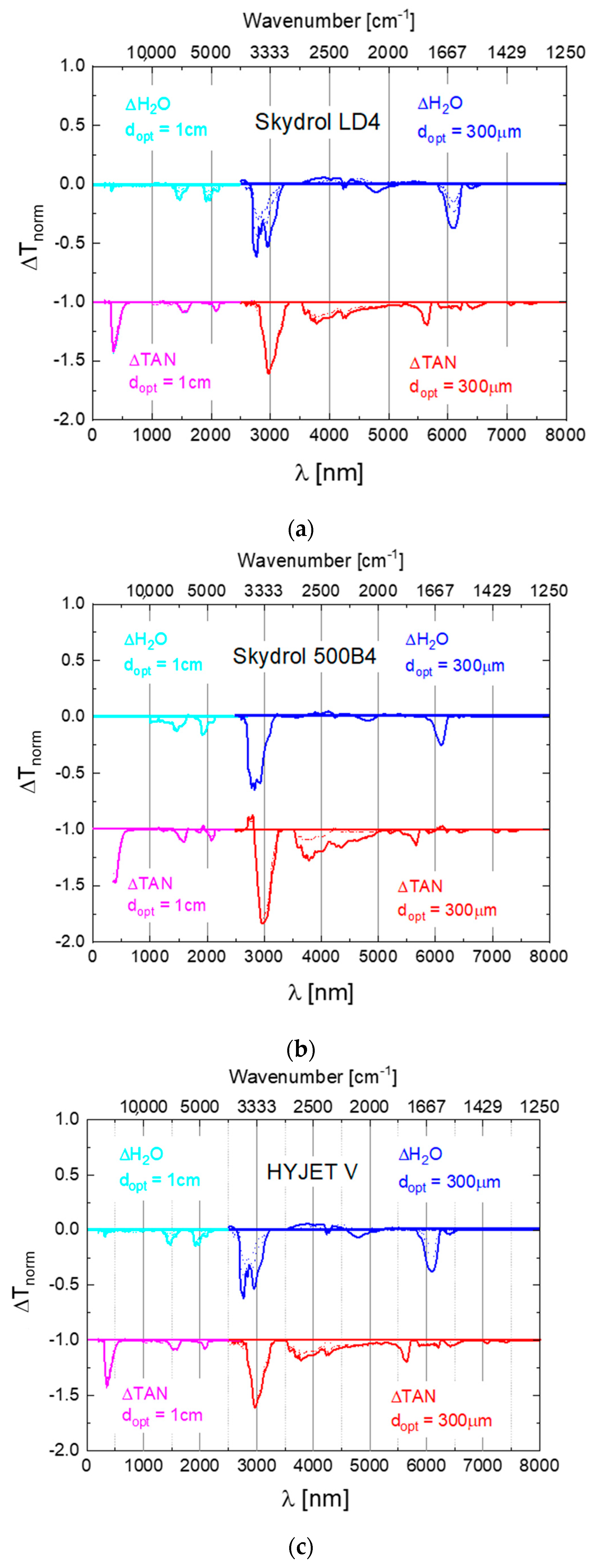
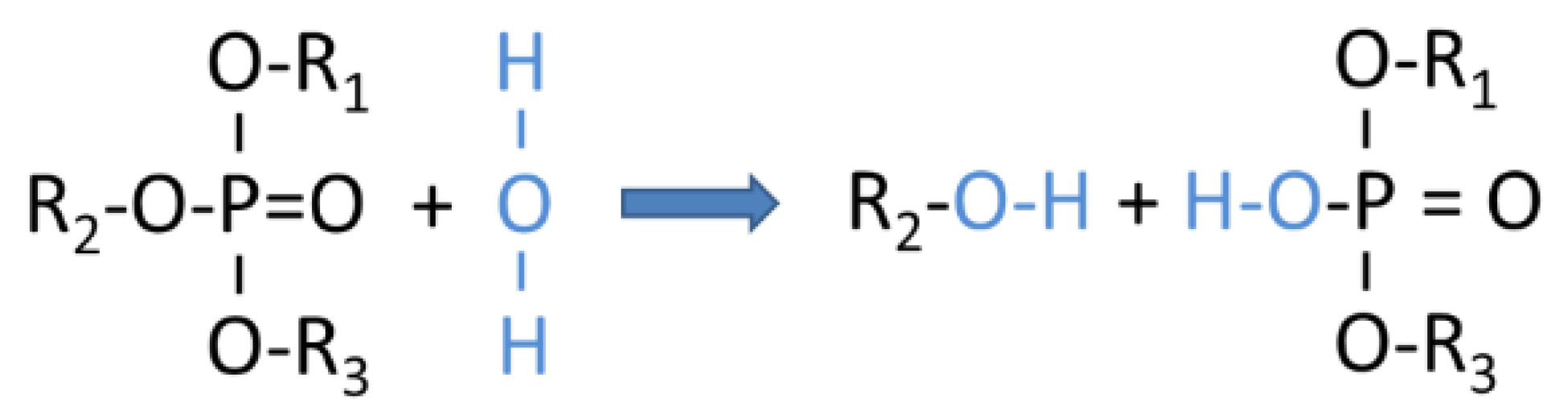
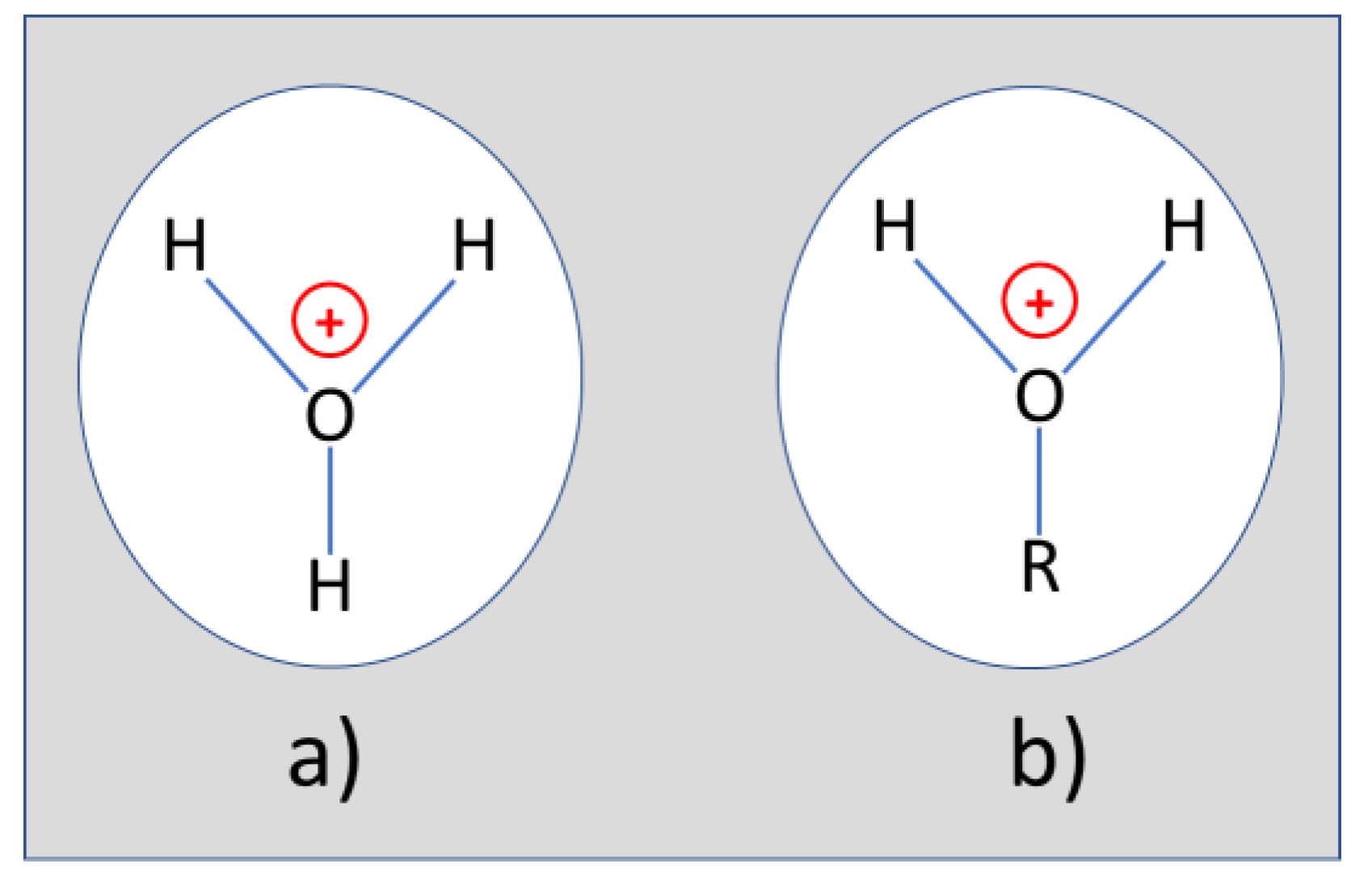
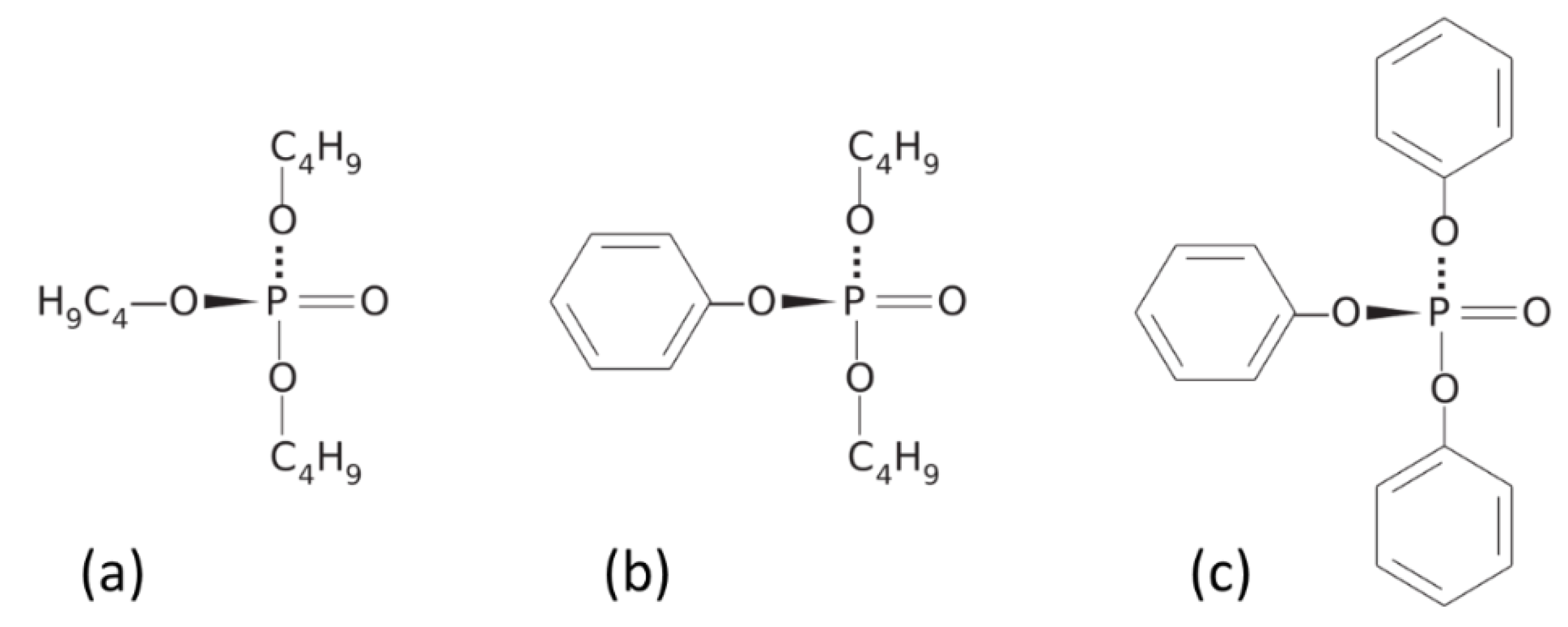
Appendix B. Assignment of Observed Optical Features to Molecular Vibration Modes
| Water Vibration Feature | λH2O [nm] | λSkydrol_H2O [nm] | Δλ [nm] | Δλ/λH2O [%] | |
|---|---|---|---|---|---|
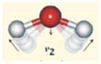 | Bend ν2 | 6080 | 6099 | 19 | 0.3 |
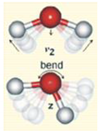 | Combination Bend ν2+L2 | 4650 | 4791 | 141 | 3.03 |
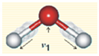 | Symmetric Stretch ν1 | 3050 | 2952 | −98 | −3.21 |
 | Asymmetric stretch ν3 | 2870 | 2766 | −104 | −3.62 |
| Combination | aν1 + ν2 + bν3. a + b = 1 | 1900 | 1917 | 17 | 0.89 |
| Combination | aν1 + bν3. a + b = 2 | 1470 | 1471 | 1 | 0.07 |
| Skydrol LD4 Vibrational Features | λSkydrol_H2O [nm] | λSkydrol_acidified [nm] | Δλ [nm] | Δλ/λ [%] |
|---|---|---|---|---|
| Bend ν2 | 6099 | 6416 | 317 | 5.2 |
| Combination Bend ν2 + L2 | 4791 | 5635 | 844 | 14.98 |
| Partial phosphates PO-H stretch POH comb. | - | (broad) 3500–5500 | - | - |
| Symmetric Stretch ν1 | 2952 | 3773 | 821 | 27.81 |
| Asymmetric stretch ν3 | 2766 | 2979 | 213 | 7.70 |
| Combination aν1 + ν2 + bν3. a + b = 1 | 1917 | 2080 | 163 | 8.50 |
| Combination aν1 + bν3. a + b = 2 | 1471 | 1518 | 47 | 3.20 |
| Aromatic decay products | - | 361 | - | - |

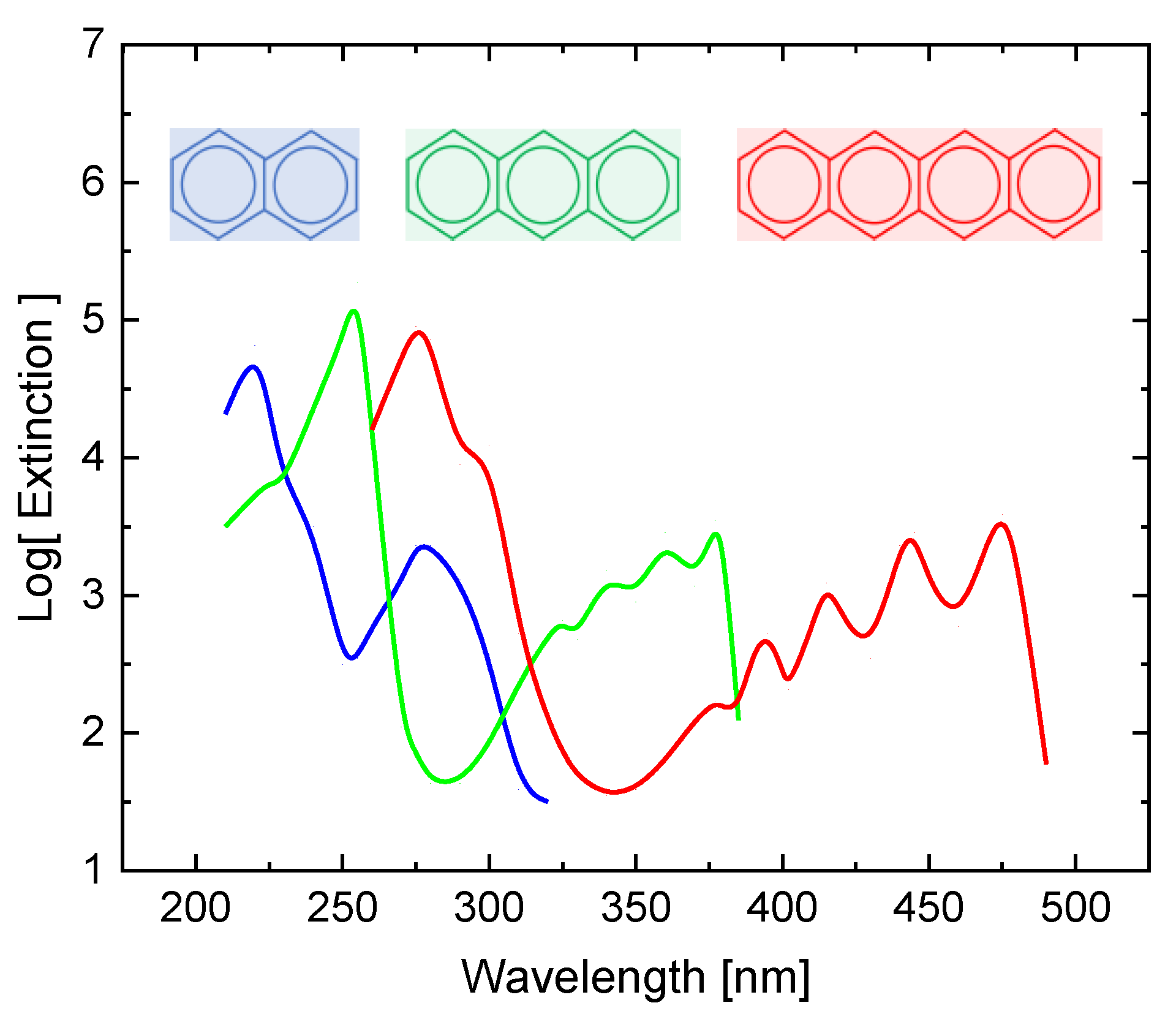
Appendix C. UV-VIS Absorption of Single- and Multi-Ring Aromatic Species
References
- Churchill, J. The Skydrol Story; Kindle Edition; 2012. Available online: https://www.amazon.com/Skydrol-Story-John-Churchill-ebook/dp/B00KGWI04K (accessed on 10 December 2020).
- Skydrol™ Aviation Hydraulic Fluids. Available online: https://www.skydrol.com (accessed on 10 December 2020).
- Totten, G.E.; De Negri, V.J. Handbook of Hydraulic Fluid Technology, 2nd ed.; CRC Press Inc.: Boca Raton, FI, USA, 2011; ISBN 978-1-4200-8526-6. [Google Scholar]
- Okazaki, M.E.; Abernathy, S.M.; Laurent, J.W. Hydrolysis of Phosphate-Based Aviation Hydraulic Fluids. J. Synth. Lubr. 1993, 10, 107–118. [Google Scholar] [CrossRef]
- Hertens, D.; Baumbach, V.; (Airbus Industries). Private communication, 2010.
- Paul, S. Optochemical Sensor Systems for Aerospace Applications. Ph.D. Thesis, Justus-Liebig-University, Giessen, Germany, 2014. [Google Scholar]
- Paul, S.; Legner, W.; Krenkow, A.; Müller, G.; Lemettais, T.; Pradat, T.; Hertens, D. Chemical Contamination Sensor for Phosphate Ester Hydraulic Fluids. Int. J. Aerosp. Eng. 2010, 2010, 156281. [Google Scholar] [CrossRef]
- Paul, S.; Legner, W.; Hackner, A.; Baumbach, V.; Müller, G. Multi-Parameter Monitoring System for Hydraulic Fluids. Tech. Mess. 2011, 78, 260–267. [Google Scholar] [CrossRef]
- Helwig, A.; Müller, G.; Rausch, J.; Luther, R.; Bley, T.; Steffensky, J.; Mannebach, H. Applikationsnahe Erprobung und Weiterentwicklungsperspektiven eines Infrarot-basierten Sensorsystems für die Fluidik—Assessment of an Infrared-Based Sensor System for Fluid Monitoring Applications. In Proceedings of the AMA Conference, Nürnberg, Germany, 4 June 2014. [Google Scholar]
- Helwig, A.; Müller, G.; Bley, T.; Steffensky, J.; Mannebach, H. An optoelectronic monitoring system for aviation hydraulic fluids. In Proceedings of the Procedia Engineering, EUROSENSORS 2015, Freiburg, Germany, 6–9 September 2015. [Google Scholar]
- Bley, T.; Steffensky, J.; Mannebach, H.; Helwig, A.; Müller, G. Degradation monitoring of aviation hydraulic fluids using non-dispersive infrared sensor systems. Sens. Actuators B Chem. 2016, 224, 539–546. [Google Scholar] [CrossRef]
- Christen, H.R. Grundlagen der Allgemeinen und Anorganischen Chemie; Salle+Sauerländer: Frankfurt, Germany; Berlin, Germany; München, Germany, 1982; ISBN 3-7935-5394-9. [Google Scholar]
- Fischer, K. Neues Verfahren zur maßanalytischen Bestimmung des Wassergehaltes von Flüssigkeiten und festen Körpern. Angew. Chem. 1935, 48, 394–396. [Google Scholar] [CrossRef]
- Lemettais, T.; Pradat, T.; Hertens, D. (EADS Innovation Works France, Airbus Industries, Toulouse). Private communication.
- Table of IR Absorptions. Available online: https://webspectra.chem.ucla.edu/irtable.html (accessed on 10 December 2020).
- Aslin, R.E.; Denisov, G.S.; Poplevchenkov, D.N.; Tokbadze, K.G.; Velikanova, T.Y. IR v(OH) Band and Dimerization of Phosphorus Acids in the Gas Phase and Solid State. Pol. J. Chem. 2002, 76, 1223–1231. [Google Scholar]
- Müller, L.; Käpplinger, I.; Biermann, S.; Brode, W.; Hofmann, M. Infrared emitting nanostructures for highly efficient micro hotplates. J. Micromech. Microeng. 2014, 24, 035014. [Google Scholar] [CrossRef]
- Available online: https://www.microhybrid.com/de/produkte/thermische-ir-detektoren/ (accessed on 10 December 2020).
- Dillner, U.; Kessler, E.; Meyer, H.G. Figures of merit of thermoelectric and bolometric thermal radiation sensors. J. Sens. Sens. Syst. 2013, 2, 85–94. [Google Scholar] [CrossRef]
- Müller, L.; Günschmann, S.; Fischer, M.; Müller, J.; Hoffmann, M.; Käpplinger, I.; Brode, W.; Magi, A.; Biermann, S.; Pignanelli, E. Entwicklung und Optimierung mikrotechnischer Silicium- und Keramikkomponenten zur Realisierung eines Fluidiksensors - Development and optimization of a fluidic sensor system based on silicon and ceramic microtechnology components. In Proceedings of the Proc. AMA Conference, Nürnberg, Germany, 4 June 2014. [Google Scholar]
- NAMIFLU (BMBMF) Final Report. Available online: https://www.tu-ilmenau.de/mne-et/projekte/namiflu/ (accessed on 10 December 2020).
- Aircraft Hydraulic GroundLab AHG. Available online: https://www.hydac.com.br/wp-content/uploads/e-76670-ahg-en-2016-03-01.pdf (accessed on 10 December 2020).
- Behr, R.; Baumbach, V. Model Based Aerospace Hydraulic Fluid Lifetime Prognostics. In Proceedings of the International Conference on Recent Advances in Aerospace Actuation Systems and Components, Toulouse, France, 5–7 May 2010. [Google Scholar]
- Available online: https://www.sciencedirect.com/book/9780120926565/handbook-of-fluorescence-spectra-of-aromatic-molecules (accessed on 11 December 2020).
- Available online: https://en.wikipedia.org/wiki/Predictive_maintenance (accessed on 11 December 2020).
- Mobley, R.K. (Ed.) An Introduction to Predictive Maintenance; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar] [CrossRef]
- Levitt, J. Complete Guide to Preventive and Predictive Maintenance, 2nd ed.; Industrial Press Inc.: New York, NY, USA, 2011; ISBN1 0831134410. ISBN2 978-0831134419. [Google Scholar]
- Lughofer, E.; Sayed-Mouchaweh, M. (Eds.) Predictive Maintenance in Dynamic Systems—Advanced Methods, Decision Support Tools and Real-World Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2019; ISBN 978-3-030-05645-2. [Google Scholar]
- Harrick, N.J. Internal Reflection Spectroscopy; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1967; ISBN 0-470-35250-7. [Google Scholar]
- Milosevic, M. Internal Reflection and ATR Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Print ISBN 9780470278321, Online ISBN 9781118309742. [Google Scholar] [CrossRef]
- Vigano, C.; Ruysschaert, J.-M.; Goormaghtigh, E. Sensor Applications of Attenuated Total Reflection Infrared Spectroscopy. Talanta 2005, 65, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Geörg, D.; Schalk, R.; Methner, F.J.; Beuermann, T. MIR-ATR sensor for process monitoring. Meas. Sci. Technol. 2015, 26, 6. [Google Scholar] [CrossRef]
- Geörg, D. Entwicklung eines MIR-ATR-Sensors zur Prozesskontrolle in der Chemischen Industrie und Biotechnologie. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 2017. [Google Scholar]
- Morhart, T.A.; Read, S.T.; Wells, G.; Jacobs, M.; Rosendahl, S.M.; Achenbach, S.; Burgess, I.J. Micromachined multigroove silicon ATR FT-IR internal reflection elements for chemical imaging of microfluidic devices. Anal. Methods 2019, 11, 5776–5783. [Google Scholar] [CrossRef]
- Haas, J.; Müller, A.; Sykora, L.; Mizaikoff, B. Analytical performance of μ-groove silicon attenuated total reflection waveguides. Analyst 2019, 144, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Ebermann, M.; Neumann, N.; Hiller, K.; Seifert, M.; Meinig, M.; Kurth, S. Tunable MEMS Fabry-Pérot filters for infrared microspectrometers: A review. In MOEMS and Miniaturized Systems XV; Piyawattanametha, W., Park, Y.-H., Eds.; SPIE: Bellingham, DC, USA, 2016. [Google Scholar] [CrossRef]
- Ghoname, A.; Sabry, Y.; Anwar, M.; Khalil, D. Attenuated total reflection (ATR) MEMS FTIR spectro-meter. In Proceedings of the SPIE 11293, MOEMS and Miniaturized Systems XIX, San Francisco, CA, USA, 1–6 February 2020. [Google Scholar]
- Pedras, B.; Orrelana, G.; Berberan-Santor, M.N. Luminescence-Based Sensors for Aeronautical Applications. In Fluorescence in Industry; Pedras, B., Ed.; Springer Series on Fluorescence (Methods and Applications); Springer: Cham, Switzerland, 2019; Volume 18. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Becker, P.; Hille, P.; Schörmann, J.; Teubert, J.; Eickhoff, M. Detection of Oxidizing Gases Using an Optochemical Sensor System Based on GaN/InGaN Nanowires. Sens. Actuators B 2014, 197, 87–94. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Teubert, J.; Eickhoff, M. Photoluminescence Probing of Complex H2O Adsorption on InGaN/GaN Nanowires. Nano Lett. 2017, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.; Helwig, A.; Müller, G.; Eickhoff, M. Luminescence Probing of Surface Adsorption Processes Using InGaN/GaN Nanowire Heterostructure Arrays. In Semiconductor Gas Sensors, 2nd ed.; Jaaniso, R., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2020; pp. 239–270. ISBN 978-0-08-102559-8. [Google Scholar] [CrossRef]
- Paul, S.; Maier, K.; Das, A.; Furtmayr, F.; Helwig, A.; Teubert, J.; Monroy, E.; Müller, G.; Eickhoff, M. III-nitride nanostructures for optical gas detection and pH sensing. In Proceedings of the SPIE 8725, Micro- and Nanotechnology Sensors, Systems, and Applications V, Baltimore, MD, USA, 29 April–3 May 2013. [Google Scholar] [CrossRef]
- Available online: http://www1.lsbu.ac.uk/water/water_vibrational_spectrum.html (accessed on 11 December 2020).
- Haken, H.; Wolf, H.C. Molecular Physics and Elements of Quantum Chemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004; ISBN1 3540407928. ISBN2 978-3540407928. [Google Scholar]
- Keller-Rudek, H.; Moortgat, G.K.; Sander, R.; Sörensen, R. The MPI-Mainz UV/VIS Spectral Atlas of Gaseous Molecules of Atmospheric Interest. Available online: www.uv-vis-spectral-atlas-mainz.org (accessed on 11 December 2020).
- Waser, R. (Ed.) Nanoelectronics and Information Technology; Wiley VCH: Weinheim, Germany, 2003; p. 131ff. [Google Scholar]
- Available online: http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/UV-Vis/spectrum.htm (accessed on 11 December 2020).
- Available online: https://www.shimadzu.com/an/service-support/technical-support/technical-information/uv-ap/apl.html (accessed on 11 December 2020).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helwig, A.; Müller, G.; Paul, S. Health Monitoring of Aviation Hydraulic Fluids Using Opto-Chemical Sensor Technologies. Chemosensors 2020, 8, 131. https://doi.org/10.3390/chemosensors8040131
Helwig A, Müller G, Paul S. Health Monitoring of Aviation Hydraulic Fluids Using Opto-Chemical Sensor Technologies. Chemosensors. 2020; 8(4):131. https://doi.org/10.3390/chemosensors8040131
Chicago/Turabian StyleHelwig, Andreas, Gerhard Müller, and Sumit Paul. 2020. "Health Monitoring of Aviation Hydraulic Fluids Using Opto-Chemical Sensor Technologies" Chemosensors 8, no. 4: 131. https://doi.org/10.3390/chemosensors8040131
APA StyleHelwig, A., Müller, G., & Paul, S. (2020). Health Monitoring of Aviation Hydraulic Fluids Using Opto-Chemical Sensor Technologies. Chemosensors, 8(4), 131. https://doi.org/10.3390/chemosensors8040131





