DFO@EVOH and 3,4-HP@EVOH: Towards New Polymeric Sorbents for Iron(III)
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Chemicals
2.1.2. Instruments
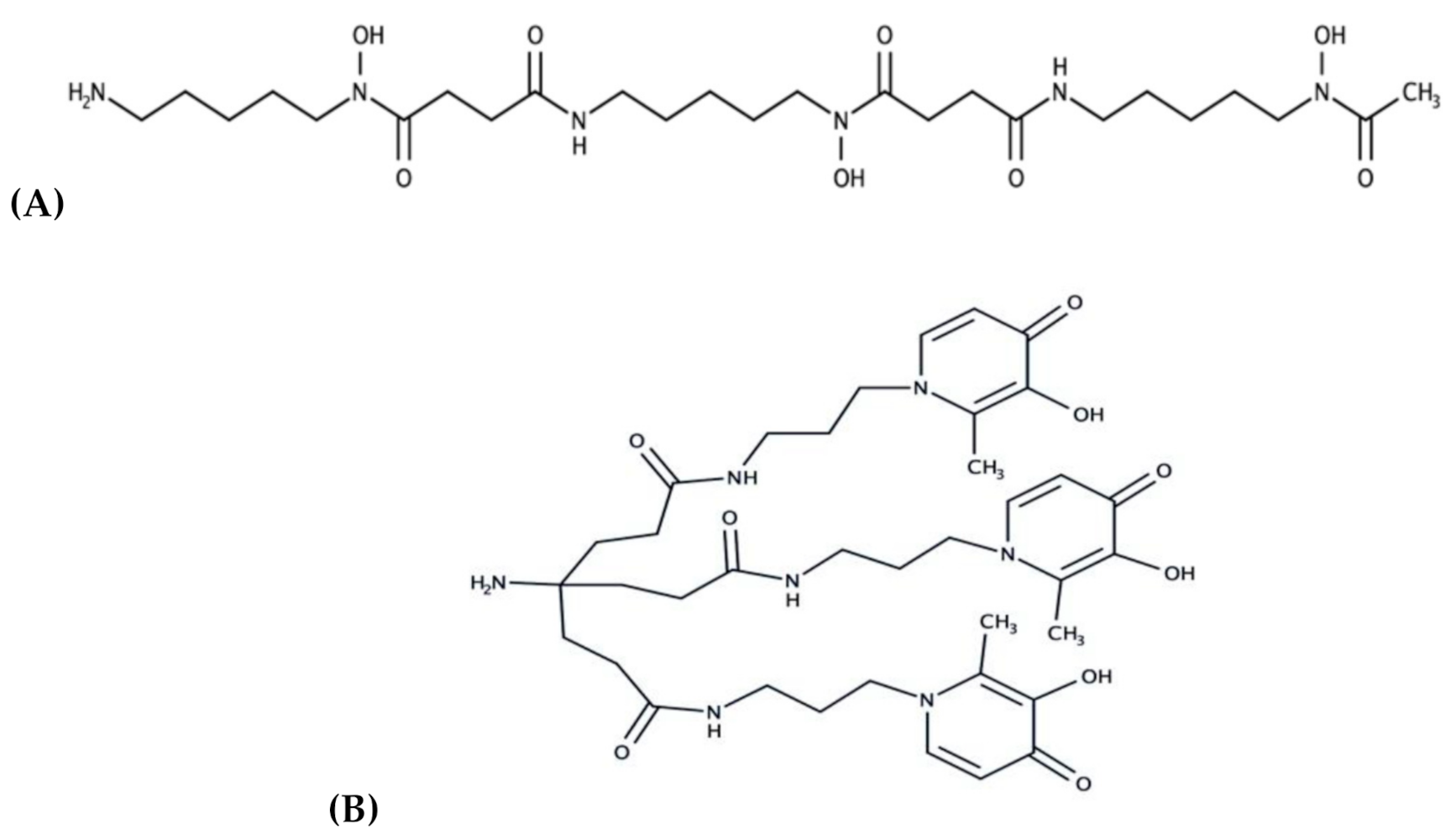
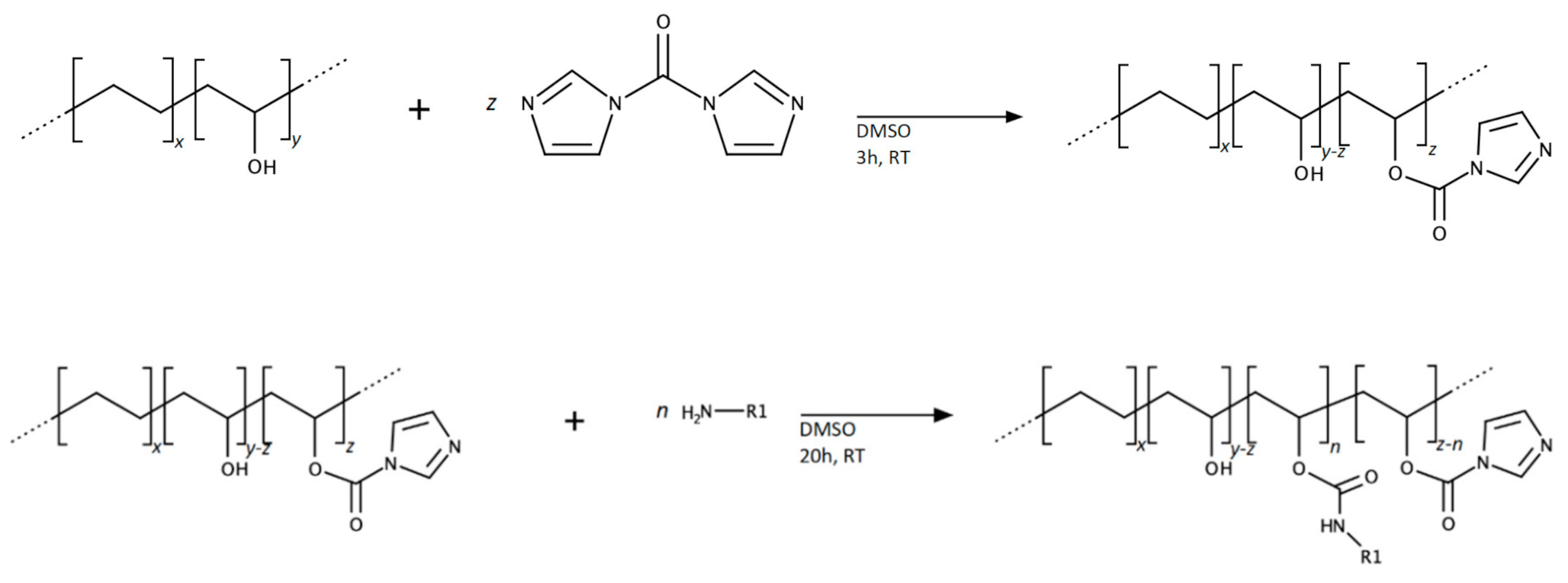
2.1.3. Synthesis
2.1.4. Isotherm Experiments
2.1.5. Desorption Experiments
3. Results and Discussion
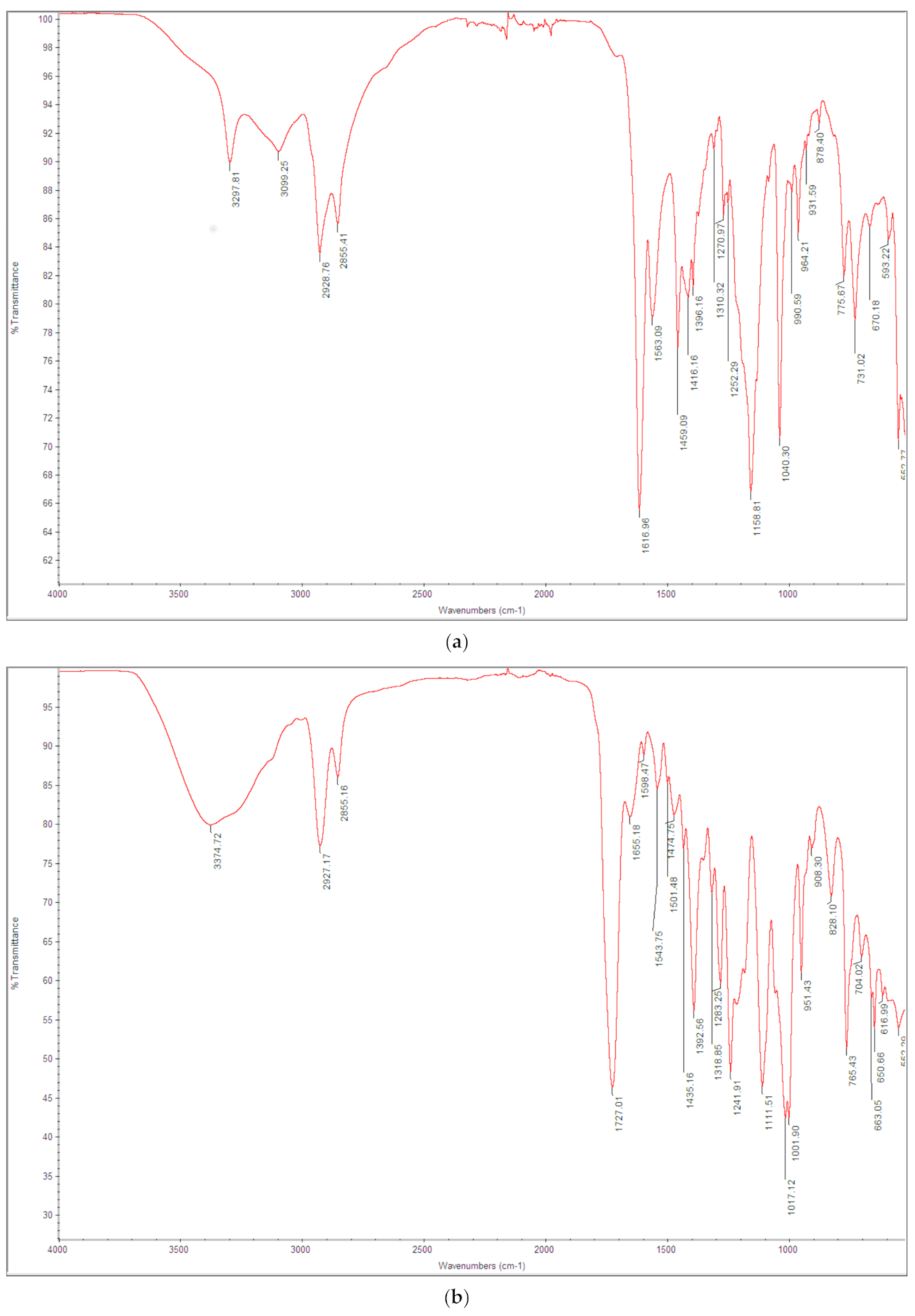
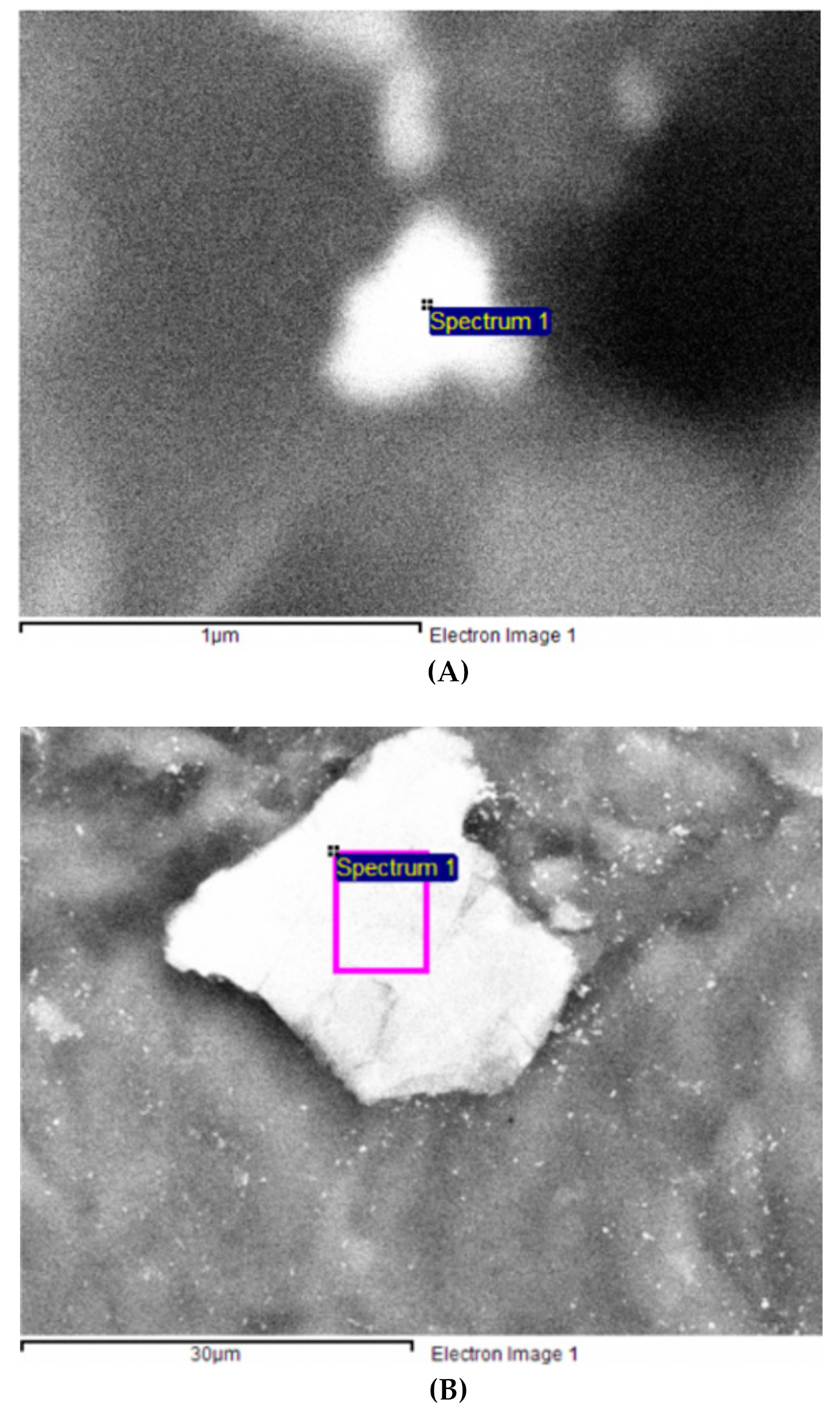
3.1. Physico-Chemical Characterization
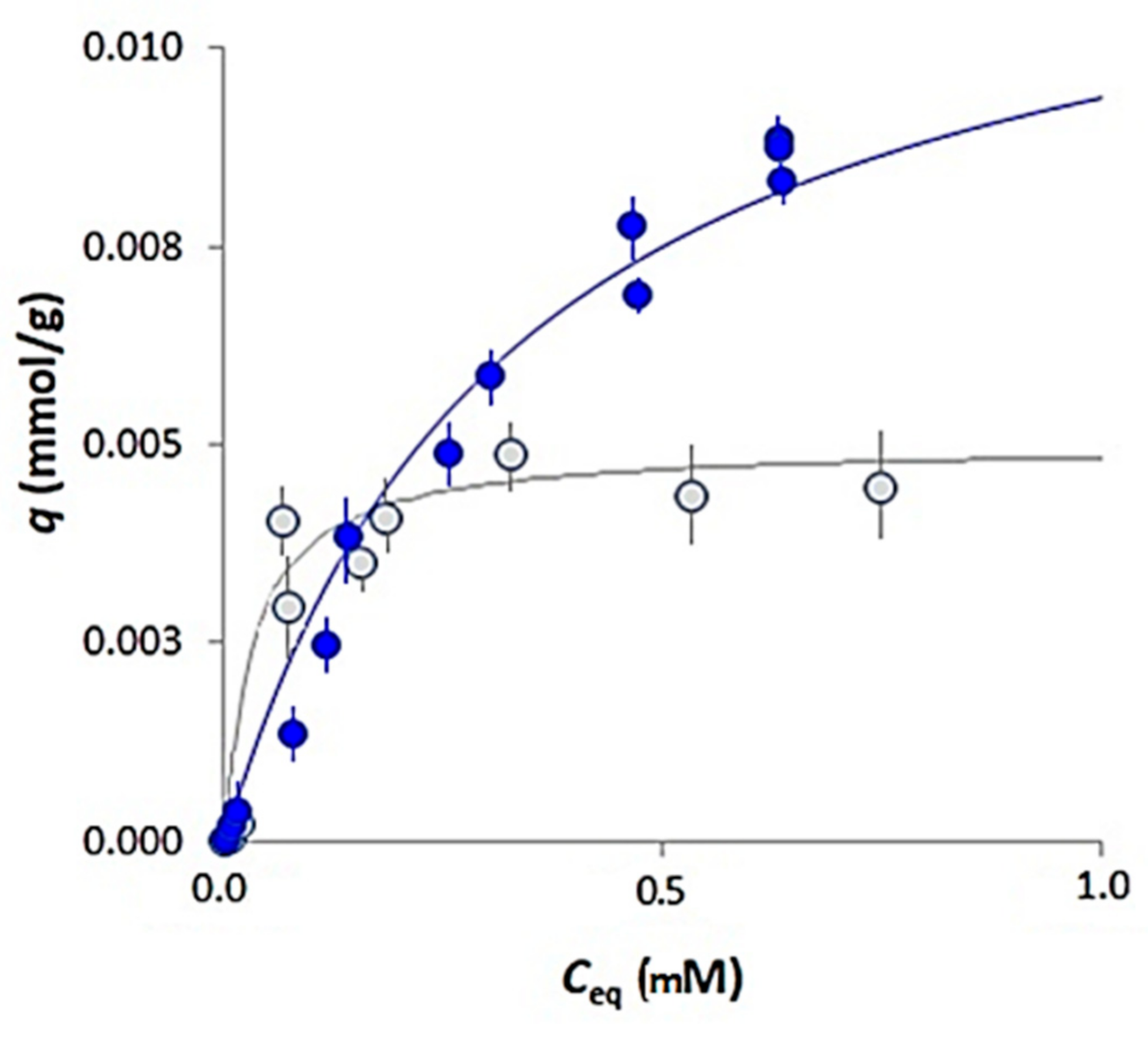
3.2. Sorption Isotherms
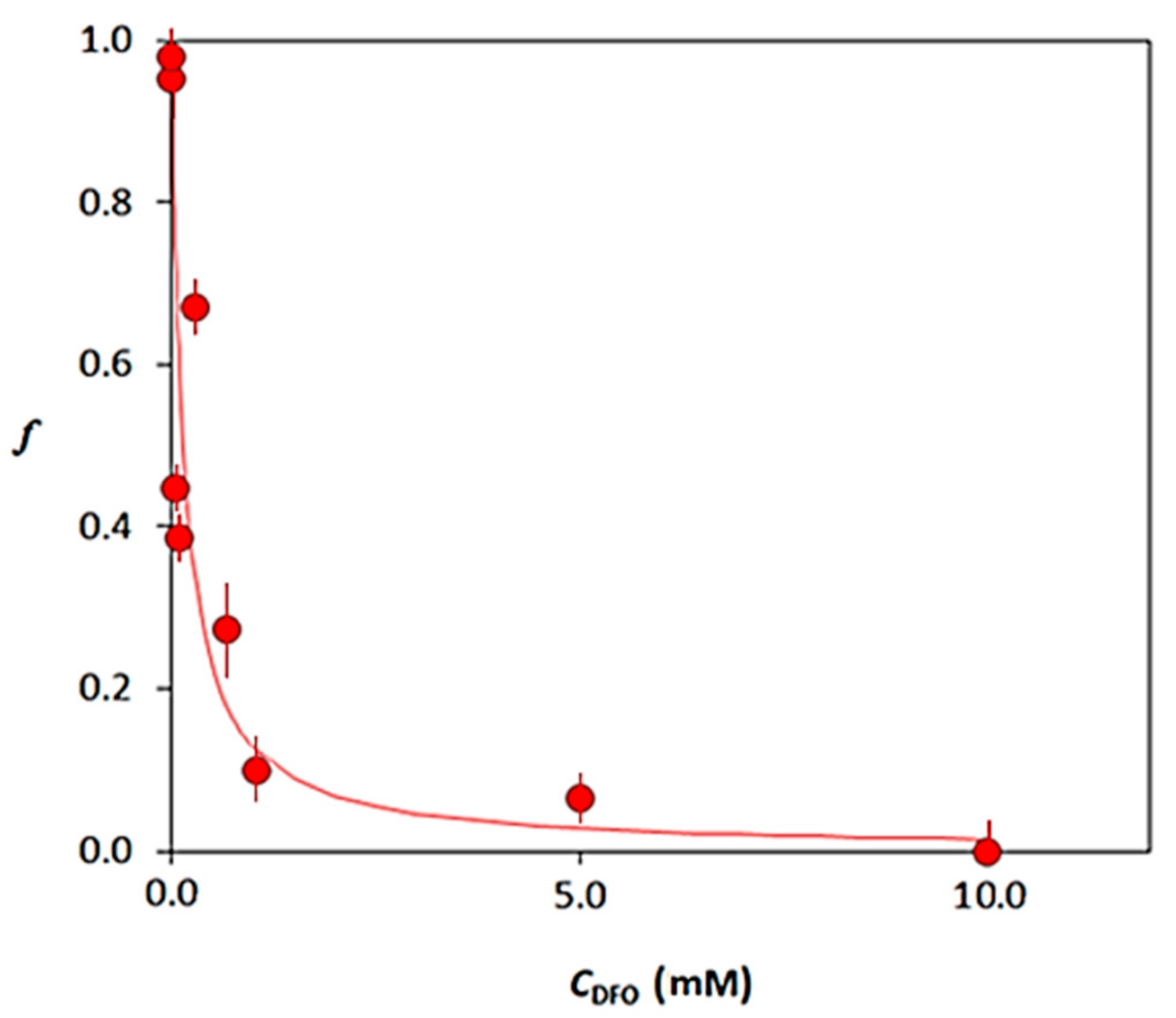
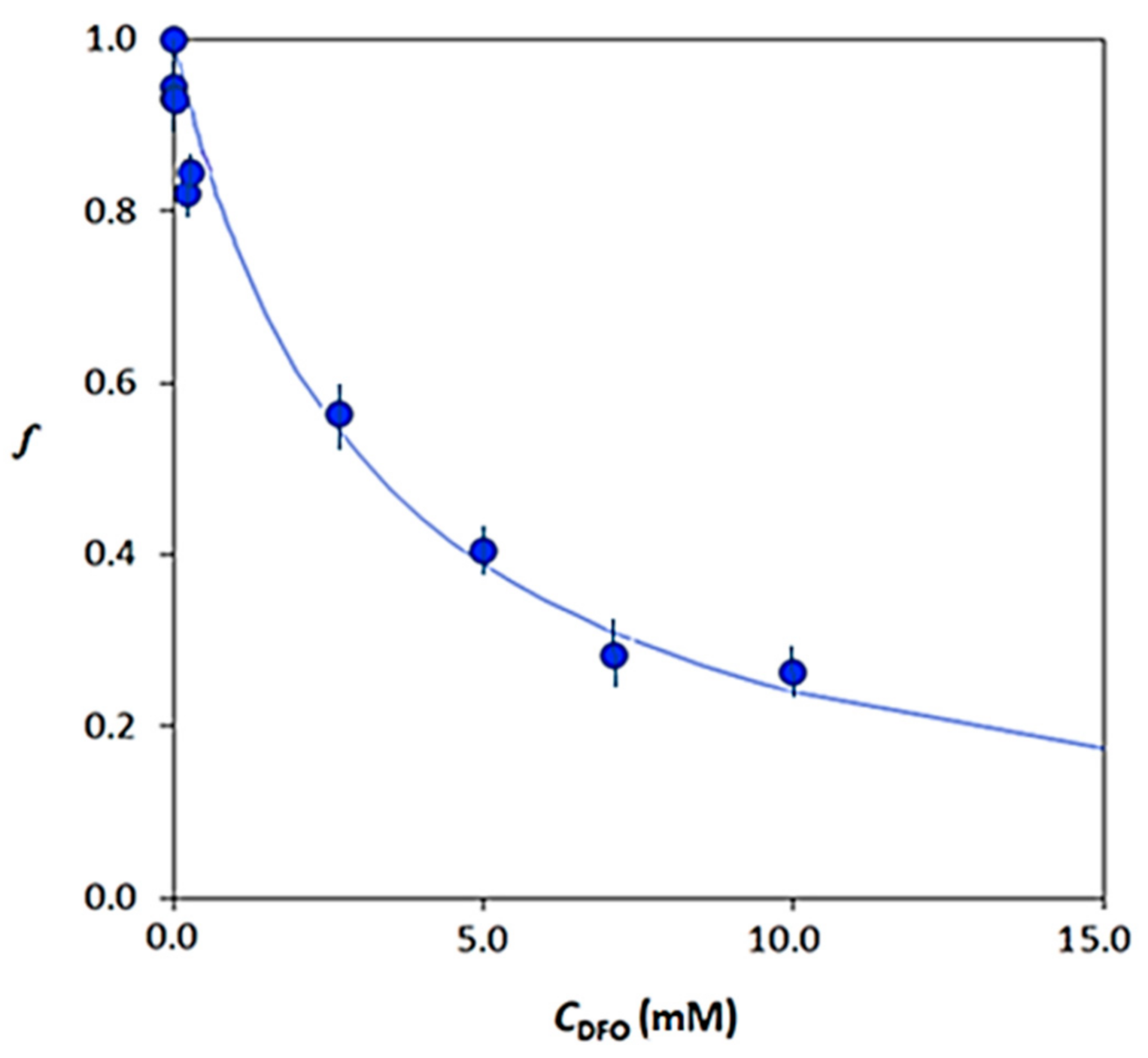
3.3. Desorption Profiles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Hu, X.; Pan, D.; Han, H.; Lin, M.; Lu, Y.; Wang, C.; Zhu, R. Speciation determination of iron and its spatial and seasonal distribution in coastal river. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oguz, M.; Kursunlu, A.N.; Yilmaz, M. Low-cost and environmentally sensitive fluorescent cellulose paper for naked-eye detection of Fe(III) in aqueous media. Dye. Pigment. 2020, 173, 107974. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed.; Clarendon Press: Oxford, UK, 2015. [Google Scholar]
- Kalinowski, D.S.; Richardson, D.R. The Evolution of Iron Chelators for the Treatment of Iron Overload Disease and Cancer. Pharmacol. Rev. 2005, 57, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Crisponi, G.; Remelli, M. Iron chelating agents for the treatment of iron overload. Co-ord. Chem. Rev. 2008, 252, 1225–1240. [Google Scholar] [CrossRef]
- Jones, G.; Goswami, S.K.; Kang, H.; Choi, H.S.; Kim, J. Combating iron overload: A case for deferoxamine-based nanochelators. Nanomedicine 2020, 15, 1341–1356. [Google Scholar] [CrossRef]
- Darmawan, W.; Nurani, D.A.; Rahayu, D.U.C.; Abdullah, I. Synthesis of Ion Imprinted Polymer for Separation and Preconcentration of Iron (III). In Proceedings of the 5th International Symposium on Current Progress in Mathematics and Sciences (Iscpms2019), Depok, Indonesia, 9–10 July 2019; Volume 2242, p. 040025. [Google Scholar] [CrossRef]
- Eyyubova, E. Baku State University ADSORPTION STUDY OF Fe(III) IONS BY MASC-2-AMINO-4-NITROPHENOL. Azerbaijan Chem. J. 2020, 2, 26–33. [Google Scholar] [CrossRef]
- Ahmad, K.; Hui, Y.Z.; Bairq, Z.A.S. Comparison of the performance of a hydrogel and hybrid graphene oxide with hydrogel to remove iron (III) and phenol from wastewater. Res. Chem. Intermed. 2020, 46, 2613–2639. [Google Scholar] [CrossRef]
- Sui, D.-P.; Chen, H.-X.; Li, D.-W. Sol–gel-derived thiocyanato-functionalized silica gel sorbents for adsorption of Fe(III) ions from aqueous solution: Kinetics, isotherms and thermodynamics. J. SolGel. Sci. Technol. 2016, 80, 504–513. [Google Scholar] [CrossRef]
- Biesuz, R.; Alberti, G.; D’Agostino, G.; Magi, E.; Pesavento, M. Determination of cadmium(II), copper(II), manganese(II) and nickel(II) species in Antarctic seawater with complexing resins. Mar. Chem. 2006, 101, 180–189. [Google Scholar] [CrossRef]
- Pesavento, M.; Biesuz, R.; Gallorini, M.; Profumo, A. Sorption mechanism of trace amounts of divalent metal ions on a chelating resin containing iminodiacetate groups. Anal. Chem. 1993, 65, 2522–2527. [Google Scholar] [CrossRef]
- Pesavento, M.; Biesuz, R.; Alberti, G.; Sturini, M. Characterization of the sorption of uranium(VI) on different complexing resins. Anal. Bioanal. Chem. 2003, 376, 1023–1029. [Google Scholar] [CrossRef]
- Ringbom, A.; Still, E. The calculation and use of a coefficients. Anal. Chim. Acta 1972, 59, 143–146. [Google Scholar] [CrossRef]
- Alberti, G.; Emma, G.; Colleoni, R.; Pesavento, M.; Nurchi, V.M.; Biesuz, R. Novel DFO-functionalized mesoporous silica for iron sensing. Part 2. Experimental detection of free iron concentration (pFe) in urine samples. Anallyze 2014, 139, 3940–3948. [Google Scholar] [CrossRef]
- Biesuz, R.; Emma, G.; Milanese, C.; Dacarro, G.; Taglietti, A.; Nurchi, V.M.; Alberti, G. Novel DFO-SAM on mesoporous silica for iron sensing. Part I. Synthesis optimization and characterization of the material. Analyze 2014, 139, 3932–3939. [Google Scholar] [CrossRef]
- Alberti, G.; Quattrini, F.; Colleoni, R.; Nurchi, V.M.; Biesuz, R. Deferoxamine–paper for iron(III) and vanadium(V) sensing. Chem. Pap. 2015, 69, 1024–1032. [Google Scholar] [CrossRef]
- Xu, D.; Lu, J.; Yan, S.; Xiao, R. Aminated EVOH nanofiber membranes for Cr(vi) adsorption from aqueous solution. RSC Adv. 2018, 8, 742–751. [Google Scholar] [CrossRef]
- Salehi-Mobarakeh, H.; Yadegari, A.; Didehvar, J.; Khakzad-Esfahlan, F. Polyamide grafting onto ethylene-vinyl alcohol copolymer. J. Polym. Eng. 2013, 33, 843–850. [Google Scholar] [CrossRef]
- Salgado, C.; Arrieta, M.P.; Chiloeches, A.; Muñoz-Bonilla, A.; Peponi, L.; López, D.; Fernández-García, M. Development of photoresponsive coumarin-modified ethylene-co-vinyl alcohol copolymers with antifouling behavior. React. Funct. Polym. 2020, 157, 104750. [Google Scholar] [CrossRef]
- Du, J.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of sodium styrene sulfonate-grafted ethylene-vinyl alcohol copolymer as adsorbent for ammonium removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 27235–27244. [Google Scholar] [CrossRef]
- Xie, K.; Dong, Z.; Wang, Y.; Qi, W.; Zhai, M.; Zhao, L. Facile Preparation of EVOH-Based Amphoteric Ion Exchange Membrane Using Radiation Grafting Technique: A Preliminary Investigation on Its Application for Vanadium Redox Flow Battery. Polymers 2019, 11, 843. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Cappai, R.; Chand, K.; Chaves, S.; Gano, L.; Crisponi, G.; Peana, M.; Zoroddu, M.A.; Santos, M.A. New strong extrafunctionalizable tris(3,4-HP) and bis(3,4-HP) metal sequestering agents: Synthesis, solution and in vivo metal chelation. Dalton Trans. 2019, 48, 16167–16183. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Hilborn, J. Poly(vinyl alcohol)-Based Hydrogels Formed by “Click Chemistry”. Macromology 2006, 39, 1709–1718. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, H.; Seo, J. EVOH nanocomposite films with enhanced barrier properties under high humidity conditions. Polym. Compos. 2013, 35, 644–654. [Google Scholar] [CrossRef]
- Sánchez-Chaves, M.; Ruiz, C.; Cerrada, M.; Fernández-García, M. Novel glycopolymers containing aminosaccharide pendant groups by chemical modification of ethylene–vinyl alcohol copolymers. Polymers 2008, 49, 2801–2807. [Google Scholar] [CrossRef]
- Alberti, G.; Amendola, V.; Pesavento, M.; Biesuz, R. Beyond the synthesis of novel solid phases: Review on modelling of sorption phenomena. Co-ord. Chem. Rev. 2012, 256, 28–45. [Google Scholar] [CrossRef]
- Farkas, E.; Enyedy, É.A.; Csóka, H. A comparison between the chelating properties of some dihydroxamic acids, desferrioxamine B and acetohydroxamic acid. Polyhedron 1999, 18, 2391–2398. [Google Scholar] [CrossRef]
- Alberti, G.; Biesuz, R.; Profumo, A.; Pesavento, M. Determination of the total concentration and speciation of Al(III) in tea infusions. J. Inorg. Biochem. 2003, 97, 79–88. [Google Scholar] [CrossRef]
- Alberti, G.; Pesavento, M.; Biesuz, R. A chelating resin as a probe for the copper(II) distribution in grape wines. React. Funct. Polym. 2007, 67, 1083–1093. [Google Scholar] [CrossRef]
- Puigdomenech, I. MEDUSA—Chemical Equilibrium Diagrams Program 32 Bit vers. Software for Windows; Royal Institute of Technology: Stockholm, Sweden, 2010. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Santos, M.A.; Nurchi, V.M.; Biesuz, R. DFO@EVOH and 3,4-HP@EVOH: Towards New Polymeric Sorbents for Iron(III). Chemosensors 2020, 8, 111. https://doi.org/10.3390/chemosensors8040111
Alberti G, Zanoni C, Magnaghi LR, Santos MA, Nurchi VM, Biesuz R. DFO@EVOH and 3,4-HP@EVOH: Towards New Polymeric Sorbents for Iron(III). Chemosensors. 2020; 8(4):111. https://doi.org/10.3390/chemosensors8040111
Chicago/Turabian StyleAlberti, Giancarla, Camilla Zanoni, Lisa Rita Magnaghi, Maria Amélia Santos, Valeria Marina Nurchi, and Raffaela Biesuz. 2020. "DFO@EVOH and 3,4-HP@EVOH: Towards New Polymeric Sorbents for Iron(III)" Chemosensors 8, no. 4: 111. https://doi.org/10.3390/chemosensors8040111
APA StyleAlberti, G., Zanoni, C., Magnaghi, L. R., Santos, M. A., Nurchi, V. M., & Biesuz, R. (2020). DFO@EVOH and 3,4-HP@EVOH: Towards New Polymeric Sorbents for Iron(III). Chemosensors, 8(4), 111. https://doi.org/10.3390/chemosensors8040111








