Opto-Electronic Nose Coupled to a Silicon Micro Pre-Concentrator Device for Selective Sensing of Flavored Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Opto-Electronic Nose
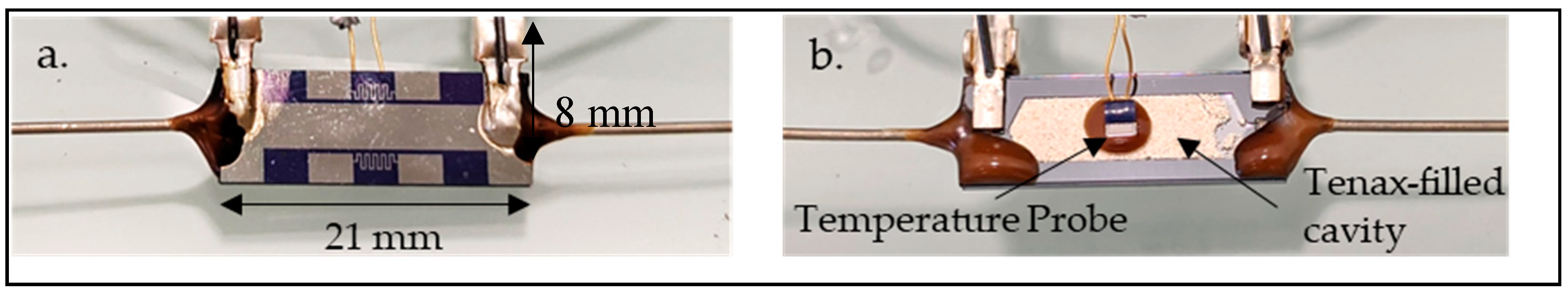
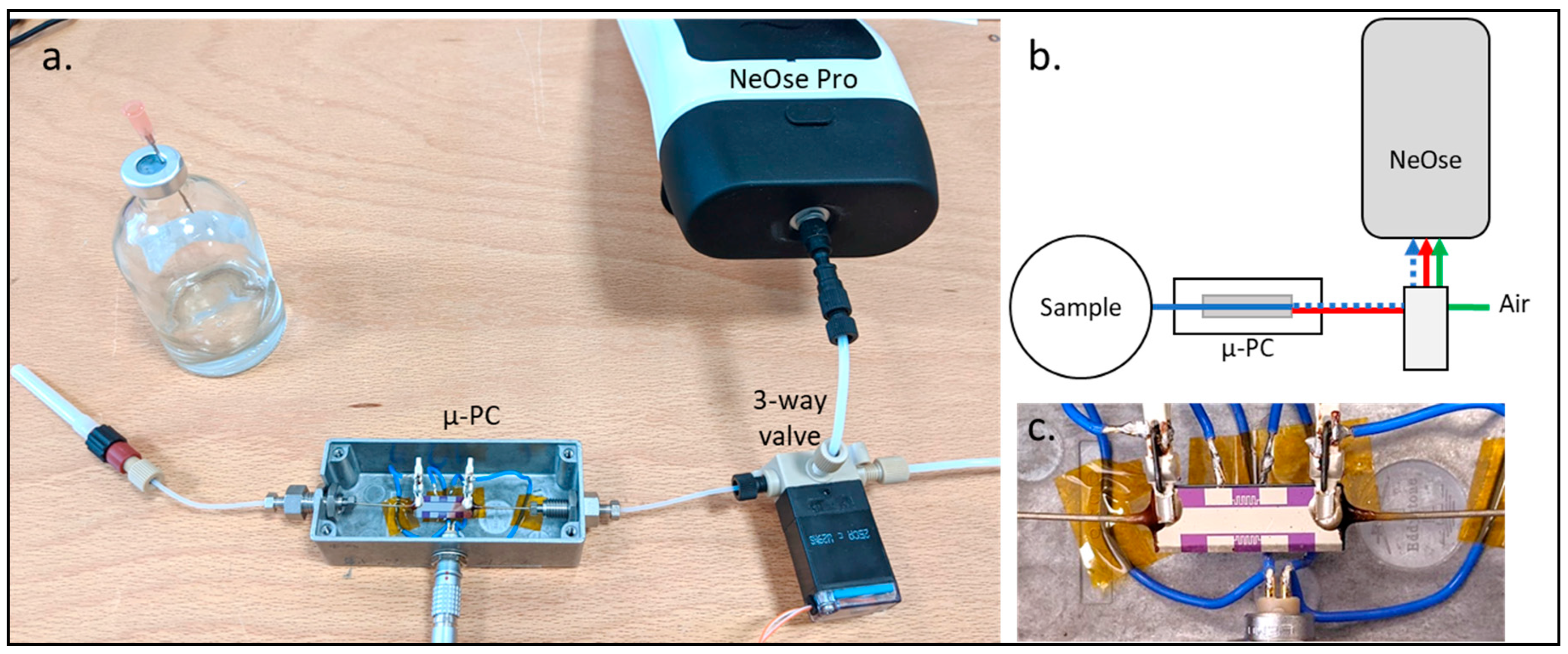
2.2. Silicon Micro Pre-Concentrator and Coupling to NeOse Pro Device
2.3. Detection Limit Assays of NeOse Pro Direct and Coupled to the Silicon Micro Pre-Concentrator
2.4. Signal Interpretation of n-Nonane Assays
2.5. Flavored Water Samples
2.6. NeOse Pro Direct Analysis of Flavored Waters
2.7. NeOse Pro/ATD Analysis of Flavored Waters
2.8. Data Processing for Multivariate Analysis of Water Samples
2.9. Total VOC Measurement
3. Results and Discussion
3.1. NeOse Pro Direct and Thermo-Desorbed Sensing of Nonane Dilutions
3.2. Flavored Water Sensing
3.2.1. Total VOC Measurements
3.2.2. NeOse Pro Direct and ATD Sensing of Flavored Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wardencki, W.; Chmiel, T.; Dymerski, T. Gas chromatography-olfactometry (GC-O), electronic noses (e-noses) and electronic tongues (e-tongues) for in vivo food flavour measurement. In Instrumental Assessment of Food Sensory Quality; Elsevier: Amsterdam, The Netherlands, 2013; pp. 195–229. [Google Scholar]
- Wilson, A.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, D.; Ulucan, O.; Turkan, M. Electronic Nose and Its Applications: A Survey. Int. J. Autom. Comput. 2020, 17, 179–209. [Google Scholar] [CrossRef]
- Rocco, G. Every breath you take: The value of the electronic nose (e-nose) technology in the early detection of lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 155, 2622–2625. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2700–2710. [Google Scholar] [CrossRef]
- Wilson, A.; Oberle, C.; Oberle, D. Detection of Off-Flavor in Catfish Using a Conducting Polymer Electronic-Nose Technology. Sensors 2013, 13, 15968–15984. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses: Powerful tools in meat quality assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef]
- Karlshøj, K.; Nielsen, P.V.; Larsen, T.O. Prediction of Penicillium expansum Spoilage and Patulin Concentration in Apples Used for Apple Juice Production by Electronic Nose Analysis. J. Agric. Food Chem. 2007, 55, 4289–4298. [Google Scholar] [CrossRef]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokozik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil.pdf. Int. J. Food Sci. Technol. 2020, 55, 2161–2170. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M.; Balasubramanian, S. Meat Quality Assessment by Electronic Nose (Machine Olfaction Technology). Sensors 2009, 9, 6058–6083. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, H.; Turner, C.; Spooner, A.; Chambers, M. Methodological variation in headspace analysis of liquid samples using electronic nose. Sens. Actuators B Chem. 2009, 139, 353–360. [Google Scholar] [CrossRef]
- Cellini, A.; Blasioli, S.; Biondi, E.; Bertaccini, A.; Braschi, I. Francesco Spinelli Potential Applications and Limitations of Electronic Nose Devices for Plant Disease Diagnosis. Sensors 2017, 17, 2596. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Recent progress in the design and clinical development of electronic-nose technologies. Nanobiosens. Dis. Diagn. 2016, 5. [Google Scholar] [CrossRef]
- Saktiawati, A.M.I.; Stienstra, Y.; Subronto, Y.W.; Rintiswati, N.; Gerritsen, J.-W.; Oord, H.; Akkerman, O.W.; van der Werf, T.S. Sensitivity and specificity of an electronic nose in diagnosing pulmonary tuberculosis among patients with suspected tuberculosis. PLoS ONE 2019, 14, e0217963. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sun, J.; Jin, H.; Khatib, M.; Li, X.; Wei, Z.; Wang, F.; Horev, Y.D.; Wu, W.; Haick, H. Chemically Modified Polyaniline for the Detection of Volatile Biomarkers of Minimal Sensitivity to Humidity and Bending. Adv. Healthc. Mater. 2018, 7, 1800232. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, H.J.; Quoc Vuong, P.; Kim, S.S. A Novel X-Ray Radiation Sensor Based on Networked SnO2 Nanowires. Appl. Sci. 2019, 9, 4878. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, W.; Han, Y.; Yang, Z.; Huang, Y. Single-Nanowire Fuse for Ionization Gas Detection. Sensors 2019, 19, 4358. [Google Scholar] [CrossRef]
- Rudnitskaya, A. Calibration Update and Drift Correction for Electronic Noses and Tongues. Front. Chem. 2018, 6, 433. [Google Scholar] [CrossRef]
- Liang, Z.; Tian, F.; Yang, S.; Zhang, C.; Sun, H.; Liu, T. Study on Interference Suppression Algorithms for Electronic Noses: A Review. Sensors 2018, 18, 1179. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, J.; Yang, S.; Zhao, Z.; Liang, Z.; Liu, Y.; Wang, D. Suppression of Strong Background Interference on E-Nose Sensors in an Open Country Environment. Sensors 2016, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Sweelssen, J.; Blokland, H.; Rajamäki, T.; Boersma, A. Capacitive and Infrared Gas Sensors for the Assessment of the Methane Number of LNG Fuels. Sensors 2020, 20, 3345. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodriguez, E.; Guzman-Chavez, A.; Baeza-Serrato, R. Tailored Algorithm for Sensitivity Enhancement of Gas Concentration Sensors Based on Tunable Laser Absorption Spectroscopy. Sensors 2018, 18, 1808. [Google Scholar] [CrossRef] [PubMed]
- Gancarz, M.; Nawrocka, A.; Rusinek, R. Identification of Volatile Organic Compounds and Their Concentrations Using a Novel Method Analysis of MOS Sensors Signal. J. Food Sci. 2019, 84, 2077–2085. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzański, B.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef]
- Lu, C.-J.; Zellers, E.T. A Dual-Adsorbent Preconcentrator for a Portable Indoor-VOC Microsensor System. Anal. Chem. 2001, 73, 3449–3457. [Google Scholar] [CrossRef]
- Chae, M.-S.; Kim, J.; Yoo, Y.; Kang, J.; Lee, J.; Hwang, K. A Micro-Preconcentrator Combined Olfactory Sensing System with a Micromechanical Cantilever Sensor for Detecting 2,4-Dinitrotoluene Gas Vapor. Sensors 2015, 15, 18167–18177. [Google Scholar] [CrossRef]
- Thomas, C.M.; Schneider, J.F. Odor Concentration to Enhance Electronic Nose Performance. Process Anal. Chem. 2007, 10, 43–55. [Google Scholar]
- Cáceres, J.M.; Durán, C.M.; Gualdron, O.E. Thermal Desorption System for Breath Samples Analysis from Colombian Patients with Gastric Cancer. Chem. Eng. Trans. 2018, 6, 427–432. [Google Scholar] [CrossRef]
- Biondi, E.; Blasioli, S.; Galeone, A.; Spinelli, F.; Cellini, A.; Lucchese, C.; Braschi, I. Detection of potato brown rot and ring rot by electronic nose: From laboratory to real scale. Talanta 2014, 129, 422–430. [Google Scholar] [CrossRef]
- Fujioka, K.; Shimizu, N.; Manome, Y.; Ikeda, K.; Yamamoto, K.; Tomizawa, Y. Discrimination Method of the Volatiles from Fresh Mushrooms by an Electronic Nose Using a Trapping System and Statistical Standardization to Reduce Sensor Value Variation. Sensors 2013, 13, 15532–15548. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Covington, J.A.; Gardner, J.W. Combined electronic nose and tongue for a flavour sensing system. Sens. Actuators B Chem. 2011, 156, 832–839. [Google Scholar] [CrossRef]
- Muenchmeyer, W.; Walte, A.; Matz, G. Improving electronic noses using a trap and thermal desorption unit. Sens. Actuators B Chem. 2000, 69, 379–383. [Google Scholar] [CrossRef]
- Camara, E.H.M.; Breuil, P.; Briand, D.; de Rooij, N.F.; Pijolat, C. A micro gas preconcentrator with improved performance for pollution monitoring and explosives detection. Anal. Chim. Acta 2011, 688, 175–182. [Google Scholar] [CrossRef] [PubMed]
- McCartney, M.M.; Zrodnikov, Y.; Fung, A.G.; LeVasseur, M.K.; Pedersen, J.M.; Zamuruyev, K.O.; Aksenov, A.A.; Kenyon, N.J.; Davis, C.E. An Easy to Manufacture Micro Gas Preconcentrator for Chemical Sensing Applications. ACS Sens. 2017, 2, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- James, F.; Breuil, P.; Pijolat, C.; Camara, M.; Briand, D.; Bart, A.; Cozic, R. Development of a MEMS Preconcentrator for Micro-gas Chromatography Analyses. Procedia Eng. 2014, 87, 500–503. [Google Scholar] [CrossRef]
- Yeom, J. Micro-preconcentrator Technology for Portable Gas Chromatography System. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, Netherlands, 2015; pp. 1–8. ISBN 978-94-007-6178-0. [Google Scholar]
- Xu, X.; Tian, F.; Yang, S.; Li, Q.; Yan, J.; Machacek, J. A Solid Trap and Thermal Desorption System with Application to a Medical Electronic Nose. Sensors 2008, 8, 6885–6898. [Google Scholar] [CrossRef]
- Bourlon, B.; Ho, B.-A.P.; Ricoul, F.; Chappuis, T.; Bellemin Comte, A.; Constantin, O.; Icard, B. Revisiting gas sampling and analysis with microtechnology: Feasability of low cost handheld gas chromatographs. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Bourlon, B.; Ricoul, F.; Beghi, S.; Bellemin-Comte, A.; David, N.; Bordy, T.; Icard, B.; Salette, A.; Petitjean, M.; Barattin, R.; et al. Silicon Based Micro-Preconcentrators For Portable Gas Analysis Systems. In Proceedings of the 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2014, San Antonio, Texas, TX, USA, 26–30 October 2014; p. 3. [Google Scholar]
- Brenet, S.; John-Herpin, A.; Gallat, F.-X.; Musnier, B.; Buhot, A.; Herrier, C.; Rousselle, T.; Livache, T.; Hou, Y. Highly-Selective Optoelectronic Nose Based on Surface Plasmon Resonance Imaging for Sensing Volatile Organic Compounds. Anal. Chem. 2018, 90, 9879–9887. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Hurot, C.; Weerakkody, J.S.; Mathey, R.; Buhot, A.; Mascini, M.; Hou, Y.; Compagnone, D. Development of an optoelectronic nose based on surface plasmon resonance imaging with peptide and hairpin DNA for sensing volatile organic compounds. Sens. Actuators B Chem. 2020, 303, 127188. [Google Scholar] [CrossRef]
- Maho, P.; Herrier, C.; Livache, T.; Rolland, G.; Comon, P.; Barthelmé, S. Reliable chiral recognition with an optoelectronic nose. Biosens. Bioelectron. 2020, 159, 112183. [Google Scholar] [CrossRef]
- Garbacz, M.; Malec, A.; Duda-Saternus, S.; Suchorab, Z.; Guz, Ł.; Łagód, G. Methods for Early Detection of Microbiological Infestation of Buildings Based on Gas Sensor Technologies. Chemosensors 2020, 8, 7. [Google Scholar] [CrossRef]
- Aishima, T. Analysis of responses from a gas sensor array. Anal. Chim. Acta 1991, 243, 293–300. [Google Scholar] [CrossRef]
- Schieweck, A.; Gunschera, J.; Varol, D.; Salthammer, T. Analytical procedure for the determination of very volatile organic compounds (C3–C6) in indoor air. Anal. Bioanal. Chem. 2018, 410, 3171–3183. [Google Scholar] [CrossRef] [PubMed]





| Nonane/Oil (m/m) % | 0 | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 5 | 10 | 50 | 100 |
| [TVOC] (ppmv) | 1.3 | 3.6 | 9 | 14 | 62 | 106 | 462 | 646 | 780 | 805 |
| Sample Code | Flavored Water | Descriptive Flavor | PID (ppmv) |
|---|---|---|---|
| Water | - | Non-flavored | <0.001 |
| A | Volvic Juicy Agrumade Pamplemousse | Grapefruit | 111.0 |
| B | Volvic Juicy Citronade | Lemonade | 17.0 |
| C | Volvic ZEST Citron | Lemon | 10.5 |
| D | Volvic Thé Vert Pêche Blanche | White Peach | 12.5 |
| E | Volvic Juicy Bio Fraise | Organic Strawberry | 11.0 |
| F | Volvic Juicy Fraise | Strawberry | 7.0 |
| G | Volvic Juicy Bio Pomme | Organic Apple | 66.5 |
| H | Volvic Infusion Rooibos Mangue & Fruits de la passion | Mango & Passion fruit | 17.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slimani, S.; Bultel, E.; Cubizolle, T.; Herrier, C.; Rousselle, T.; Livache, T. Opto-Electronic Nose Coupled to a Silicon Micro Pre-Concentrator Device for Selective Sensing of Flavored Waters. Chemosensors 2020, 8, 60. https://doi.org/10.3390/chemosensors8030060
Slimani S, Bultel E, Cubizolle T, Herrier C, Rousselle T, Livache T. Opto-Electronic Nose Coupled to a Silicon Micro Pre-Concentrator Device for Selective Sensing of Flavored Waters. Chemosensors. 2020; 8(3):60. https://doi.org/10.3390/chemosensors8030060
Chicago/Turabian StyleSlimani, Sami, Etienne Bultel, Thomas Cubizolle, Cyril Herrier, Tristan Rousselle, and Thierry Livache. 2020. "Opto-Electronic Nose Coupled to a Silicon Micro Pre-Concentrator Device for Selective Sensing of Flavored Waters" Chemosensors 8, no. 3: 60. https://doi.org/10.3390/chemosensors8030060
APA StyleSlimani, S., Bultel, E., Cubizolle, T., Herrier, C., Rousselle, T., & Livache, T. (2020). Opto-Electronic Nose Coupled to a Silicon Micro Pre-Concentrator Device for Selective Sensing of Flavored Waters. Chemosensors, 8(3), 60. https://doi.org/10.3390/chemosensors8030060





