Phage-Based Sensors in Medicine: A Review
Abstract
1. Introduction
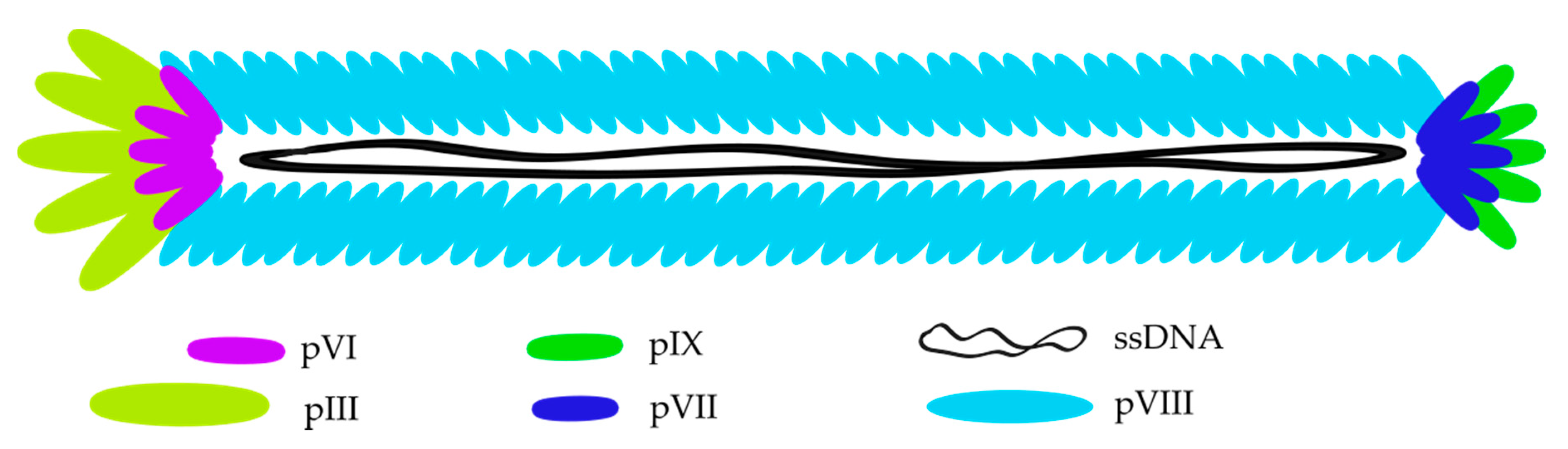
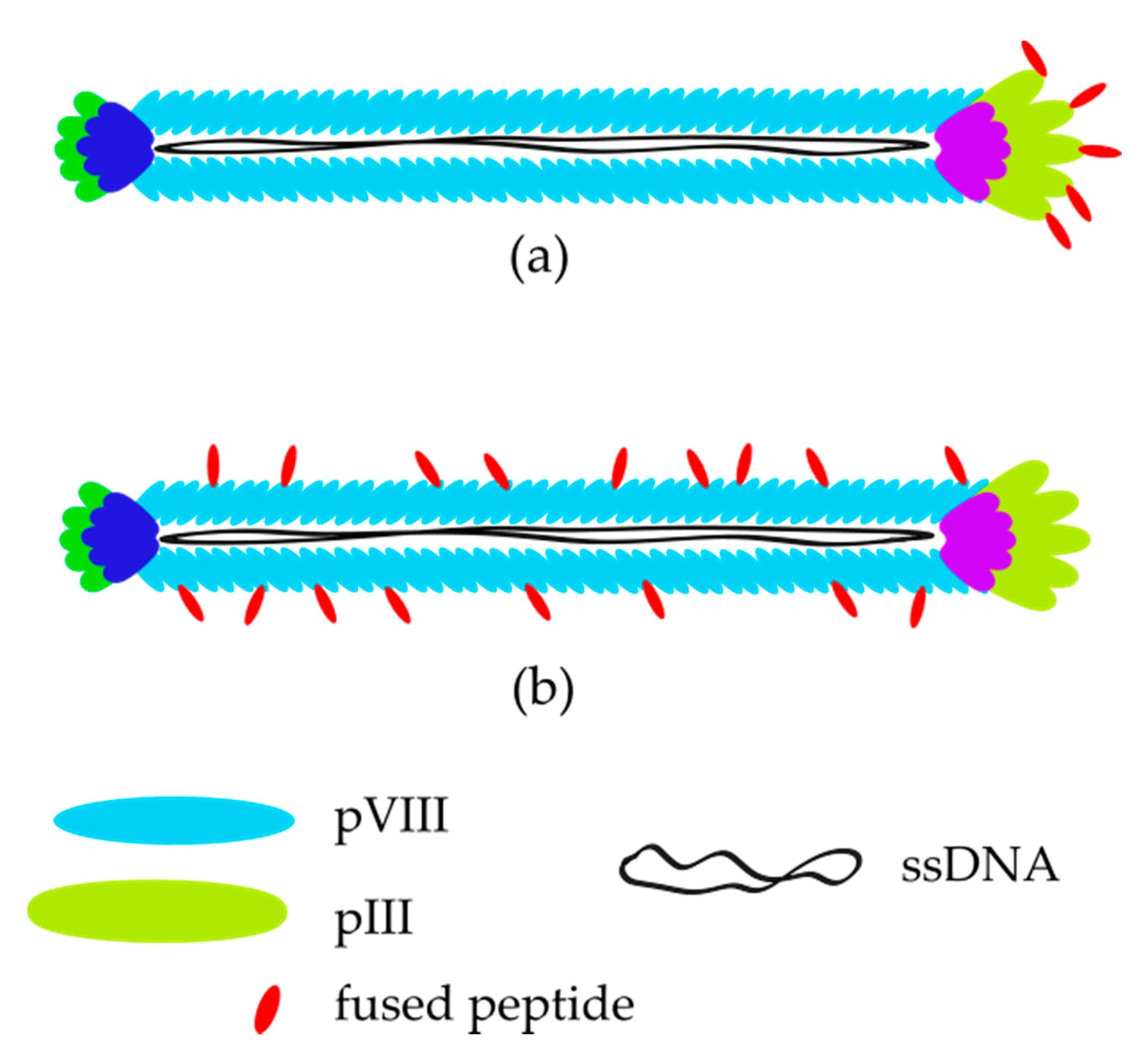
2. Bacteriophages—Biology and Their Application in Phage Display Technology
3. M13 Bacteriophage-Based and Peptide-Based Sensors
| Target | Binding Sequence | Affinity (Kd) 1 | Method | LOD | Reference |
|---|---|---|---|---|---|
| Whole M13 bacteriophage applied in sensor | |||||
| Prostate-specific membrane antigen (PSMA) | CALCEFGL | (n.a.) | QCM | (n.a.) | [55] |
| EIS | 120 nM | ||||
| CALCEFGL LDCVEVFQNSCDW | (n.a.) | VBR | 100 pM | [56] | |
| Prostate-specific antigen (PSA) | ATRSANGM | (n.a.) | ELISA | 1.6 ng/mL | [57,58] |
| DPV | 3 pg/mL | ||||
| EIS | 4 fg/mL (t-PSA) | ||||
| Human serum albumin (HSA) | (n.a.) | EIS: 300 nM (PBS) | VBR | 100 nM | [59] |
| EIS: 1036 nM (urine) | |||||
| (n.a.) | 7.5 nM | [60] | |||
| DJ-1 (bladder cancer marker) | KYRYVCHDVGGTYCIRDGV | 14 pM or 206 nM | VBR | 10 pM | [61] |
| VBR: 39 nM | |||||
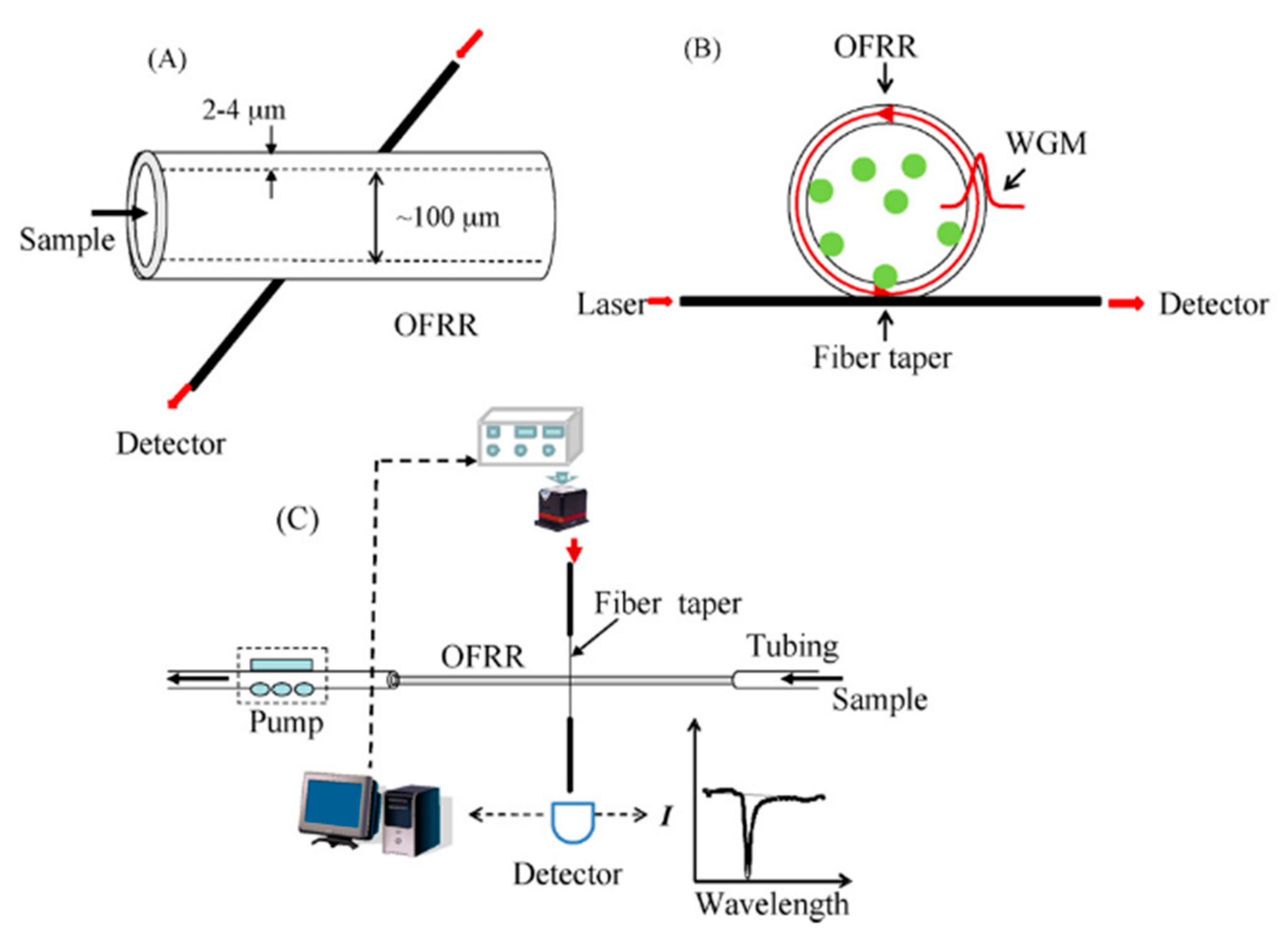
| Streptavidin (as a model protein) | ANRLPCHPQFPCTSHE | OFRR: 25 pM | OFRR | 100 pM | [62] |
| Human phosphatase of regenerating liver-3 (hPRL-3) | (n.a.) | (n.a.) | LAPS | (n.a.) | [63] |
| Β-galactosidase | (n.a.) | SPR: 1.3 nM or 26 nM | SPR | (n.a.) | [64] |
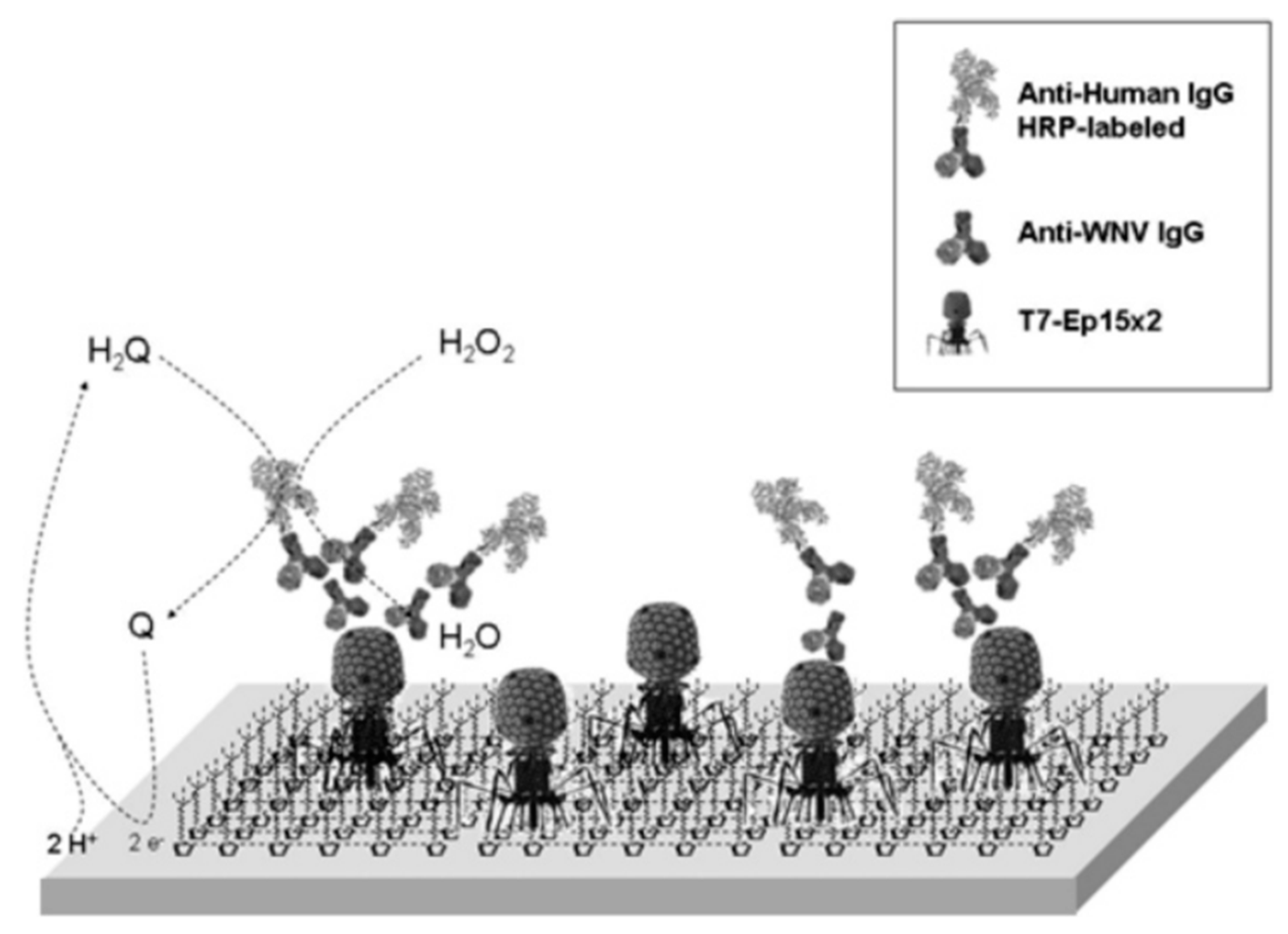
| Dengue virus type 2 marker | EHDRMHAYYLTR | EIS: 3.9 nM | EIS | 0.025 µg/mL | [53] |
| Peptide-based sensors | |||||
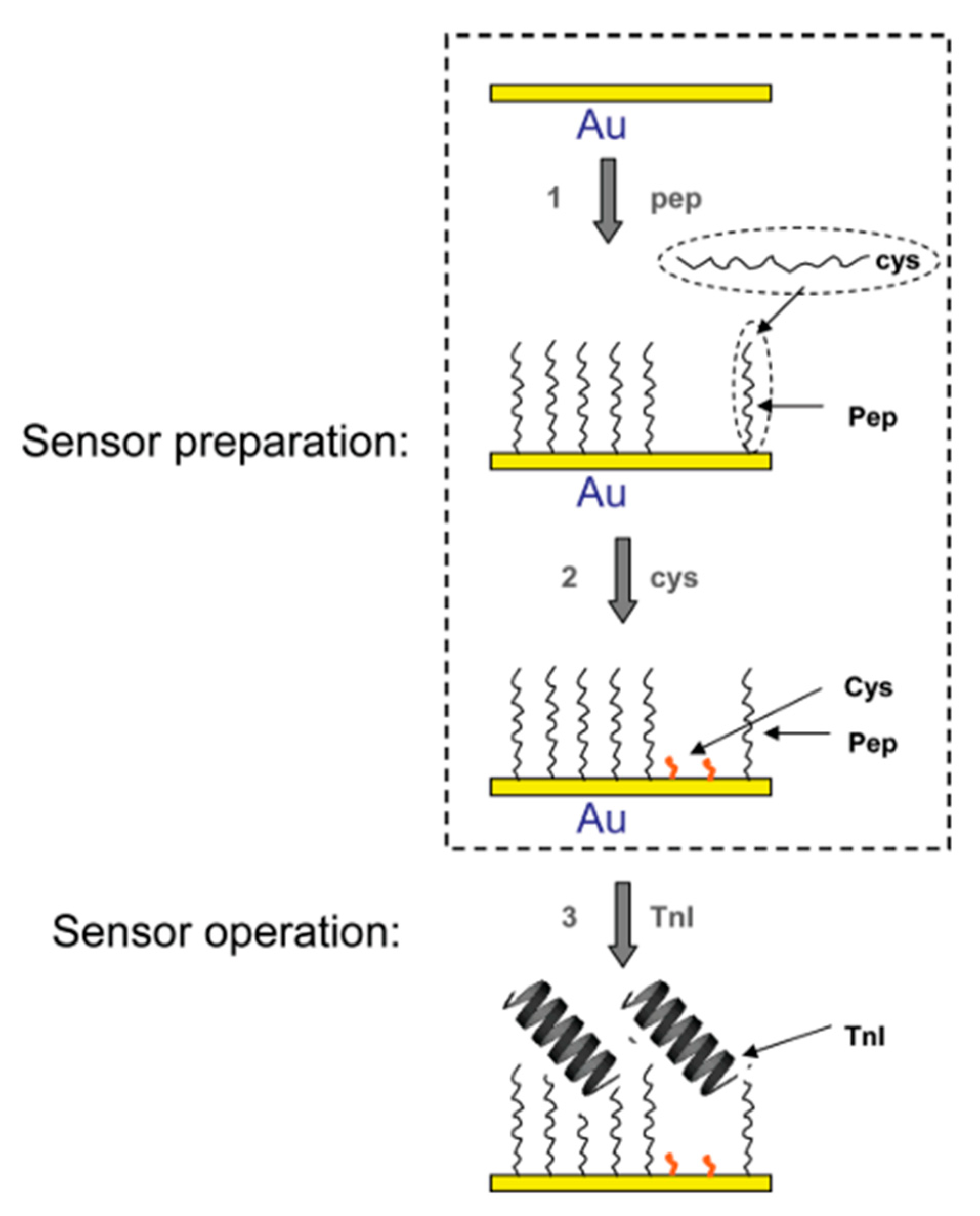
| Troponin I (TnI) | FYSHSFHENWPS | 2.5 nM | EIS | 0.34 µg/mL | [51,65] |
| QCM: 17 nM and 66 nM | QCM | 0.11 µg/mL | |||
| Alanine aminotransferase (ALT) | WHWRNPDFWYLK | 80 nM | EIS | 92 ng/mL | [66] |
| QCM | 60 ng/mL | ||||
| Norovirus P2 protein | QHIMHLPHINTL | 185 nM | EIS | 99.8 ng/mL (P2) | [67,68] |
| 7.8 virions/mL (whole Norovirus) | |||||
| Myoglobin (Mb) | 3R1: CNLSSSWIC | 125 nM | DPV | 3R7: 9.8 ng/mL | [69,70] |
| 3R7: CPSTLGASC | 57 nM | ||||
| 3R10: CVPRLSAPC | 293 nM | ||||
| Procalcitonin (proCT) | MSCAGHMCTRFV | 1.9 nM | EIS | 12.5 ng/mL | [71,72] |
| EIS: 0.39 nM | |||||
| Cholera toxin B | VQCRLGPPWCAK | 6.7 nM | LSPR | 1.89 ng/mL | [73] |
| SERS | 3.51 pg/mL | ||||
| Neutrophil gelatinase-associated lipocalin (NGAL) | DRWVARDPASIF | (n.a.) | SWV | 3.93 ng/mL | [74] |
| EIS | 1.74 ng/mL | ||||
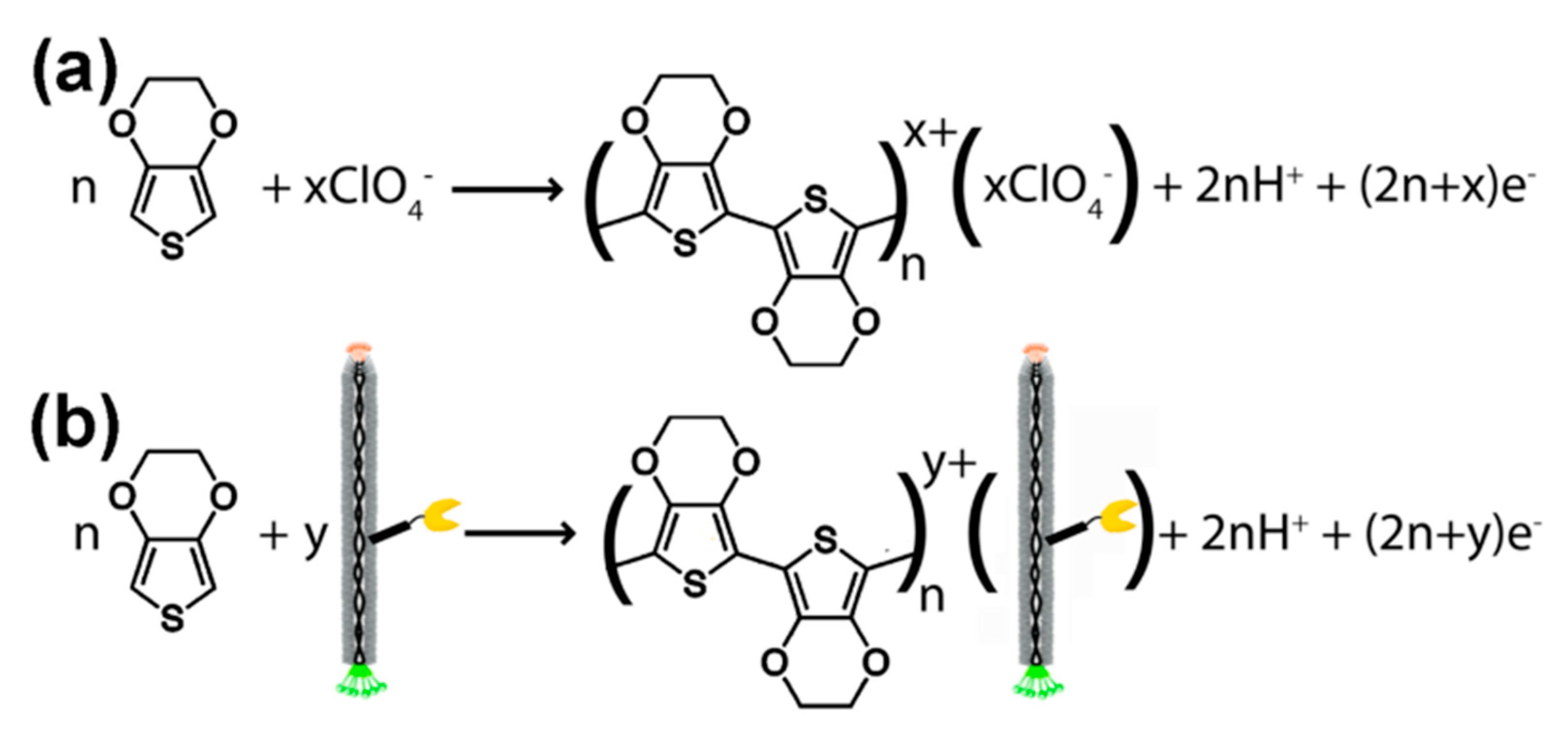
3.1. Voltammetric and Impedimetric
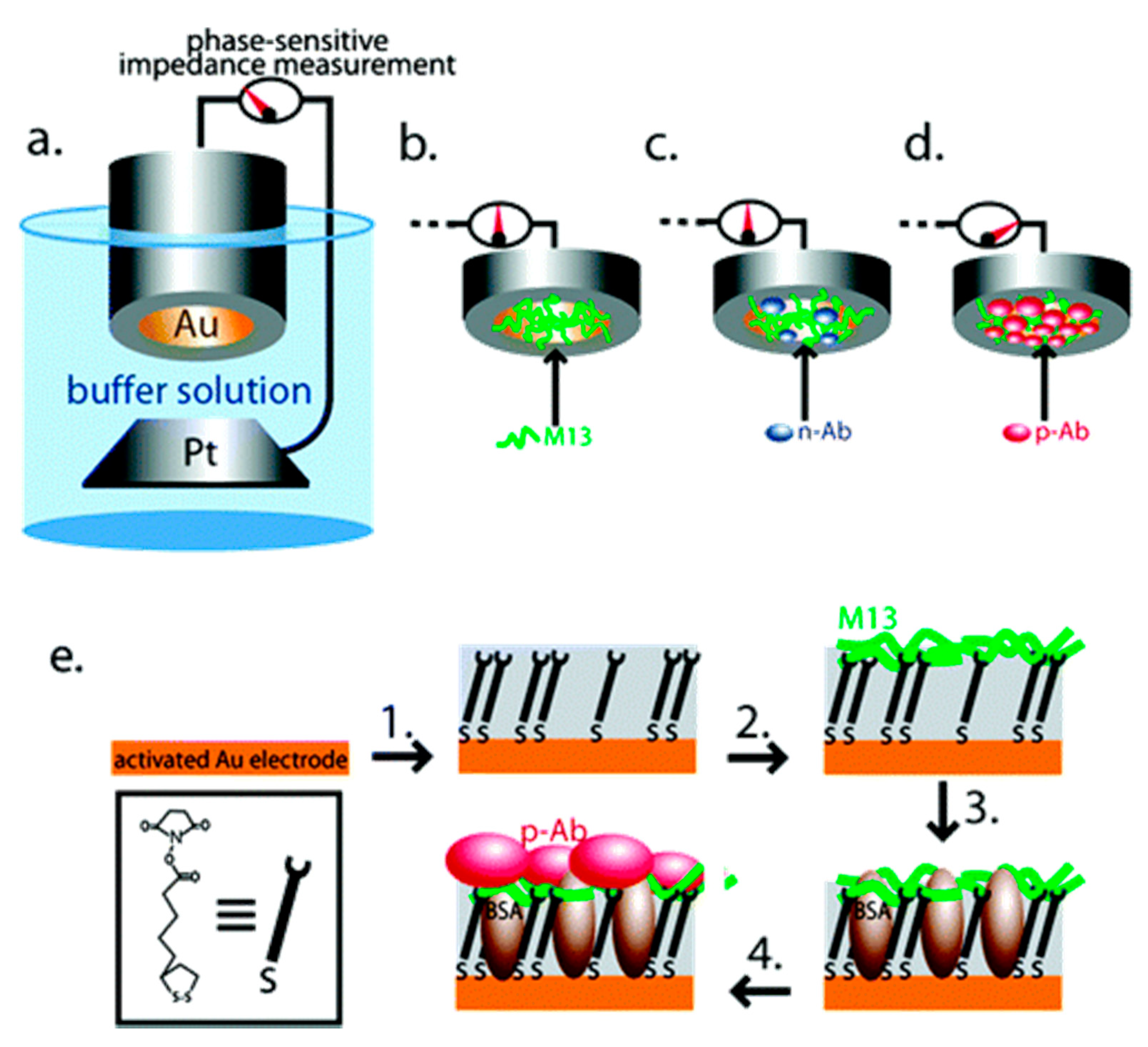
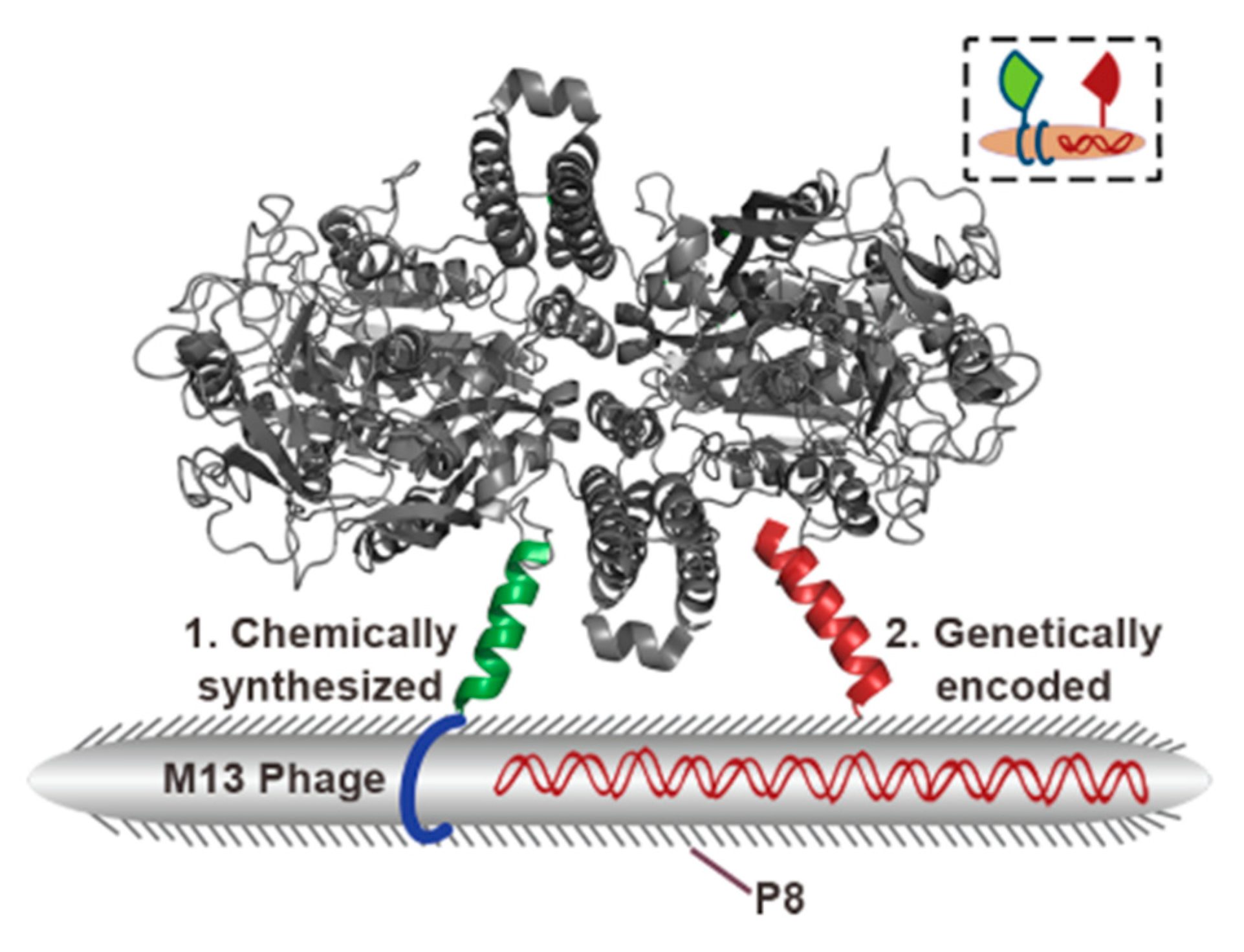
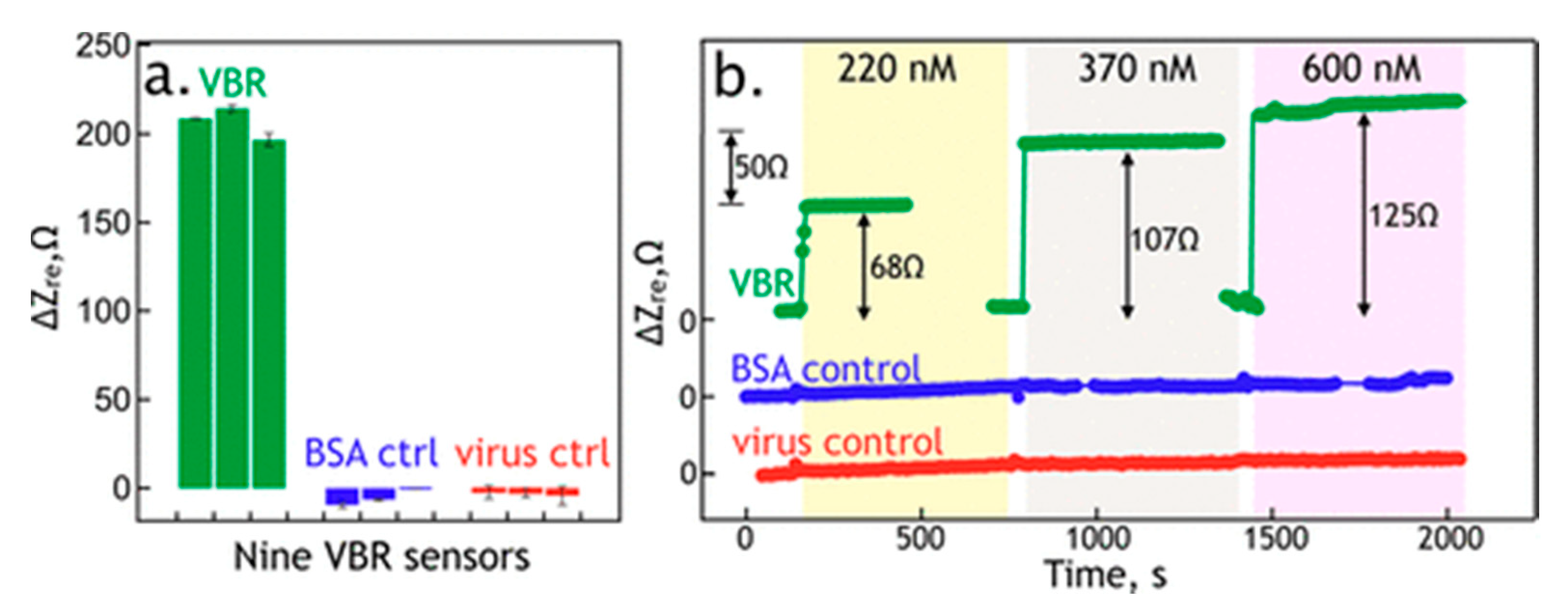
3.2. Virus Bio-Resistor (VBR) Sensing Devices
3.3. Light Addressable Potentiometric Sensors
3.4. Surface Plasmon Resonance and Surface-Enhanced Raman Spectroscopy Based on M13 Bacteriophage/Peptide Sensors
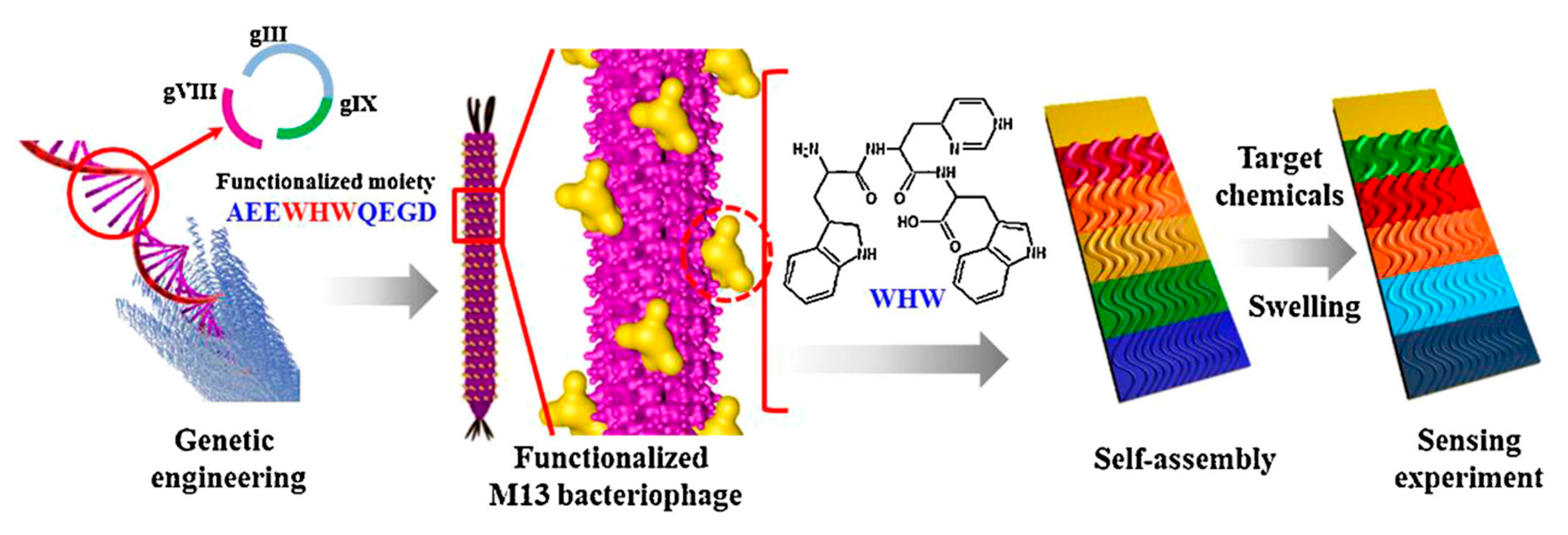
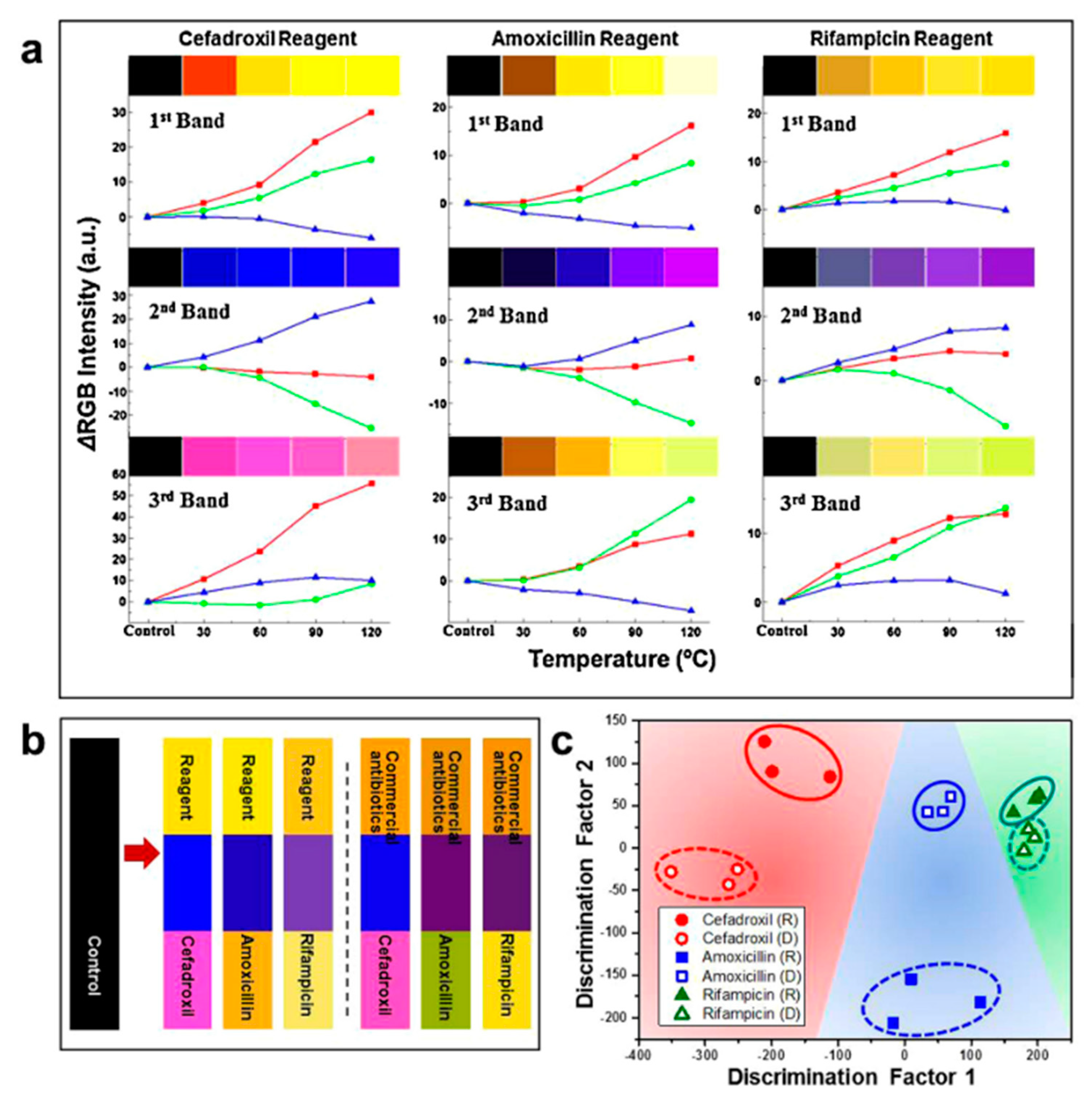
3.5. Virus-Based Colorimetric Sensors
3.6. Optofluidic Ring Resonator
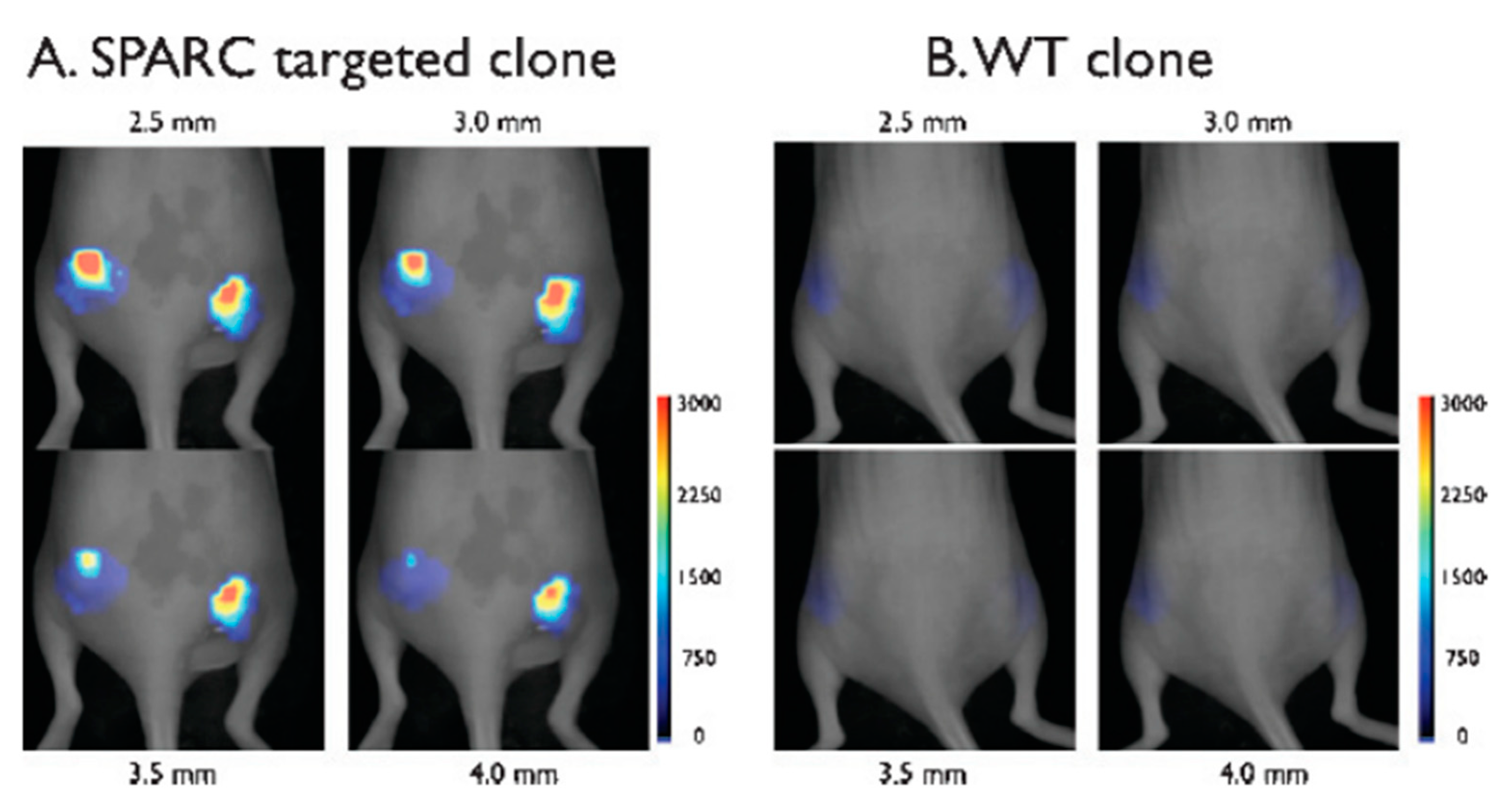
4. In Vivo Imaging by M13-Labeling of Cells
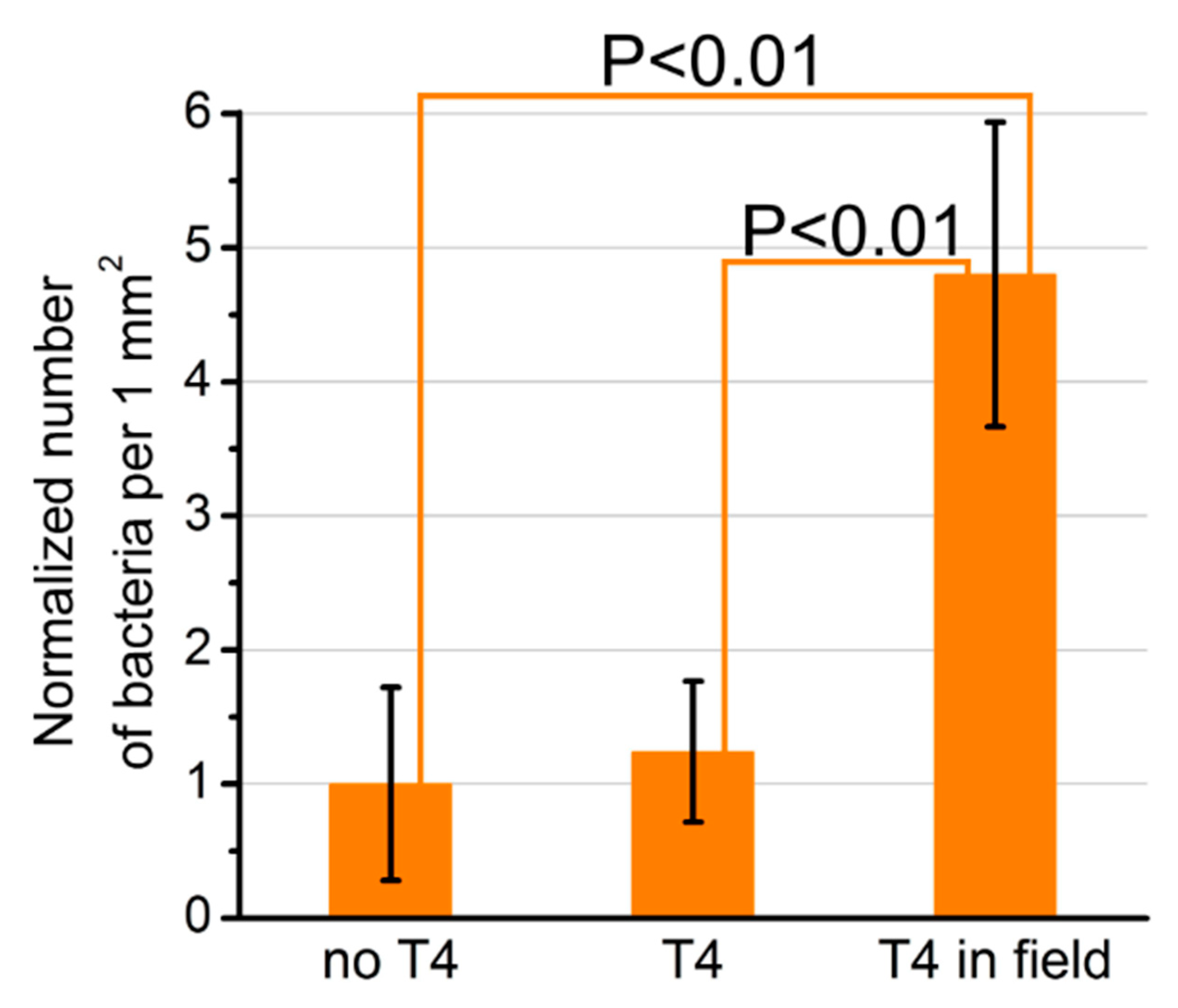
5. Other Bacteriophages Applications
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Alpert, J.S.; Thygesen, K.; Antman, E.; Bassand, J.P. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J. Am. Coll. Cardiol. 2000, 36, 959–969. [Google Scholar] [CrossRef]
- Adigun, O.O.; Khetarpal, S. Alpha Fetoprotein (AFP, Maternal Serum Alpha Fetoprotein, MSAFP). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430750/ (accessed on 24 July 2020).
- Davies, R.J.; Eapen, S.S.; Carlisle, S.J. Lateral-Flow Immunochromatographic Assays. In Handbook of Biosensors and Biochips; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Brigati, J.R.; Petrenko, V.A. Thermostability of landscape phage probes. Anal. Bioanal. Chem. 2005, 382, 1346–1350. [Google Scholar] [CrossRef]
- Geysen, H.M.; Meloen, R.H.; Barteling, S.J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 1984, 81, 3998–4002. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Bakhshinejad, B.; Ghiasvand, S. Bacteriophages in the human gut: Our fellow travelers throughout life and potential biomarkers of heath or disease. Virus Res. 2017, 240, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Wojciechowska, R.; Górski, A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Benito-Peña, E.; Barderas, R.; Moreno-Bondi, M.C. Phage display in the quest for new selective recognition elements for biosensors. ACS Omega 2019, 4, 11569–11580. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, C.; Ylera, F.; Frisch, C.; Ten Haaf, A.; Knappik, A. Chapter 3—Monoclonal Antibody Generation by Phage Display: History, State-of-the-Art, and Future. In Handbook of Immunoassay Technologies; Academic Press: Cambridge, MA, USA, 2018; pp. 47–80. [Google Scholar]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage display—A powerful technique for immunotherapy. Hum. Vaccines Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef]
- Hammers, C.M.; Stanley, J.R. Antibody phage display: Technique and applications. J. Investig. Dermatol. 2014, 134, 1–5. [Google Scholar] [CrossRef]
- Larman, H.B.; Zhao, Z.; Laserson, U.; Li, M.Z.; Ciccia, A.; Gakidis, M.A.M.; Church, G.M.; Kesari, S.; Leproust, E.M.; Solimini, N.L.; et al. Autoantigen discovery with a synthetic human peptidome. Nat. Biotechnol. 2011, 29, 535–541. [Google Scholar] [CrossRef]
- Mimmi, S.; Maisano, D.; Quinto, I.; Iaccino, E. Phage Display: An Overview in Context to Drug Discovery. Trends Pharmacol. Sci. 2019, 40, 87–91. [Google Scholar] [CrossRef]
- Hyde-Deruyscher, R.; Paige, L.A.; Christensen, D.J.; Hyde-Deruyscher, N.; Lim, A.; Fredericks, Z.L.; Kranz, J.; Gallant, P.; Zhang, J.; Rocklage, S.M.; et al. Detection of small-molecule enzyme inhibitors with peptides isolated from phage-displayed combinatorial peptide libraries. Chem. Biol. 2000, 7, 17–25. [Google Scholar] [CrossRef]
- Park, I.W.; Kim, K.W.; Hong, Y.; Yoon, H.J.; Lee, Y.; Gwak, D.; Heo, K. Recent developments and prospects of M13-bacteriophage based piezoelectric energy harvesting devices. Nanomaterials 2020, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Chung, W.J.; McFarland, S.; Lee, S.W. Assembly of bacteriophage into functional materials. Chem. Rec. 2013, 13, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Janczuk, M.; Niedziółka-Jönsson, J.; Szot-Karpińska, K. Bacteriophages in electrochemistry: A review. J. Electroanal. Chem. 2016, 779, 207–219. [Google Scholar] [CrossRef]
- Merzlyak, A.; Lee, S.W. Phage as templates for hybrid materials and mediators for nanomaterial synthesis. Curr. Opin. Chem. Biol. 2006, 10, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Weiss, G.A. Chemically Modifying Viruses for Diverse Applications. ACS Chem. Biol. 2016, 11, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.M.; Ratna, B.R. Virus hybrids as nanomaterials for biotechnology. Curr. Opin. Biotechnol. 2010, 21, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y. Engineered phages for electronics. Biosens. Bioelectron. 2016, 85, 964–976. [Google Scholar] [CrossRef]
- Peltomaa, R.; López-Perolio, I.; Benito-Peña, E.; Barderas, R.; Moreno-Bondi, M.C. Application of bacteriophages in sensor development. Anal. Bioanal. Chem. 2016, 408, 1805–1828. [Google Scholar] [CrossRef]
- Richter, Ł.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Paczesny, J.; Hołyst, R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov. Today 2018, 23, 448–455. [Google Scholar] [CrossRef]
- Farooq, U.; Yang, Q.; Ullah, M.W.; Wang, S. Bacterial biosensing: Recent advances in phage-based bioassays and biosensors. Biosens. Bioelectron. 2018, 118, 204–216. [Google Scholar] [CrossRef]
- Xu, J.; Chau, Y.; Lee, Y. kuen Phage-based electrochemical sensors: A review. Micromachines 2019, 10, 855. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Sullivan, M.B.; Knezevic, P.; van Zyl, L.J.; Sarkar, B.L.; Dutilh, B.E.; Alfenas-Zerbini, P.; Łobocka, M.; Tong, Y.; Brister, J.R.; et al. Taxonomy of prokaryotic viruses: 2018-2019 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2020, 165, 1253–1260. [Google Scholar] [CrossRef]
- Rakonjac, J.; Russel, M.; Khanum, S.; Brooke, S.J.; Rajic, M. Filamentous Phage: Structure and Biology. Adv. Exp. Med. Biol. 2017, 1053, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Marvin, D. Filamentous phage structure, infection and assembly. Curr. Opin. Struct. Biol. 1998, 8, 150–158. [Google Scholar] [CrossRef]
- Russel, M. Moving through the membrane with filamentous phages. Trends Microbiol. 1995, 3, 223–228. [Google Scholar] [CrossRef]
- Rakonjac, J.; Model, P. Roles of pIII in filamentous phage assembly. J. Mol. Biol. 1998, 282, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Roth, T.A.; Baldi, P.F.; Sidhu, S.S. Comprehensive mutagenesis of the C-terminal domain of the M13 gene-3 minor coat protein: The requirements for assembly into the bacteriophage particle. J. Mol. Biol. 2003, 332, 777–782. [Google Scholar] [CrossRef]
- Specthrie, L.; Bullitt, E.; Horiuchi, K.; Model, P.; Russel, M.; Makowski, L. Construction of a microphage variant of filamentous bacteriophage. J. Mol. Biol. 1992, 228, 720–724. [Google Scholar] [CrossRef]
- Qi, H.; Lu, H.; Qiu, H.J.; Petrenko, V.; Liu, A. Phagemid vectors for phage display: Properties, characteristics and construction. J. Mol. Biol. 2012, 417, 129–143. [Google Scholar] [CrossRef]
- Grant, R.A.; Lin, T.C.; Konigsberg, W.; Webster, R.E. Structure of the Filamentous Bacteriophage f1. J. Biol. Chem. 1981, 256, 539–546. [Google Scholar]
- Petrenko, V.A. Landscape phage: Evolution from phage display to nanobiotechnology. Viruses 2018, 10, 311. [Google Scholar] [CrossRef]
- Cwirla, S.E.; Peters, E.A.; Barrett, R.W.; Dower, W.J. Peptides on phage: A vast library of peptides for identifying ligands. Proc. Natl. Acad. Sci. USA 1990, 87, 6378–6382. [Google Scholar] [CrossRef]
- Noren, K.A.; Noren, C.J. Construction of high-complexity combinatorial phage display peptide libraries. Methods 2001, 23, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Cabilly, S. The basic structure of filamentous phage and its use in the display of combinatorial peptide libraries. Appl. Biochem. Biotechnol. Part B Mol. Biotechnol. 1999, 12, 143–148. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Lowman, H.B.; Cunningham, B.C.; Wells, J.A. Phage display for selection of novel binding peptides. Methods Enzymol. 2000, 328, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S. Engineering M13 for phage display. Biomol. Eng. 2001, 18, 57–63. [Google Scholar] [CrossRef]
- Scott, J.K.; Smith, G.P. Searching for peptide ligands with an epitope library. Science 1990, 249, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Knez, K.; Noppe, W.; Geukens, N.; Janssen, K.P.F.; Spasic, D.; Heyligen, J.; Vriens, K.; Thevissen, K.; Cammue, B.P.A.; Petrenko, V.; et al. Affinity comparison of p3 and p8 peptide displaying bacteriophages using surface plasmon resonance. Anal. Chem. 2013, 85, 10075–10082. [Google Scholar] [CrossRef]
- Friguet, B.; Chaffotte, A.F.; Djavadi-Ohaniance, L.; Goldberg, M.E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 1985, 77, 305–319. [Google Scholar] [CrossRef]
- Park, J.P.; Cropek, D.M.; Banta, S. High affinity peptides for the recognition of the heart disease biomarker troponin I identified using phage display. Biotechnol. Bioeng. 2010, 105, 678–686. [Google Scholar] [CrossRef]
- Petrenko, V.A.; Vodyanoy, V.J. Phage display for detection of biological threat agents. J. Microbiol. Methods 2003, 53, 253–262. [Google Scholar] [CrossRef]
- Lim, J.M.; Kim, J.H.; Ryu, M.Y.; Cho, C.H.; Park, T.J.; Park, J.P. An electrochemical peptide sensor for detection of dengue fever biomarker NS1. Anal. Chim. Acta 2018, 1026, 109–116. [Google Scholar] [CrossRef]
- Honegger, A. Engineering Antibodies for Stability and Efficient Folding BT—Therapeutic Antibodies; Chernajovsky, Y., Nissim, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–68. ISBN 978-3-540-73259-4. [Google Scholar]
- Yang, L.M.C.; Tam, P.Y.; Murray, B.J.; McIntire, T.M.; Overstreet, C.M.; Weiss, G.A.; Penner, R.M. Virus electrodes for universal biodetection. Anal. Chem. 2006, 78, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Donavan, K.C.; Arter, J.A.; Penner, R.M.; Weiss, G.A. Sub-nanomolar Detection of Prostate-Specific Membrane Antigen in Synthetic Urine by Synergistic, Dual-Ligand Phage. J. Am. Chem. Soc. 2013, 135, 7761–7767. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xia, H.; Yin, L.; Petrenko, V.A.; Liu, A. Selected landscape phage probe as selective recognition interface for sensitive total prostate-specific antigen immunosensor. Biosens. Bioelectron. 2018, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, D.; Yan, L.; Petrenko, V.A.; Liu, A. Specific phages-based electrochemical impedimetric immunosensors for label-free and ultrasensitive detection of dual prostate-specific antigens. Sens. Actuators B Chem. 2019, 297, 126727. [Google Scholar] [CrossRef]
- Ogata, A.F.; Edgar, J.M.; Majumdar, S.; Briggs, J.S.; Patterson, S.V.; Tan, M.X.; Kudlacek, S.T.; Schneider, C.A.; Weiss, G.A.; Penner, R.M. Virus-Enabled Biosensor for Human Serum Albumin. Anal. Chem. 2017, 89, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.; Ogata, A.F.; Briggs, J.S.; Tam, P.Y.; Tan, M.X.; Weiss, G.A.; Penner, R.M. The Virus Bioresistor: Wiring Virus Particles for the Direct, Label-Free Detection of Target Proteins. Nano Lett. 2018, 18, 3623–3629. [Google Scholar] [CrossRef]
- Bhasin, A.; Sanders, E.C.; Ziegler, J.M.; Briggs, J.S.; Drago, N.P.; Attar, A.M.; Santos, A.M.; True, M.Y.; Ogata, A.F.; Yoon, D.V.; et al. Virus Bioresistor (VBR) for Detection of Bladder Cancer Marker DJ-1 in Urine at 10 pM in One Minute. Anal. Chem. 2020, 92, 6654–6666. [Google Scholar] [CrossRef]
- Zhu, H.; White, I.M.; Suter, J.D.; Fan, X. Phage-based label-free biomolecule detection in an opto-fluidic ring resonator. Biosens. Bioelectron. 2008, 24, 461–466. [Google Scholar] [CrossRef]
- Jia, Y.; Qin, M.; Zhang, H.; Niu, W.; Li, X.; Wang, L.; Li, X.; Bai, Y.; Cao, Y.; Feng, X. Label-free biosensor: A novel phage-modified Light Addressable Potentiometric Sensor system for cancer cell monitoring. Biosens. Bioelectron. 2007, 22, 3261–3266. [Google Scholar] [CrossRef]
- Nanduri, V.; Balasubramanian, S.; Sista, S.; Vodyanoy, V.J.; Simonian, A.L. Highly sensitive phage-based biosensor for the detection of β-galactosidase. Anal. Chim. Acta 2007, 589, 166–172. [Google Scholar] [CrossRef]
- Wu, J.; Cropek, D.M.; West, A.C.; Banta, S. Development of a troponin i biosensor using a peptide obtained through phage display. Anal. Chem. 2010, 82, 8235–8243. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Park, J.P.; Dooley, K.; Cropek, D.M.; West, A.C.; Banta, S. Rapid development of new protein biosensors utilizing peptides obtained via phage display. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Ryu, M.Y.; Park, J.P. Identification of high affinity peptides for capturing norovirus capsid proteins. RSC Adv. 2015, 5, 55300–55302. [Google Scholar] [CrossRef]
- Hwang, H.J.; Ryu, M.Y.; Park, C.Y.; Ahn, J.; Park, H.G.; Choi, C.; Ha, S.D.; Park, T.J.; Park, J.P. High sensitive and selective electrochemical biosensor: Label-free detection of human norovirus using affinity peptide as molecular binder. Biosens. Bioelectron. 2017, 87, 164–170. [Google Scholar] [CrossRef]
- Padmanaban, G.; Park, H.; Choi, J.S.; Cho, Y.W.; Kang, W.C.; Moon, C.I.; Kim, I.S.; Lee, B.H. Identification of peptides that selectively bind to myoglobin by biopanning of phage displayed-peptide library. J. Biotechnol. 2014, 187, 43–50. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, J.S.; Guruprasath, P.; Lee, B.-H.; Cho, Y.W. An Electrochemical Biosensor Based on a Myoglobin-specific Binding Peptide for Early Diagnosis of Acute Myocardial Infarction. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2015, 31, 699–704. [Google Scholar] [CrossRef]
- Park, J.P.; Park, C.Y.; Park, A.Y.; Ryu, M.Y. Evolutionary identification of affinity peptides for the detection of sepsis biomarker procalcitonin. RSC Adv. 2015, 5, 90531–90533. [Google Scholar] [CrossRef]
- Lim, J.M.; Ryu, M.Y.; Kim, J.H.; Cho, C.H.; Park, T.J.; Park, J.P. An electrochemical biosensor for detection of the sepsis-related biomarker procalcitonin. RSC Adv. 2017, 7, 36562–36565. [Google Scholar] [CrossRef]
- Lim, J.M.; Heo, N.S.; Oh, S.Y.; Ryu, M.Y.; Seo, J.H.; Park, T.J.; Huh, Y.S.; Park, J.P. Selection of affinity peptides for interference-free detection of cholera toxin. Biosens. Bioelectron. 2018, 99, 289–295. [Google Scholar] [CrossRef]
- Cho, C.H.; Kim, J.H.; Song, D.K.; Park, T.J.; Park, J.P. An affinity peptide-incorporated electrochemical biosensor for the detection of neutrophil gelatinase-associated lipocalin. Biosens. Bioelectron. 2019, 142, 111482. [Google Scholar] [CrossRef]
- Bravaccini, S.; Puccetti, M.; Bocchini, M.; Ravaioli, S.; Celli, M.; Scarpi, E.; De Giorgi, U.; Tumedei, M.M.; Raulli, G.; Cardinale, L.; et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E.; Bodor, G.S.; Davila-Roman, V.G.; Delmez, J.A.; Apple, F.S.; Ladenson, J.H.; Jaffe, A.S. Cardiac troponin I: A marker with high specificity for cardiac injury. Circulation 1993, 88, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Bagai, A.; Alexander, K.P.; Berger, J.S.; Senior, R.; Sajeev, C.; Pracon, R.; Mavromatis, K.; Lopez-Sendón, J.L.; Gosselin, G.; Diaz, A.; et al. Use of troponin assay 99th percentile as the decision level for myocardial infarction diagnosis. Am. Heart J. 2017, 190, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Gibler, W.B.; Gibler, C.D.; Weinshenker, E.; Abbottsmith, C.; Hedges, J.R.; Barsan, W.G.; Sperling, M.; Chen, I.W.; Embry, S.; Kereiakes, D. Myoglobin as an early indicator of acute myocardial infarction. Ann. Emerg. Med. 1987, 16, 851–856. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Arter, J.A.; Taggart, D.K.; McIntire, T.M.; Penner, R.M.; Weiss, G.A. Virus-PEDOT nanowires for biosensing. Nano Lett. 2010, 10, 4858–4862. [Google Scholar] [CrossRef]
- Donavan, K.C.; Arter, J.A.; Pilolli, R.; Cioffi, N.; Weiss, G.A.; Penner, R.M. Virus−Poly(3,4-ethylenedioxythiophene) Composite Films for Impedance-Based Biosensing. Anal. Chem. 2011, 83, 2420–2424. [Google Scholar] [CrossRef]
- Arter, J.A.; Diaz, J.E.; Donavan, K.C.; Yuan, T.; Penner, R.M.; Weiss, G.A. Virus–Polymer Hybrid Nanowires Tailored to Detect Prostate-Specific Membrane Antigen. Anal. Chem. 2012, 84, 2776–2783. [Google Scholar] [CrossRef]
- Donavan, K.C.; Arter, J.A.; Weiss, G.A.; Penner, R.M. Virus-PEDOT Biocomposite Films. Langmuir 2012, 28, 12581–12587. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Özgür, E.; Derazshamshir, A.; Bereli, N.; Yavuz, H.; Denizli, A. Surface Plasmon Resonance Sensors for Medical Diagnosis. In Nanotechnology Characterization Tools for Biosensing and Medical Diagnosis; Springer-Verlag GmbH: Heidelberg, Germany, 2018; pp. 425–458. [Google Scholar]
- Dudak, F.C.; Boyaci, I.H. Rapid and label-free bacteria detection by surface plasmon resonance (SPR) biosensors. Biotechnol. J. 2009, 4, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Karoonuthaisiri, N.; Charlermroj, R.; Morton, M.J.; Oplatowska-Stachowiak, M.; Grant, I.R.; Elliott, C.T. Development of a M13 bacteriophage-based SPR detection using Salmonella as a case study. Sens. Actuators B Chem. 2014, 190, 214–220. [Google Scholar] [CrossRef]
- Ding, X.; Yang, K.L. Development of an oligopeptide functionalized surface plasmon resonance biosensor for online detection of glyphosate. Anal. Chem. 2013, 85, 5727–5733. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Moon, J.-S.; Kim, W.-G.; Kim, C.; Park, G.-T.; Heo, J.; Yoo, S.Y.; Oh, J.-W. M13 Bacteriophage-Based Self-Assembly Structures and Their Functional Capabilities. Mini-Rev. Org. Chem. 2015, 12, 271–281. [Google Scholar] [CrossRef]
- Oh, J.W.; Chung, W.J.; Heo, K.; Jin, H.E.; Lee, B.Y.; Wang, E.; Zueger, C.; Wong, W.; Meyer, J.; Kim, C.; et al. Biomimetic virus-based colourimetric sensors. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Moon, J.S.; Lee, Y.; Shin, D.M.; Kim, C.; Kim, W.G.; Park, M.; Han, J.; Song, H.; Kim, K.; Oh, J.W. Identification of Endocrine Disrupting Chemicals using a Virus-Based Colorimetric Sensor. Chem. Asian J. 2016, 11, 3097–3101. [Google Scholar] [CrossRef]
- Kim, W.G.; Song, H.; Kim, C.; Moon, J.S.; Kim, K.; Lee, S.W.; Oh, J.W. Biomimetic self-templating optical structures fabricated by genetically engineered M13 bacteriophage. Biosens. Bioelectron. 2016, 85, 853–859. [Google Scholar] [CrossRef]
- Moon, J.S.; Park, M.; Kim, W.G.; Kim, C.; Hwang, J.; Seol, D.; Kim, C.S.; Sohn, J.R.; Chung, H.; Oh, J.W. M-13 bacteriophage based structural color sensor for detecting antibiotics. Sens. Actuators B Chem. 2017, 240, 757–762. [Google Scholar] [CrossRef]
- Moon, J.S.; Kim, W.G.; Shin, D.M.; Lee, S.Y.; Kim, C.; Lee, Y.; Han, J.; Kim, K.; Yoo, S.Y.; Oh, J.W. Bioinspired M-13 bacteriophage-based photonic nose for differential cell recognition. Chem. Sci. 2017, 8, 921–927. [Google Scholar] [CrossRef]
- Moon, J.S.; Choi, J.; Hwang, Y.H.; Oh, J.W. Liquid Sensing of a M-13 Bacteriophage-Based Colorimetric Sensor. Macromol. Res. 2018, 26, 775–779. [Google Scholar] [CrossRef]
- Tronolone, J.J.; Orrill, M.; Song, W.; Kim, H.S.; Lee, B.Y.; Leblanc, S. Electric field assisted self-assembly of viruses into colored thin films. Nanomaterials 2019, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, H.; Devaraj, V.; Kim, W.G.; Lee, Y.; Kim, Y.; Jeong, N.N.; Choi, E.J.; Baek, S.H.; Han, D.W.; et al. Hierarchical cluster analysis of medical chemicals detected by a bacteriophage-based colorimetric sensor array. Nanomaterials 2020, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Oh, J.W.; Kwak, K.; Lee, B.Y.; Meyer, J.; Wang, E.; Hexemer, A.; Lee, S.W. Biomimetic self-templating supramolecular structures. Nature 2011, 478, 364–368. [Google Scholar] [CrossRef]
- Zhu, H.; White, I.M.; Suter, J.D.; Zourob, M.; Fan, X. Opto-fluidic micro-ring resonator for sensitive label-free viral detection. Analyst 2008, 133, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Suter, J.D.; Fan, X. Overview of the optofluidic ring resonator: A versatile platform for label-free biological and chemical sensing. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC, Minneapolis, MN, USA, 3–6 September 2009; pp. 1042–1044. [Google Scholar] [CrossRef]
- Chen, L.; Zurita, A.J.; Ardelt, P.U.; Giordano, R.J.; Arap, W.; Pasqualini, R. Design and validation of a bifunctional ligand display system for receptor targeting. Chem. Biol. 2004, 11, 1081–1091. [Google Scholar] [CrossRef][Green Version]
- Kim, I.; Moon, J.-S.; Oh, J.-W. Recent advances in M13 bacteriophage-based optical sensing applications. Nano Converg. 2016, 3. [Google Scholar] [CrossRef]
- Sun, Y.; Shukla, G.S.; Weaver, D.; Pero, S.C.; Krag, D.N. Phage-display selection on tumor histological specimens with laser capture microdissection. J. Immunol. Methods 2009, 347, 46–53. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Cao, B.; Li, Y.; Mao, C. Phage nanofibers in nanomedicine: Biopanning for early diagnosis, targeted therapy, and proteomics analysis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 1–33. [Google Scholar] [CrossRef]
- Kelly, K.A.; Waterman, P.; Weissleder, R. In vivo imaging of molecularly targeted phage. Neoplasia 2006, 8, 1011–1018. [Google Scholar] [CrossRef]
- Ghosh, D.; Lee, Y.; Thomas, S.; Kohli, A.G.; Yun, D.S.; Belcher, A.M.; Kelly, K.A. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnol. 2012, 7, 677–682. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.E.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the Immune Response to Phage Therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef]
- Bábíčková, J.; Tóthová, Ľ.; Boor, P.; Celec, P. In vivo phage display—A discovery tool in molecular biomedicine. Biotechnol. Adv. 2013, 31, 1247–1259. [Google Scholar] [CrossRef]
- Trepel, M.; Arap, W.; Pasqualini, R. In vivo phage display and vascular heterogeneity: Implications for targeted medicine. Curr. Opin. Chem. Biol. 2002, 6, 399–404. [Google Scholar] [CrossRef]
- Deng, X.; Wang, L.; You, X.; Dai, P.; Zeng, Y. Advances in the T7 phage display system (Review). Mol. Med. Rep. 2018, 17, 714–720. [Google Scholar] [CrossRef]
- Nicastro, J.; Sheldon, K.; Slavcev, R.A. Bacteriophage lambda display systems: Developments and applications. Appl. Microbiol. Biotechnol. 2014, 98, 2853–2866. [Google Scholar] [CrossRef]
- Gamkrelidze, M.; Dąbrowska, K. T4 bacteriophage as a phage display platform. Arch. Microbiol. 2014, 196, 473–479. [Google Scholar] [CrossRef]
- Sharma, S.C.; Memic, A.; Rupasinghe, C.N.; Duc, A.C.E.; Spaller, M.R. T7 phage display as a method of peptide ligand discovery for PDZ domain proteins. Biopolymers 2009, 92, 183–193. [Google Scholar] [CrossRef]
- Maruyama, I.N.; Maruyama, H.I.; Brenner, S. λfoo: A λ phage vector for the expression of foreign proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8273–8277. [Google Scholar] [CrossRef]
- van Wezenbeek, P.M.; Hulsebos, T.J.; Schoenmakers, J.G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: Comparison with phage fd. Gene 1980, 11, 129–148. [Google Scholar] [CrossRef]
- Sternberg, N.; Hoess, R.H. Display of peptides and proteins on the surface of bacteriophage λ. Proc. Natl. Acad. Sci. USA 1995, 92, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, V.; Sorokulova, I.B.; Samoylov, A.M.; Simonian, A.L.; Petrenko, V.A.; Vodyanoy, V. Phage as a molecular recognition element in biosensors immobilized by physical adsorption. Biosens. Bioelectron. 2007, 22, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Richter, Ł.; Matuła, K.; Leśniewski, A.; Kwaśnicka, K.; Łoś, J.; Łoś, M.; Paczesny, J.; Hołyst, R. Ordering of bacteriophages in the electric field: Application for bacteria detection. Sens. Actuators B Chem. 2016, 224, 233–240. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Cosnier, S.; Herrmann, S.; Marks, R.S. Amperometric immunosensor for the detection of anti-West Nile virus IgG. Anal. Chem. 2007, 79, 8662–8668. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Smith, G.P.; Gong, X.; Quinn, T. A library of organic landscapes on filamentous phage. Protein Eng. Des. Sel. 1996, 9, 797–801. [Google Scholar] [CrossRef]
- Evoy, S.; Singh, A.; Glass, N. Bacteriophage Immobilization for Biosensors. U.S. Patent 2010/0291541 A1, 18 November 2010. [Google Scholar]
- Ramasamy, R.P.; Zhou, Y. Bacteriophage-Based Electrochemical Bacterial Sensors, Systems, and Methods. U.S. Patent 2020/0033340 A1, 30 January 2020. [Google Scholar]
- Albrecht, J.; Conrad, A.J. Biologic Machines for the Detection of Biomolecules. U.S. Patent 10,662,459 B2, 26 May 2020. [Google Scholar]
- Weiss, G.A.; Penner, R.M.; Tam, P.Y.; Yang, L.-M.; Brigham, T. Method and Apparatus for Target Detection Using Electrode-Bound Viruses. U.S. Patent 8,513,001 B2, 20 August 2013. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machera, S.J.; Niedziółka-Jönsson, J.; Szot-Karpińska, K. Phage-Based Sensors in Medicine: A Review. Chemosensors 2020, 8, 61. https://doi.org/10.3390/chemosensors8030061
Machera SJ, Niedziółka-Jönsson J, Szot-Karpińska K. Phage-Based Sensors in Medicine: A Review. Chemosensors. 2020; 8(3):61. https://doi.org/10.3390/chemosensors8030061
Chicago/Turabian StyleMachera, Sebastian J., Joanna Niedziółka-Jönsson, and Katarzyna Szot-Karpińska. 2020. "Phage-Based Sensors in Medicine: A Review" Chemosensors 8, no. 3: 61. https://doi.org/10.3390/chemosensors8030061
APA StyleMachera, S. J., Niedziółka-Jönsson, J., & Szot-Karpińska, K. (2020). Phage-Based Sensors in Medicine: A Review. Chemosensors, 8(3), 61. https://doi.org/10.3390/chemosensors8030061







