A Sensitive Impedimetric Sensor Based on Biosourced Polyphosphine Films for the Detection of Lead Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Monomer Synthesis
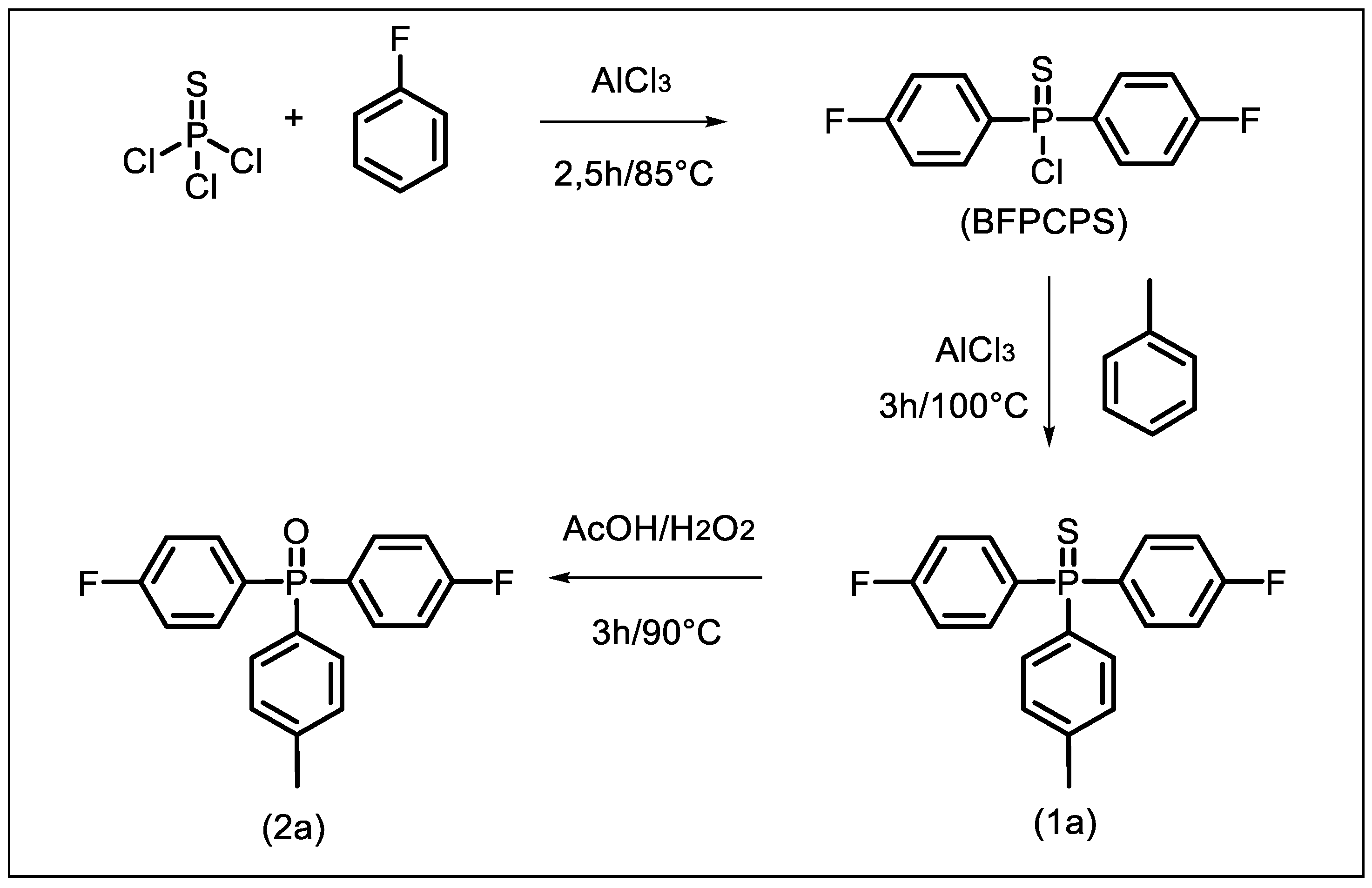
2.2.1. Bis(4-fluorophenyl)chlorophosphine sulfide (BFPCPS)
2.2.2. Bis(4-fluorophenyl)(4-methylphenyl)phosphine sulfide (1a)
2.2.3. Bis(4-fluorophenyl)(4-methylphenyl)phosphine oxide (2a)
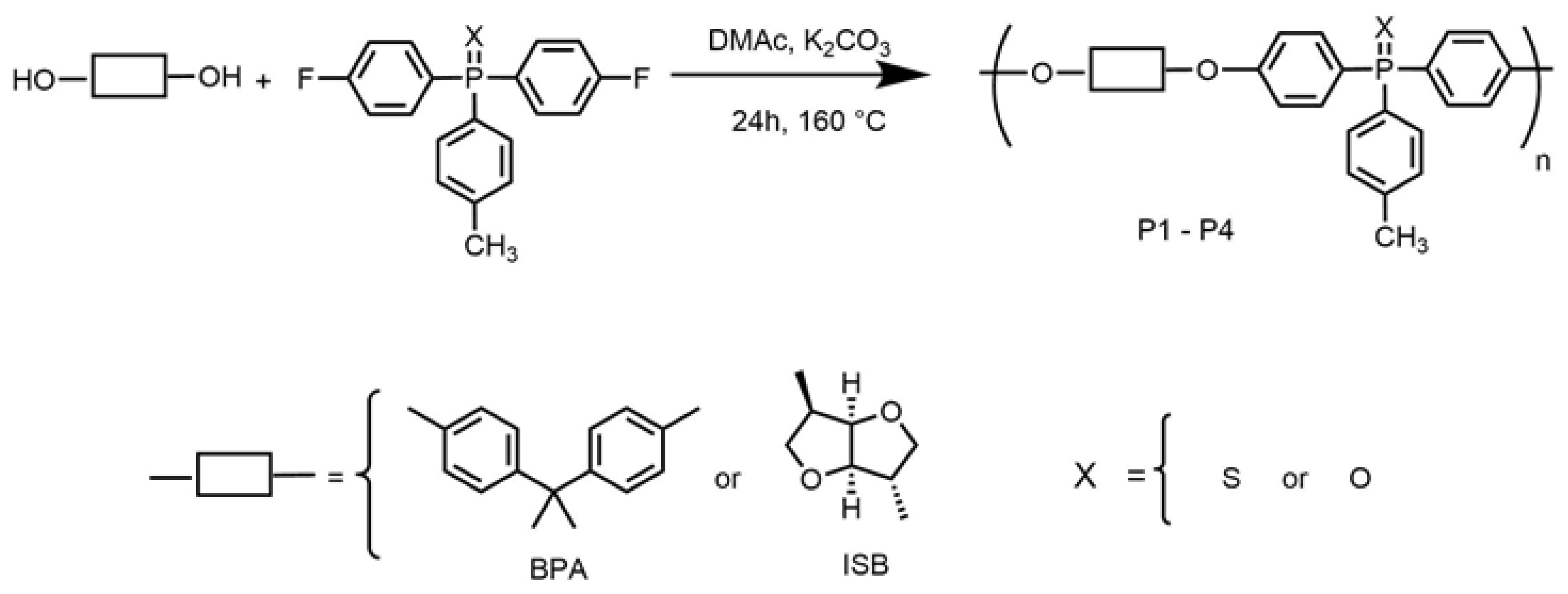
2.3. Synthesis of Polymers
2.4. Charaterization Methods
2.5. Preparation of Polymer-Modified Gold Electrodes and Impedimetric Measurements
3. Results and Discussion
3.1. Physicochemical Characterization of the Resulting Polymers P1–P4
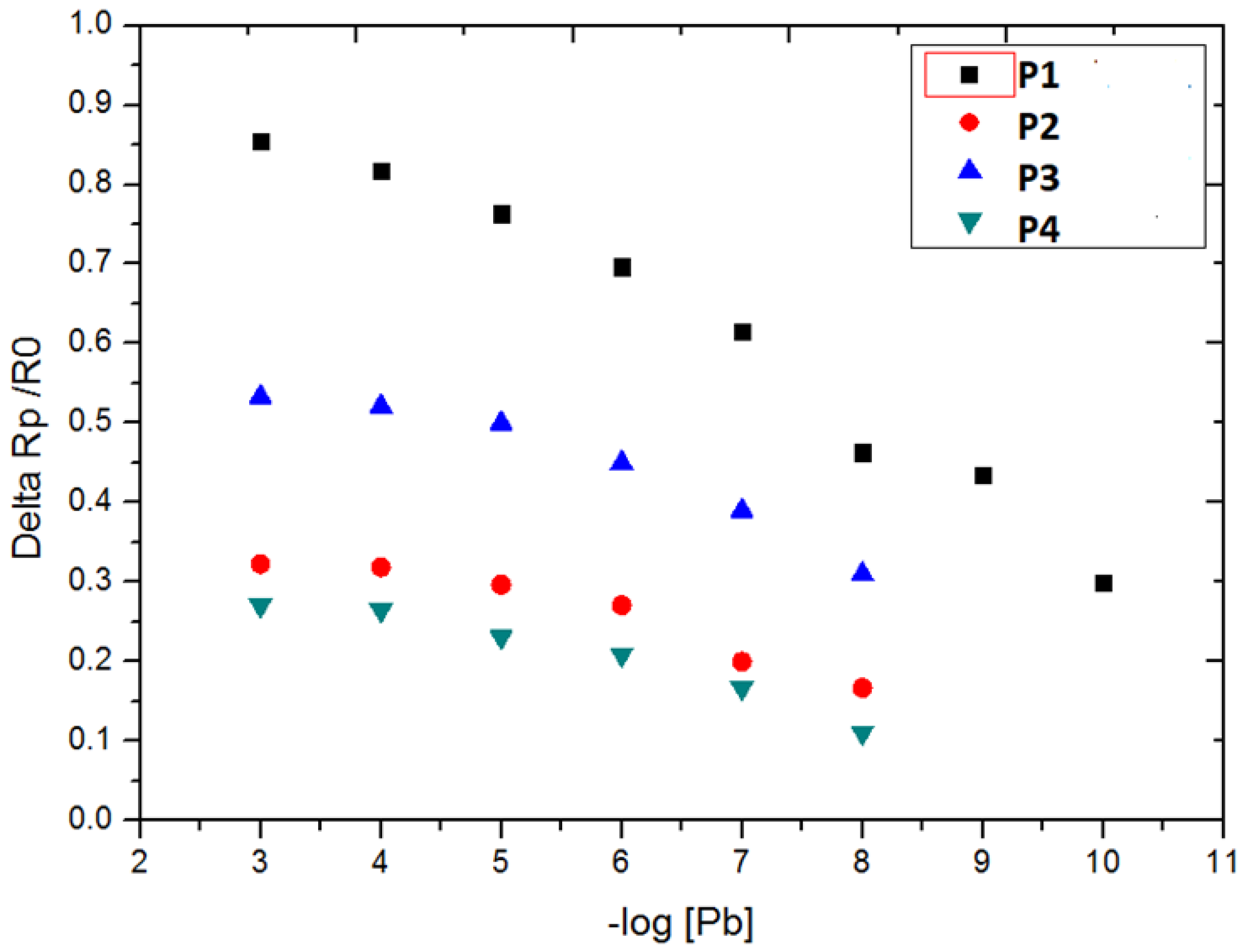
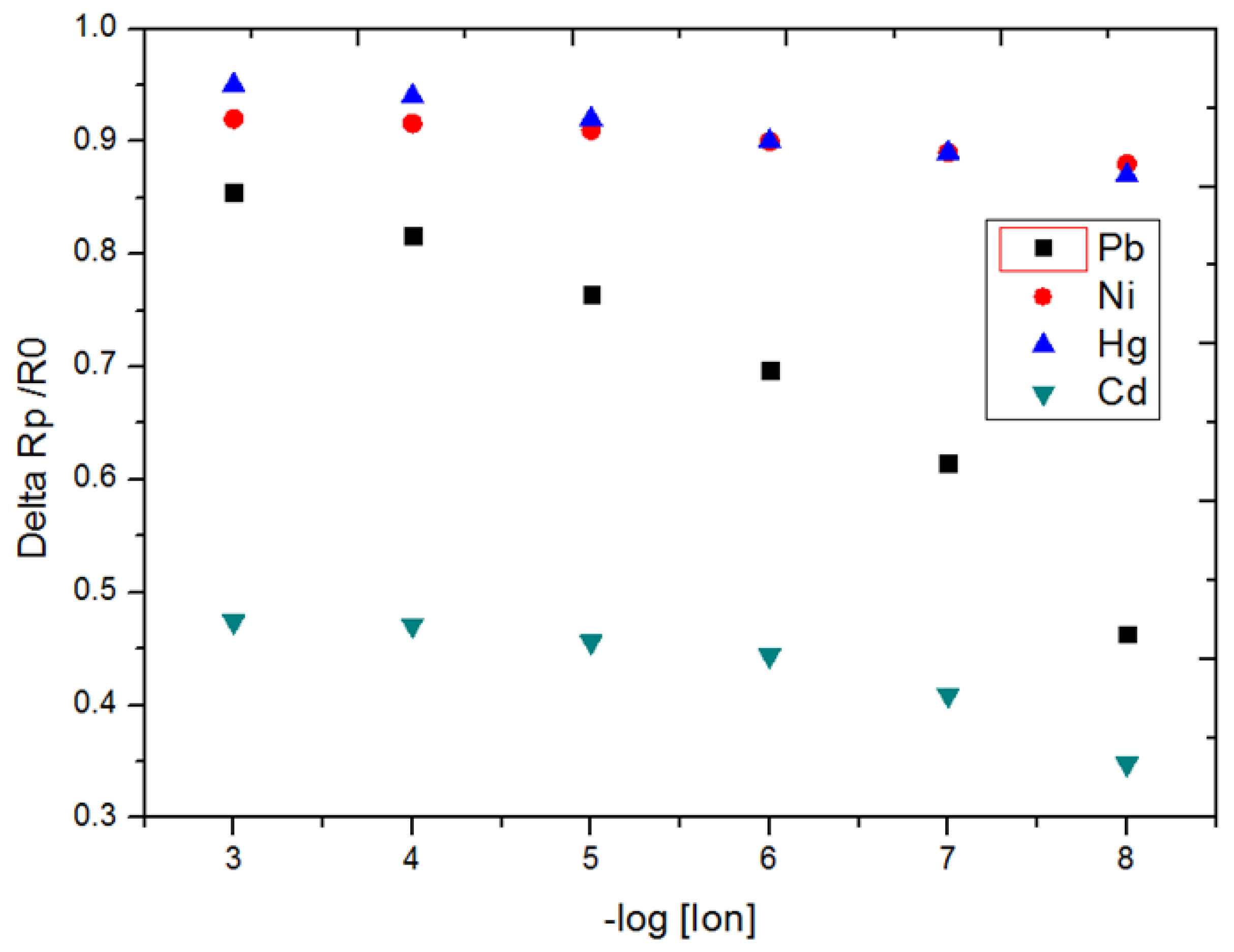
3.2. Detection of Pb2+ Ion by EIS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ. Pollut. 2010, 158, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Diarra, I.; Prasad, S. The current state of heavy metal pollution in Pacific Island Countries: A review. Appl. Spectrosc. Rev. 2020. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Yoshinaga, M.; Ninomiya, H.; Al Hossain, M.M.A.; Sudo, M.; Akhand, A.A.; Ahsan, N.; Alim, M.A.; Khalequzzaman, M.; Iida, M.; Yajima, I.; et al. A Comprehensive Study Including Monitoring, Assessment of Health Effects and Development of a Remediation Method for Chromium Pollution. Chemosphere 2018, 201, 667–675. [Google Scholar] [CrossRef]
- Commission Implementaing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0840&from=fr (accessed on 10 May 2020).
- Trindade, A.S.N.; Dantas, A.F.; Lima, D.C.; Ferreira, S.L.C.; Teixeira, L.S.G. Multivariate optimization of ultrasound-assisted extraction for determination of Cu, Fe, Ni and Zn in vegetable oils by high-resolution continuum source atomic absorption spectrometry. Food Chem. 2015, 185, 145–150. [Google Scholar] [CrossRef]
- Sitko, R.; Janik, P.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I. Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal. Chem. 2015, 87, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Steinnes, E. Atmospheric deposition of heavy metals in Norway studied by the analysis of moss samples using neutron activation analysis and atomic absorption spectrometry. J. Radioanal. Chem. 1980, 58, 387–391. [Google Scholar] [CrossRef]
- Cui, C.; He, M.; Hu, B. Membrane solid phase microextraction with alumina hollow fiber on line coupled with ICP-OES for the determination of trace copper, manganese and nickel in environmental water samples. J. Hazard. Mater. 2011, 187, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sbartai, A.; Namour, P.; Errachid, A.; Krejci, J.; Sejnohova, R.; Renaud, L.; Hamlaoui, M.L.; Loir, A.S.; Garrelie, F.; Donnet, C.; et al. Electrochemical boron-doped diamond film microcells micromachined with femtosecond laser: Application to the determination of Water Framework Directive metals. Anal. Chem. 2012, 84, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Sbartai, A.; Namour, P.; Barbier, F.; Krejci, J.; Kunceriva, R.; Krejci, T.; Nedela, V.; Sobota, J.; Jaffrezic-Renault, N. Electrochemical performances of diamond like carbon for Pb (II) detection in tap water using differential pulse anodic stripping voltammetry technique. Sens. Lett. 2013, 11, 1524–1529. [Google Scholar] [CrossRef]
- Ben Mefteh, W.; Touzi, H.; Chevalier, Y.; Ben Ouada, H.; Othmane, A.; Kalfat, R.; Jaffrezic Renault, N. Comparison of polysiloxane films substituted by undecenyl-cyclam and by naphthyl-cyclam for the design of ISFET devices sensitive to Fe3+ ions. Sens. Actuators B Chem. 2014, 204, 723–733. [Google Scholar] [CrossRef]
- Ben Mefteh, W.; Chevalier, Y.; Bala, C.; Jaffrezic-Renault, N. Voltammetric Detection of Copper Ions on a Gold Electrode Modified with a N-methyl-2-naphthyl-cyclam film. Anal. Lett. 2018, 51, 971–982. [Google Scholar] [CrossRef]
- Ben Mefteh, W.; Touzi, H.; Chevalier, Y.; Bessueille, F.; Kalfat, R.; Jaffrezic Renault, N. Gold electrodes functionalized by methyl-naphthyl substituted cyclam films for the detection of metal ions. Sens. Actuators B Chem. 2015, 213, 334–342. [Google Scholar] [CrossRef]
- Zazoua, A.; Morakchi, K.; Kherrat, R.; Samar, M.H.; Errachid, A.; Jaffrezic-Renault, N.; Boubellout, R. Electrochemical characterization of an EIS sensor functionalized with a TOPO doped polymeric layer for Cr(VI) detection. ITBM-RBM 2008, 29, 187–191. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Parinejad, M.; Matt, D. A lead-selective membrane electrode based upon a phosphorylated hexahomotrioxacalix[3]arene. J. Chin. Chem. Soc. 2007, 54, 1535–1542. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus: Chemistry, Biochemistry and Technology, 6th ed.; CRC Press: New York, NY, USA, 2013; pp. 1139–1164. [Google Scholar]
- Mayer, H.A.; Kaska, W.C. Stereochemical control of transition metal complexes by polyphosphine ligands. Chem. Rev. 1994, 94, 1239–1272. [Google Scholar] [CrossRef]
- Iliescu, S.; Zubizarreta, L.; Plesu, N.; Macarie, L.; Popa, A.; Ilia, G. Polymers containing phosphorus groups and polyethers: From synthesis to application. Chem. Cent. J. 2012, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.J. Phosphorus-containing polymers a great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Sun, J.; Wu, B.; Zhou, Q. Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar] [CrossRef]
- Petreus, O.; Vlad-Bubulac, T.; Hamciuc, C. Synthesis and characterization of new polyesters with enhanced phosphorus content. Eur. Polym. J. 2005, 41, 2663–2670. [Google Scholar] [CrossRef]
- Chang, Y.L.; Wang, Y.Z.; Ban, D.M.; Yang, B.; Zhao, G.M. A Novel phosphorus-containing polymer as a highly effective flame retardant. Macromol. Mater. Eng. 2004, 289, 703–707. [Google Scholar] [CrossRef]
- Chauveau, E.; Marestin, C.; Mercier, R.; Brunaux, A.; Martin, V.; Nogueira, R.P.; Percheron, A.; Roche, V.; Waton, H. Phosphonic acid-containing polysulfones as anticorrosive layers. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Biles, J.E.; McNeal, T.P.; Begley, T.H.; Hollifield, H.C. Determination of bisphenol-A in reusable polycarbonate food-contact plastics and migration to food-simulating liquids. J. Agric. Food Chem. 1997, 45, 3541–3544. [Google Scholar] [CrossRef]
- Hoekstra, E.J.; Simoneau, C. Release of bisphenol A from polycarbonate-A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402. [Google Scholar] [CrossRef]
- Plazek, D.J.; Choy, I.C. The physical properties of bisphenol-A-based epoxy resins during and after curing. II. Creep behaviour above and below the glass transition temperature. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 307–324. [Google Scholar] [CrossRef]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and properties of a bisphenol a based phthalonitrile resin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4136–4143. [Google Scholar] [CrossRef]
- Ben Abderrazak, H.; Fildier, A.; Ben Romdhane, H.; Chatti, S.; Kricheldorf, H.R. Synthesis of new poly(ether ketone)s derived from biobased diols. Macromol. Chem. Phys. 2013, 214, 1423–1433. [Google Scholar] [CrossRef]
- Chatti, S.; Hani, M.A.; Bornhorst, K.; Kricheldorf, H.R. Poly(ether sulfone) of isosorbide, isomannide and isoidide. High Perform. Polym. 2009, 21, 105–118. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Chatti, S.; Schwarz, G.; Krüger, R.P. Macrocycles 27: Cyclic aliphatic polyesters of isosorbide. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3414–3424. [Google Scholar] [CrossRef]
- Hani, M.; Chatti, S.; Kricheldorf, H.R.; Zarrouk, H. Polycondensation of isosorbide and various diols by means of diphosgene characterization by a combination of MALDI and NMR. Recent Res. Dev. Org. Chem. 2007, 661, 1–11. [Google Scholar]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.P.; Boutevin, B. Synthesis of isosorbide-based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Nelson, A.M.; Long, T.E. A Perspective on emerging polymer technologies for bisphenol-A replacement. Polym. Int. 2012, 61, 1485–1491. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J. Progress in polymer science (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Feng, X.; East, A.J.; Hammond, W.B.; Zhang, Y.; Jaffe, M. Overview of advances in sugar-based polymers. Polym. Adv. Technol. 2011, 22, 139–150. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications-Quo Vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef]
- Belgacem, C.; Medimagh, R.; Kricheldorf, H.; Ben Romdhane, H.; Chatti, S. Copolyethersulfones of 1,4:3,6-dianhydrohexitols and bisphenol A. Des. Monomers Polym. 2016, 19, 248–255. [Google Scholar] [CrossRef]
- Gomri, M.; Abderrazak, H.; Souissi, R.; Saint-Martin, P.; Casabianca, H.; Chatti, S.; Mercier, R.; Errachid, A.; Jaffrezic-Renault, N. Combination of partially biosourced poly(ether-pyridine)s films and gold electrodes for the detection of metal ions. under review.
| Ref. Polymer | Yield a) (%) | Tg b) (°C) | Td5% c) (°C) | Mn d) (Da) | Mw d) (Da) | PD d) |
|---|---|---|---|---|---|---|
| P1 | 80 | 202 | 408 | 1967 | 3826 | 2.72 |
| P2 | 82 | 196 | 403 | 4252 | 8575 | 2.02 |
| P3 | 78 | 184 | 431 | 2856 | 8034 | 2.86 |
| P4 | 89 | 185 | 431 | 3508 | 9798 | 2.79 |
| Sensing Polymers Ref. | Sensitivity | LOD [g/L] | Linear Range [g/L] |
|---|---|---|---|
| P1 | 80.7 ± 2.0 | 10−10 | [10−10–10−3] |
| P2 | 33.2±0.8 | 10−8 | [10−8–10−5] |
| P3 | 44.5 ± 1.1 | 10−8 | [10−8–10−5] |
| P4 | 31.9 ± 0.8 | 10−8 | [10−8–10−5] |
| Ion | Sensitivity | LOD [g/L] | Linear Range [g/L] |
|---|---|---|---|
| Pb2+ | 80.7 ± 2.0 | 10−10 | [10−10–10−3] |
| Hg2+ | 16.3 ± 0.4 | 10−8 | [10−10–10−5] |
| Ni2+ | 10.0 ± 0.2 | 10−8 | [10−10–10−5] |
| Cd2+ | 35.9 ± 0.9 | 10−8 | [10−10–10−5] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabbah, T.; Abderrazak, H.; Souissi, R.; Saint-Martin, P.; Casabianca, H.; Chatti, S.; Mercier, R.; Rassas, I.; Errachid, A.; Hammami, M.; et al. A Sensitive Impedimetric Sensor Based on Biosourced Polyphosphine Films for the Detection of Lead Ions. Chemosensors 2020, 8, 34. https://doi.org/10.3390/chemosensors8020034
Chabbah T, Abderrazak H, Souissi R, Saint-Martin P, Casabianca H, Chatti S, Mercier R, Rassas I, Errachid A, Hammami M, et al. A Sensitive Impedimetric Sensor Based on Biosourced Polyphosphine Films for the Detection of Lead Ions. Chemosensors. 2020; 8(2):34. https://doi.org/10.3390/chemosensors8020034
Chicago/Turabian StyleChabbah, Taha, Houyem Abderrazak, Radhia Souissi, Patrice Saint-Martin, Herve Casabianca, Saber Chatti, Regis Mercier, Ilhem Rassas, Abdelhamid Errachid, Mohamed Hammami, and et al. 2020. "A Sensitive Impedimetric Sensor Based on Biosourced Polyphosphine Films for the Detection of Lead Ions" Chemosensors 8, no. 2: 34. https://doi.org/10.3390/chemosensors8020034
APA StyleChabbah, T., Abderrazak, H., Souissi, R., Saint-Martin, P., Casabianca, H., Chatti, S., Mercier, R., Rassas, I., Errachid, A., Hammami, M., & Jaffrezic-Renault, N. (2020). A Sensitive Impedimetric Sensor Based on Biosourced Polyphosphine Films for the Detection of Lead Ions. Chemosensors, 8(2), 34. https://doi.org/10.3390/chemosensors8020034









