Synthesis of a 5-Carboxy Indole-Based Spiropyran Fluorophore: Thermal, Electrochemical, Photophysical and Bovine Serum Albumin Interaction Investigations
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
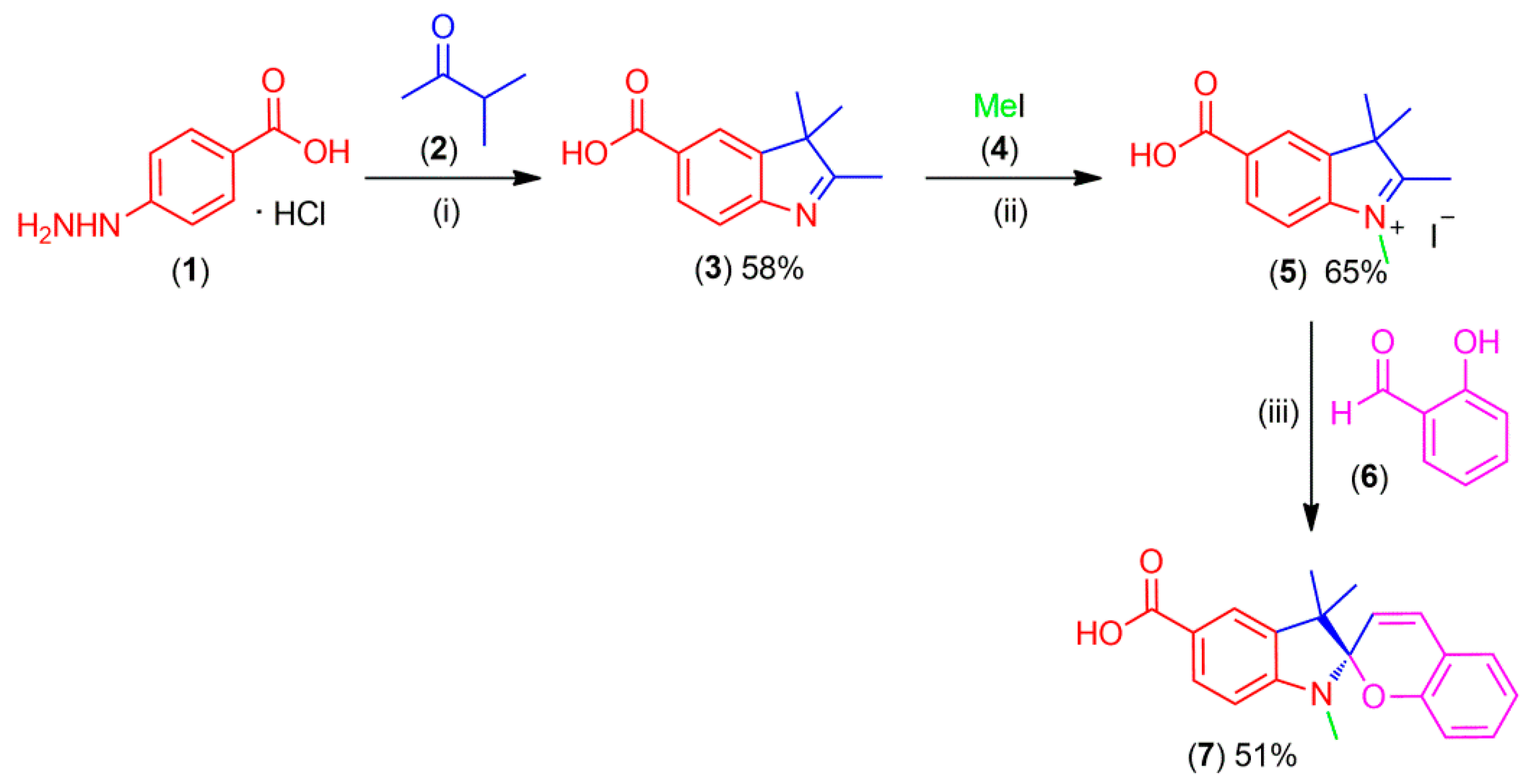
3.1. Synthesis of Spiropyran
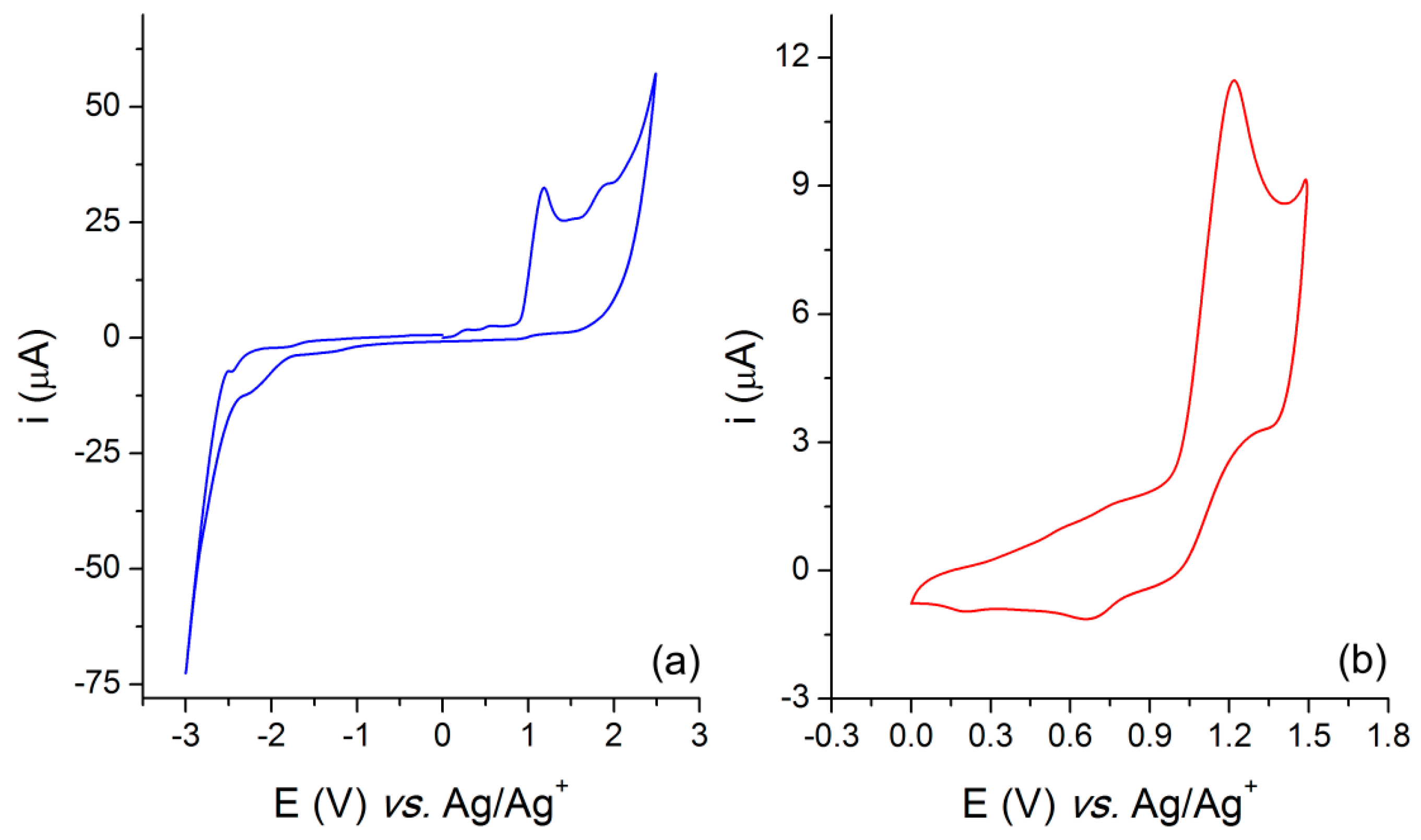
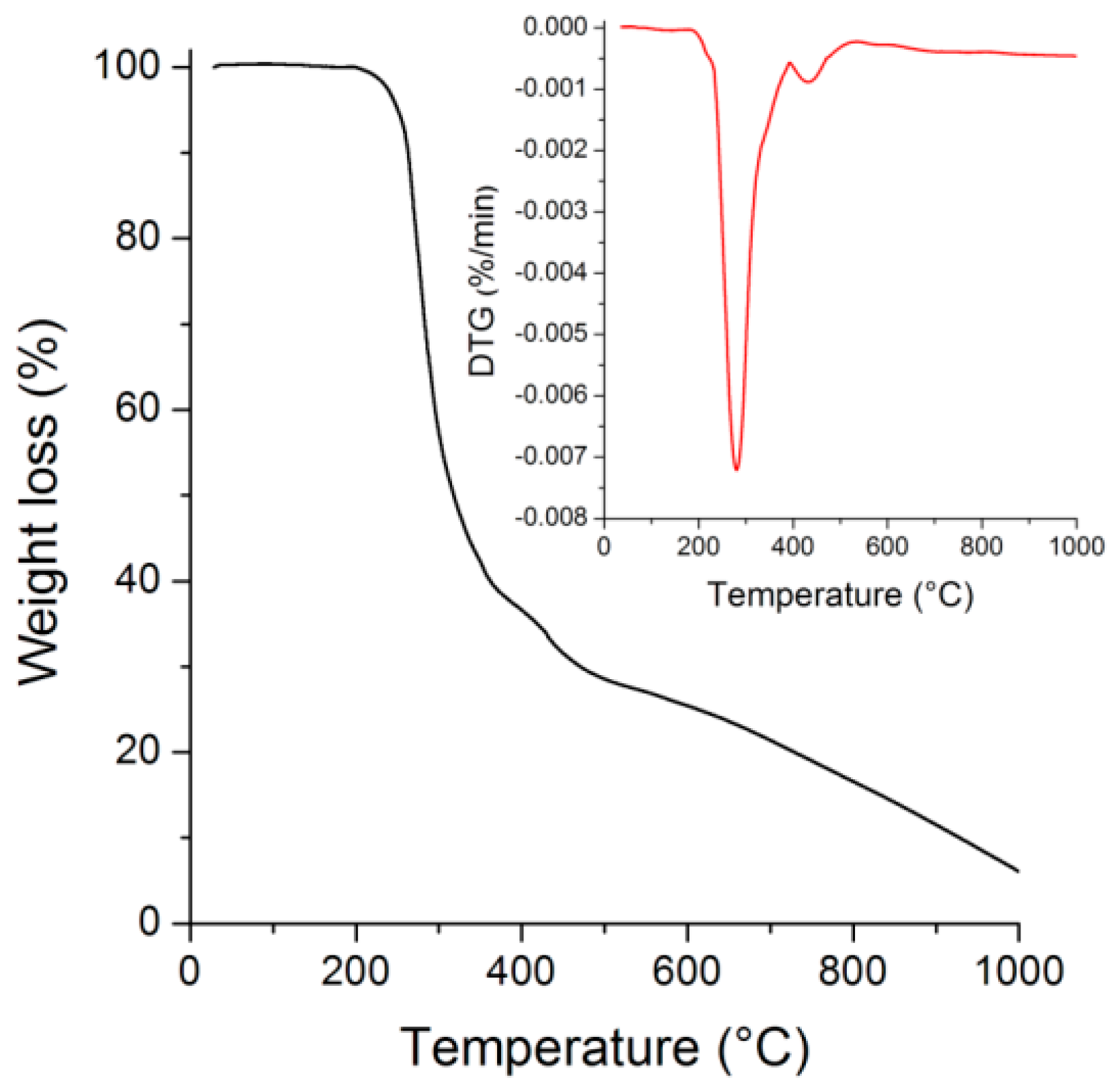
3.2. Electrochemical and Thermal Characterization
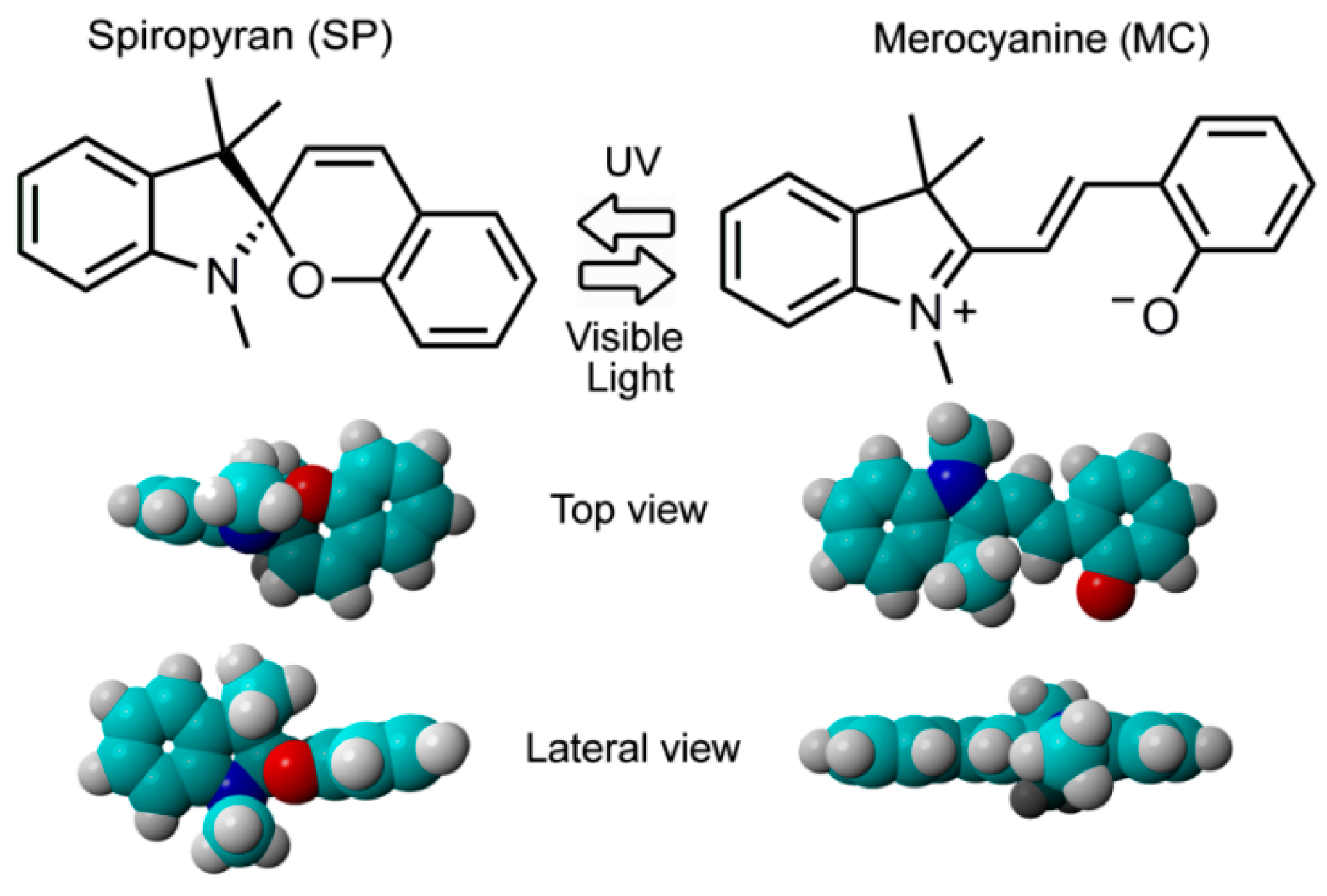
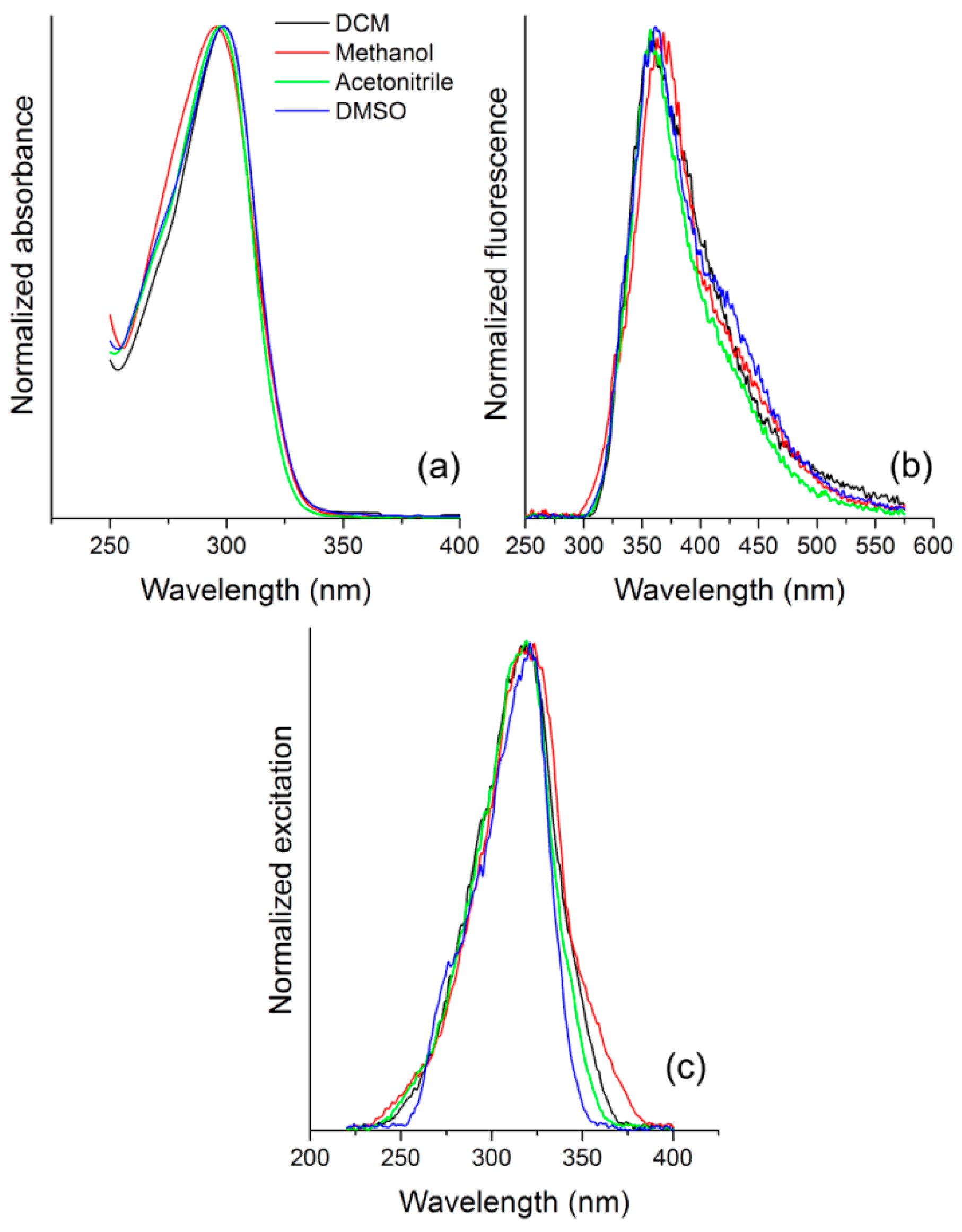
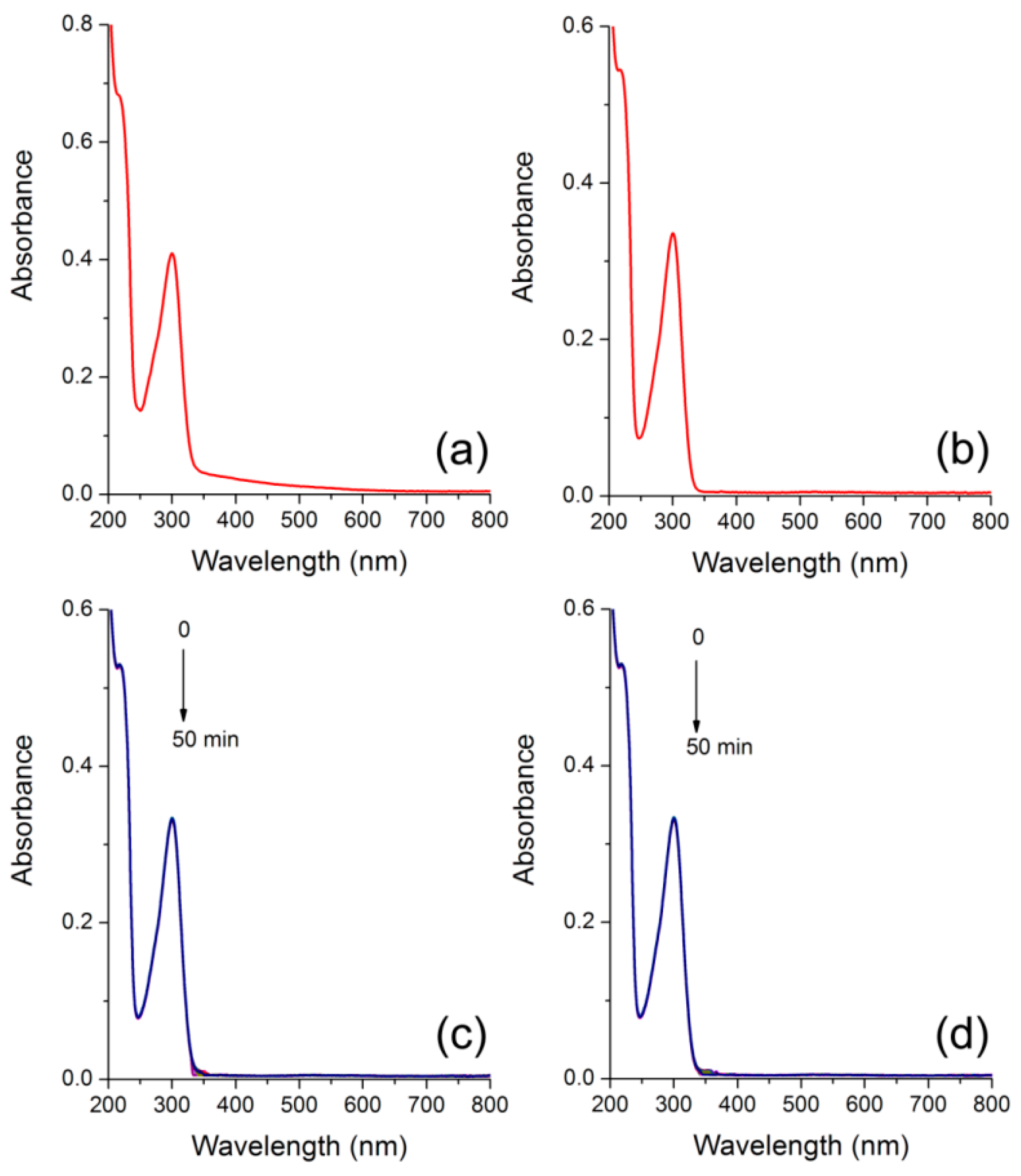
3.3. Photophysics
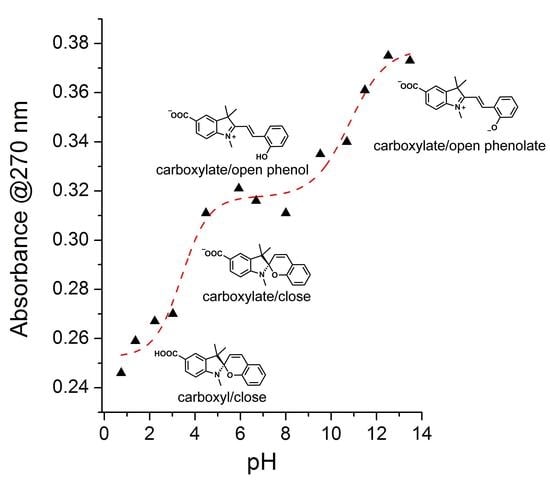
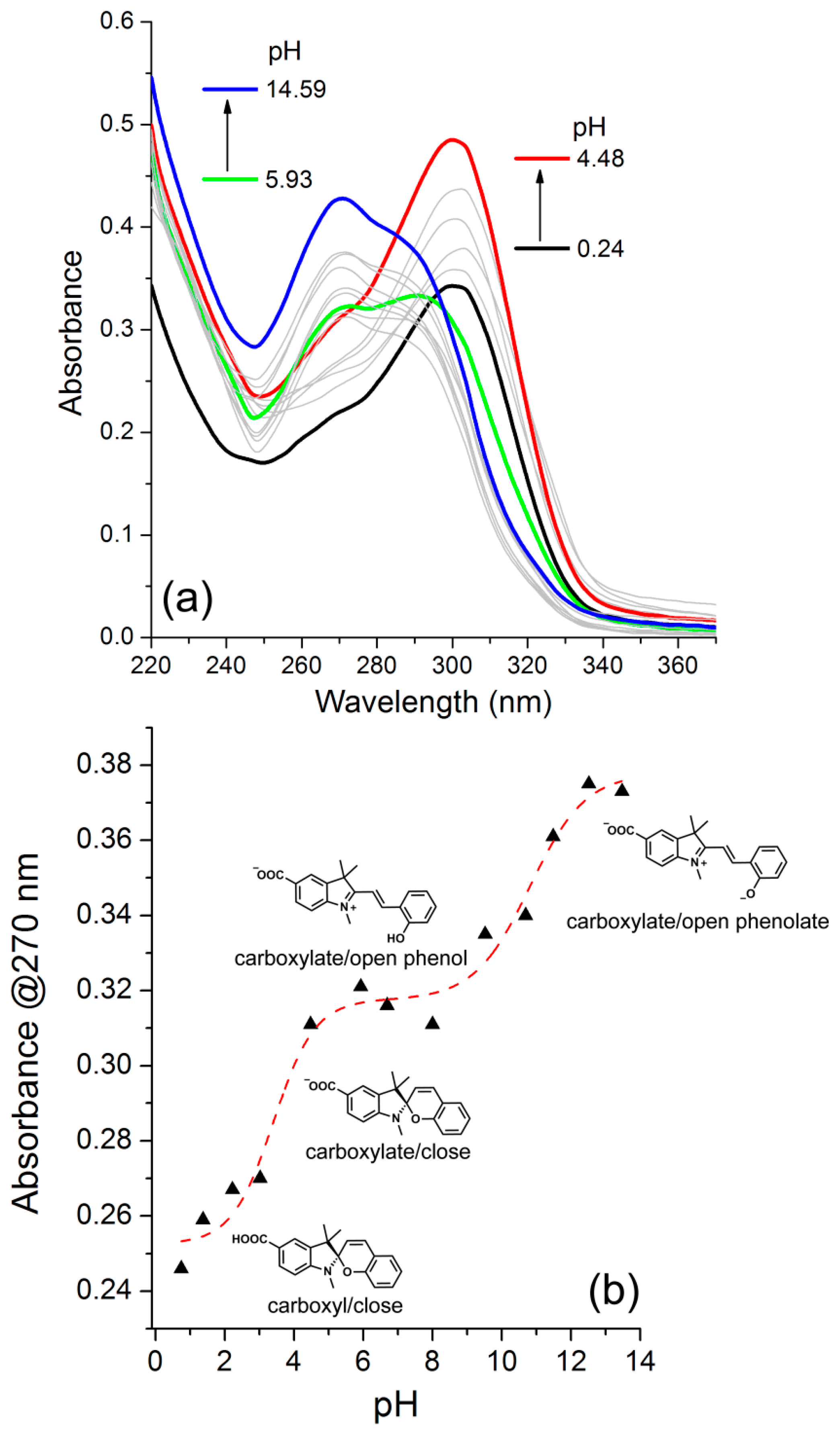
3.4. Ionization Constants
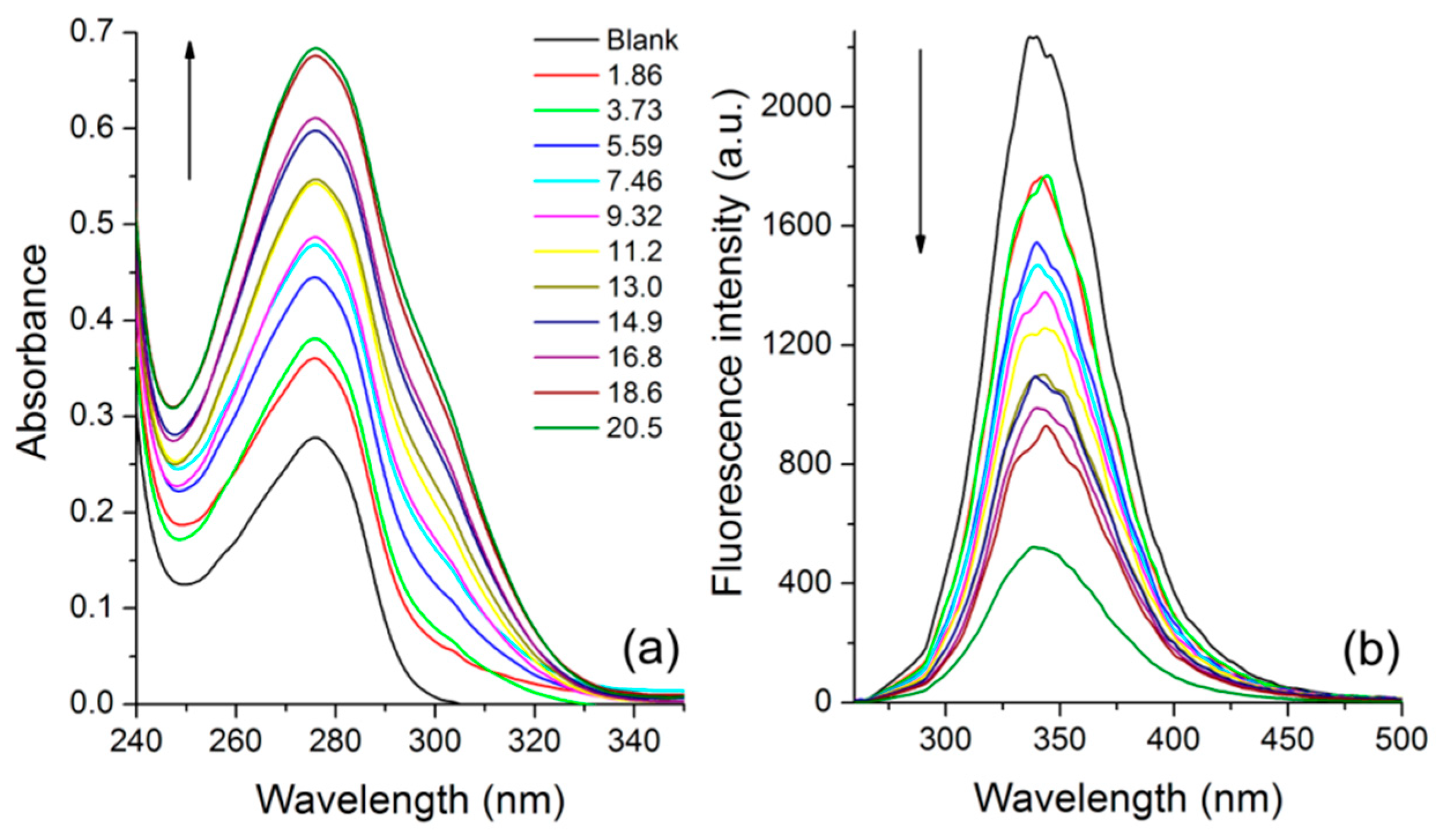
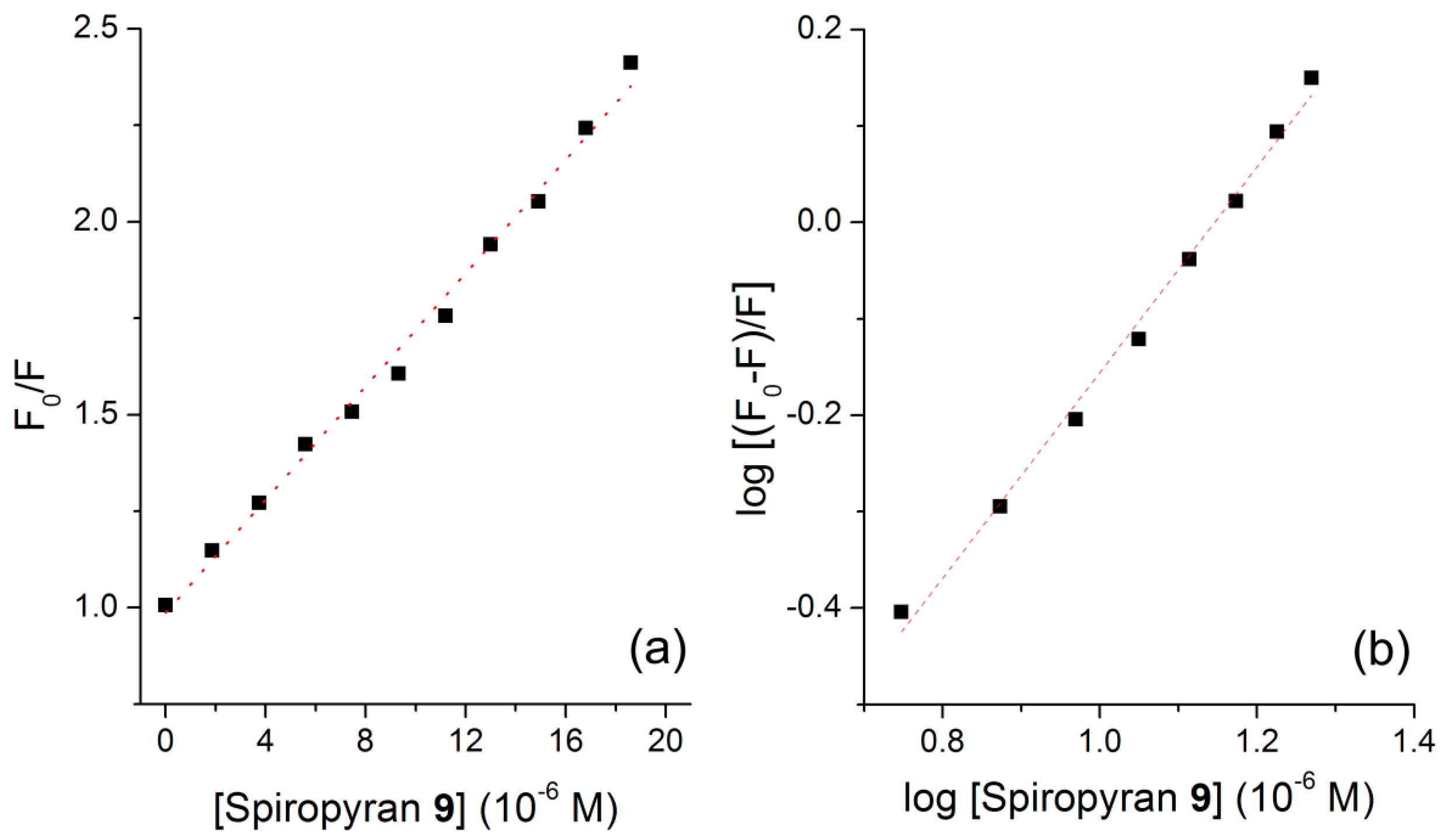
3.5. BSA Interaction Study
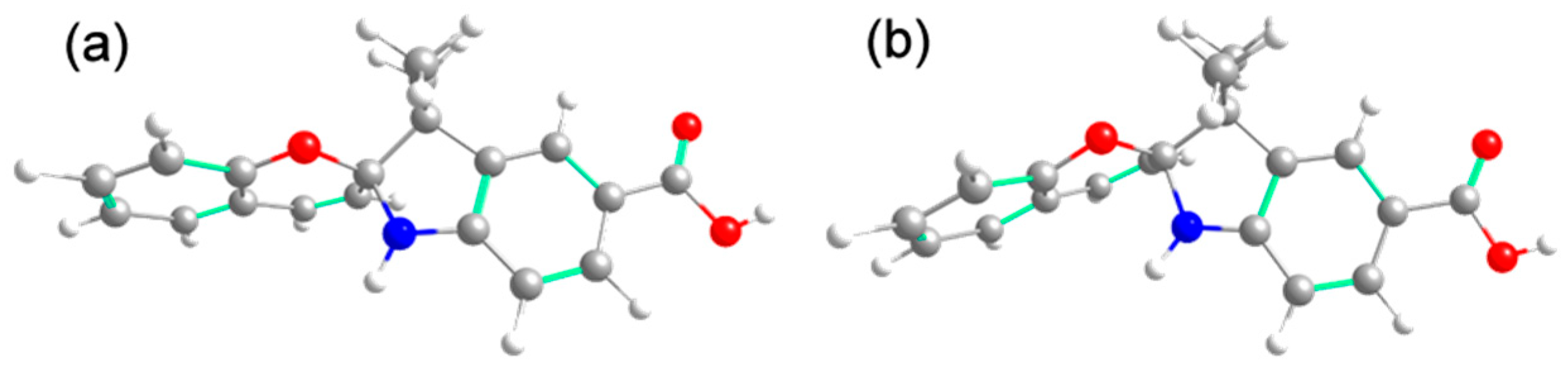
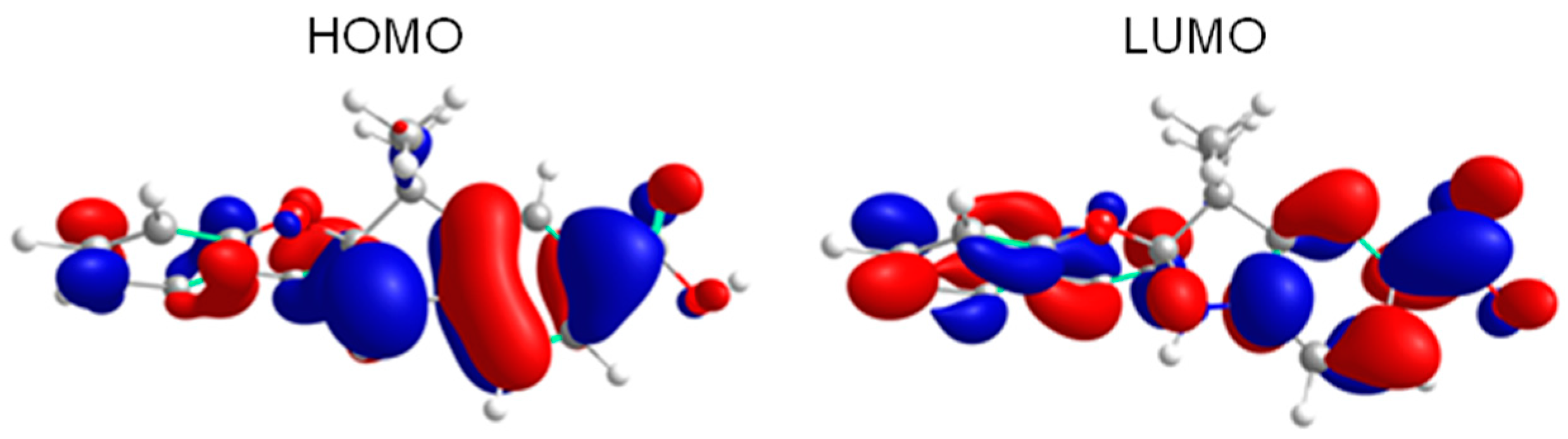
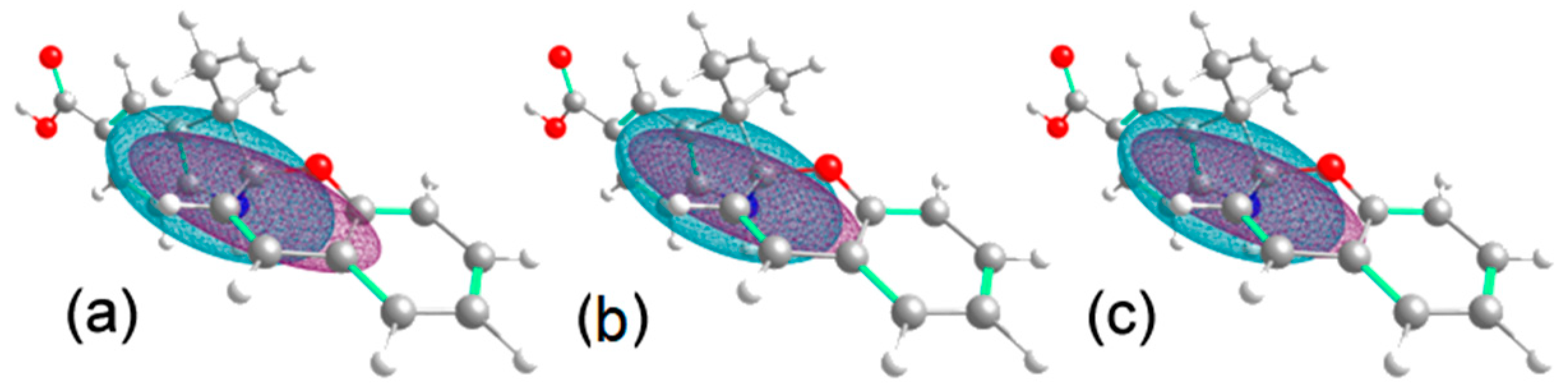
3.6. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [PubMed]
- Feeney, M.J.; Thomas, S.W. Tuning the Negative Photochromism of Water-Soluble Spiropyran Polymers. Macromol. 2018, 51, 8027–8037. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, Y.; Matsuura, T.; Meng, J.-B. Synthesis of functionalized spiropyran and spirooxazine derivatives and their photochromic properties. J. Photochem. Photobiol. A: Chem. 2004, 161, 201–213. [Google Scholar] [CrossRef]
- Samanta, D.; Galaktionova, D.; Gemen, J.; Shimon, L.J.W.; Diskin-Posner, Y.; Avram, L.; Král, P.; Klajn, R. Reversible chromism of spiropyran in the cavity of a flexible coordination cage. Nat. Commun. 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Q.; Wang, Y.; Hong, C.-Y.; Pan, C.-Y. Spiropyran-Based Polymeric Vesicles: Preparation and Photochromic Properties. Macromol. Rapid Commun. 2011, 32, 1174–1179. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Gallina, M.E.; Zhang, X.; Wu, I.-C.; Jin, Y.; Sun, W.; Chiu, D.T. Reversible Photoswitching of Spiropyran-Conjugated Semiconducting Polymer Dots. Anal. Chem. 2012, 84, 9431–9438. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, H.; Zhang, G.; Zhou, Y.; Fan, L.; Shi, L.; Zhang, C.; Shuang, S.; Dong, C. Substituent effect on the acid-induced isomerization of spiropyran compounds. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 202, 13–17. [Google Scholar] [CrossRef]
- Byrne, R.; Fraser, K.J.; Izgorodina, E.; Macfarlane, D.R.; Forsyth, M.; Diamond, D. Photo- and solvatochromic properties of nitrobenzospiropyran in ionic liquids containing the [NTf2]− anion. Phys. Chem. Chem. Phys. 2008, 10, 5919–5924. [Google Scholar] [CrossRef]
- Crano, J.C.; Guglielmetti, R.J. (Eds.) Organic photochromic and thermochromic compounds, 1st ed.; Springer US: New York, NY, USA, 2002. [Google Scholar]
- Kobatake, S.; Irie, M. 8 Photochromism. Annu. Rep. Prog. Chem. Sect. C: Phys. Chem. 2003, 99, 277–313. [Google Scholar] [CrossRef]
- Paramonov, S.; Lokshin, V.; Fedorova, O.A. Spiropyran, chromene or spirooxazine ligands: Insights into mutual relations between complexing and photochromic properties. J. Photochem. Photobiol. C: Photochem. Rev. 2011, 12, 209–236. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Levitus, M.; Glasser, G.; Neher, D.; Aramendía, P.F. Direct measurement of the dipole moment of a metastable merocyanine by electromechanical interferometry. Chem. Phys. Lett. 1997, 277, 118–124. [Google Scholar] [CrossRef]
- Bletz, M.; Pfeifer-Fukumura, U.; Kolb, U.; Baumann, W. Ground- and First-Excited-Singlet-State Electric Dipole Moments of Some Photochromic Spirobenzopyrans in Their Spiropyran and Merocyanine Form†. J. Phys. Chem. A 2002, 106, 2232–2236. [Google Scholar] [CrossRef]
- Casades, I.; Constantine, S.; Cardin, D.; García, H.; Gilbert, A.; Márquez, F. ‘Ship-in-a-Bottle’ Synthesis and Photochromism of Spiropyrans Encapsulated within Zeolite Y Supercages. Tetrahedron 2000, 56, 6951–6956. [Google Scholar] [CrossRef]
- Alonso, M.; Reboto, V.; Guiscardo, L.; Martin, A.S.; Rodríguez-Cabello, J.C. Spiropyran Derivative of an Elastin-like Bioelastic Polymer: Photoresponsive Molecular Machine to Convert Sunlight into Mechanical Work. Macromol. 2000, 33, 9480–9482. [Google Scholar] [CrossRef]
- Xia, H.; Chen, Y.; Yang, G.; Zou, G.; Zhang, Q.; Zhang, D.; Wang, P.; Ming, H. Optical Modulation of Waveguiding in Spiropyran-Functionalized Polydiacetylene Microtube. ACS Appl. Mater. Interfaces 2014, 6, 15466–15471. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Shirakawa, E.; Tanaka, K.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Spiropyran-Modified Gold Nanoparticles: Reversible Size Control of Aggregates by UV and Visible Light Irradiations. ACS Appl. Mater. Interfaces 2014, 6, 7554–7562. [Google Scholar] [CrossRef]
- Tian, W.; Tian, J. Synergy of Different Fluorescent Enhancement Effects on Spiropyran Appended onto Cellulose. Langmuir 2014, 30, 3223–3227. [Google Scholar] [CrossRef]
- Schenderlein, H.; Voss, A.; Stark, R.W.; Biesalski, M. Preparation and Characterization of Light-Switchable Polymer Networks Attached to Solid Substrates. Langmuir 2013, 29, 4525–4534. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Miyamoto, R.; Hirai, T. Spiropyran-Conjugated Thermoresponsive Copolymer as a Colorimetric Thermometer with Linear and Reversible Color Change. Org. Lett. 2009, 11, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Li, M.; Zhu, S.; Li, H.; Xi, G.; Li, Y.-G.; Wang, Y.; Li, Q.; Liang, S.; Zhong, K.; et al. Hydrochromic molecular switches for water-jet rewritable paper. Nat. Commun. 2014, 5, 3044. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zheng, Y.; Shen, J.; Yang, W.; Yin, M. “On–off–on” Switchable Sensor: A Fluorescent Spiropyran Responds to Extreme pH Conditions and Its Bioimaging Applications. ACS Appl. Mater. Interfaces 2014, 6, 19515–19519. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Alinejad, Z.; Mahdavian, A.R. Facile and fast photosensing of polarity by stimuli-responsive materials based on spiropyran for reusable sensors: a physico-chemical study on the interactions. J. Mater. Chem. C 2017, 5, 6588–6600. [Google Scholar] [CrossRef]
- Parker, R.; Gates, J.; Rogers, H.L.; Smith, P.; Grossel, M.C. Using the photoinduced reversible refractive-index change of an azobenzene co-polymer to reconfigure an optical Bragg grating. J. Mater. Chem. 2010, 20, 9118–9125. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Shen, Y.; Chen, M.; Shao, X. Spiropyran-azobenzene-DBU system as solvent indicator. Tetrahedron Lett. 2018, 59, 3829–3832. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Wu, S.; Su, J.; Tong, Z. Photoreversible Fluorescent Modulation of Nanoparticles via One-Step Miniemulsion Polymerization. Small 2009, 5, 970–978. [Google Scholar] [CrossRef]
- Cui, H.; Liu, H.; Chen, S.; Wang, R. Synthesis of amphiphilic spiropyran-based random copolymer by atom transfer radical polymerization for Co2+ recognition. Dye. Pigment. 2015, 115, 50–57. [Google Scholar] [CrossRef]
- Streib, M.; Kräling, K.; Richter, K.; Xie, X.; Steuber, H.; Meggers, E. An organometallic inhibitor for the human repair enzyme 7,8-dihydro-8-oxoguanosine triphosphatase. Angew. Chem. Int. Ed. 2014, 53, 305–309. [Google Scholar] [CrossRef]
- Wang, L.-F.; Chen, J.-W.; Chen, J.-W. Cyclodextrin effects on the photochromism of spiropyrans/β-cyclodextrin inclusion polymers. Mater. Chem. Phys. 2012, 136, 151–159. [Google Scholar] [CrossRef]
- Raymo, F.M.; Alvarado, R.J.; Giordani, S.; Cejas, M.A. Memory Effects Based on Intermolecular Photoinduced Proton Transfer. J. Am. Chem. Soc. 2003, 125, 2361–2364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-Q.; Zhang, G.-F.; Li, C.; Aldred, M.; Chang, E.; Drezek, R.A.; Li, A.D.Q. Reversible Two-Photon Photoswitching and Two-Photon Imaging of Immunofunctionalized Nanoparticles Targeted to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Dai, X.; Li, H.; Yu, S.; Meng, W. A New Spiropyran-Based Sensor for Colorimetric and Fluorescent Detection of Divalent Cu2+ and Hg2+ Ions and Trivalent Ce3+, Cr3+ and Al3+ Ions. J. Fluoresc. 2019, 29, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Lafuma, A.; Quinn, F.X.; Sanchez, C.; Chodorowski-Kimmes, S. Photochromic Properties of a Spirooxazine and a Spiropyran in Alcoholic Solutions of Zirconium and Aluminium Alkoxides: Influence of the Ethyl Acetoacetate Chelating Agent on the Optical Properties. Eur. J. Inorg. Chem. 2003, 2003, 331–338. [Google Scholar] [CrossRef]
- Lee, C.H.; Yun, H.J.; Jung, M.R.; Lee, J.G.; Kim, J.H.; Kim, J.H. Preparation and Characterization of Squaraine Dyes containing Mono- and Bis-Anchoring Groups as the Light Absorber in Dye Sensitized Solar Cells. Electrochimica Acta 2014, 138, 148–154. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Avdeef, A.; Comer, J.E.A.; Thomson, S.J. pH-Metric log P. 3. Glass electrode calibration in methanol-water, applied to pKa determination of water-insoluble substances. Anal. Chem. 1993, 65, 42–49. [Google Scholar] [CrossRef]
- Herrero-Martínez, J.M.; Repollés, C.; Bosch, E.; Rosés, M.; Ràfols, C. Potentiometric determination of aqueous dissociation constants of flavonols sparingly soluble in water. Talanta 2008, 74, 1008–1013. [Google Scholar] [CrossRef]
- Duarte, L.G.T.A.; Germino, J.C.; de Ávila Braga, C.; Barboza, C.A.; Atvars, T.D.Z.; Santos, F.D.S.; Rodembusch, F.S. Photoacidity as a tool to rationalize excited state intramolecular proton transfer reactivity in flavonols. Photochem. Photobiol. Sci. 2018, 17, 231–238. [Google Scholar] [CrossRef]
- Coelho, F.L.; de Ávila Braga, C.; Zanotto, G.M.; Gil, E.S.; Campo, L.F.; Gonçalves, P.F.B.; Rodembusch, F.S.; Santos, F.D.S. Low pH optical sensor based on benzothiazole azo dyes. Sensors Actuators B: Chem. 2018, 259, 514–525. [Google Scholar] [CrossRef]
- Castro, G.T.; Giordano, O.S.; Blanco, S.E. Determination of the pKa of hydroxy-benzophenones in ethanol-water mixtures. Solvent effects. J. Mol. Struct. THEOCHEM 2003, 626, 167–178. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/ (accessed on 29 April 2020).
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D. Perspectives on Basis Sets Beautiful: Seasonal Plantings of Diffuse Basis Functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Menucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chemin- 2005, 36, 2999–3093. [Google Scholar] [CrossRef]
- Le Bahers, T.; Adamo, C.; Ciofini, I. A Qualitative Index of Spatial Extent in Charge-Transfer Excitations. J. Chem. Theory Comput. 2011, 7, 2498–2506. [Google Scholar] [CrossRef]
- Lu, T. Computer Program, Multiwfn. 2017. Available online: https://www.multiwfn.codeplex.com (accessed on 24 March 2020).
- Chemcraft - graphical software for visualization of quantum chemistry computations. Available online: https://www.chemcraftprog.com (accessed on 24 March 2020).
- Allen, C.F.H.; Wilson, C. V The use of N15 as a tracer element in chemical reactions. The mechanism of the Fischer indole synthesis. J. Am. Chem. Soc. 1943, 65, 611–612. [Google Scholar] [CrossRef]
- Zhang, X.; Heng, S.; Abell, A.D. Photoregulation of α-chymotrypsin activity by spiropyran-based inhibitors in solution and attached to an optical fiber. Chem. Eur. J. 2015, 21, 10703–10713. [Google Scholar] [CrossRef]
- Armendáriz-Vidales, G.; Martínez-González, E.; Hernández-Melo, D.; Tiburcio, J.; Frontana, C. Electrochemical Characterization of Spiropyran Structures. Procedia Chem. 2014, 12, 41–46. [Google Scholar] [CrossRef][Green Version]
- Ivashenko, O.; Van Herpt, J.T.; Rudolf, P.; Feringa, B.L.; Browne, W.R. Oxidative electrochemical aryl C–C coupling of spiropyrans. Chem. Commun. 2013, 49, 6737–6739. [Google Scholar] [CrossRef]
- Gerkman, M.A.; Yuan, S.; Duan, P.; Taufan, J.; Schmidt-Rohr, K.; Han, G.G.D. Phase transition of spiropyrans: impact of isomerization dynamics at high temperatures. Chem. Commun. 2019, 55, 5813–5816. [Google Scholar] [CrossRef]
- Tangso, K.J.; Fong, W.-K.; Darwish, T.A.; Kirby, N.; Boyd, B.J.; Hanley, T.L. Novel Spiropyran Amphiphiles and Their Application as Light-Responsive Liquid Crystalline Components. J. Phys. Chem. B 2013, 117, 10203–10210. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Wang, S.; Dong, H.; Ma, X.; Cao, D. Synthesis and properties of photochromic spirooxazine with aggregation-induced emission fluorophores polymeric nanoparticles. Dye. Pigment. 2017, 142, 481–490. [Google Scholar] [CrossRef]
- Strickler, S.J.; Berg, R.A. Relationship between Absorption Intensity and Fluorescence Lifetime of Molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef]
- Turro, N.J.; Scaiano, J.C.; Ramamurthy, V. Principles of molecular photochemistry: An introduction, 1st ed.; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- He, T.; Zhang, J. (Eds.) Photochromic Materials: Preparation, Properties and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Metelitsa, A.V.; Micheau, J.C.; Besugliy, S.O.; Gaeva, E.B.; Voloshin, N.A.; Voloshina, E.N.; Samat, A.; Minkin, V.I. Photochromic properties of six 5-On-alkyl, 6′-CN substituted spironaphthoxazines. Int. J. Photoenergy 2004, 6, 199–204. [Google Scholar] [CrossRef]
- Tiwari, S.; Ghosh, K.K.; Marek, J.; Ramalho, T.C. Spectrophotometric Determination of the Acidity Constants of Some Oxime-Based α-Nucleophiles. J. Chem. Eng. Data 2010, 55, 1153–1157. [Google Scholar] [CrossRef]
- Islamoglu, F.; Kahveci, B.; Ozil, M.; Akyuz, E. Studies on the protonation constants of some triazoles in ethanol-water mixtures. Asian J. Chem. 2008, 20, 3569–3578. [Google Scholar]
- Islamoglu, F.; Kahveci, B.; Ozil, M.; Akyuz, E.; Mentesf, E.; Ekinci, A.P. Determination of the protonation constants of triazole derivatives in non-aqueous solvents. Asian J. Chem. 2009, 21, 1453–1459. [Google Scholar]
- Ferreira, Q.; Gomes, P.J.; Ribeiro, P.; Jones, N.C.; Hoffmann, S.V.; Mason, N.J.; Oliveira, O.N.; Raposo, M. Determination of Degree of Ionization of Poly(allylamine hydrochloride) (PAH) and Poly[1-[4-(3-carboxy-4 hydroxyphenylazo)benzene sulfonamido]-1,2-ethanediyl, sodium salt] (PAZO) in Layer-by-Layer Films using Vacuum Photoabsorption Spectroscopy. Langmuir 2012, 29, 448–455. [Google Scholar] [CrossRef]
- Zheng, T.; Xu, Z.; Zhao, Y.; Li, H.; Jian, R.; Lu, C. Multiresponsive polysiloxane bearing photochromic spirobenzopyran for sensing pH changes and Fe3+ ions and sequential sensing of Ag+ and Hg2+ ions. Sensors Actuators B: Chem. 2018, 255, 3305–3315. [Google Scholar] [CrossRef]
- Roxburgh, C.J.; Sammes, P.G.; Abdullah, A. Steric and electronically biasing substituent effects on the Photoreversibility of novel, 3′-, 5′- and 3-substituted indolospirobenzopyrans. Thermal evaluation using 1H NMR spectroscopy and Overhauser enhancement studies. Dye. Pigment. 2009, 83, 31–50. [Google Scholar] [CrossRef]
- Brauer, G.M.; Durany, G.; Argentar, H. Ionization Constants of Substituted Benzoic Acids in Ethanol-Water. J. Res. Natl. Bur. Stand. Sect. A: Phys. Chem. 1967, 71, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of fluorescence spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Feng, X.-Z.; Lin, Z.; Yang, L.-J.; Wang, C.; Bai, C. Investigation of the interaction between acridine orange and bovine serum albumin. Talanta 1998, 47, 1223–1229. [Google Scholar] [CrossRef]
- Scatchard, G. THE ATTRACTIONS OF PROTEINS FOR SMALL MOLECULES AND IONS. Ann. New York Acad. Sci. 1949, 51, 660–672. [Google Scholar] [CrossRef]
| Parameters | Experimental Data |
|---|---|
| (V) a | 1.17 |
| (V) b | −1.54 |
| λonset (nm) c | 332.90 |
| (eV) d | 3.72 |
| IP (HOMO) (eV) e | −5.61 |
| EA (LUMO) (eV) f | −1.89 |
| Solvent | λabs | ε | f | ke0 | τ0 | λexc a) | λem b) | ΔλST | QY |
|---|---|---|---|---|---|---|---|---|---|
| Dichloromethane | 299 | 2.49 | 0.62 | 6.93 | 1.44 | 319 | 358 | 59/5512 | 0.4 |
| Methanol | 296 | 2.33 | 0.61 | 7.01 | 1.43 | 321 | 367 | 71/6536 | 0.4 |
| Acetonitrile | 298 | 2.42 | 0.57 | 6.41 | 1.56 | 317 | 359 | 61/5702 | 0.4 |
| Dimethylsulfoxide | 298 | 2.21 | 0.60 | 6.73 | 1.49 | 321 | 359 | 61/5702 | 0.5 |
| Solvent | λabs | Δabs | μGS | f | λem | f | Δem | μES | ΔλST |
|---|---|---|---|---|---|---|---|---|---|
| Dichloromethane | 309 | 10 | 4.81 | 0.7811 | 402 | 0.1832 | 44 | 11.37 | 7487 |
| Acetonitrile | 311 | 13 | 4.99 | 0.8724 | 395 | 0.2407 | 36 | 11.31 | 6838 |
| Methanol | 311 | 15 | 4.99 | 0.8694 | 395 | 0.2384 | 32 | 11.31 | 6838 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa Duarte, R.; da Silveira Santos, F.; Bercini de Araújo, B.; Cercena, R.; Brondani, D.; Zapp, E.; Fernando Bruno Gonçalves, P.; Severo Rodembusch, F.; Gonçalves Dal-Bó, A. Synthesis of a 5-Carboxy Indole-Based Spiropyran Fluorophore: Thermal, Electrochemical, Photophysical and Bovine Serum Albumin Interaction Investigations. Chemosensors 2020, 8, 31. https://doi.org/10.3390/chemosensors8020031
da Costa Duarte R, da Silveira Santos F, Bercini de Araújo B, Cercena R, Brondani D, Zapp E, Fernando Bruno Gonçalves P, Severo Rodembusch F, Gonçalves Dal-Bó A. Synthesis of a 5-Carboxy Indole-Based Spiropyran Fluorophore: Thermal, Electrochemical, Photophysical and Bovine Serum Albumin Interaction Investigations. Chemosensors. 2020; 8(2):31. https://doi.org/10.3390/chemosensors8020031
Chicago/Turabian Styleda Costa Duarte, Rodrigo, Fabiano da Silveira Santos, Bruno Bercini de Araújo, Rodrigo Cercena, Daniela Brondani, Eduardo Zapp, Paulo Fernando Bruno Gonçalves, Fabiano Severo Rodembusch, and Alexandre Gonçalves Dal-Bó. 2020. "Synthesis of a 5-Carboxy Indole-Based Spiropyran Fluorophore: Thermal, Electrochemical, Photophysical and Bovine Serum Albumin Interaction Investigations" Chemosensors 8, no. 2: 31. https://doi.org/10.3390/chemosensors8020031
APA Styleda Costa Duarte, R., da Silveira Santos, F., Bercini de Araújo, B., Cercena, R., Brondani, D., Zapp, E., Fernando Bruno Gonçalves, P., Severo Rodembusch, F., & Gonçalves Dal-Bó, A. (2020). Synthesis of a 5-Carboxy Indole-Based Spiropyran Fluorophore: Thermal, Electrochemical, Photophysical and Bovine Serum Albumin Interaction Investigations. Chemosensors, 8(2), 31. https://doi.org/10.3390/chemosensors8020031