Abstract
Chemosensors have attracted considerable attention among the numerous strategies for detecting organic molecules in water. A turn-off mechanism was previously employed for the construction of a cyclodextrin (CD) chemosensor. This mechanism is greatly effective but has several shortcomings. In order to overcome these shortcomings, new fluorescent chemosensors NC0αCD, NC0βCD, and NC0γCD, which were (7-nitrobenz-2-oxa-1,3-diazol-4-yl)amine-modified α-CD, β-CD, and γ-CD, respectively, were prepared. Their guest selectivities were different from those of previously reported CD chemosensors. Here, the mechanism of new CD chemosensors was investigated using nuclear magnetic resonance (NMR) spectroscopy and molecular mechanics calculations. The fluorescence intensity of NC0βCD and NC0γCD slightly decreased and largely increased, respectively, upon the addition of ursodeoxycholic acid as a guest. This is due to the fact that the fluorophore of NC0βCD moved away to the hydrophilic bulk water to form hydrogen bonds between the host and the guest, while the fluorophore of NC0γCD remained located at the primary hydroxy side of the γ-CD unit to form a stable inclusion complex with hydrogen bonds between the fluorophore and the guest. NC0αCD also acted as a turn-on chemosensor for small guests, which could not be detected by the previous CD chemosensors. The motion restriction of the fluorophore through the generation of inclusion complexes could also contribute to increase in fluorescence intensity.
1. Introduction
The development of methods for the detection of organic molecules in water is an important avenue of research in many fields including environmental, chemical, biological, clinical, and security areas. Chemosensors have attracted considerable attention among the numerous strategies investigated for detecting chemical species, owing to advantages such as portability, operational simplicity, rapid response and cost effectiveness [1,2,3,4,5,6,7]. Colorless neutral molecules can be detected in water by following changes in fluorescence intensity or color changes of chemosensors. Colored photophysically active guests can be detected by some methods using a photoinduced electron transfer, charge-transfer, etc., but the methods for the detection of colorless neutral molecules are quite few. A new method for the detection of colorless neutral molecules is desired.
Ueno employed a turn-off mechanism for the construction of a cyclodextrin (CD) chemosensor to detect organic molecules. This turn-off mechanism was due to the positional change in a fluorophore from the inside to the outside of the CD cavity. [8,9,10,11,12]. CDs are cyclic oligosaccharides, consisting of six, seven, and eight D-glucopyranose units named as α-CD, β-CD, and γ-CD, respectively [13,14,15,16]. CDs can accommodate a variety of organic compounds in their central cavities in aqueous solution. A chromophore was connected to the CD framework through a suitable spacer unit. The mechanism of this chemosensor is shown in Figure 1a. Fluorophore-appended CDs with a spacer unit exist as an equilibrium of two kinds of observable conformational isomers [9]. Species for which fluorescence intensity is strong and weak are the ones with the fluorophore inside and outside the cavity, respectively, as shown in Figure 1a. A self-inclusion state, in which the fluorophore is located in the interior of the CD cavity, is usually the predominant conformation in aqueous solution. An induced-fit conformational change in a fluorophore-modified CD may occur in conjunction with the accommodation of a guest, which displaces the fluorophore from the inside to the outside of the CD cavity; this displacement induces a change in the intensity of its fluorescence (Figure 1a). A fluorescent CD exhibits strong fluorescence in the self-inclusion state due to the hydrophobic environment of the CD cavity. The exclusion of the fluorophore from the cavity to the bulk water weakens its fluorescence intensity.
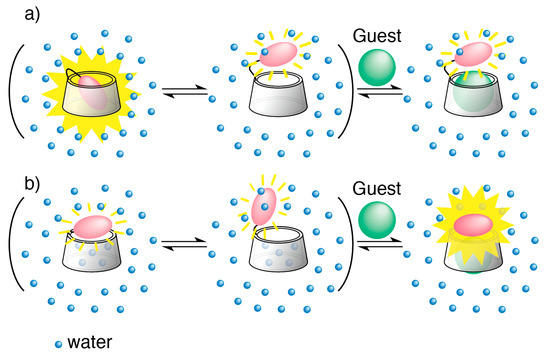
Figure 1.
Turn-off and turn-on chemosensors: (a) An equilibrium model for guest-induced conformational change in a conventional chromophore-modified cyclodextrin (turn-off CD chemosensor) in aqueous solution; (b) An equilibrium model for guest-induced conformational change in a new type of chromophore-modified cyclodextrin (turn-on CD chemosensor) in aqueous solution.
A turn-off mechanism is greatly effective for producing a detectable change in a signal upon guest accommodation into a CD cavity, however, it has several shortcomings. Firstly, a stable self-inclusion state of the turn-off CD chemosensor can inhibit the accommodation of a guest. For example, the self-inclusion state of N-dansyl-D-leucine-appended β-CD was twice as stable as that of N-dansyl-L-leucine-appended β-CD, and the binding ability of the former for a guest was about half that of the latter [9]. Secondly, the effect due to variations in a chromophore or spacer unit is not sufficiently significant to alter the selectivity of a turn-off CD chemosensor. The guest selectivity of a turn-off CD chemosensor mainly depends on the selectivity of the CD itself. For instance, the affinities of both β-CD and γ-CD for bile acid derivatives are greater than those for adamantane or borneol derivatives. Therefore, turn-off CD chemosensors cannot discriminate whether adamantanol occurs in a mixed solution of a bile acid and adamantanol or not. If chemosensors with different selectivities for guests could be obtained, chemical nose array sensing systems could be prepared [17,18]. Thirdly, although the cavity size of α-CD is suitable for small guests such as halomethanes or alkanols, it is difficult to use the turn-off mechanism to construct chemosensors using α-CD. There are only few chromophores whose size and shape are suitable for the self-inclusion state in the narrow cavity of α-CD. The chromophore in the self-inclusion state is often not excluded upon the addition of a guest, since the binding affinity of α-CD is not sufficient for most guests. Therefore, few chemosensors based on α-CDs have been reported, and their sensing properties have not been well studied. Finally, the detection of a guest is accompanied by a decrease in the fluorescence intensity of a turn-off CD chemosensor, however, an increase in the emission intensity caused by a guest response would be more effective for chemical sensing systems [19].
Therefore, in order to overcome the issues associated with the currently available turn-off CD chemosensors, new fluorescent chemosensors (NC0βCD, NC0γCD) were prepared (Figure 2) [20,21]. (7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amine (NBDamine) was selected as a fluorophore for the generation of this new type of chemosensors, as it possesses the interesting property of fluorescing weakly in water and strongly in organic solvents, membranes, or hydrophobic environments [22]. The NBDamine unit was directly connected to the CD framework without a spacer unit. The responses of these new chemosensors to guests mostly consisted of increases in fluorescence intensity. In other words, they served as turn-on CD chemosensors. A valuable α-CD chemosensor (NC0αCD) could also be constructed using this method, since it was not necessary for the fluorophore to be self-included [23,24]. The guest selectivities of NC0βCD were quite different from those of previous turn-off CD chemosensors. For example, the responses of NC0βCD to bile acid derivatives were quite small, while the fluorescence intensities of previous turn-off CD chemosensors were largely decreased upon the addition of bile acid derivatives [20]. Notably, the fluorescence intensity of NC0βCD was increased upon the addition of 1-adamantanol (1-AdOH) even in the presence of a bile acid, although the binding affinities of bile acid derivatives to native β-CD were over 10 times higher than that of 1-AdOH [20]. This is the first example of the detection of adamantanol in the presence of a bile acid by a CD-based chemosensor.
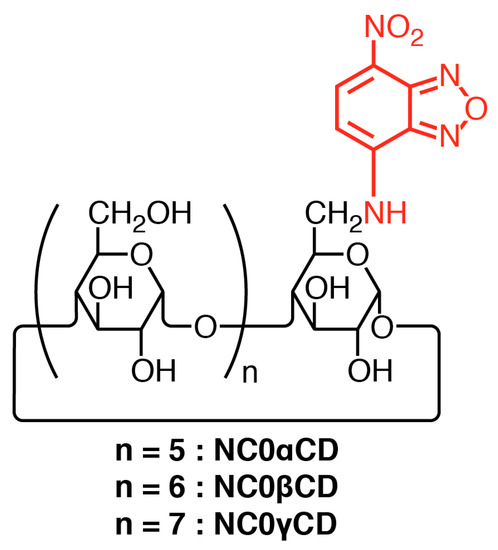
Figure 2.
Structures of NBDamine-modified cyclodextrins.
The mechanism of these new turn-on chemosensors remains unclear, although they have different properties to turn-off CD chemosensors. Here, we demonstrated the guest selectivities of a turn-on α-CD chemosensor (NC0αCD) in order to clarify the properties of this type of chemosensor using the same set of guests as those employed for NC0βCD and NC0γCD. The structure of the inclusion complexes of the turn-on CD chemosensors with guests were estimated using nuclear magnetic resonance (NMR) spectroscopy and MMFF94 molecular mechanics calculations, aiming to shed light on their mechanisms.
2. Materials and Methods
2.1. Materials
CDs were kindly donated by Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan), and were used without further purification. Reagents were purchased from Sigma-Aldrich Japan Co. (Tokyo, Japan), Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and FUJIFILM Wako Pure Chemical Co. (Osaka, Japan), and were used without further purification. Deuterium oxide for NMR measurements was obtained from Merck Co (Tokyo, Japan).
2.2. Measurements
Reverse phase HPLC was performed using a HPLC system comprising a L-7100 Intelligent Pump, D-7500 Chromato-Integrator and L-7400 UV-Vis Detector (Hitachi High-Tech Corporation (Tokyo, Japan)). 1H NMR spectra were measured on a Avance 600 (Bruker Japan (Yokohama, Japan)) and VXR-500S (Varian Japan (Tokyo, Japan) spectrometer operating at 600.13 and 499.843 MHz, respectively. HDO (δ = 4.70) was used as an internal standard. Matrix-assisted laser desorption/ionization and time-of-flight mass spectrometry (MALDITOF MS) was performed on a KRATOS KOMPACT MALDI III mass spectrometer (SHIMADZU CO. (Kyoto, Japan)) using α-cyano-4-hydroxycinnamic acid as matrix. Thin-layer chromatography (TLC, n-butanol–ethanol–water = 5:4:3, and conc. NH3(aq.)–ethyl acetate–2-propanol–water = 1:3:5:4) was carried out with silica gel F254 (Merck Co (Tokyo, Japan)). Absorption spectra were measured on a UV-Vis spectrophotometer UV-2550 (SHIMADZU CO. (Kyoto, Japan)). Fluorescence spectra were measured on a fluorescence spectrophotometer F-2500 (Hitachi High-Tech Corporation (Tokyo, Japan)).
2.3. Synthesis of NC0αCD, NC0βCD, and NC0γCD
The synthetic methods of NC0αCD, NC0βCD, and NC0γCD were already reported [20,23].
2.4. Molecular Mechanics Calculations
In order to elucidate the plausible structure of NC0αCD, NC0βCD, NC0γCD and their inclusion complexes, molecular mechanics calculations were performed using the ChemBio3D Ultra 16.0 (Perkin Elmer, 2016) software with an MMFF94 force field [25,26]. The molecular dynamics simulation and conformational search included in Chem3D Pro 16.0 were also used.
2.4.1. α-CD, β-CD, and γ-CD
The cyclic molecular structures of the α-CD, β-CD, and γ-CD were generated by the linking of six, seven, or eight units of α-D-glucopyranose (4C1 form), respectively. Energy minimizations of the CDs were performed by using MMFF94 molecular mechanics calculations. The obtained CD structures were highly regular, as all O4 atoms were almost coplanar, more so than the crystalline structures obtained by the X-ray and neutron diffraction data available at the Cambridge Structural Data Base [27]. We chose this highly symmetrical structure rather than the crystalline counterpart, as the latter was slightly distorted due to the hydration water present inside and outside the CD cavity. When a stable structure of an NBDamine-modified CD was estimated, its dependence on the selection of the glucose unit which was modified by the NBDamine unit could be avoided by using the symmetric CD structure.
2.4.2. Ursodeoxycholic Acid (UDCA)
The aliphatic side chain of ursodeoxycholic acid (UDCA) consists of four C-C bonds and the number of conformers is, at minimum, 34 = 81. The number of conformers for linear alkanes is generally greater than 3n [28].
A stable structure was obtained by combining the conformational search and the MMFF94 molecular mechanics calculations. The obtained structure was similar to the crystalline structure that was obtained from X-ray analysis [27].
3. Results and Discussion
3.1. Guest Selectivities of NC0αCD, NC0βCD, and NC0γCD
Previously reported data about guest selectivities of NC0βCD, and NC0γCD were summarized and new data about guest selectivities of NC0αCD were shown. The absorption intensities of NC0αCD, NC0βCD, and NC0γCD were decreased by increasing the concentration of guests. This result indicates that the guest increased the hydrophobicity near the NBDamine moiety of the host, because the molar absorption coefficient (ε) of NBD derivatives rises with increasing solvent polarity. The isosbestic points were observed at 495, 510, and 500 nm for NC0αCD, NC0βCD, and NC0γCD respectively. This suggests the formation of a 1:1 inclusion complex. The wavelength at the isosbestic point was chosen as the excitation wavelength for the fluorescence measurement.
The guest selectivities of NC0αCD, NC0βCD, and NC0γCD are shown in Figure 3 as the sensitivity parameters expressed by ΔI/I0 (where ΔI = I − I0, with I and I0 being the fluorescence intensities in the presence and absence of the guest, respectively). The guest structures are shown in Figure 4. The response of NC0βCD to the selected guests was quite different from that of previous turn-off CD chemosensors. NC0βCD exhibited a large increase in fluorescence intensity upon the addition of 1-adamantanol (1-AdOH) and borneol (Bor) derivatives, which have a comparatively spherical shape that fits the β-CD cavity. This indicates that the accommodation of 1-AdOH led to an increase in hydrophobicity nearby the NBDamine unit. The fluorescence intensity of NC0βCD slightly decreased upon the addition of bile acid derivatives, although the binding affinities of bile acid derivatives to native β-CD were over 10 times larger than that of 1-AdOH [14]. The responses of previous turn-off CD chemosensors to bile acid derivatives consisted of large decreases in fluorescence intensity.
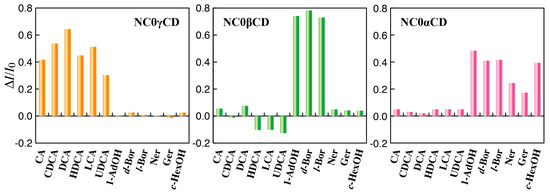
Figure 3.
Sensitivity parameters (ΔI/I0) of NC0αCD, NC0βCD, and NC0γCD for various guests in phosphate buffer (200 mM, pH 7.0) at 25 °C: [NC0αCD] = [NC0βCD] = [NC0γCD] = 5 × 10−6 M, [guest] = 1 × 10−5 M, ΔI/I0 = (I − I0)/I0 (where I and I0 are the fluorescence intensities in the presence and absence of a guest, respectively). The excitation wavelength was 495, 510, and 500 nm for NC0αCD, NC0βCD, and NC0γCD, respectively. The emission wavelength was 550, 570, and 566 nm for NC0αCD, NC0βCD, and NC0γCD, respectively.
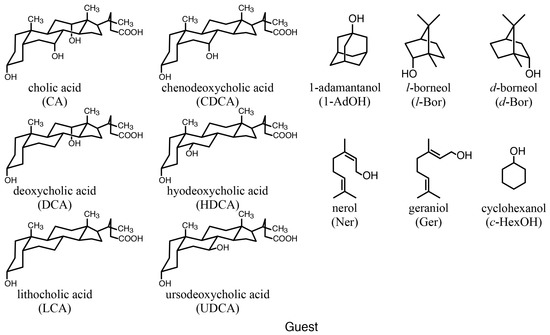
Figure 4.
Structures of guests.
The line width of the 1H resonances for the NBD unit changed with the addition of both 1-AdOH and ursodeoxycholic acid (UDCA) (Figure 5). Upon the addition of 1-AdOH and UDCA, the 1H resonances for the NBD unit broadened and sharpened, respectively. This suggests that both 1-AdOH and UDCA interacted with NC0βCD. This change suggested that the motion of the NBD unit was restricted and not restricted, after forming an inclusion complex with 1-AdOH and UDCA, respectively. The motion of the NBD unit of NC0βCD might be restricted even in the absence of a guest based on comparison of the line width of the 1H resonances for the NBD unit in the absence and presence of UDCA.
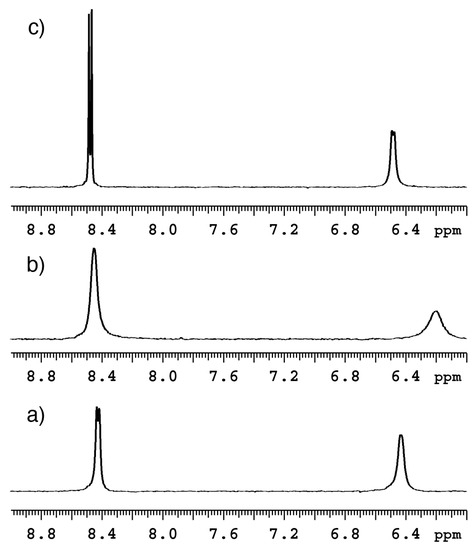
Figure 5.
1H NMR spectra of NC0βCD; (a) alone and in the presence of (b) 1-AdOH or (c) UDCA showing the region of the NBD protons; [NC0βCD] = [1-AdOH] = [UDCA] = 5 × 10−4 M (Adapted from [20] with permission from the Royal Society of Chemistry).
On the other hand, NC0γCD responded only to bile acid derivatives with large increases in fluorescence intensity (Figure 3).
The guest selectivities of NC0αCD about halomethanes and cyclic and acyclic alcohols were previously provided [23,24]. The new data about the guest selectivities of NC0αCD about the same set of guests as those employed for NC0βCD and NC0γCD were investigated in order to clarify properties of this new type of chemosensor. The fluorescence intensity of NC0αCD significantly increased upon the addition of 1-AdOH, Bor derivatives, and cyclohexanol (c-HexOH). The responses of previous turn-off CD chemosensors based on the β-CD framework to Bor derivatives and c-HexOH led to a slight decrease in fluorescence intensity. The new turn-on mechanism could be applied to the α-CD framework for generating an α-CD chemosensor, which could sensitively detect small molecules. It should be noted that the synthesis of α-CD chemosensor cannot be realized using the previously described turn-off mechanism and small molecules could not be detected. Since the guest selectivities of the newly developed turn-on CD chemosensors are quite different from those of turn-off CD chemosensors, it is necessary to determine their mechanism from the standpoint of the structure of their inclusion complexes of the new turn-on CD chemosensors with the guests.
3.2. The Structure of NC0βCD
At first, the stable structure of NC0βCD was studied. There are three rotatable bonds between the β-CD framework and the NBD unit, i.e., the C5-C6 bond of the glucose unit, C6-NH bond, and NH-NBD bond (Figure 6). Three local-minimum conformers existed about each bond and the number of total local-minimum conformer was at least 27. The key conformer that can change the location between the β-CD framework and NBD unit was the C5-C6 bond, i.e., gt, gg, and tg conformers (Figure 7a). The initial structures for molecular mechanics calculations were obtained by rotating the dihedral angles about the C6-NH and HN-NBD bond by 30° intervals from 0° to 360°, after the dihedral angle about the C5-C6 bond was rotated to give the gt, gg, or tg conformer. All obtained initial structures were subjected to energy minimization by MMFF94 molecular mechanics calculations.
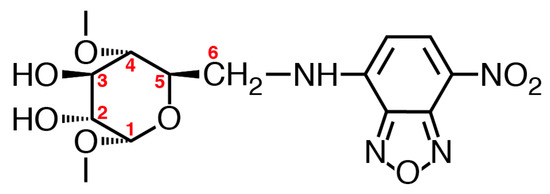
Figure 6.
Structure of the NBDamine-modified glucose unit.
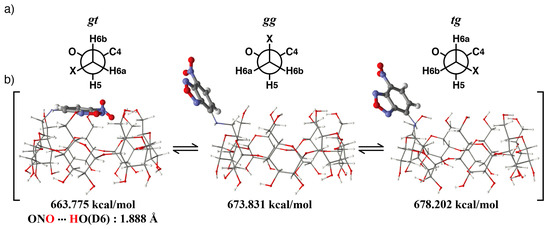
Figure 7.
Estimated conformational equilibrium of NC0βCD in the gt, gg, and tg conformers. (a) Three kinds of conformers about the C5-C6 bond, (b) Estimated structures of NC0βCD in the gt, gg, and tg conformers.
The most stable structures for each conformer about the C5-C6 bond are shown in Figure 7. For the gt conformer, the primary hydroxy side was covered by the NBD unit. The nitro group of the NBD unit formed a hydrogen bond with the primary hydroxy group of the β-CD. The distance between the oxygen atom of the nitro group and the hydrogen atom of the primary hydroxy group (D6) was 1.89 Å, which is smaller than the sum of the van der Waals radii of an oxygen atom and hydrogen atom (2.04 Å). This interaction might stabilize the structure of NC0βCD at the gt conformer, thus causing the broadening of the 1H resonances of the NBD unit. In the case of the gg and tg conformers, the structures in which the primary hydroxy sides were covered by the NBD unit were not stable. It was necessary to either distort the CD framework or take the eclipsed form of the C6-NH bond in order to cover the primary hydroxy sides with the NBD unit. The ratio of the gt, gg, and tg conformers was 33.8:33.2:33.0 based on a Boltzmann distribution.
3.3. Structure of the βCD/UDCA Complex
The NC0βCD/UDCA complex was examined as an example in order to determine the mechanism of these chemosensors. Before estimating the structure of the inclusion complex of NC0βCD with UDCA, the structure of the inclusion complex consisting of the native β-CD and UDCA was constructed using restraints derived from the ROESY spectra and MMFF94 molecular mechanics calculations. Since UDCA could enter into the cavity of the β-CD on the side of the secondary hydroxy groups, either with the A-ring of the steroid nucleus or the aliphatic side chain, two types of inclusion complexes were possible (Figure 8). The optimized structure of UDCA obtained by the method shown in the experimental section was used for the estimation of its inclusion complex. The aliphatic side chain or the A-ring of UDCA was initially turned near the secondary hydroxy side of the β-CD and UDCA was placed on the C7 axis of the β-CD. The position of UDCA on the C7 axis was slightly changed until a minimum interaction energy was found. This position was taken as the starting point to minimize the complex. UDCA was then slightly tilted against the C7 axis in all directions and all obtained initial structures were subjected to energy minimization by the MMFF94 molecular mechanics calculations. A large angle tilt was not used to escape the distortion of the β-CD framework during the MMFF94 molecular mechanics calculations. UDCA was also rotated around the C7 axis by 30° intervals, and all obtained initial structures were subjected to energy minimization. MMFF94 molecular mechanics calculations suggested that the complex in which the carboxy group of UDCA was directed towards the primary hydroxy side of the β-CD was more stable than the other complex (Figure 9). The carboxy group of UDCA formed hydrogen bonds with two primary hydroxy groups of the β-CD. The distance between the hydrogen atom of the carboxy group and the oxygen atom of the primary hydroxy group (C6) was 1.72 Å, while the distance between the oxygen atom of the carbonyl group and the hydrogen atom of the primary hydroxy group (F6) was 1.89 Å in the more stable complex (A). The hydroxy group of the A-ring of UDCA also formed hydrogen bonds with a secondary hydroxy group (E2) of the β-CD in the case of more stable complex (A). The hydroxy group of the A-ring of UDCA weakly interacted with the primary hydroxy groups of the β-CD, while the carboxy group of UDCA formed a hydrogen bond with the secondary hydroxy group (F3) of β-CD in the case of the less stable complex (B). The distances between the hydrogen atom of the hydroxy group of the A-ring of UDCA and the oxygen atom of the primary hydroxy group (E6) were 2.46 Å. The distance between the oxygen atom of the hydroxy group of the A-ring of UDCA and the hydrogen atom of the primary hydroxy group (E6) was 2.08 Å. The hydroxy group of the A-ring could not come close to a primary hydroxy group of the β-CD due to steric hindrance. The distance between the hydrogen atom of the carboxy group of UDCA and the oxygen atom of the secondary hydroxy group (F3) was 1.70 Å.
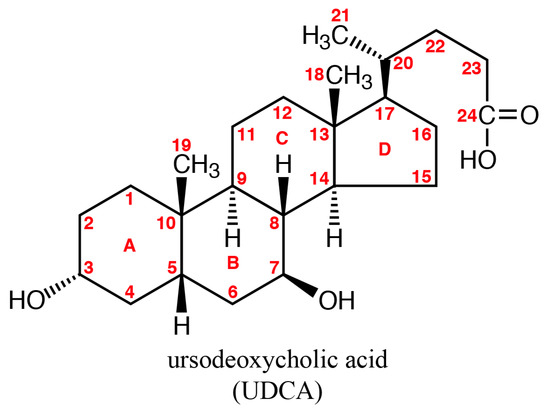
Figure 8.
Structure of ursodeoxycholic acid (UDCA).
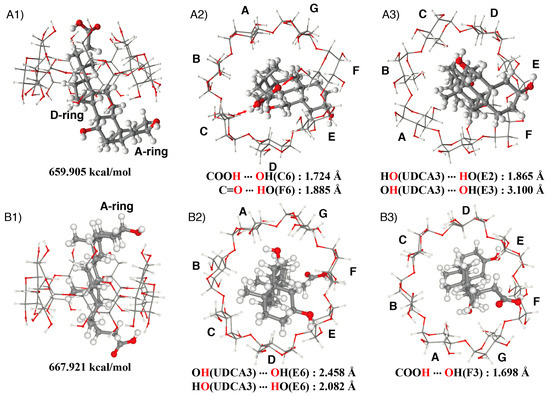
Figure 9.
Estimated structure of the β-CD/UDCA complex; (A) the UDCA enters on the side of the aliphatic side chain: (A1) side view, (A2) view from the primary hydroxy side, (A3) view from the secondary hydroxy side; (B) the UDCA enters on the side of the A-ring: (B1) side view, (B2) view from the primary hydroxy side, (B3) view from the secondary hydroxy side.
This estimated structure was supported with ROESY data (Figure 10). The ROESY spectrum indicated that the H-21, H-22, H-15, and H-16 of UDCA were closer to the H-5 of the β-CD (the primary hydroxy side), whereas the H-18, H-15, H-16, and H-12 of UDCA were closer to the H-3 of the β-CD (the secondary hydroxy side). Since the H-15 and H-16 of UDCA were located near the center of the β-CD cavity, NOE signals of both H-15 and H-16 of UDCA with both H-3 and H-5 of the β-CD were observed. The NOE signals of the H-15 and H-16 of UDCA with H-3 of the β-CD were larger than those with the H-5 of the β-CD. Although the difference between their calculated energies was small and two types of the complexes could exist in a solution, the ROESY data clearly indicated that complex (A) was the main species, while complex (B) is the minor species.
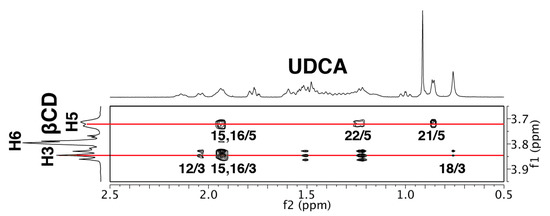
Figure 10.
ROESY spectrum of the β-CD/UDCA complex in D2O with mixing time of 300 ms.
3.4. Structure of the NC0βCD/UDCA Complex
Stable structures of the NC0βCD/UDCA complex were obtained by a similar method to that employed for the βCD/UDCA complex. Since the primary hydroxy side was not covered by the NBD unit in the gg and tg conformers of NC0βCD, UDCA could easily generate a stable complex with NC0βCD, similar to its inclusion complex with native β-CD. The estimated stable structure of the inclusion complex of the gg conformer of NC0βCD with UDCA is shown in Figure 11B as an example. On the other hand, UDCA could not directly form a stable complex with NC0βCD in the stable gt conformer because the primary hydroxy side was covered by the NBD unit. The gt conformer of NC0βCD was also in equilibrium with many other conformers. The rotation around the C6-NH bond could move the NBD unit away from the primary hydroxy side, and UDCA could form a stable complex with NC0βCD, as shown in Figure 11A. Since the binding constant of NC0βCD for UDCA was over ten thousand, the NBD unit could not easily return to the primary hydroxy side.
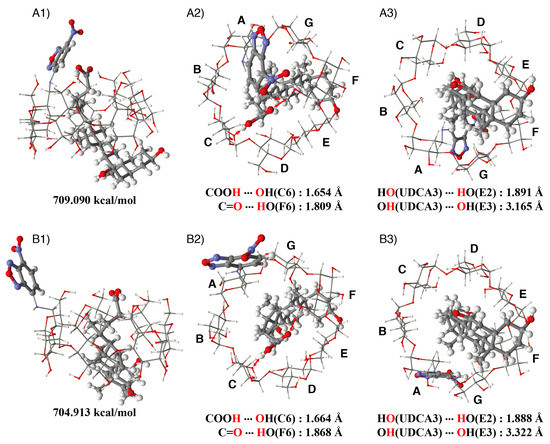
Figure 11.
Estimated structure of the NC0βCD/UDCA complex; (A) gt conformer: (A1) side view, (A2) view from the primary hydroxy side, (A3) view from the secondary hydroxy side; (B) gg conformer: (B1) side view, (B2) view from the primary hydroxy side, (B3) view from the secondary hydroxy side.
The NBDamine units of the NC0βCD/UDCA complex in both the gt and gg conformers were located in a hydrophilic environment and the fluorescence intensity of NC0βCD did not increase after the formation of the inclusion complex. The sensitivity parameter of NC0βCD for UDCA was slightly minus (Figure 3). This indicates that the primary hydroxy side of NC0βCD was slightly more hydrophobic than the bulk water.
3.5. Structure of the NC0γCD/UDCA Complex
The stable structure of the NC0γCD/UDCA complex was estimated by MMFF94 molecular mechanics calculations (Figure 12). The carboxy group could interact with the primary hydroxy groups, even in the stable gt conformer, because the diameter of the γ-CD cavity was larger than that of the β-CD cavity. The oxygen atom of the carbonyl group of UDCA formed hydrogen bonds with the hydrogen atoms of the primary hydroxy groups (D6 and E6). The hydrogen atom of the carboxy group of UDCA formed a hydrogen bond with the oxygen atom of the nitro group of the NBD unit. The distances between the oxygen atom of the carbonyl group and the hydrogen atoms of the primary hydroxy groups (D6 and E6) were 1.82 and 1.94 Å, respectively. The distance between the hydrogen atom of the carboxy group and the oxygen atom of the nitro group was 1.69 Å. This interaction stabilized the inclusion complex and restricted the motion of the NBDamine unit. The accommodation of UDCA could exclude water molecules from the γ-CD cavity and inhibit the entrance of water from the secondary hydroxy side, resulting in an increase in the hydrophobicity around the NBDamine unit, as well as in the fluorescence intensity. The restriction of the motion of the NBD unit by the hydrogen bond might contribute to the increase in the fluorescence intensity.
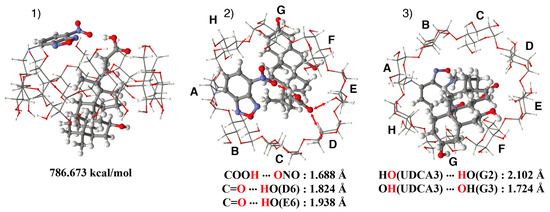
Figure 12.
Estimated structure of the NC0γCD/UDCA complex (1) side view, (2) view from the primary hydroxy side, and (3) view from the secondary hydroxy side.
3.6. Structures of the NC0βCD/1-AdOH and NC0αCD/1-AdOH Complexes
Figure 13 shows the stable structures of the complexes of 1-AdOH with NC0βCD and NC0αCD estimated by MMFF94 molecular mechanics calculations. In the complexes of 1-AdOH with NC0βCD and NC0αCD in the gt conformers, the NBD unit covered the primary hydroxy side, which was the most stable conformer for NC0βCD or NC0αCD itself. The accommodation of 1-AdOH excluded the water molecules from the β-CD or α-CD cavity, and inhibited their entrance from the secondary hydroxy side, resulting in an increase in the hydrophobicity around the NBDamine unit as well as an increase in the fluorescence intensity.
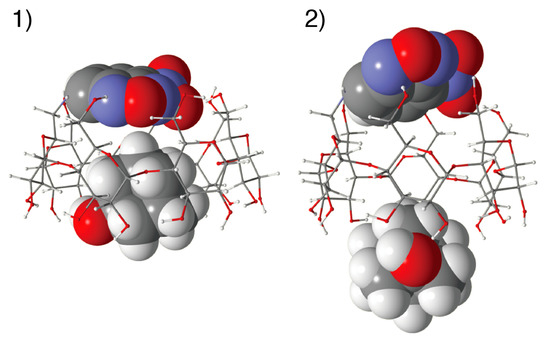
Figure 13.
Estimated structure of (1) the NC0βCD/1-AdOH and (2) NC0αCD/1-AdOH complex.
The changes of the line width of the 1H resonances for the NBD unit of NC0βCD and NC0αCD upon the addition of 1-AdOH suggested that the motion of the NBD unit was restricted by the formation of inclusion complexes with 1-AdOH (Figure 5 and Figure 14). Since the accommodation of 1-AdOH to NC0αCD was quite shallow, and the NBD unit could not directly interact with 1-AdOH, the reason for the motion restriction of the NBD unit was not its direct interaction with 1-AdOH. The structure of the CD framework is not rigid but flexible, and it exists as equilibrium between many types of conformers; moreover, the conformational interconversion occurs too rapidly to be followed by NMR spectroscopy. This fluctuation causes fluctuations in the NBD unit, thus reducing its fluorescence intensity.
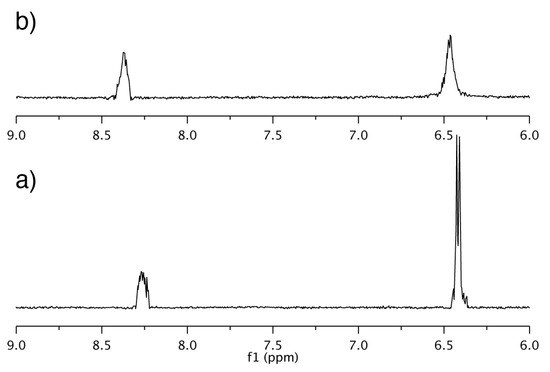
Figure 14.
1H NMR spectra of NC0αCD; (a) alone and (b) in the presence of 1-AdOH showing the region of NBD protons; [NC0αCD] = [1-AdOH] = 5 × 10−4 M.
1-AdOH has a spherical shape and could fix the CD cavity as a Cn symmetrical structure. Even if 1-AdOH spun in the cavity, the conformational change in the CD framework was quite small. This reduction in fluctuations in the CD framework could reduce the fluctuations in the NBD unit and contribute to the large increase in the fluorescence intensity of NC0αCD upon the addition of 1-AdOH, although the accommodation of 1-AdOH was quite shallow. Since the cavity of NC0γCD was too large to lead to the formation of a stable inclusion complex with 1-AdOH, NC0γCD showed no response to 1-AdOH. The conventional turn-off γ-CD chemosensors also did not respond to 1-AdOH.
4. Conclusions
Although the solvent effects were not considered in the chemosensor-guest complexation process, MMFF94 molecular mechanics calculations aided in shedding light on the mechanism of new turn-on CD chemosensors. This indicated that energy minimized interactions between chemosensor and guest essentially governed a bimodal complexation process. In the case of UDCA, the formation of hydrogen bonds by the carboxy group of UDCA is important for generating stable inclusion complexes with a chemosensor. The NBD unit of NC0βCD was moved away from the primary hydroxy side in order to form stable inclusion complex with UDCA. On the other hand, the primary hydroxy side remained covered by the NBD unit of NC0γCD, and the carboxy group could interact with the nitro group of the NBD unit and the primary hydroxy groups of NC0γCD, thus UDCA could afford a stable inclusion complex with NC0γCD. These structural differences between the NC0βCD and NC0γCD complexes led to different changes in their fluorescence intensity upon the accommodation of UDCA into the CD cavity. Although 1-AdOH was too large to be suitable for making a stable inclusion complex with α-CD, the fluorescence intensity of NC0αCD significantly increased upon the addition of 1-AdOH. This new finding suggests that even shallow accommodation can support detection of the guest. It was also suggested that the motion restriction of the NBD unit was important to increase the fluorescence intensity, although it was not clear how much the reduction in fluctuation in the NBD unit contributed to increases in the fluorescence intensity. This new mechanism is expected to assist in the design of alternative chemosensors with a diverse responsiveness to many types of guests.
Funding
This work was supported by JSPS KAKENHI Grant Number JP18550120.
Conflicts of Interest
The author declares no conflict of interest.
References
- Czarnik, A.W. Fluorescent Chemosensors for Ion and Molecule Recognition; American Chemical Society: Washington, DC, USA, 1993. [Google Scholar]
- Desvergne, J.P.; Czarnik, A.W. Chemosensors of Ion and Molecule Recognition; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.; Anslyn, E.V. Sensing A Paradigm Shift in the Field of Molecular Recognition: From Selective to Differential Receptors. Angew. Chem. Int. Ed. 2001, 40, 3118–3130. [Google Scholar] [CrossRef]
- Mirsky, V.M.; Yatsimirsky, A.K. Artificial Receptors for Chemical Sensors; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- You, L.; Zha, D.; Anslyn, E.V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Suzuki, I.; Osa, T. Host–guest sensory system for detecting a variety of organic compounds by variations in pyrene excimer and monomer fluorescence intensities. Chem. Lett. 1989, 18, 1059–1062. [Google Scholar] [CrossRef]
- Ikeda, H.; Nakamura, M.; Ise, N.; Oguma, N.; Nakamura, A.; Ikeda, T.; Toda, F.; Ueno, A. Fluorescent cyclodextrins for molecule sensing: Fluorescent properties, NMR characterization, and inclusion phenomena of N-dansylleucine-modified cyclodextrins. J. Am. Chem. Soc. 1996, 118, 10980–10988. [Google Scholar] [CrossRef]
- Ogoshi, T.; Harada, A. Chemical sensors based on cyclodextrin derivatives. Sensors 2008, 8, 4961–4982. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H. Fluorescent Cyclodextrins as chemosensors for molecule detection in Water. In Artificial Receptors for Chemical Sensors; Mirsky, V.M., Yatsimirsky, A.K., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 113–134. [Google Scholar]
- Ikeda, H. Chemosensors for water contaminants based on chromophore-appended cyclodextrins. In Environmental Chemistry for a Sustainable World; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 17, pp. 125–148. [Google Scholar]
- Szejtli, J. Cyclodextrin Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Szejtli, J.; Osa, T. Comprehensive Supramolecular Chemistry; Elsevier: Eastbourne, UK, 1996; Volume 3. [Google Scholar]
- Dodziuk, H. Cyclodextrins and Their Complexes-Chemistry, Analytical Methods, Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.L.; Anslyn, E.V. Array sensing using optical methods for detection of chemical and biological hazards. Chem. Soc. Rev. 2013, 42, 8596–8611. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Peveler, W.J.; Rotello, V.M. Array-based “chemical nose” sensing in diagnostics and drug discovery. Angew. Chem. Int. Ed. 2019, 58, 5190–5200. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraju, S.; Umadevi, D.; González-Barcia, L.M.; Delente, J.M.; Byrne, K.; Schmitt, W.; Watson, G.W.; Gunnlaugsson, T. “Turn-on” fluorescence sensing of volatile organic compounds using a 4-amino-1,8-naphthalimide Tröger’s base functionalised triazine organic polymer. Chem. Commun. 2019, 55, 12140–12143. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Murayama, T.; Ueno, A. Skeleton-selective fluorescent chemosensor based on cyclodextrin bearing a 4-amino-7-nitrobenz-2-oxa-1,3-diazole moiety. Org. Biomol. Chem. 2005, 3, 4262–4267. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Murayama, T.; Ueno, A. Bile acids-selective chemosensors based on NBD-amine-modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 101–105. [Google Scholar] [CrossRef]
- Uchiyama, S.; Santa, T.; Okiyama, N.; Fukushima, T.; Imai, K. Fluorogenic and fluorescent labeling reagents with a benzodfurazan skeleton. Biomed. Chromatogr. 2001, 15, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Ueno, A. Fluorescent α-cyclodextrin as a chemosensor for halomethanes. Chem. Commun. 2009, 4281–4283. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H. Fluorescent cyclodextrins as chemosensors for the detection of cyclic and acyclic alcohols. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 71–75. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J. Comput. Chem. 1996, 17, 520–552. [Google Scholar] [CrossRef]
- Crystal Structure Database; The Cambridge Crystallographic Data Centre: Cambridge, UK, 1965.
- Gotō, H.; Ōawa, E.; Yamato, M. How many conformers are there for small n-alkanes? Consequences of asymmetric deformation in GG’ segment. Tetrahedron 1993, 49, 387–396. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).