Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production
Abstract
1. Introduction
2. Applications of Voltammeric Biosensors in Wine Production
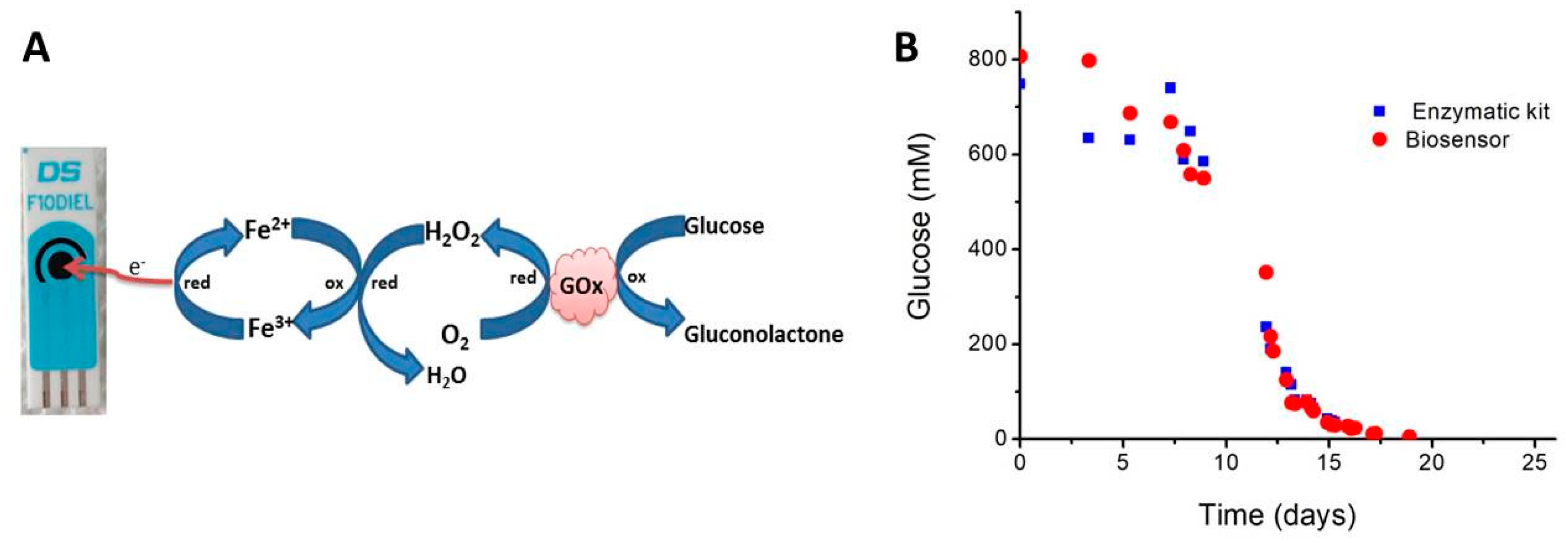
2.1. Biosensors for Monitoring Alcoholic Fermentation
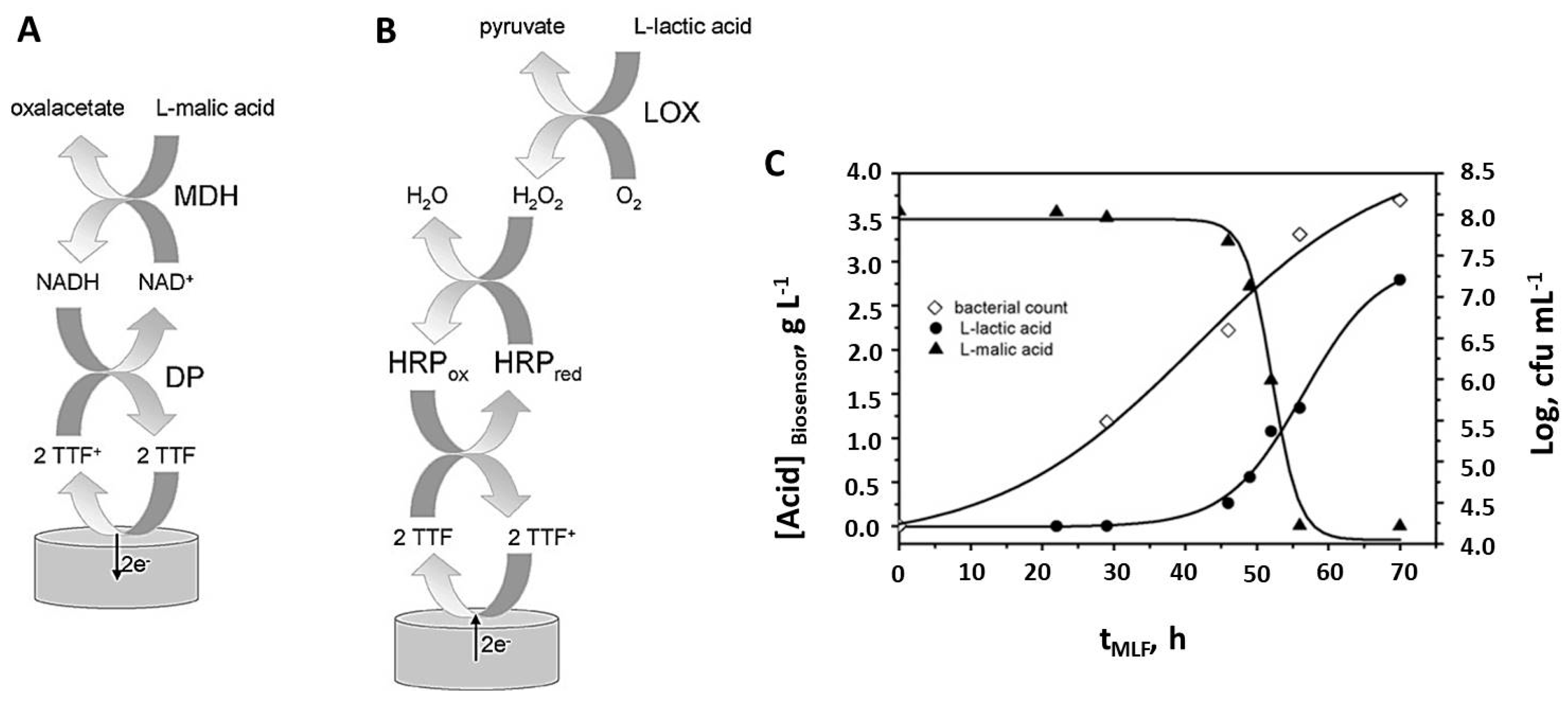
2.2. Biosensors for Monitoring the Malolactic Fermentation
2.3. Biosensors for Phenolic Compounds and Antioxidant Capacity
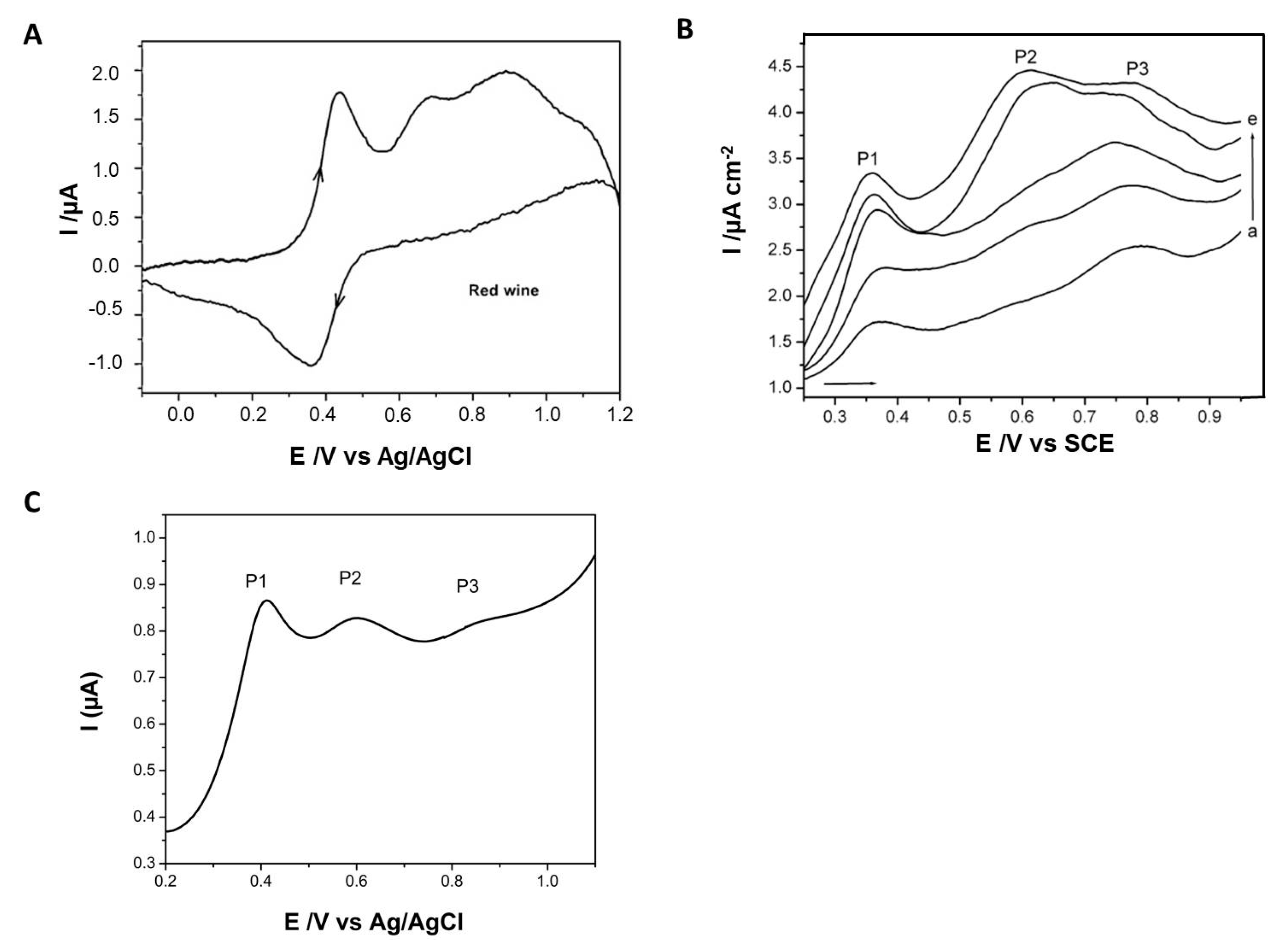
2.3.1. Biosensors for the Quantitative Determination of Phenolic Compounds
Direct Oxidation of Phenolic Compounds from Wines on Bare or Chemically/Nanomaterial-Modified Electrodes
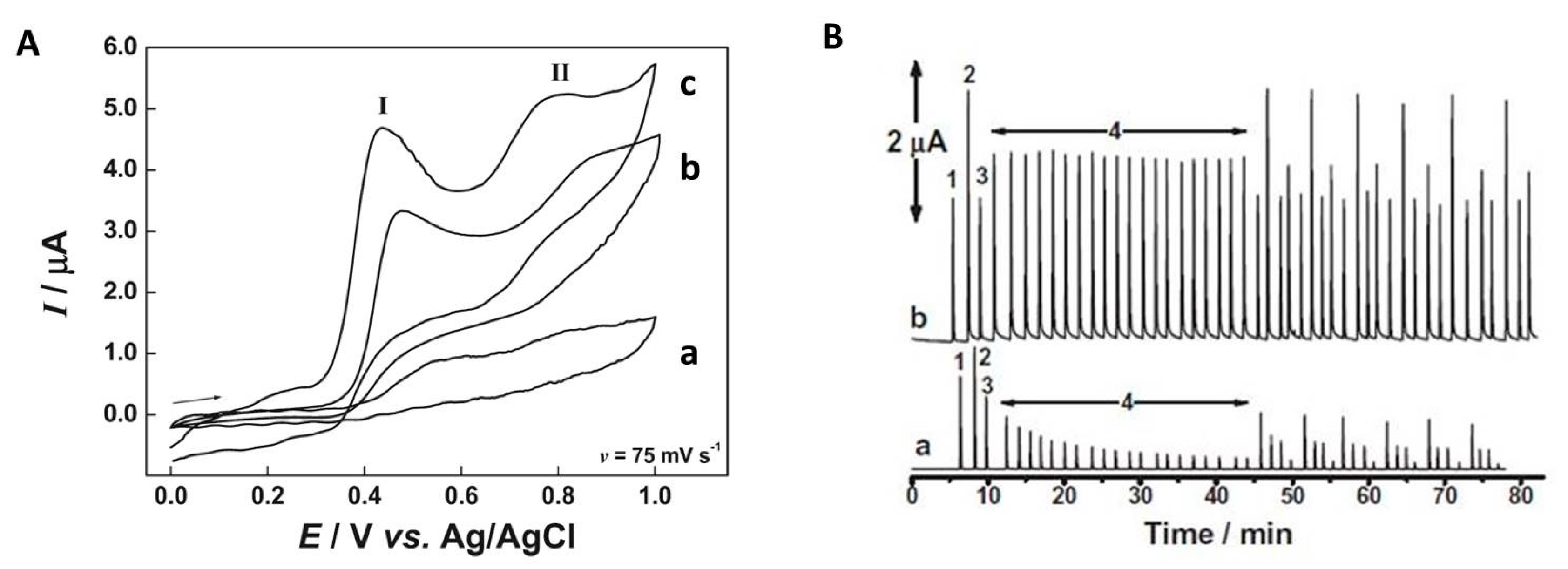
Enzyme-Mediated Amperometric Detection
- (1)
- Phenol + O2 o-quinone + H2OLaccase catalyzes the oxidation of substituted mono- and polyphenols, aromatic amines and thiol compounds, leading to phenoxy radicals that can be further oxidized to quinones. In the same reaction, oxygen is reduced to water [88]:
- (2)
- AH2 + O2 A + H2O, where AH2 and A represent the reduced and oxidized form of the polyphenolic compound, respectively.Horseradish peroxidase (HRP) acts as a catalyst in the oxidation of phenols in the presence of hydrogen peroxide.
- (3)
- Phenol red + H2O2 Phenol ox + 2 H2OThe phenoxyradicals and the quinones formed in the enzymatic reactions can be reduced electrochemically on the surface of a suitably polarized electrode and the magnitude of the reduction current is proportional to the amount of phenolic compounds in the sample. Alternatively, the oxygen or hydrogen peroxide consumed in the reaction can be determined electrochemically.
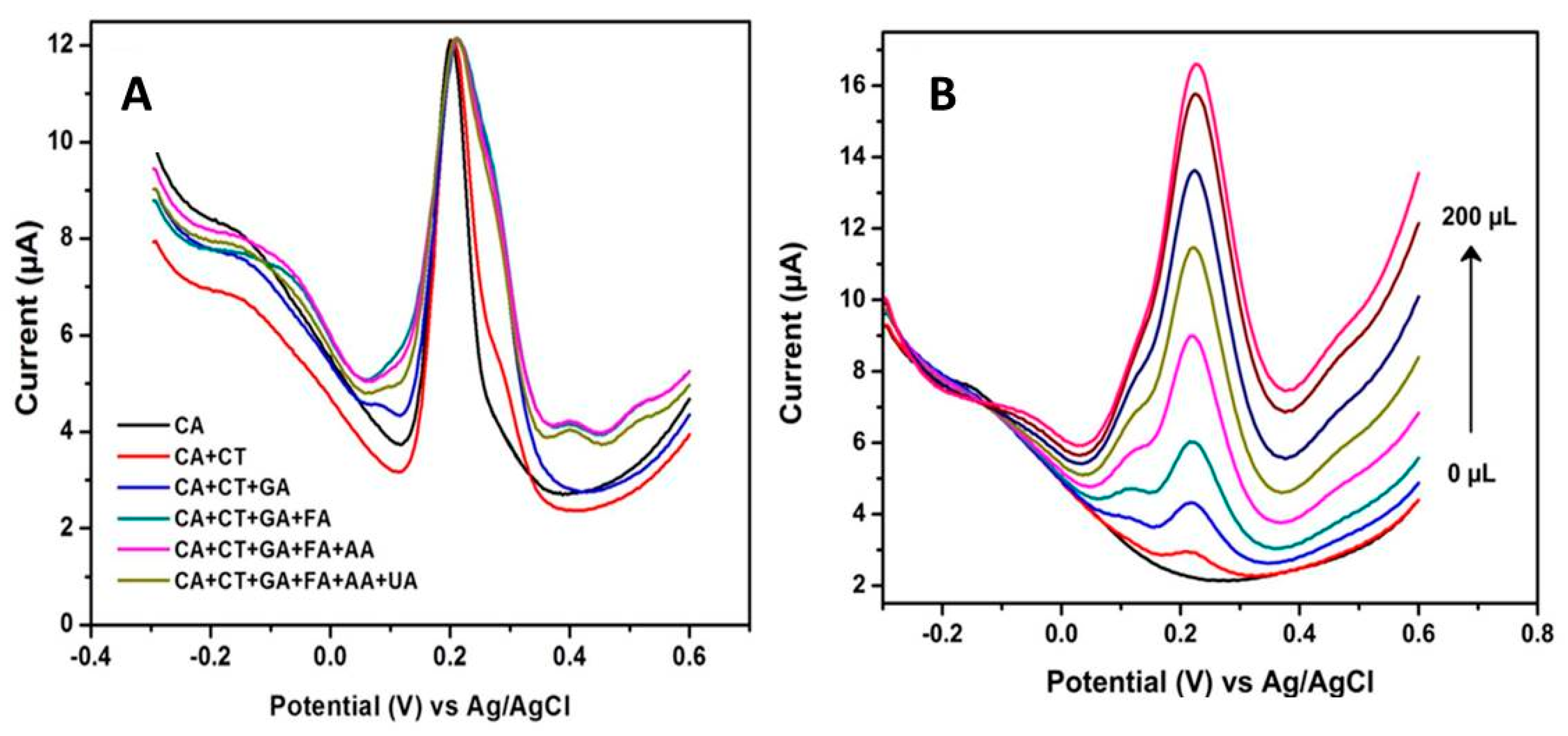
Nanomaterial–Enabled Biomimetic Detection of Polyphenolic Compounds
2.3.2. Biosensors for the Total Antioxidant Capacity (TAC)
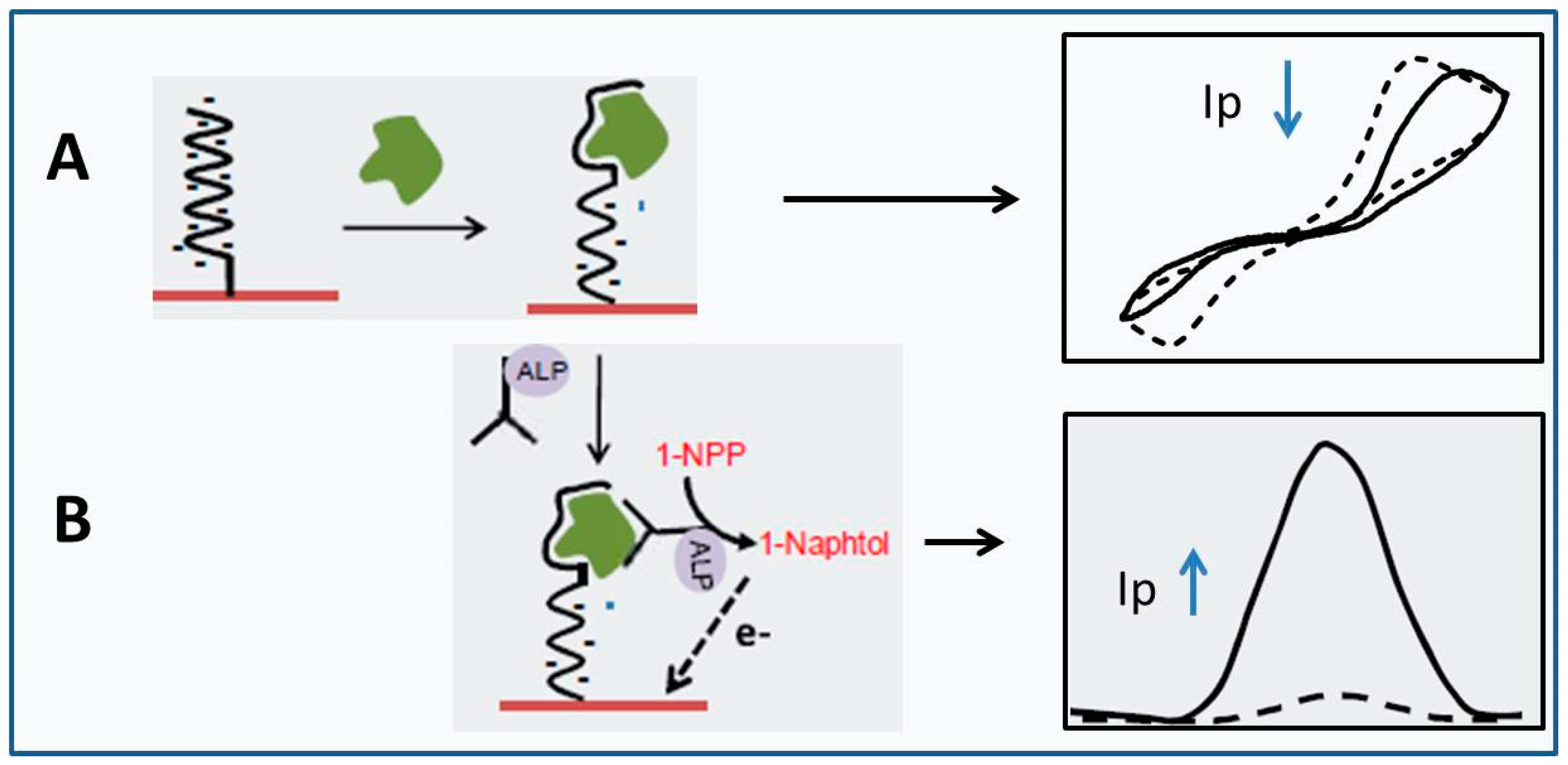
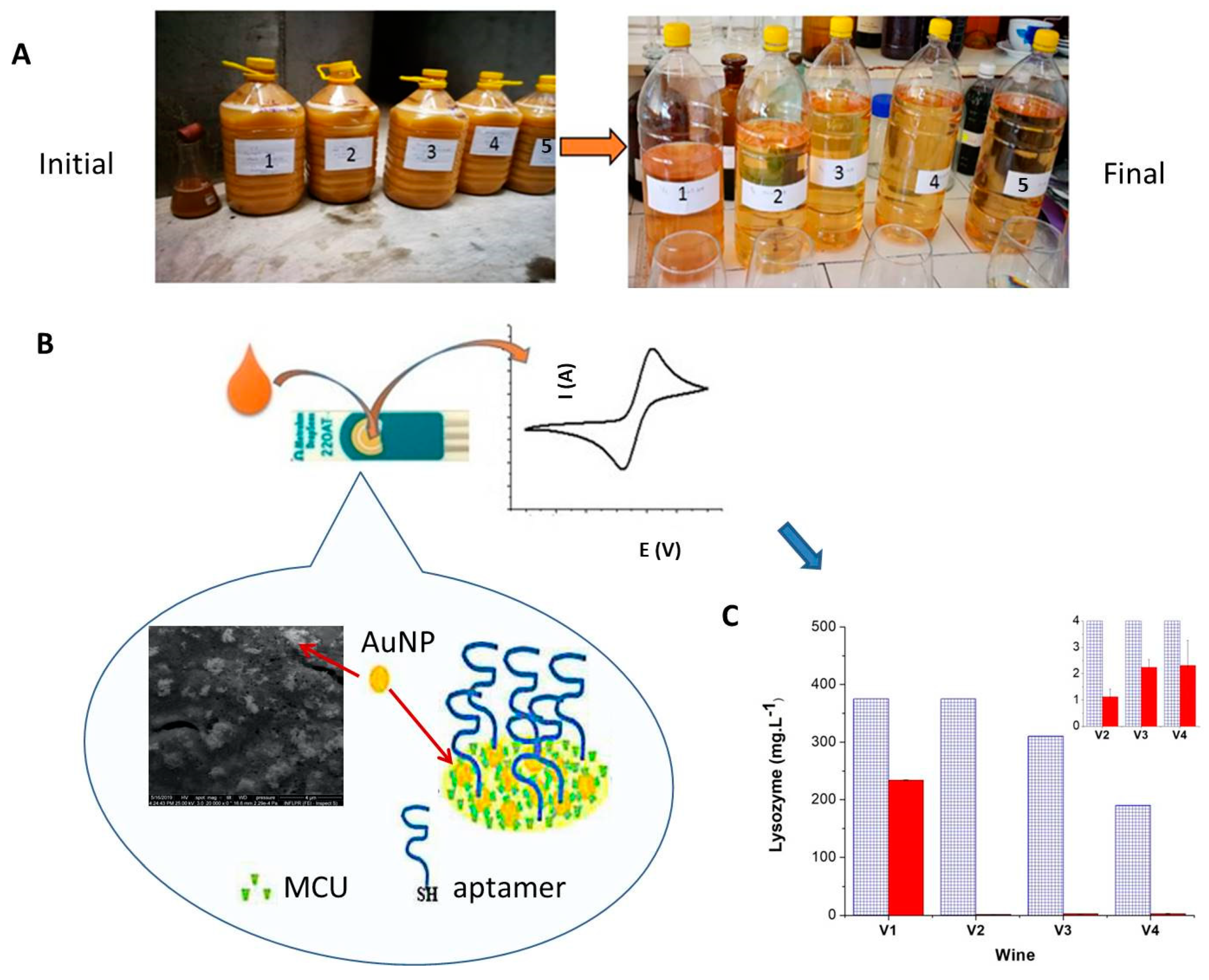
2.4. Biosensors for Allergens
2.5. Commercial Electrochemical Biosensors for Wine Monitoring
2.6. Case Study: A Biosensor-Based System for Monitoring Wine Fermentation
3. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Jiao, Q.; Zhang, C.; Zuo, X.; Xiao, X.; Liang, Y.; Nan, J. Amperometric nonenzymatic determination of glucose based on a glassy carbon electrode modified with nickel(II) oxides and graphene. Microchim. Acta 2013, 180, 477–483. [Google Scholar]
- Ampelli, C.; Leonardi, S.G.; Genovese, C.; Lanzafame, P.; Perathoner, S.; Centi, G.; Neri, G. Monitoring of glucose in fermentation processes by using Au/TiO2 composites as novel modified electrodes. J. Appl. Electrochem. 2015, 45, 943–951. [Google Scholar] [CrossRef]
- Sánchez Arribas, A.; Martínez-Fernández, M.; Moreno, M.; Bermejo, E.; Zapardiel, A.; Chicharro, M. Analysis of total polyphenols in wines by FIA with highly stable amperometric detection using carbon nanotube-modified electrodes. Food Chem. 2013, 136, 1183–1192. [Google Scholar]
- Kilmartin, P.A. Electrochemistry applied to the analysis of wine: A mini-review. Electrochem. Commun. 2016, 67, 39–42. [Google Scholar] [CrossRef]
- De Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Roginsky, V.; Barsukova, T.; Adams, D.O.; Waterhouse, A.L. Phenolics: A Comparison of Diverse Analytical Methods. Am. J. Enol. Vitic. 2004, 55, 389–400. [Google Scholar]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. Correlation of Wine Phenolic Composition versus Cyclic Voltammetry Response. Am. J. Enol. Vitic. 2002, 53, 294–302. [Google Scholar]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef]
- Schneider, M.; Türke, A.; Fischer, W.J.; Kilmartin, P.A. Determination of the wine preservative sulphur dioxide with cyclic voltammetry using inkjet printed electrodes. Food Chem. 2014, 159, 428–432. [Google Scholar] [CrossRef]
- Titoiu, A.M.; Porumb, R.; Fanjul-Bolado, P.; Epure, P.; Zamfir, M.; Vasilescu, A. Detection of Allergenic Lysozyme during Winemaking with an Electrochemical Aptasensor. Electroanalysis 2019, 31, 2262–2273. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Negulescu, G.P.; Pisoschi, A. Determination of ascorbic acid content of some fruit juices and wine by voltammetry performed at pt and carbon paste electrodes. Molecules 2011, 16, 1349–1365. [Google Scholar] [CrossRef]
- Newair, E.F.; Kilmartin, P.A.; Garcia, F. Square wave voltammetric analysis of polyphenol content and antioxidant capacity of red wines using glassy carbon and disposable carbon nanotubes modified screen-printed electrodes. Eur. Food Res. Technol. 2018, 244, 1225–1237. [Google Scholar] [CrossRef]
- Scampicchio, M.; Lawrence, N.S.; Arecchi, A.; Mannino, S. Determination of Sulfite in Wine by Linear Sweep Voltammetry. Electroanalysis 2008, 20, 444–447. [Google Scholar] [CrossRef]
- Pigani, L.; Rioli, C.; Foca, G.; Ulrici, A.; Seeber, R.; Terzi, F.; Zanardi, C. Determination of polyphenol content and colour index in wines through PEDOT-modified electrodes. Anal. Bioanal. Chem. 2016, 408, 7329–7338. [Google Scholar] [CrossRef]
- Petrovic, S.C. Correlation of Perceived Wine Astringency to Cyclic Voltammetric Response. Am. J. Enol. Vitic. 2009, 60, 373–378. [Google Scholar]
- Martins, R.C.; Oliveira, R.; Bento, F.; Geraldo, D.; Lopes, V.V.; Guedes de Pinho, P.; Oliveira, C.; Silva Ferreira, M.A.C. Oxidation management of white wines using cyclic voltammetry and multivariate process monitoring. J. Agric. Food Chem. 2008, 56, 12092–12098. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Muena, J.P.; Gonzalez-Ulianoff, D.; Miranda, A. Fast electrochemical detection of non-desirable aging in bottled white wine. J. Chil. Chem. Soc. 2014, 59, 2526–2528. [Google Scholar] [CrossRef][Green Version]
- Arribas, A.S.; Martínez-Fernández, M.; Chicharro, M. The role of electroanalytical techniques in analysis of polyphenols in wine. TrAC 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar]
- Cetó, X.; Voelcker, N.H.; Prieto-Simón, B. Bioelectronic tongues: New trends and applications in water and food analysis. Biosens. Bioelectron. 2016, 79, 608–626. [Google Scholar]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic noses and tongues in wine industry. Front. Bioeng. Biotechnol. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Parra, V.; Hernando, T.; Rodríguez-Méndez, M.L.; de Saja, J.A. Electrochemical sensor array made from bisphthalocyanine modified carbon paste electrodes for discrimination of red wines. Electrochim. Acta 2004, 49, 5177–5185. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; Del Valle, M. BioElectronic Tongue for the quantification of total polyphenol content in wine. Talanta 2012, 99, 544–551. [Google Scholar] [CrossRef]
- Gonzalez, A.; Vidal, S.; Ugliano, M. Untargeted voltammetric approaches for characterization of oxidation patterns in white wines. Food Chem. 2018, 269, 1–8. [Google Scholar] [CrossRef]
- Antonacci, A.; Arduini, F.; Moscone, D.; Palleschi, G.; Scognamiglio, V. Commercially Available (Bio)sensors in the Agrifood Sector. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 74, pp. 315–340. [Google Scholar]
- Yan, C.; Dong, F.; Chun-Yuan, B.; Si-Rong, Z.; Jian-Guo, S. Recent Progress of Commercially Available Biosensors in China and Their Applications in Fermentation Processes. J. Northeast Agric. Univ. (Engl. Ed.) 2014, 21, 73–85. [Google Scholar] [CrossRef]
- Barthelmebs, L.; Calas-Blanchard, C.; Istamboulie, G.; Marty, J.L.; Noguer, T. Biosensors as Analytical Tools in Food Fermentation Industry. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Berra, B., Eds.; Springer: Boston, MA, USA, 2010; pp. 293–307. [Google Scholar]
- Mao, X.L.; Wu, J.; Ying, Y.B. Application of Electrochemical Biosensors in Fermentation. Chin. J. Anal. Chem. 2008, 36, 1749–1755. [Google Scholar] [CrossRef]
- Goriushkina, T.B.; Soldatkin, A.P.; Dzyadevych, S.V. Application of Amperometric Biosensors for Analysis of Ethanol, Glucose, and Lactate in Wine. J. Agric. Food Chem. 2009, 57, 6528–6535. [Google Scholar] [CrossRef]
- Piermarini, S.; Volpe, G.; Esti, M.; Simonetti, M.; Palleschi, G. Real time monitoring of alcoholic fermentation with low-cost amperometric biosensors. Food Chem. 2011, 127, 749–754. [Google Scholar] [CrossRef]
- Kamanin, S.S.; Arlyapov, V.A.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Biosensors based on modified screen-printed enzyme electrodes for monitoring of fermentation processes. Russ. J. Appl. Chem. 2015, 88, 463–472. [Google Scholar] [CrossRef]
- Acevedo-Restrepo, I.; Blandón-Naranjo, L.; Hoyos-Arbeláez, J.; Flavio, D.; Della Pelle, F.; Vazquez, M. Electrochemical Glucose Quantification as a Strategy for Ethanolic Fermentation Monitoring. Chemosensors 2019, 7, 14. [Google Scholar] [CrossRef]
- Samphao, A.; Butmee, P.; Saejueng, P.; Pukahuta, C.; Švorc, Ľ.; Kalcher, K. Monitoring of glucose and ethanol during wine fermentation by bienzymatic biosensor. J. Electroanal. Chem. 2018, 816, 179–188. [Google Scholar] [CrossRef]
- Ulasova, E.A.; Micheli, L.; Vasii, L.; Moscone, D.; Palleschi, G.; Vdovichev, S.V.; Zorin, A.V.; Krutovertsev, S.A.; Karyakina, E.E.; Karyakin, A.A. Flow-Injection Analysis of Residual Glucose in Wines Using a Semiautomatic Analyzer Equipped with a Prussian Blue-Based Biosensor. Electroanalysis 2003, 15, 447–451. [Google Scholar] [CrossRef]
- Albanese, D.; Sannini, A.; Malvano, F.; Pilloton, R.; Marisa, D.; Matteo, M. Optimisation of Glucose Biosensors Based on Sol–Gel Entrapment and Prussian Blue-Modified Screen-Printed Electrodes for Real Food Analysis. Food Anal. Methods 2014, 7, 1002–1008. [Google Scholar] [CrossRef]
- Serban, S.; Danet, A.F.; El Murr, N. Rapid and Sensitive Automated Method for Glucose Monitoring in Wine Processing. J. Agric. Food Chem. 2004, 52, 5588–5592. [Google Scholar] [CrossRef]
- Niculescu, M.; Erichsen, T.; Sukharev, V.; Kerenyi, Z.; Csöregi, E.; Schuhmann, W. Quinohemoprotein alcohol dehydrogenase-based reagentless amperometric biosensor for ethanol monitoring during wine fermentation. Anal. Chim. Acta 2002, 463, 39–51. [Google Scholar] [CrossRef]
- Giménez-Gómez, P.; Gutiérrez-Capitán, M.; Capdevila, F.; Puig-Pujol, A.; Fernández-Sánchez, C.; Jiménez-Jorquera, C. Robust l-malate bienzymatic biosensor to enable the on-site monitoring of malolactic fermentation of red wines. Anal. Chim. Acta 2017, 954, 105–113. [Google Scholar] [CrossRef]
- Gamella, M.; Campuzano, S.; Conzuelo, F.; Curiel, J.A.; Muñoz, R.; Reviejo, A.J.; Pingarrón, J.M. Integrated multienzyme electrochemical biosensors for monitoring malolactic fermentation in wines. Talanta 2010, 81, 925–933. [Google Scholar] [CrossRef]
- Giménez-Gómez, P.; Gutiérrez-Capitán, M.; Capdevila, F.; Puig-Pujol, A.; Fernández-Sánchez, C.; Jiménez-Jorquera, C. Monitoring of malolactic fermentation in wine using an electrochemical bienzymatic biosensor for l-lactate with long term stability. Anal. Chim. Acta 2016, 905, 126–133. [Google Scholar] [CrossRef]
- Sannini, A.; Albanese, D.; Malvano, F.; Crescitelli, A.; Matteo, M. An amperometric biosensor for the determination of lactic acid during malolactic fermentation. Chem. Eng. Trans. 2015, 44, 283–288. [Google Scholar]
- Esti, M.; Volpe, G.; Micheli, L.; Delibato, E.; Compagnone, D.; Moscone, D.; Palleschi, G. Electrochemical biosensors for monitoring malolactic fermentation in red wine using two strains of Oenococcus oeni. Anal. Chim. Acta 2004, 513, 357–364. [Google Scholar] [CrossRef][Green Version]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Thornton, A.J.; Brown, D.E. Fermentation glucose assay using the Exactech blood glucose biosensor. Biotechnol. Tech. 1991, 5, 363–366. [Google Scholar] [CrossRef]
- Lidgren, L.; Lilja, O.; Krook, M.; Kriz, D. Automatic fermentation control based on a real-time in situ SIRE® biosensor regulated glucose feed. Biosens. Bioelectron. 2006, 21, 2010–2013. [Google Scholar] [CrossRef]
- Schuhmann, W.; Wohlschläger, H.; Huber, J.; Schmidt, H.L.; Stadler, H. Development of an extremely flexible automatic analyzer with integrated biosensors for on-line control of fermentation processes. Anal. Chim. Acta 1995, 315, 113–122. [Google Scholar] [CrossRef]
- Esti, M.; Volpe, G.; Compagnone, D.; Mariotti, G.; Moscone, D.; Palleschi, G. Monitoring alcoholic fermentation of red wine by electrochemical biosensors. Am. J. Enol. Vitic. 2003, 54, 39–45. [Google Scholar]
- Albanese, D.; Liguori, C.; Paciello, V.; Pietrosanto, A. Winemaking Process Monitoring Based on a Biosensor Automatic System. IEEE Trans. Instrum. Meas. 2011, 60, 1909–1916. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wines and Musts. 2018. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 2 September 2019).
- Di Gennaro, S.F.; Matese, A.; Primicerio, J.; Genesio, L.; Sabatini, F.; Di Blasi, S.; Vaccari, F.P. Wireless real-time monitoring of malolactic fermentation in wine barrels: the Wireless Sensor Bung system. Aust. J. Grape Wine Res. 2013, 19, 20–24. [Google Scholar] [CrossRef]
- Bucur, B.; Mallat, E.; Gurban, A.M.; Gocheva, Y.; Velasco, C.; Marty, J.L.; Noguer, T. Strategies to develop malic acid biosensors based on malate quinone oxidoreductase (MQO). Biosens. Bioelectron. 2006, 21, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Compagnone, D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef]
- Cortina-Puig, M.; Noguer, T.; Marty, J.; Calas-Blanchard, C. Electrochemical Biosensors as a Tool for the Determination of Phenolic Compounds and Antioxidant Capacity. In Foods and Beverages in Biosensors in Food Processing, Safety, and Quality Control; Mutlu, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 257–272. [Google Scholar]
- José Jara-Palacios, M.; Luisa Escudero-Gilete, M.; Miguel Hernández-Hierro, J.; Heredia, F.J.; Hernanz, D. Cyclic voltammetry to evaluate the antioxidant potential in winemaking by-products. Talanta 2017, 165, 211–215. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical Methods for Total Antioxidant Capacity and its Main Contributors Determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef]
- Rauhut, R.T.; Bülbül, G.; Andreescu, S. Nanotechnology-enabled approaches for the detection of antioxidants by spectroscopic and electrochemical methods. In Measurement of Antioxidant Activity & Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 187–207. [Google Scholar]
- Di Fusco, M.; Tortolini, C.; Deriu, D.; Mazzei, F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 2010, 81, 235–240. [Google Scholar] [CrossRef]
- Lanzellotto, C.; Favero, G.; Antonelli, M.L.; Tortolini, C.; Cannistraro, S.; Coppari, E.; Mazzei, F. Nanostructured enzymatic biosensor based on fullerene and gold nanoparticles: Preparation, characterization and analytical applications. Biosens. Bioelectron. 2014, 55, 430–437. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.V.; Kusko, M.; Radoi, A.; Vasile, E.; Radu, G.L. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016, 75, 232–237. [Google Scholar] [CrossRef]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.G.; Marcolino-Júnior, L.H.; Bergamini, M.F. Voltammetric Determination of the Antioxidant Capacity in Wine Samples Using a Carbon Nanotube Modified Electrode. J. Agric. Food Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, D.; Tanaka, H.; Zhan, F.; Yuan, X.; Gao, F.; Wang, Q. An electrochemical sensor for gallic acid based on Fe(2)O(3)/electro-reduced graphene oxide composite: Estimation for the antioxidant capacity index of wines. Mater. Sci. Eng. C Mater. 2015, 57, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Andrei, V.; Sharpe, E.; Vasilescu, A.; Andreescu, S. A single use electrochemical sensor based on biomimetic nanoceria for the detection of wine antioxidants. Talanta 2016, 156–157, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Cetó, X.; Céspedes, F.; Pividori, M.I.; Gutiérrez, M.J.; del Valle, M. Resolution of phenolic antioxidant mixtures employing a voltammetric bio-electronic tongue. Analyst 2012, 137, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Andrei, V.; Bunea, A.I.; Tudorache, A.; Gáspár, S.; Vasilescu, A. Simple DPPH.-Based Electrochemical Assay for the Evaluation of the Antioxidant Capacity: a Thorough Comparison with Spectrophotometric Assays and Evaluation with Real-World Samples. Electroanalysis 2014, 26, 2677–2685. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Teixeira, M.F.S.; Caetano, F.R.; Bergamini, M.F.; Marcolino-Júnior, L.H. A Simple and Rapid Estimation of Totals Polyphenols Based On Carbon Paste Electrode Modified with Ruthenium Oxo-Complex. Electroanalysis 2015, 27, 2371–2376. [Google Scholar] [CrossRef]
- Carralero Sanz, V.; Mena, M.L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes: Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal. Chim. Acta 2005, 528, 1–8. [Google Scholar] [CrossRef]
- Gamella, M.; Campuzano, S.; Reviejo, A.J.; Pingarrón, J.M. Electrochemical Estimation of the Polyphenol Index in Wines Using a Laccase Biosensor. J. Agric. Food Chem. 2006, 54, 7960–7967. [Google Scholar] [CrossRef]
- Blasco, A.J.; Rogerio, M.C.; González, M.C.; Escarpa, A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Badea, M.; di Modugno, F.; Floroian, L.; Tit, D.M.; Restani, P.; Bungau, S.; Iovan, C.; Badea, G.E.; Aleya, L. Electrochemical strategies for gallic acid detection: Potential for application in clinical, food or environmental analyses. Sci. Total. Environ. 2019, 672, 129–140. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Martin, F.; Švorc, Ľ.; Mariano, J.F.M.L.; Antić, B. Design of titanium nitride- and wolfram carbide-doped RGO/GC electrodes for determination of gallic acid. Anal. Biochem. 2017, 539, 104–112. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Prado, T.M.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Machado, S.A.S. Synergy between Printex nano-carbons and silver nanoparticles for sensitive estimation of antioxidant activity. Anal. Chim. Acta 2016, 926, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.; Rodrigues Santos, W.; Kubota, L. Selective determination of caffeic acid in wines with electrochemical sensor based on molecularly imprinted siloxanes. Sens. Actuators B Chem. 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. Sensitive Electrochemical Detection of Caffeic Acid in Wine Based on Fluorine-Doped Graphene Oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [PubMed]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Chen, H.A. A voltammetric determination of caffeic acid in red wines based on the nitrogen doped carbon modified glassy carbon electrode. Sci. Rep. 2017, 7, 45924. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Brett, A.M.O. Redox Behavior of Anthocyanins Present in Vitis vinifera L. Electroanalysis 2007, 19, 1779–1786. [Google Scholar] [CrossRef]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenols removal in musts: Strategy for wine stabilization by laccase. J. Mol. Catal. B Enzym. 2007, 45, 102–107. [Google Scholar] [CrossRef]
- Türke, A.; Fischer, W.J.; Beaumont, N.; Kilmartin, P.A. Electrochemistry of sulfur dioxide, polyphenols and ascorbic acid at poly(3,4-ethylenedioxythiophene) modified electrodes. Electrochim. Acta 2012, 60, 184–192. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; García-Cabezón, C.; García-Hernández, C.; Bramorski, C.; Blanco-Val, Y.; Martín-Pedrosa, F.; Kawai, T.; de Saja, J.A.; Rodríguez-Méndez, M.L. Analysis of organic acids and phenols of interest in the wine industry using Langmuir–Blodgett films based on functionalized nanoparticles. Anal. Chim. Acta 2015, 853, 572–578. [Google Scholar] [CrossRef]
- Thangavelu, K.; Palanisamy, S.; Chen, S.M.; Velusamy, V.; Chen, T.W.; Ramaraj, S.K. Electrochemical Determination of Caffeic Acid in Wine Samples Using Reduced Graphene Oxide/Polydopamine Composite. J. Electrochem. Soc. 2016, 163, B726–B731. [Google Scholar] [CrossRef]
- Mannino, S.; Brenna, O.; Buratti, S.; Cosio, M.S. A New Method for the Evaluation of the ‘Antioxidant Power’ of Wines. Electroanalysis 1998, 10, 908–912. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Uncovering the influence of antioxidants on polyphenol oxidation in wines using an electrochemical method: Cyclic voltammetry. J. Electroanal. Chem. 2009, 633, 165–174. [Google Scholar] [CrossRef]
- Pigani, L.; Foca, G.; Ulrici, A.; Ionescu, K.; Martina, V.; Terzi, F.; Vignali, M.; Zanardi, C.; Seeber, R. Classification of red wines by chemometric analysis of voltammetric signals from PEDOT-modified electrodes. Anal. Chim. Acta 2009, 643, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: a never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC 2015, 74, 21–45. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, M.J.; Bellido-Milla, D. Assessment of the Polyphenol Indices and Antioxidant Capacity for Beers and Wines Using a Tyrosinase-Based Biosensor Prepared by Sinusoidal Current Method. Sensors 2018, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Tortolini, C.; Bollella, P.; Zumpano, R.; Favero, G.; Mazzei, F.; Antiochia, R. Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples. Biosensors 2018, 8, 108. [Google Scholar] [CrossRef]
- Hayat, A.; Cunningham, J.; Bulbul, G.; Andreescu, S. Evaluation of the oxidase like activity of nanoceria and its application in colorimetric assays. Anal. Chim. Acta 2015, 885, 140–147. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007; pp. 230–240. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a tool for studying antioxidant properties. Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Pisoschi, A.; Petre Negulescu, G. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2011, 1, 1000106. [Google Scholar] [CrossRef]
- Lino, F.M.A.; De Sá, L.Z.; Torres, I.M.S.; Rocha, M.L.; Dinis, T.C.P.; Ghedini, P.C.; Somerset, V.S.; Gil, E.S. Voltammetric and spectrometric determination of antioxidant capacity of selected wines. Electrochim. Acta 2014, 128, 25–31. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Maia, M.A.; Reis, S.; Segundo, M.A. Rapid assessment of endpoint antioxidant capacity of red wines through microchemical methods using a kinetic matching approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Bonanni, A.; Finotti, E.; Tomassetti, M. Biosensors for determination of total and natural antioxidant capacity of red and white wines: comparison with other spectrophotometric and fluorimetric methods. Biosens. Bioelectron. 2004, 19, 641–651. [Google Scholar] [CrossRef]
- Peñas, E.; Di Lorenzo, C.; Uberti, F.; Restani, P. Allergenic Proteins in Enology: A Review on Technological Applications and Safety Aspects. Molecules 2015, 20, 13144–13164. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). International Code of Oenological Practices; International Organisation of Vine and Wine (OIV): Paris, France, 2017; Available online: http://www.oiv.int/public/medias/5119/code-2017-en.pdf (accessed on 2 September 2019).
- International Organisation of Vine and Wine (OIV). Criteria for the Quantification of Potentially Allergenic Residues of Fining Agent Proteins in Wine; OIV-OENO 427-2010 Modified by OIV-COMEX 502-2012; International Organisation of Vine and Wine (OIV): Paris, France, 2012. [Google Scholar]
- Patzl-Fischerleitner, E.; Eder, R. Detection of residues of chicken ovalbumin and casein in wine by means of Enzyme-linked Immunosorbent Assay (ELISA) immediately after fining and during storage. Mitt. Klosterneubg. 2012, 62, 10–12. [Google Scholar]
- Alves, R.C.; Barroso, M.F.; Gonzalez-Garcia, M.B.; Oliveira, M.B.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. 2016, 56, 2304–2319. [Google Scholar] [CrossRef]
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.L. Electrochemical Affinity Biosensors Based on Disposable Screen-Printed Electrodes for Detection of Food Allergens. Sensors 2016, 16, 1863. [Google Scholar] [CrossRef]
- Wessels, H.; Paschke-Kratzin, A. New SPR-based methods for analysis of allergenic agents used in wine treatment. In Proceedings of the BIO Web of Conferences, 39th World Congress of Vine and Wine, Bento Gonçalves, Brazil, 24–28 October 2016; p. 7. [Google Scholar] [CrossRef]
- Mihai, I.; Vezeanu, A.; Polonschii, C.; Albu, C.; Radu, G.L.; Vasilescu, A. Label-free detection of lysozyme in wines using an aptamer based biosensor and SPR detection. Sens. Actuators B Chem. 2015, 206, 198–204. [Google Scholar] [CrossRef]
- Ocaña, C.; Hayat, A.; Mishra, R.K.; Vasilescu, A.; del Valle, M.; Marty, J.L. Label free aptasensor for Lysozyme detection: A comparison of the analytical performance of two aptamers. Bioelectrochem 2015, 105, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Pilolli, R.; Visconti, A.; Monaci, L. Rapid and label-free detection of egg allergen traces in wines by surface plasmon resonance biosensor. Anal. Bioanal. Chem. 2015, 407, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; del Valle, M.; Marty, J.L. A novel electrochemical aptamer–antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Wang, Q.; Li, M.; Boukherroub, R.; Szunerits, S. Aptamer-Based Electrochemical Sensing of Lysozyme. Chemosensors 2016, 4, 10. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Electrochemical oxidation of wine polyphenols in the presence of sulfur dioxide. J. Agric. Food Chem. 2013, 61, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
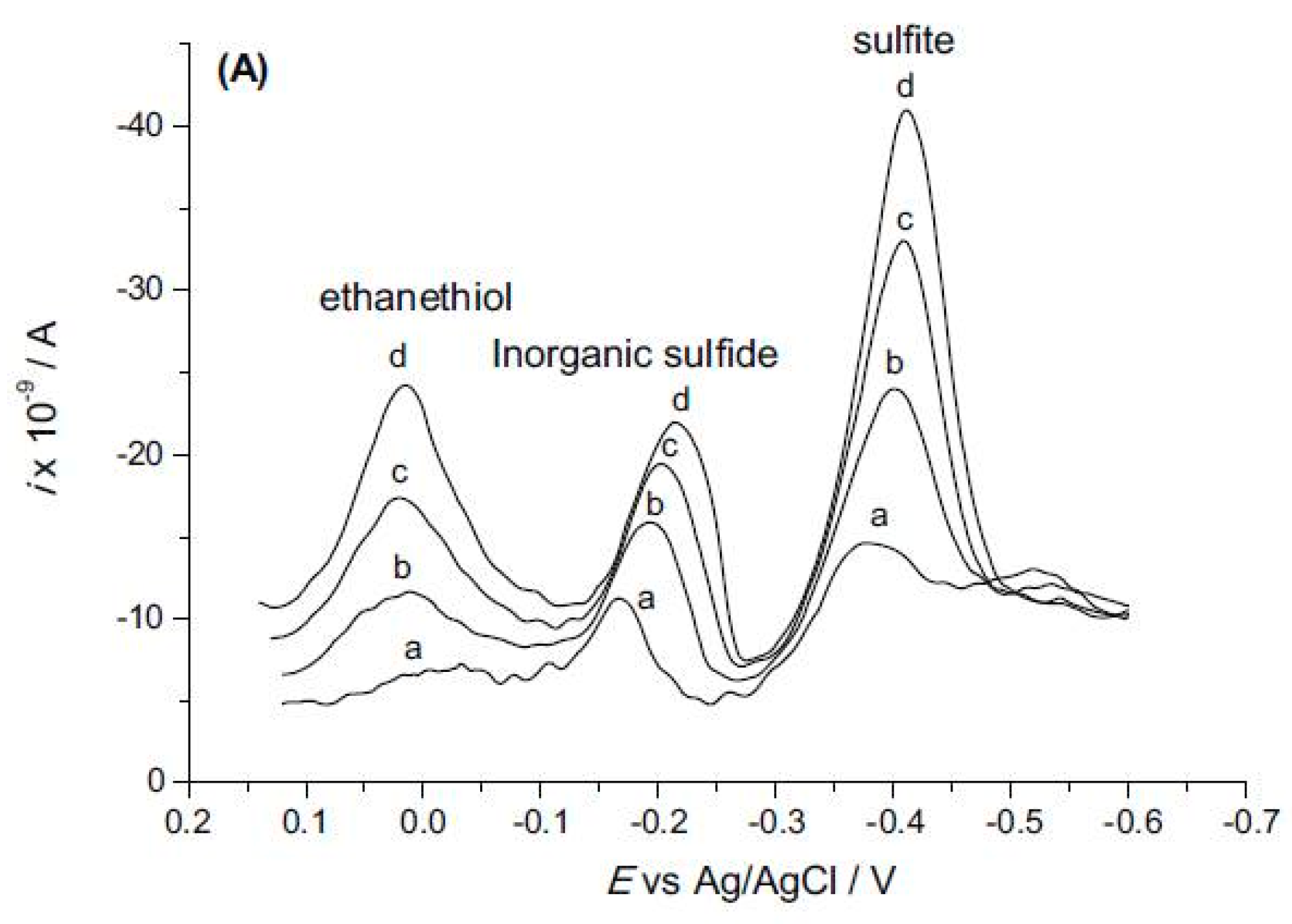
- Guarda, A.; Maciel, J.V.; Wiethan, B.A.; Schneider, A.; do Nascimento, P.C.; Dias, D. Simultaneous Determination of Ethanethiol, Inorganic Sulfide, and Sulfite in Wines by Cathodic Stripping Voltammetry. Food Anal. Methods 2017, 10, 837–844. [Google Scholar] [CrossRef]
- Molinero-Abad, B.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Sulfite oxidase biosensors based on tetrathiafulvalene modified screen-printed carbon electrodes for sulfite determination in wine. Anal. Chim. Acta 2014, 812, 41–44. [Google Scholar] [CrossRef]
- Spricigo, R.; Richter, C.; Leimkühler, S.; Gorton, L.; Scheller, F.W.; Wollenberger, U. Sulfite biosensor based on osmium redox polymer wired sulfite oxidase. Colloid Surf. A 2010, 354, 314–319. [Google Scholar] [CrossRef]
- Scrimgeour, N. Evaluating the Viability of Process Sensor Technologies for Measurement of Sugar Levels During Fermentation, Project Final Report to the Australian Grape and Wine Authority, Project Number: AWR 1401. 2015. Available online: www.wineaustralia.com/getmedia/d0e6cb2f-102e-4783-88f1-d291acbb169a/AWR-1401-Final-Report1 (accessed on 2 September 2019).
- Wang, Q.; Li, Z.; Ma, Z.; Liang, L. Real time monitoring of multiple components in wine fermentation using an on-line auto-calibration Raman spectroscopy. Sens. Actuators B Chem. 2014, 202, 426–432. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. I1.1-1–I1.1-8. [Google Scholar]


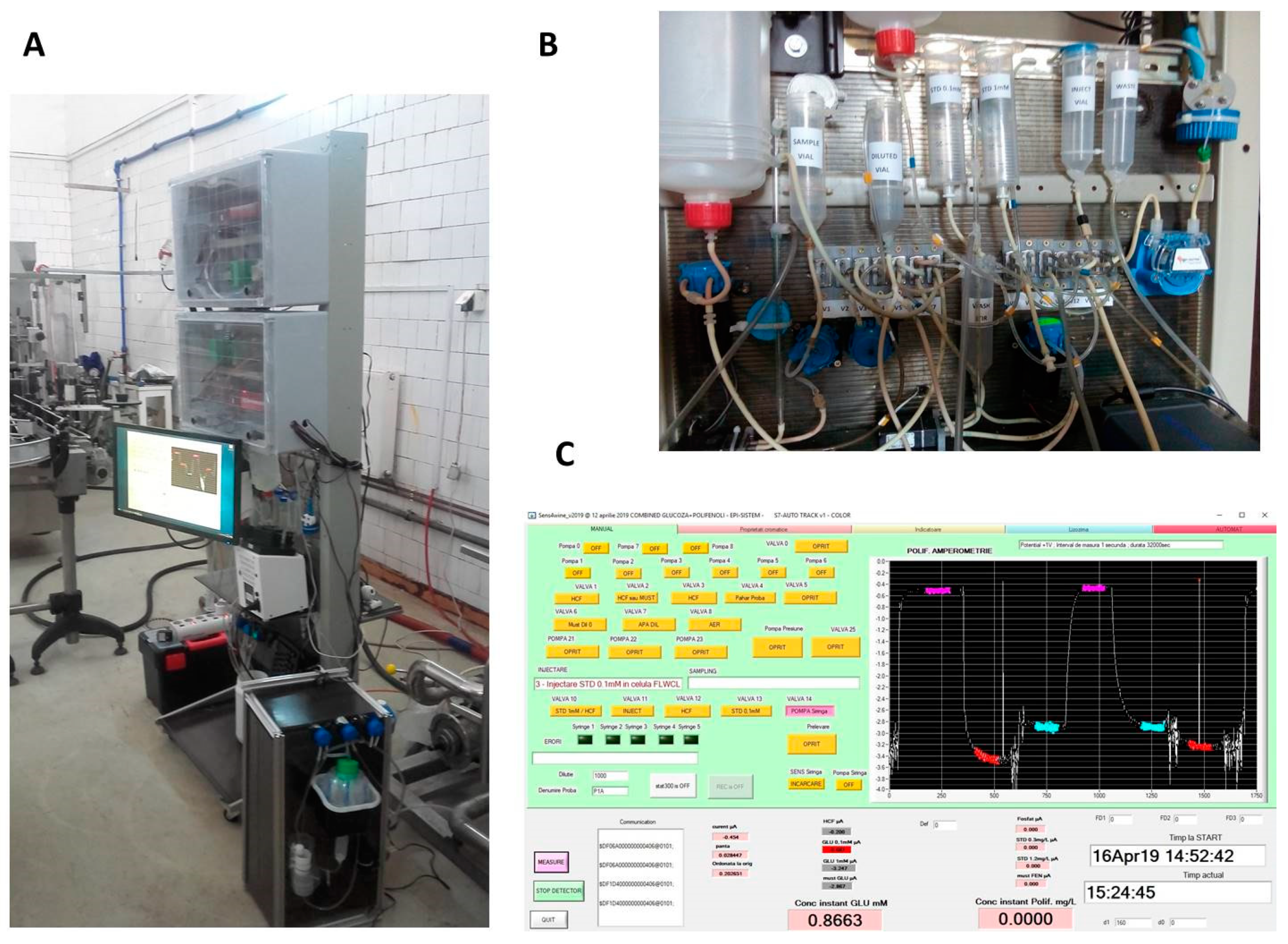
| Target Analyte | Application/Matrix | Analytical Parameters | Biosensor Configuration | Reference Method | Ref. |
|---|---|---|---|---|---|
| Glucose, ethanol, lactate | 12 wines (red and white; dry and sweet) | LR (glucose): 0.04–2.5 mM, LR (ethanol): 0.3–20 mM, LR (lactate): 0.008–1 mM; Storage stability: >2 months (ethanol, glucose); 4 days (lactate) | Pt printed electrodes/GOx (AOx, LOx) Batch | HPLC | [30] |
| Glucose, Fructose, Ethanol | microalcoholic fermentations 23 red wine samples | LR (glucose): 0.02–0.7 mM LR (fructose): 0.02–0.7 mM, LR (ethanol): 0.05–0.5 mM Stability: 90% after 6 months (glucose), 1 month (ethanol) and 15 days (fructose) Recovery in spiked wines: 95–105% | SPGE/PB/GOx (AOx) (glucose, ethanol) SPGE/PMS/FDH (fructose) Batch. | Spectrophotometric kit | [31] |
| Glucose, ethanol, lactate | Wines, milk, Fermentation media | LR (glucose): 0.05–1.10 mM LR (ethanol): 0.09–0.90 mM LR (lactate): 1–53 mM | Biosensor array SPGE/PB/GOx (AOx, LOx) Batch | HPLC | [32] |
| Glucose | Lab-scale fermentation (7 samples) | LR: 5-200 mg/L LOD:16.2 mg/L LOQ: 54.1 mg/L | H), G6P-DH, NAD+ from commercial kit, detection of NADH with SPE/O-MWCNT Batch | Glucose kit | [33] |
| Glucose, ethanol | Fermentation broth-24 wines | LR: 0.3-7.8 mM LOD: 0.1 mM Stability: 50% after 30 days | GOx/ADH/Fe3O4@Au/MnO2-CPE Batch | Glucose meter | [34] |
| Glucose | 10 red and white wines | LR: 10−6–10−3 M | GC/PB/GOx/Nafion Semiautomatic FIA analyzer | Spectrophotometric assay | [35] |
| Glucose | Commercial red/white wine | LR: 5–1000 µM LOD: 20 µM Operational stability: No decrease after 8 h of 55 injection of glucose | SPGE/PB/GOx/TEOS– PVA/Nafion FIA | HPLC | [36] |
| Glucose | 9 wines (red, rose, white, dry and sweet) | LR1: 0.3–2 g.L−1 LR2: 2–10 g.L−1 LR3: 10–50 g.L−1 | GOx/HRP/Fc/CPE FIA | Enzymatic kit | [37] |
| Glucose | 2 red wines | LR: 0.02–4.5 mM LOD: 0.005 mM Stability: 92% after 25 days | NiO-GR/GCE Batch | HPLC | [1] |
| Glucose | N/A | LR: 10–25 mM No interference from ethanol | SPGE/Au/TiO2 Batch | N/A | [2] |
| Ethanol | Alcoholic fermentation of wines (6 days) | LR: 1–250 µM LOD: 1 µM | QH-ADH + PVI13dmeOs + PEGDGE SPE On-line SIA analyzer OLGA | Enzymatic kit | [38] |
| Malate | MLF of 3 red wines | LR: 1 × 10−7–1 × 10−6 M LOD: 6.3 × 10−8M Retains 90% of sensitivity after 37 days | Thick film Au/MDH-DP, NAD+/Ppy-HAR Batch | Colorimetry | [39] |
| L-malate, L-lactate | MLF of synthetic wine induced by Lactobacillus plantarum CECT 748T | LR (malate):5.2 × 10−7–2.0 × 10−5 M LR (lactate):4.2 × 10−7–2.0 × 10−5 M LOD (malate): 5.2 × 10−7 M LOD (lactate): 4.2 × 10−7 M Stability: 90% of sensitivity after 7 days (malate); 91% of sensitivity after 5 days (lactate) | DM/MDH-DP/TTF/MPA-Au (malate) DM/Lox-HRP/TTF/MPA-Au (lactate) Batch | Enzymatic kits | [40] |
| Lactate | MLF of 3 red wines | LR: 1 × 10−6–1 × 10−4 M LOD: 5.2 × 10−7 M 90% of sensitivity after 40 days | Thick film Au/Lox-HRP/PPy Batch | Colorimetry | [41] |
| Lactate | MLF of red wine (11 samples) | LR: (0.005–1 mM; LOD: 0.005 mM Operational stability: 8 h Lifetime:30 days | SPGE/PB/Lox-TEOS (PVA) FIA | Ion chromatography | [42] |
| L-malic acid, L-lactic acid, citric acid | MLF induced by 2 strains of Oenococcus oeni (16 samples) | LR (malate): 10−5–4 × 10−4 M LR (lactate):5 × 10−6–10−3 M LOD (malate): 3 × 10−6 M LOD(lactate): 2 × 10−6 M Stability after 150 injections: 90% of response (malate), 65% of response (lactate) | Pt/LOx on Nylon membrane Pt/ME enzymatic reactor; PMS FIA | Spectrophotometric | [43] |
| Work Aim | Electrochemical Technique/Conditions | Sensor Design Details | Principle | Performance Characteristics | Ref. |
|---|---|---|---|---|---|
| TP estimation | FIA-amperometric | GCE-MWCNTs | Polyphenols oxidation. | Phenolic acids: LR: 1.0 × 10−7 1 × 10−4 mol L−1 | [3] |
| TP | DPV, 0.1 M sodium acetate–acetic acid buffer pH 3.6 | GCE | Polyphenol oxidation | Catechin LR: 1–15 mg.L−1 LOD: 0.53 mg.L−1 catechin | [11] |
| TP index (as gallic acid) | FIA-Amperometry (−0.1 V vs. Ag/AgCl) 0.1 mol L−1 Britton–Robinson buffer, pH 5 | SWCNT/MWNCT; TvL or ThL immobilized by PAP cross-linking | Detection of phenols derived quinones | Gallic Acid: LR: 0.1–17.0 mgL−1 LOD: 0.1 mgL−1 | [62] |
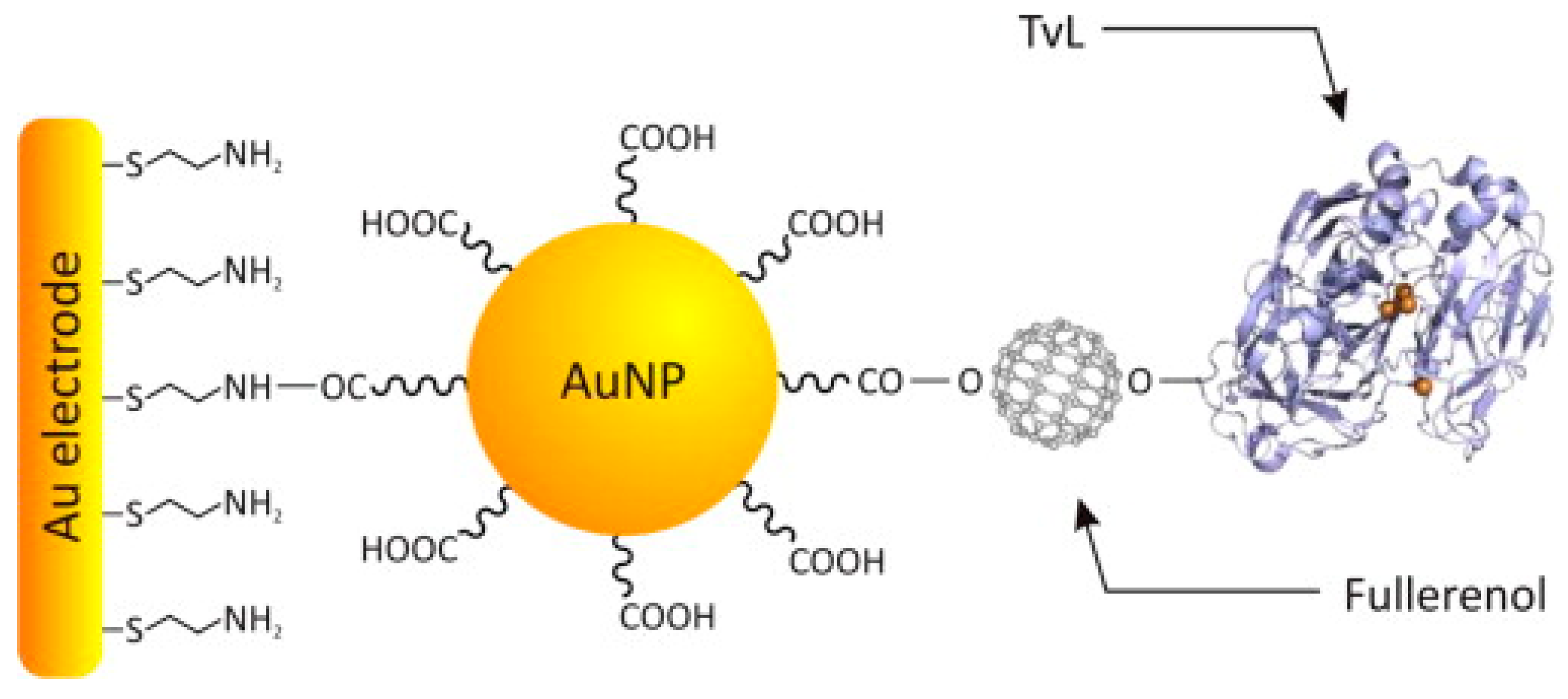
| TP evaluation | FIA-Amperometry (−100 mV vs. Ag/AgCl) Buffer: acetate 0.1 M, pH 4.5 | Au-SAM/AuNPs-Linker/Fullerenols/TvL | Detection of phenols derived quinones | Gallic acid: LR: 3.0 × 10−5–3.0 × 10−4 mol L−1; LOD: 6.0 × 10−6 mol L−1 | [63] |
| TP evaluation | Chronoamperometry (50 mV vs. Ag) 0.1 M acetate buffer with 0.1 M KCl pH 5 | GRQDs-MoS2/nanoflakes; TvL immobilized by electrostatic interaction | Detection of phenols derived quinones | Caffeic acid: LR: 3.8 × 10−7–1.0 × 10−4 mol L−1 LOD: 3.2 × 10−7 mol.L−1 | [64] |
| TAC (as gallic acid) | DPV 0.35 V vs. Ag/AgCl; 0.1 mol L−1 phosphate buffer pH 2.5 | GCE-SWCNTs | Polyphenols oxidation | Gallic acid LR: 5.0 × 10−7 to 1.5 × 10−5 mol L−1 LOD: 3.0 × 10−7 mol L−1 | [65] |
| TAC | DPV | GCE-GR reduced-Fe2O3/Chit | Polyphenol oxidation | Gallic acid: LR: 1.0 × 10−6–1.0 × 10−4 mol L−1 LOD:1.5 × 10−7 mol L−1 | [66] |
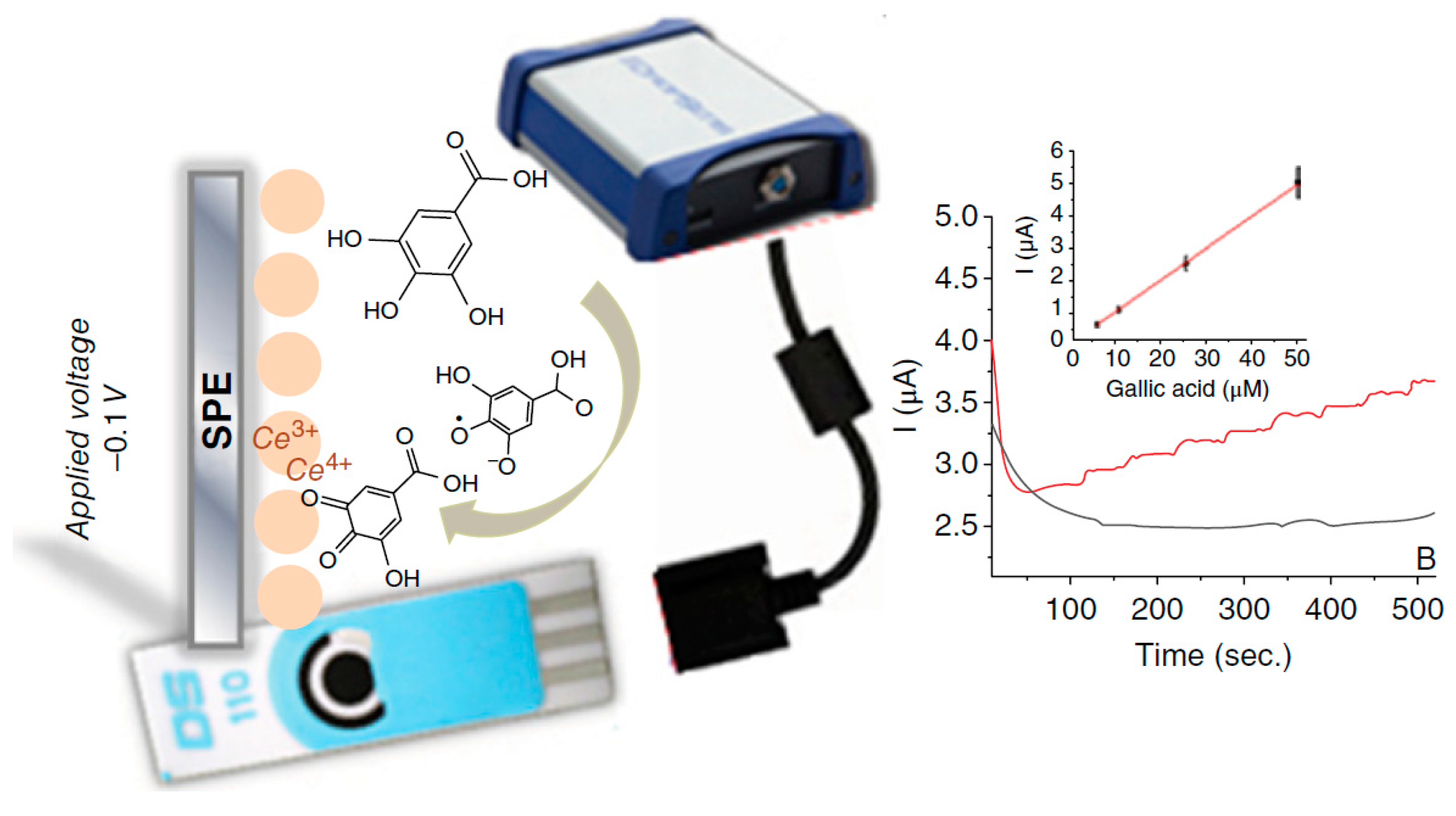
| TAC | Amperometry at −0.1 V vs. Ag/AgCl | SPCE-ceria NPs | Nanoceria mediated polyphenols oxidation to quinones and quinones electrochemical reduction | Gallic acid: 2.0 × 10−6–2.0 × 10−5 mol L−1; LOD: 1.5 × 10−6 mol L−1; Caffeic acid: LR:5 × 10−5–2 × 10−4 mol L−1; LOD: 1.5 × 10−5 molL−1 Quercetin: LR: 2 × 10−5–2 × 10−4 molL−1; LOD: 8.6 × 10−6 molL−1 Ascorbic acid: LR: 5 × 10−7–2 × 10−5 mol L−1; LOD:4 × 10−7 mol L−1 | [67] |
| Catechol, caffeic acid and catechin | CV | Cu NPs/epoxy–graphite-enzyme (tyrosinase, laccase) bioelectronics array | CV and data interpretation by artificial neural network | Average recoveries of 104% (catechol), 117% (caffeic acid) and 122% (catechin) | [68] |
| TAC | Amperometry, FIA system at −0.100 V a 1: 1 mixture of phosphate buffer pH 6 and ethanol | SPAuE | DPPHC electrochemical reduction of DPPH• | Trolo× LR: 2 × 10−6-3 × 10−5 mol L−1; LOD: 4.5 10−7 mol L−1. Sensitivity: 20.1 µA Lcm−2 µmol | [69] |
| TP based on gallic acid | Chronoamperometryat +0.45 V 0.5 mol L−1 KCl, pH 5 | CPME/Ruthenium oxo-complex | Polyphenol oxidation | Gallic acid LR: 1.12–32.5 mg L−1 LOD: 0.08 mg L−1 More than 100 measurements RSD < 5.0%, (n = 10) | [70] |
| TP Polyphenols index | Batch-Amperometry, −0.1 V; 0.1 M phosphate buffer, pH 7.4 | Tyr-nAu-GCE | Reduction of quinones formed in the enzymatic reaction | Caffeic acid LR: 2.5 10−5–9.0 10−5 mol L−1 LSensitivity: 82 µA/mM Stability: 18 days | [71] |
| Polyphenol index | FIA, −0.1 V Amperometry 0.1 mol L−1 citrate buffer of pH 5 | GCE/TvL | Reduction of quinones formed in the enzymatic reaction | Gallic acid LR: 0.04–2.0 mg L−1 LOD: 0.04 0.001 mg L−1 Caffeic acid LR: 0.001–0.100 mg L−1 LOD: 0.001 mg L−1 | [72] |
| TP index (as (+) Catechin | FIA-Amperometry +0.8 V buffer pH 7.5 | GCE | Polyphenol oxidation | LR: 1-16 mg L−1 LOD: 0.30 mg L−1 LOQ: 0.99 mg L−1 | [73] |
| Gallic acid | DPV phosphate buffer pH 5.8 | SPCE | Polyphenol oxidation | Gallic acid LR: 0.1–2.0 mM LOD: 33 µM | [74] |
| Gallic acid | DPV Supporting electrolyte: 0.1 nitric acid and 0.1 M sulfuric acid | TNrGO-modified GC electrode WCrGO-modified GC | Polyphenol oxidation | Gallic acid: TNrGO-GCE LR: 4.5–76 µM LOD: 1.1 µM WCrGO-GCE LR: 10–100 µM LOD: 3.1 µM | [75] |
| TAC (as gallic acid) | DPV; 0.1 mol L−1 phosphate buffer pH 7.0. | GC modified with Printex L6 nano-carbon and AgNPs | Polyphenol oxidation | Gallic acid: LR: 5.0 × 10−7–8.5 × 10−6 mol L−1, LOD: 6.63 × 10−8 mol L−1 Stability: 50 tests | [76] |
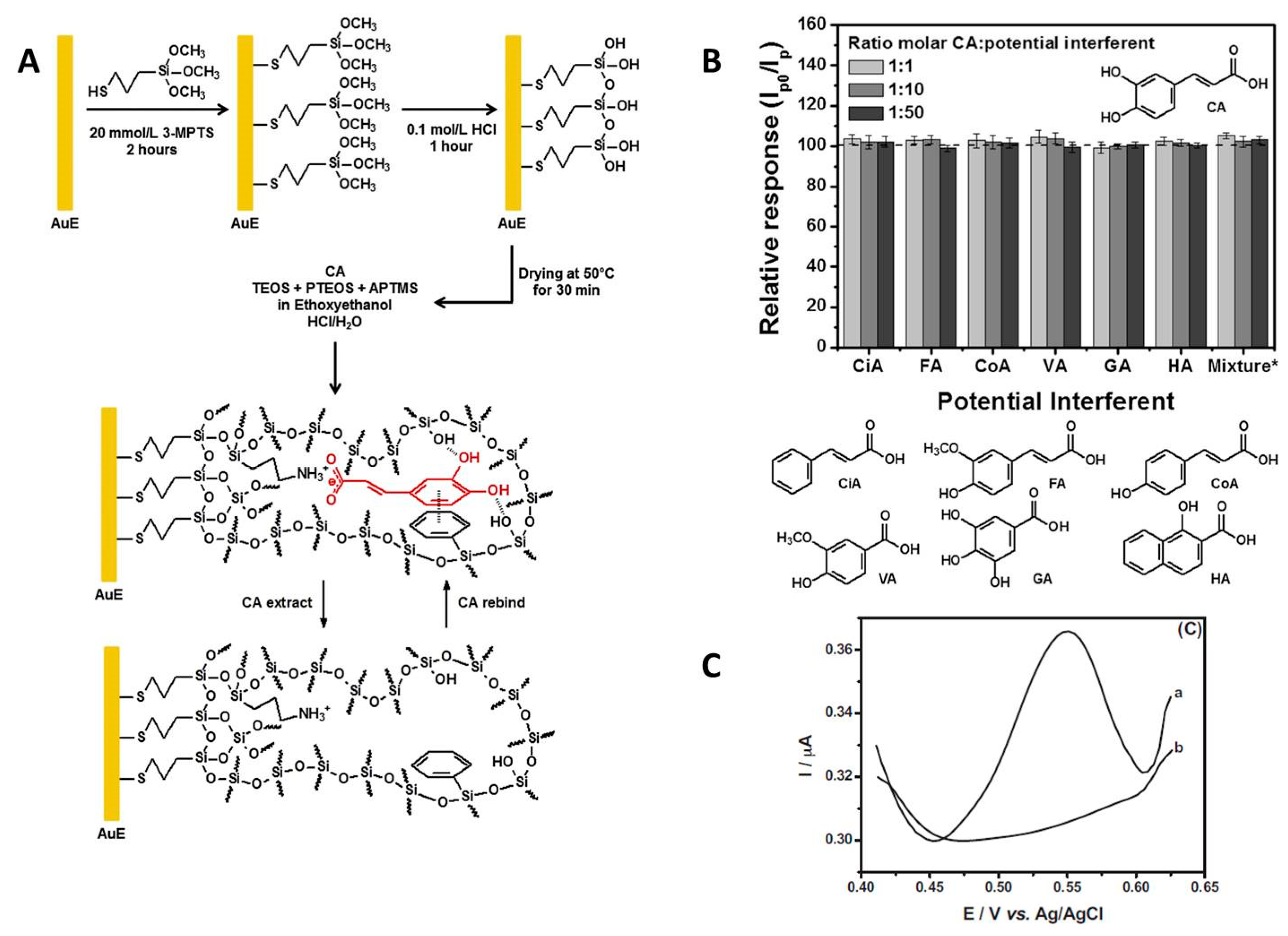
| Caffeic acid | DPV 0.4 mol L−1 sulfuric acid | Au/MIS made from TEOS; PTEOS; APTMS | Polyphenol oxidation | Caffeic acid LR: 0.15–60.0 µmol L−1 LOD: 0.15 µmol L−1 Stability: RSD = 3.2% (n = 30) Storage: no significant change after 70 days at room temperature | [77] |
| Caffeic acid | DPV 0.1 M Britton-Robinson buffer pH 2.65 | F-GO/GCE | Polyphenol oxidation | Caffeic Acid: LR: 0.5–100 µmol L−1 LOD: 0.18 µmol L−1 Stability: 94.7% of response after 30 tests Storage: 95% of activity after 10 days | [78] |
| Caffeic acid | DPV 0.05 M PB solution pH 7 | nitrogen doped carbon modified glassy carbon electrode (NDC/GCE) | Polyphenol oxidation | Caffeic acid: LR: 0.010–350 µmol L−1 LOD: 2.4 nmol L−1 Stability: 93% of the initial response after 20 test Storage: 93.5% of response 6 weeks of storage | [79] |
| Biosensor Based System | Manufacturer | Detection Principle | Parameters | Type of Equipment |
|---|---|---|---|---|
| Biowine300, Biowine500, Biowine700, | Biolan (Bizkaia, Spain, www.biolanmb.com) | Amperometry | Gluconic acid, malic acid, lactic acid, sugars, sucrose, histamine | Single to repeated use; portable + bench top |
| YSI 2900 Series Biochemistry Analyzer | Yellow Spring Instruments (Yellow Springs, Ohio, USA, https://www.ysi.com) | Amperometry, platinum electrode; membrane with immobilized enzyme | Glucose, Lactate, Glutamate, Glutamine, Glycerol, Xylose, Choline, Hydrogen Peroxide, Sucrose, Ethanol, Methanol, Lactose, Galactose | Bench top |
| OLGA-The On-Line General Analyser | Sensolytics GmbH (Bochum, Germany, www.sensolytics.com) | Amperometry | Glucose, Lactate Sucrose, Ethanol Glutamate | Sequential Injection Analysis (SIA)-system |
| LM5 lactate analyser; GL6 | Analox Instruments Ltd. (Stourbridge, UK, www.analox.com) | Amperometry, Clark-type oxygen electrode | Lactate, Ethanol, Glucose, Glycerol, Lactate, Methanol, Sucrose or Lactose. | Bench top |
| Handi-Lab biosensor measurement system | Gwent Group Advanced Materials systems (Pontypool, UK, www.gwent.org) | Amperometry | Glucose, Fructose | Single use sensors Portable |
| AMP Biosens | Biosensor SRL (Formello, Italy, www.biosensor-srl.eu) | Amperometry | Phenols, glucose, antioxidants | Bench top |
| Senzytec 2 | Tectronik srl (Limena, Italy, www.tectronik.it) | Amperometry | Ethanol, Malic acid D-Lactate, L-Lactate Glucose, Fructose | Portable |
| e-BQC | Bioquochem (Asturias, Spain, www.bioquochem.com) | Electrochemical | Antioxidant capacity | Portable |
| Enzymatic Sensor GLU10 | Catalytic Sensor 220AT | Colorimetric Kit | Labeled Value | |||
|---|---|---|---|---|---|---|
| Sample | Glucose (mM) | Total Sugars a (mM) | Reducing Sugars b (mM) | Glucose (mM) | Total Sugars a (mM) | Reducing Sugars b (mM) |
| White must | 455 ± 27 | 910±54 | 998 ± 78 | 496 ± 62 | 992±124 | 944 |
| Red must | 444 ± 29 | 888±58 | 928 ± 85 | 475 ± 32 | 950±64 | 944 |
| Grape juice | 448 ± 13 | 896±26 | 852 ± 86 | 440 ± 14 | 880±28 | 779 |
| White grape | 337 ± 37 | 674±74 | 684 ± 59 | 367 ± 10 | 734±20 | n/a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. https://doi.org/10.3390/chemosensors7040066
Vasilescu A, Fanjul-Bolado P, Titoiu A-M, Porumb R, Epure P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors. 2019; 7(4):66. https://doi.org/10.3390/chemosensors7040066
Chicago/Turabian StyleVasilescu, Alina, Pablo Fanjul-Bolado, Ana-Maria Titoiu, Roxana Porumb, and Petru Epure. 2019. "Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production" Chemosensors 7, no. 4: 66. https://doi.org/10.3390/chemosensors7040066
APA StyleVasilescu, A., Fanjul-Bolado, P., Titoiu, A.-M., Porumb, R., & Epure, P. (2019). Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors, 7(4), 66. https://doi.org/10.3390/chemosensors7040066







