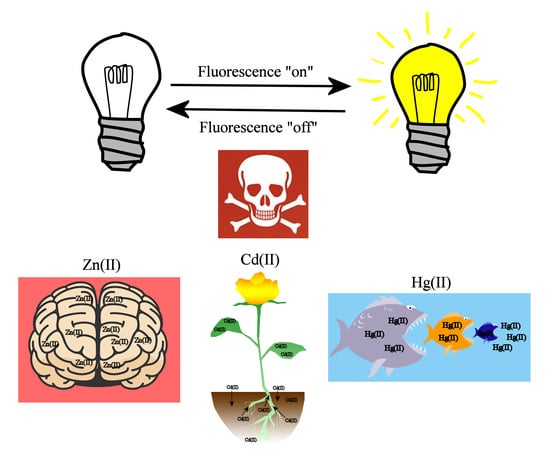
Low Molecular Weight Fluorescent Probes (LMFPs) to Detect the Group 12 Metal Triad
Abstract
1. Introduction
2. Chemosensors for the Group 12 Triad (Zinc, Cadmium, and Mercury)
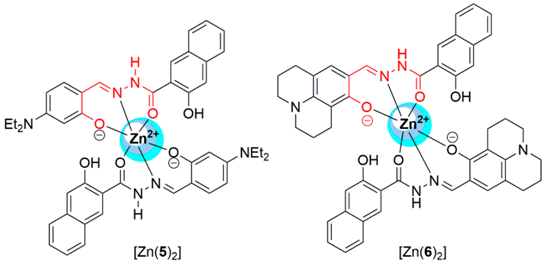
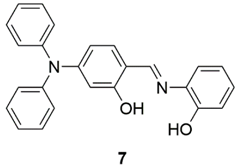
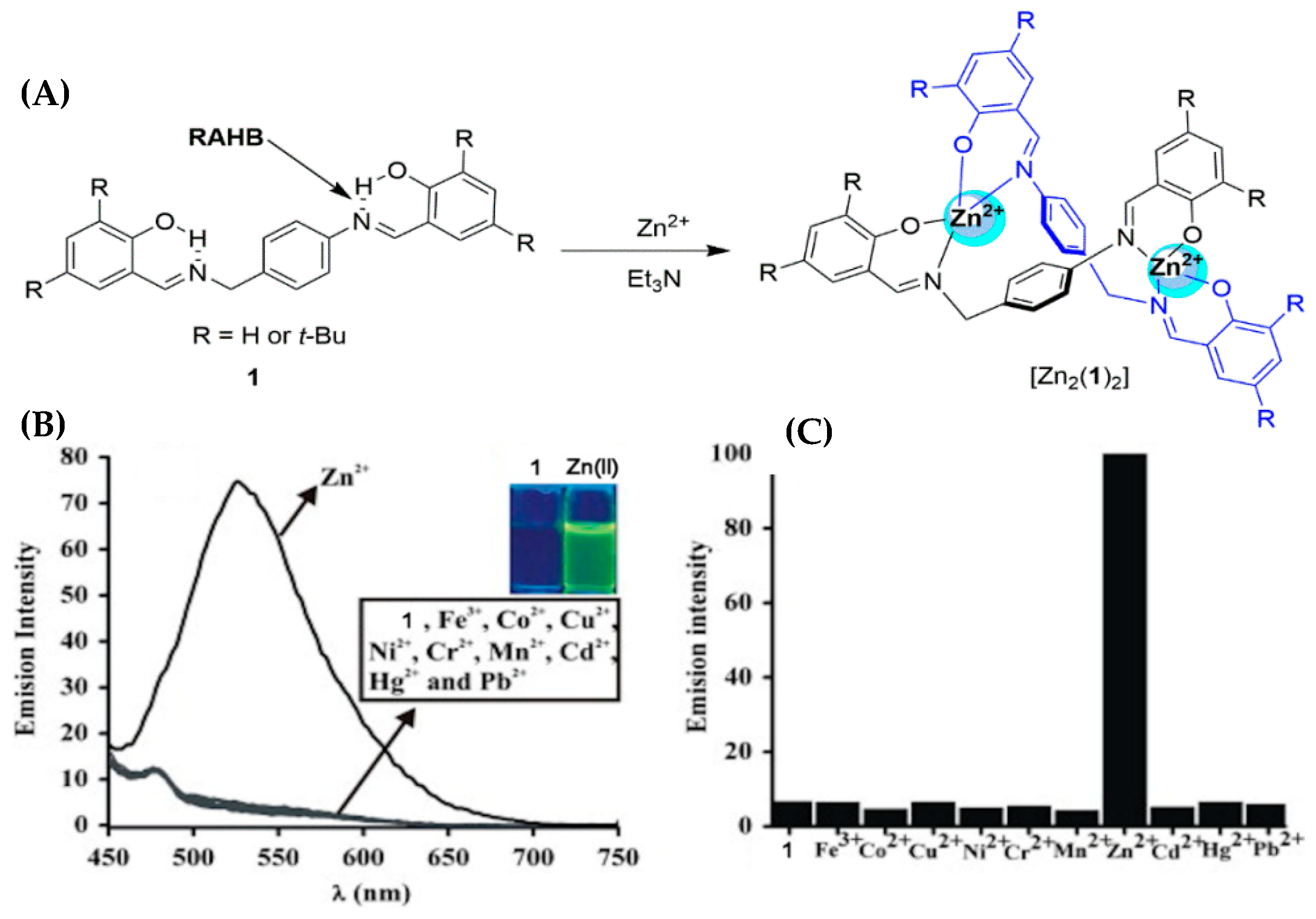
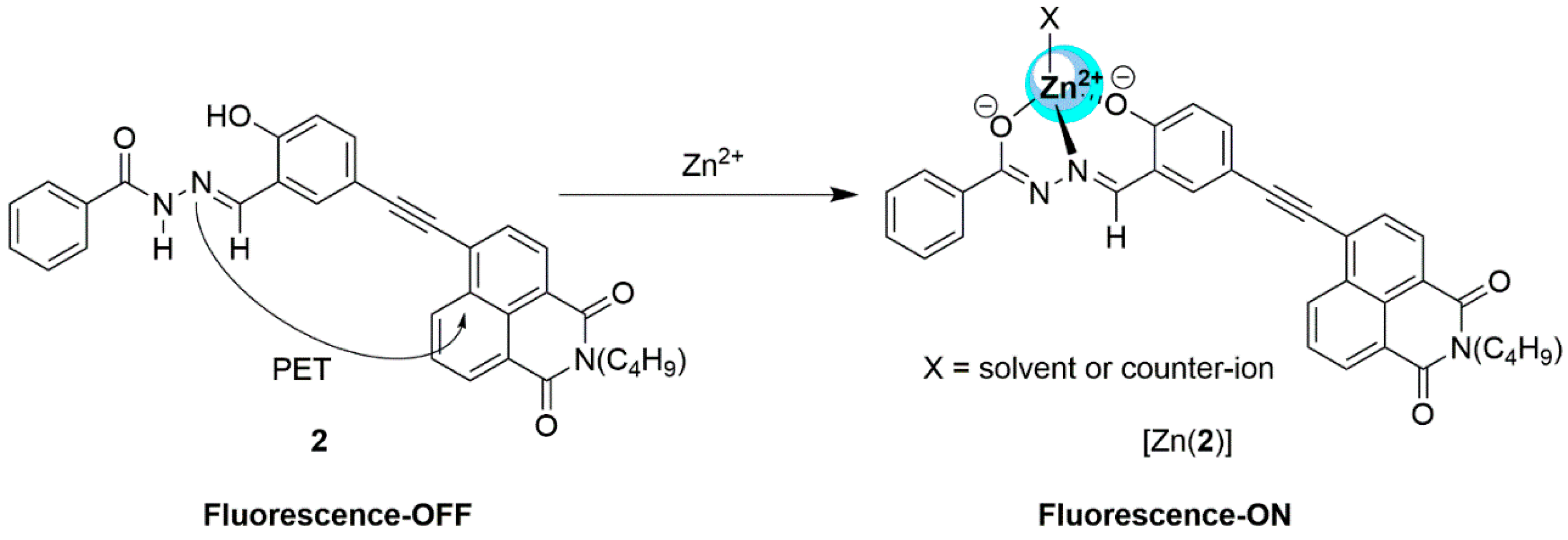
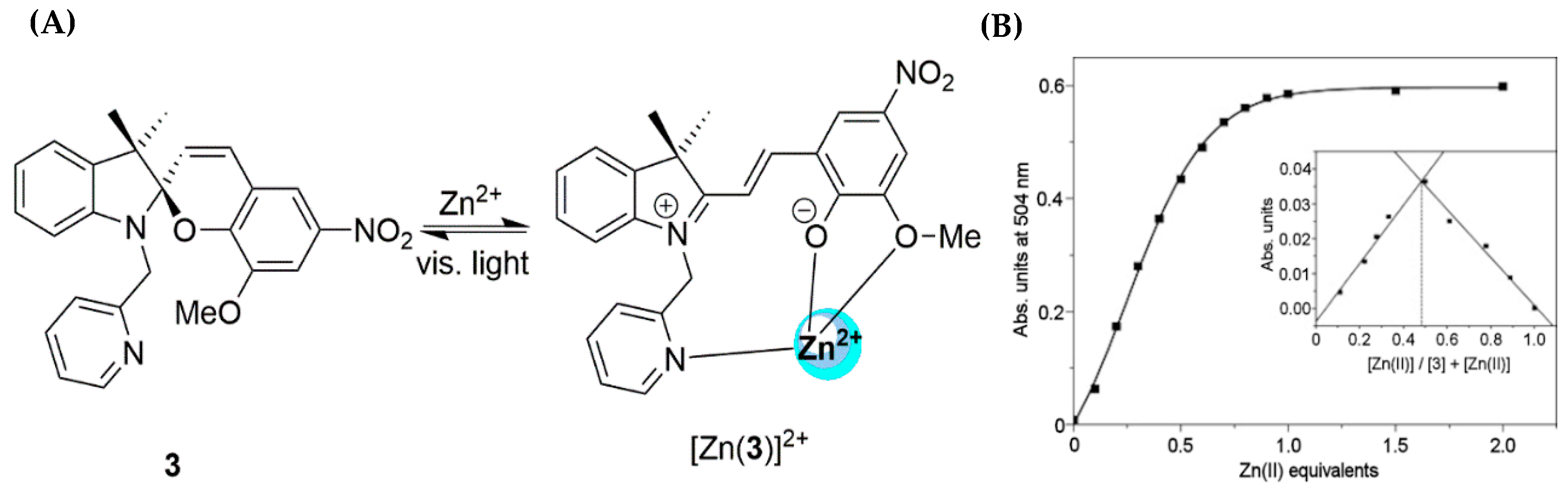
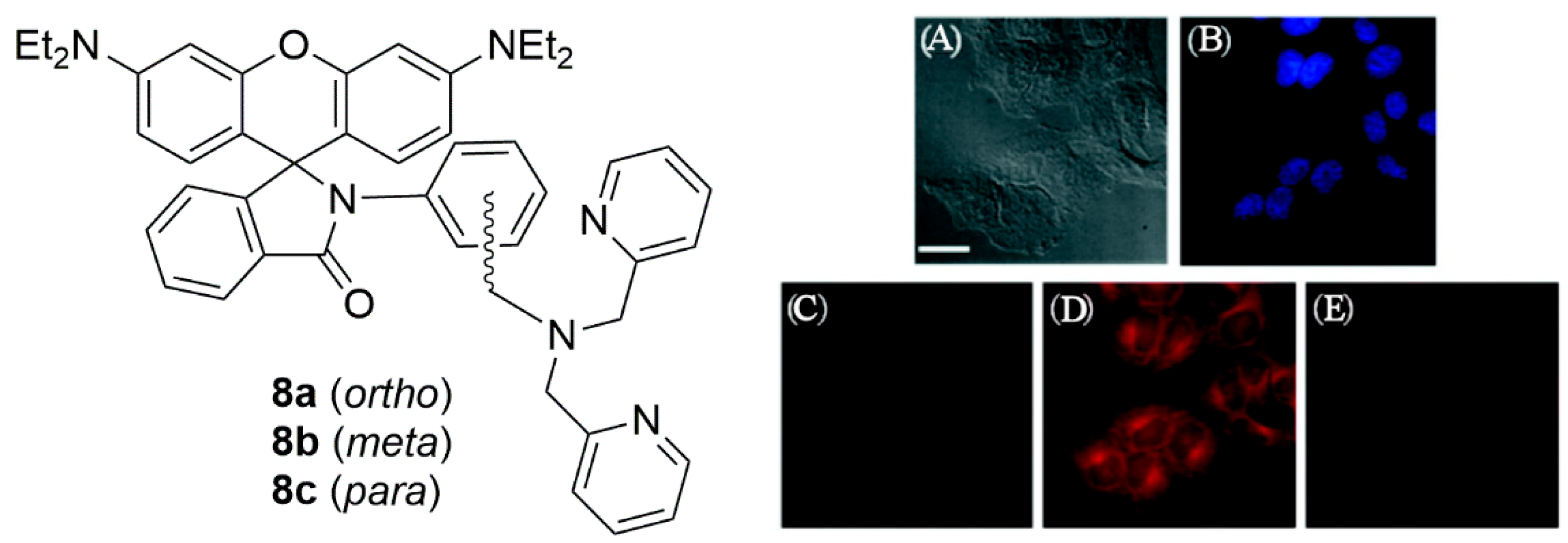
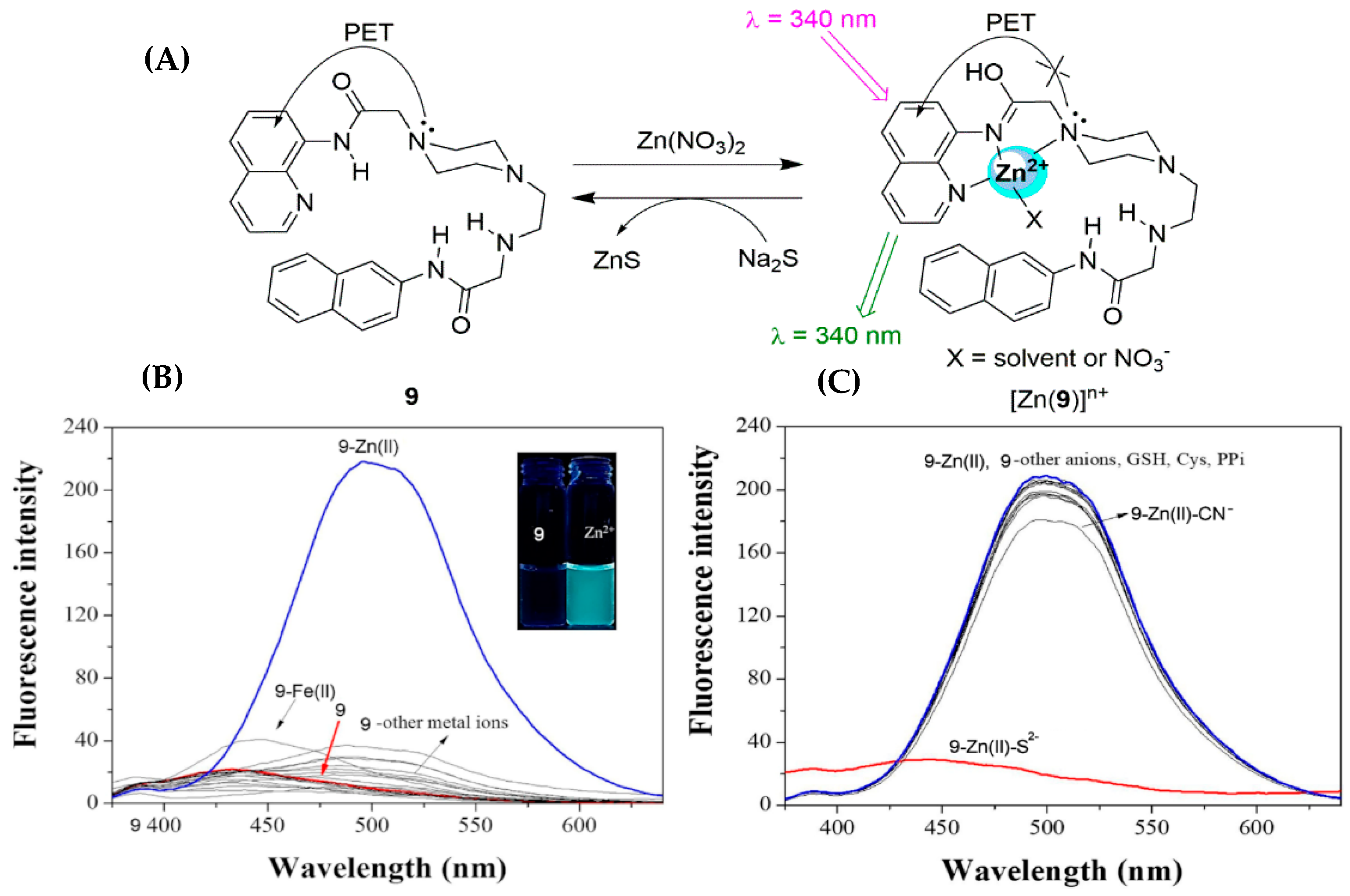
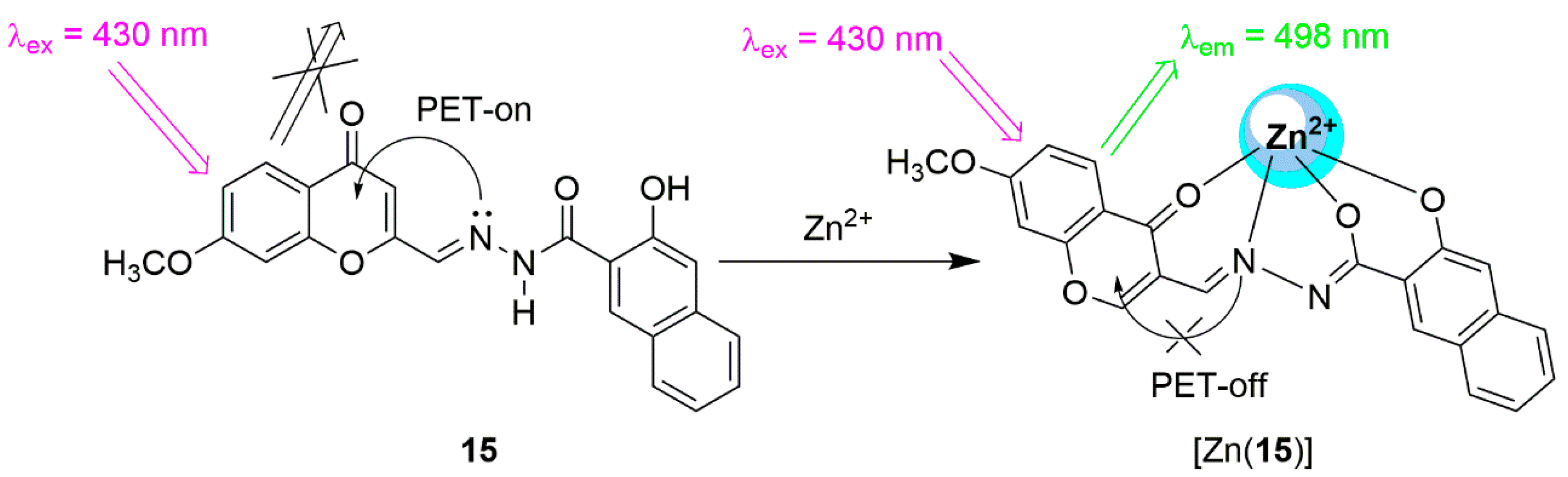
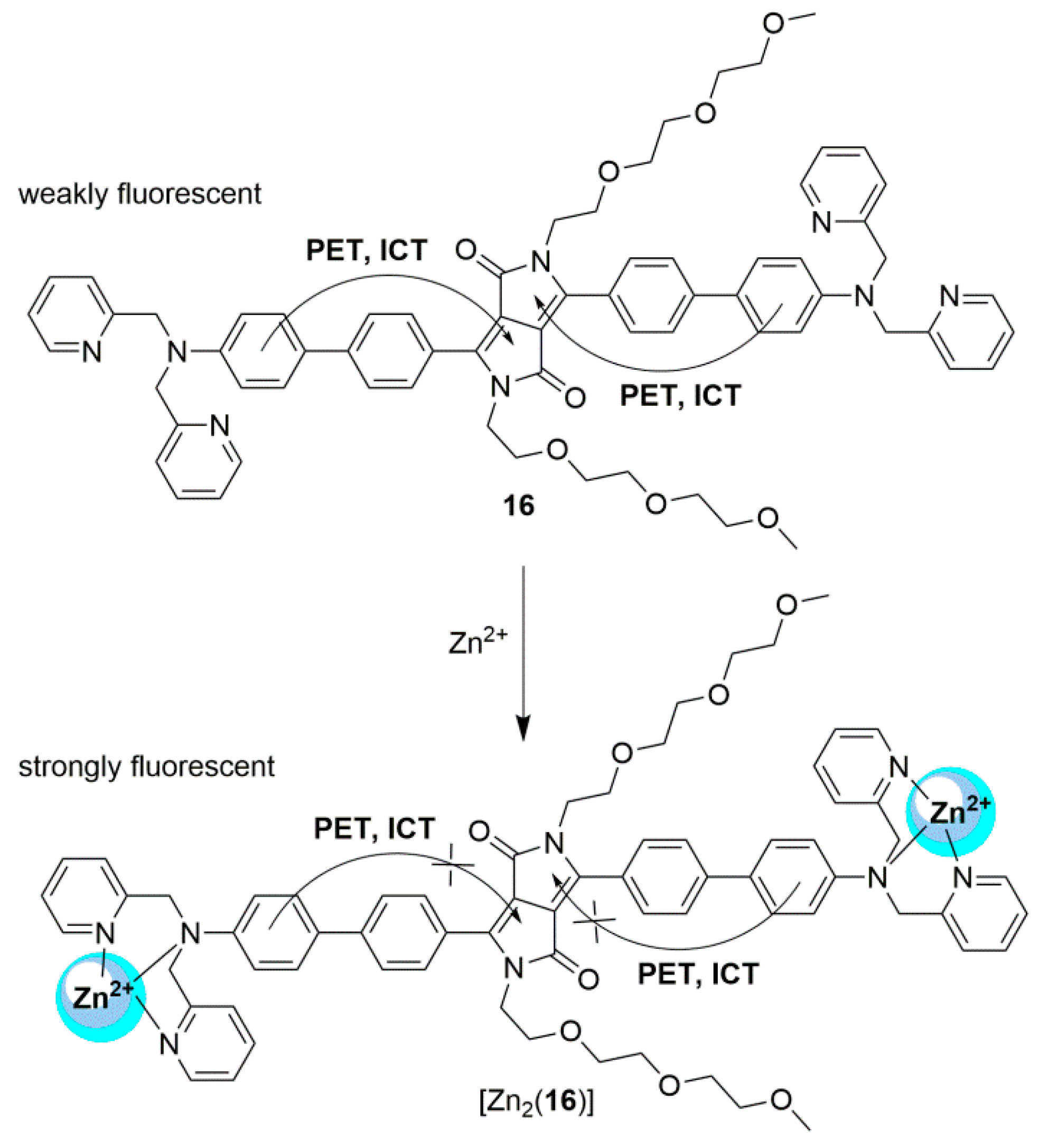
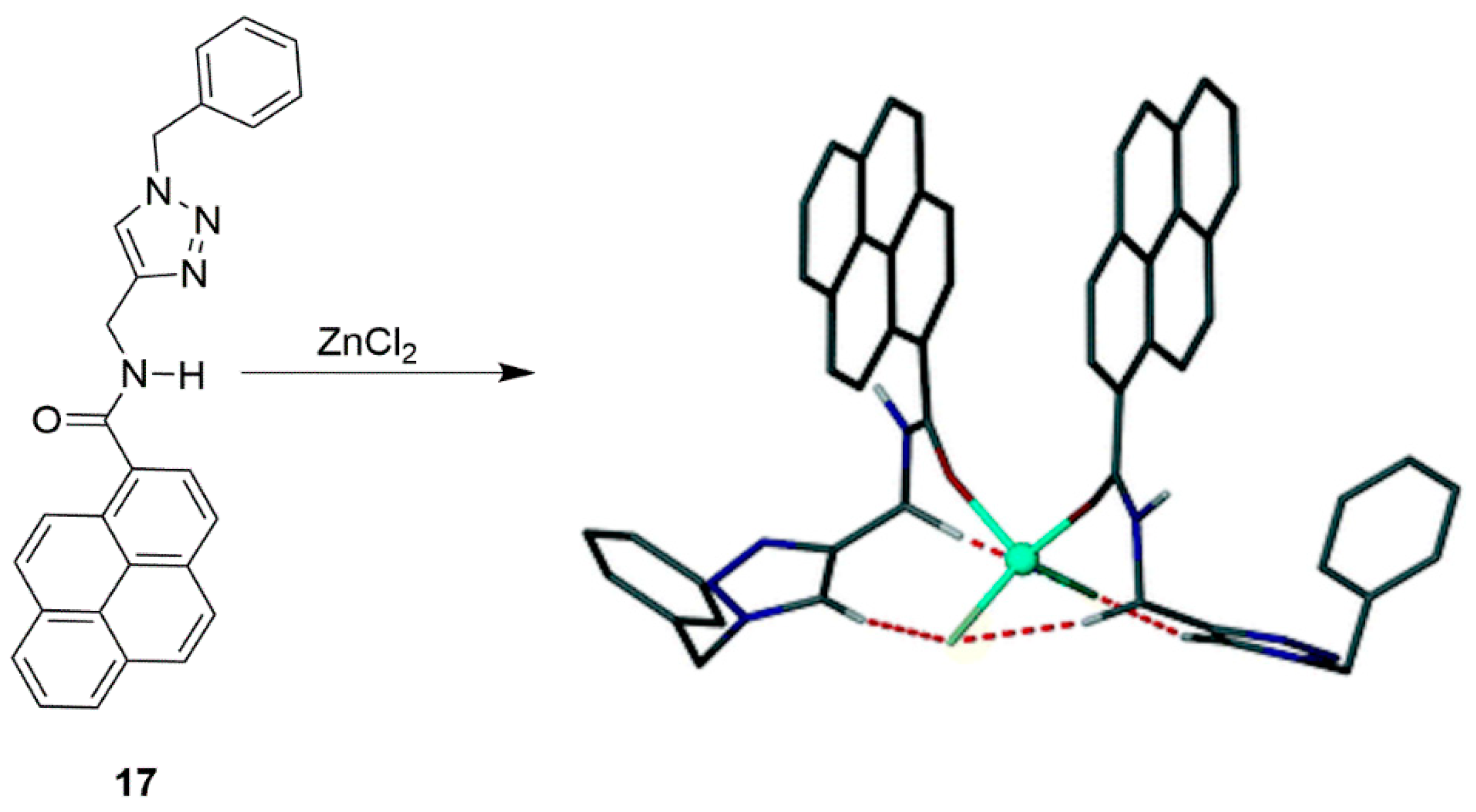
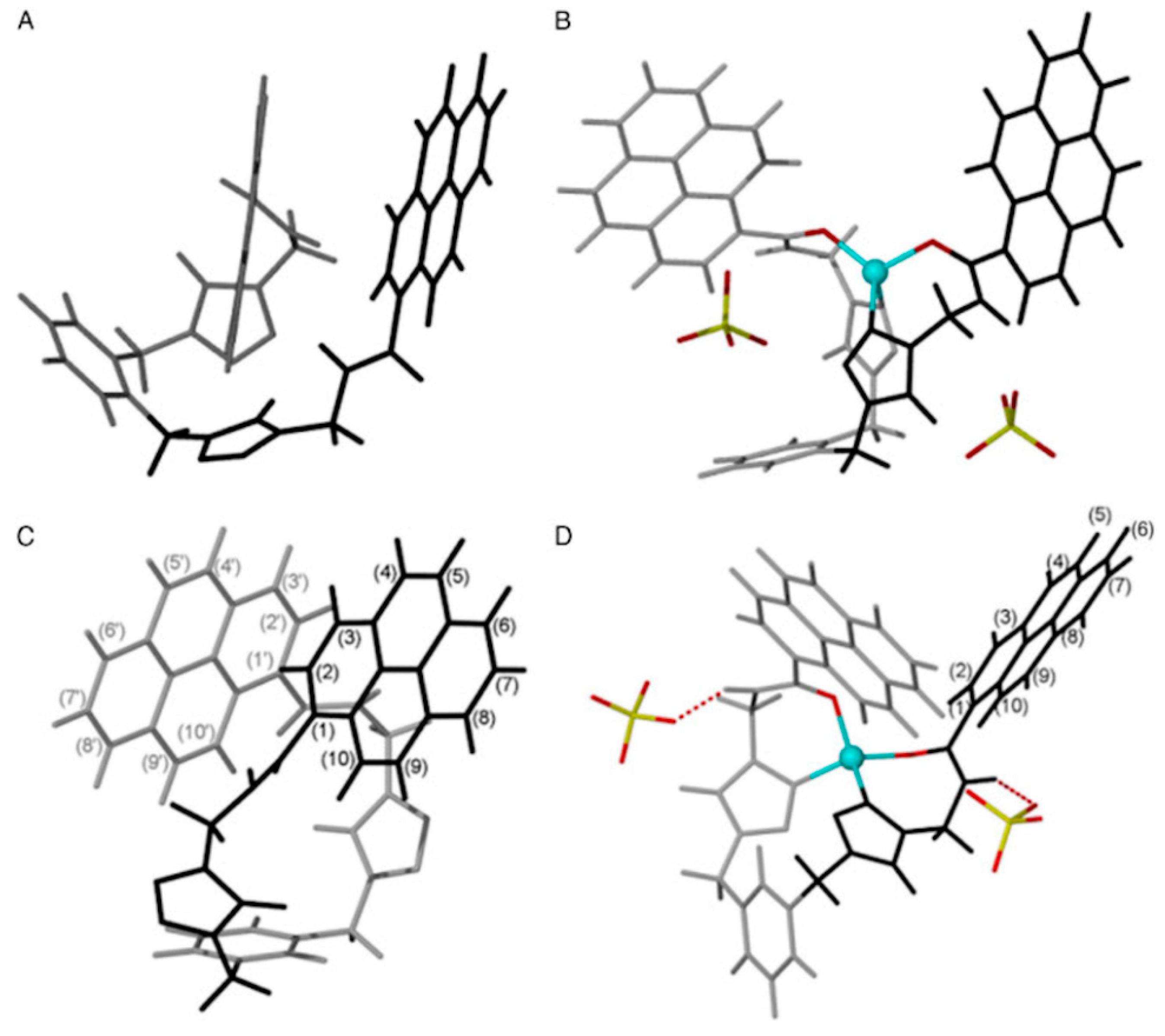
2.1. Zinc Probes
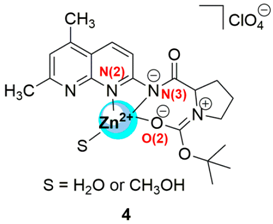
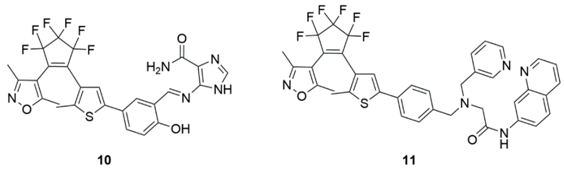
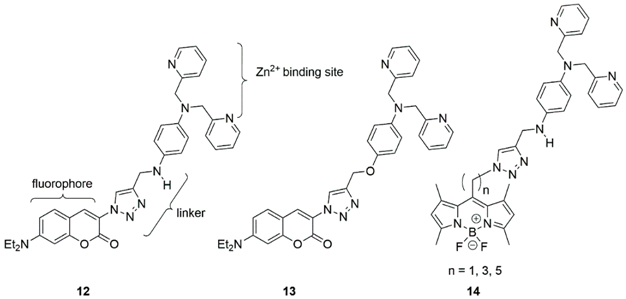
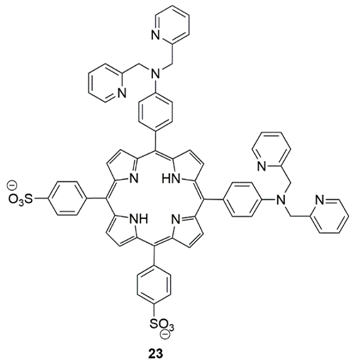
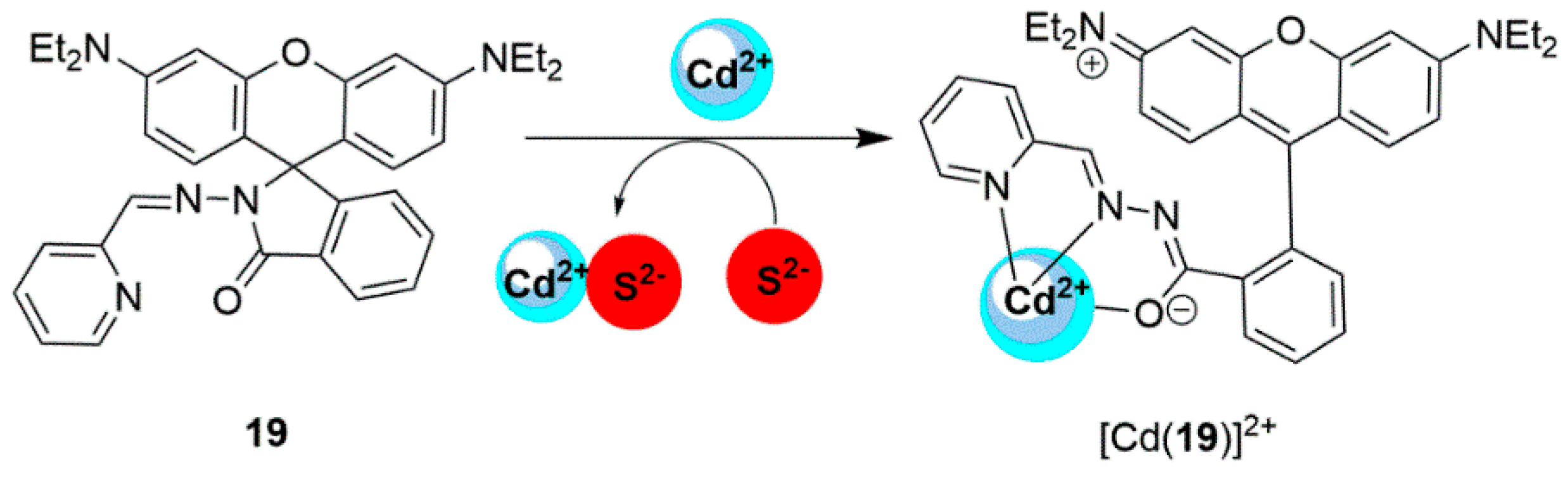
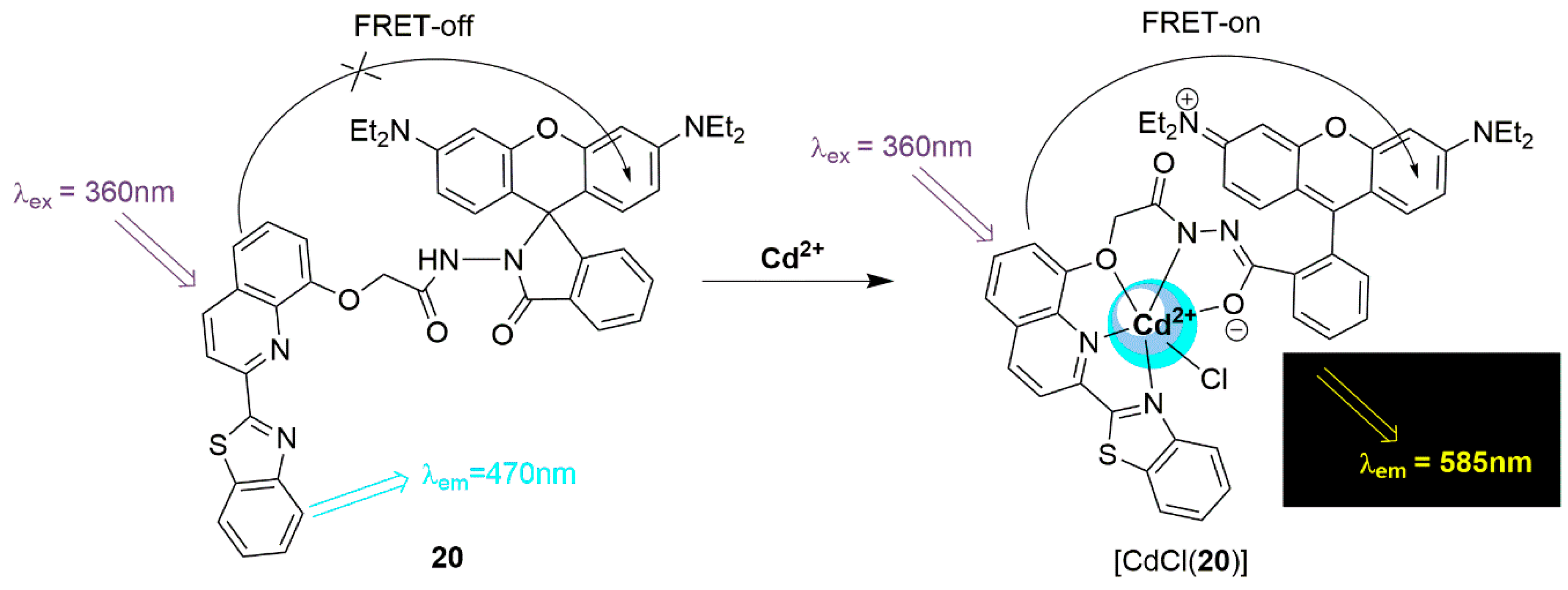
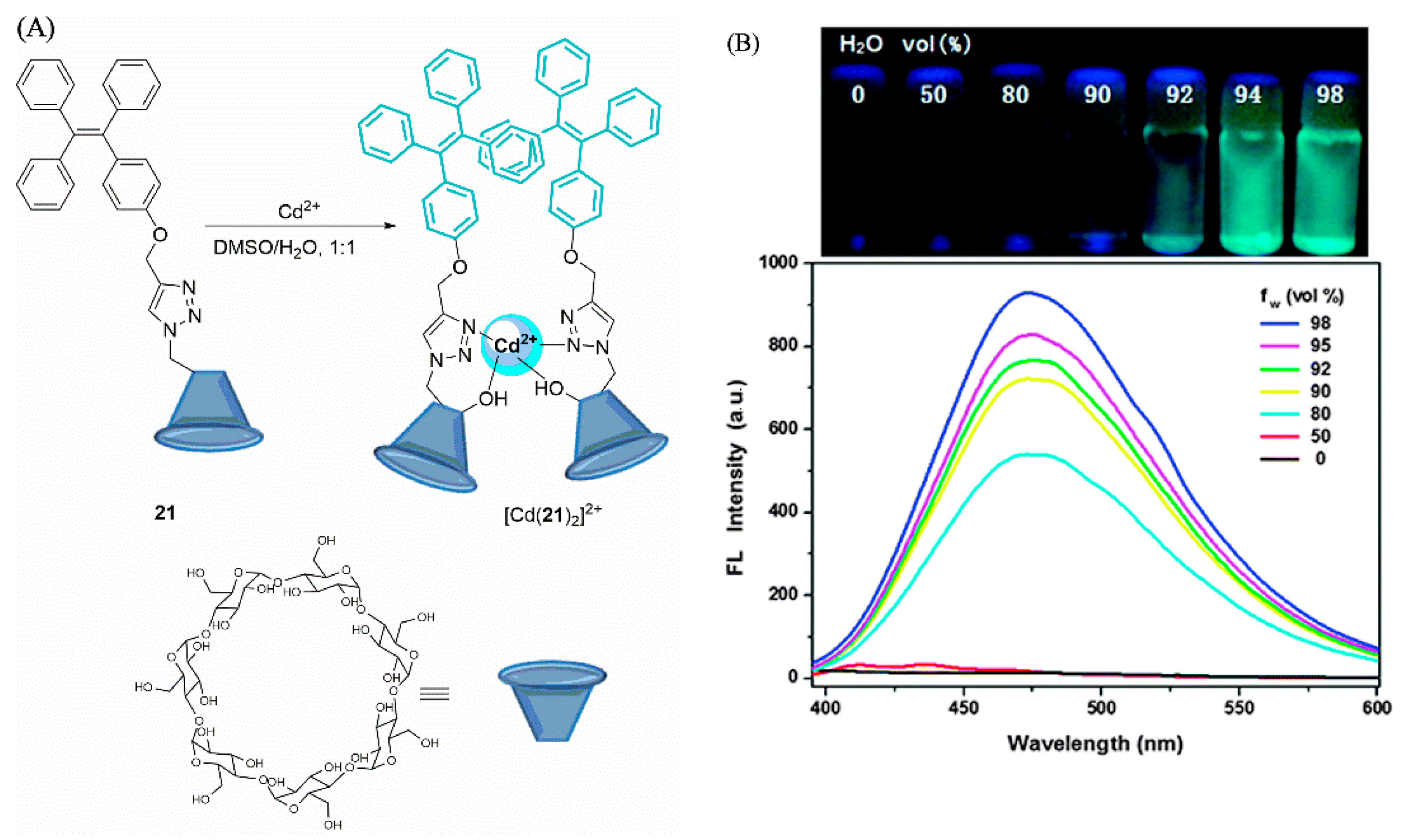
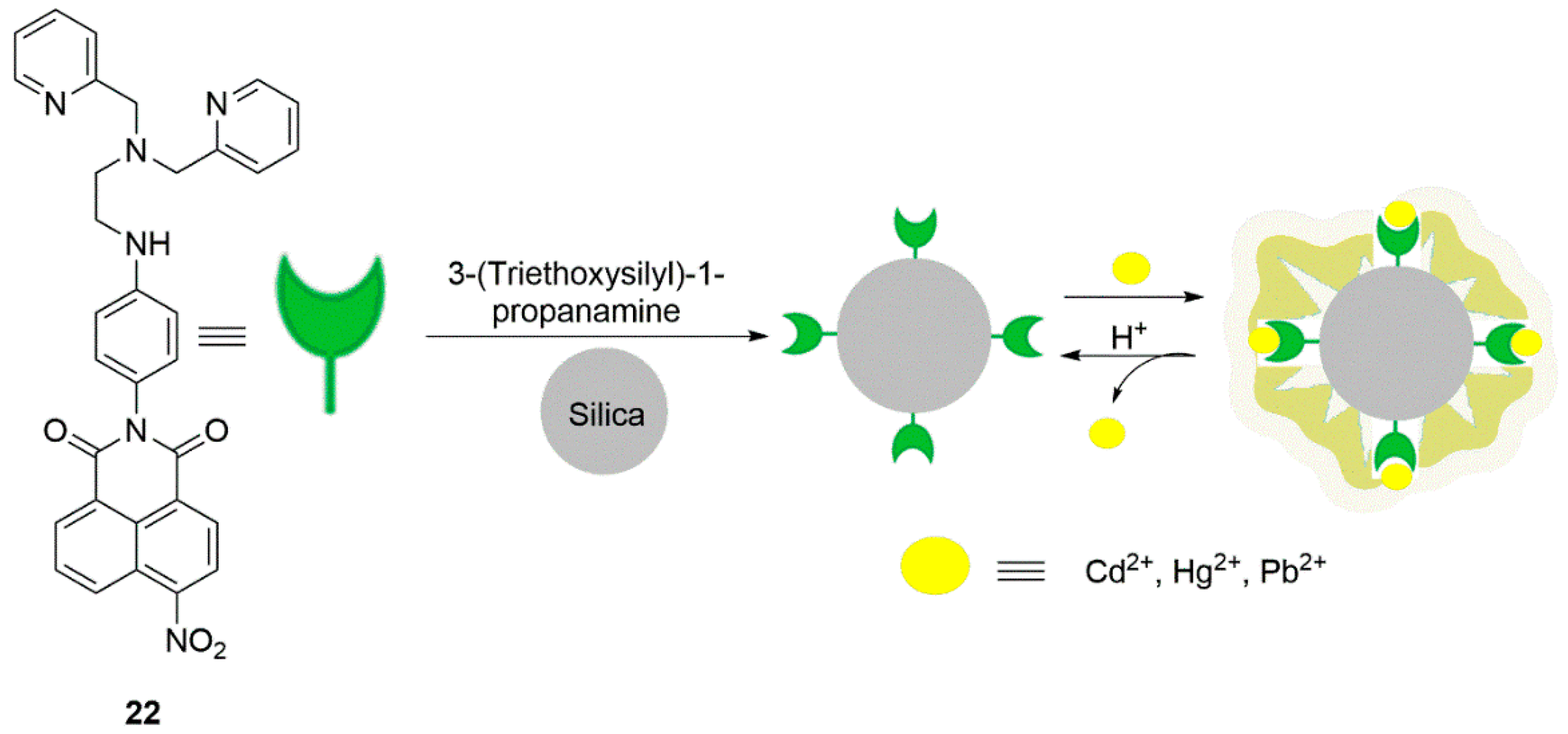
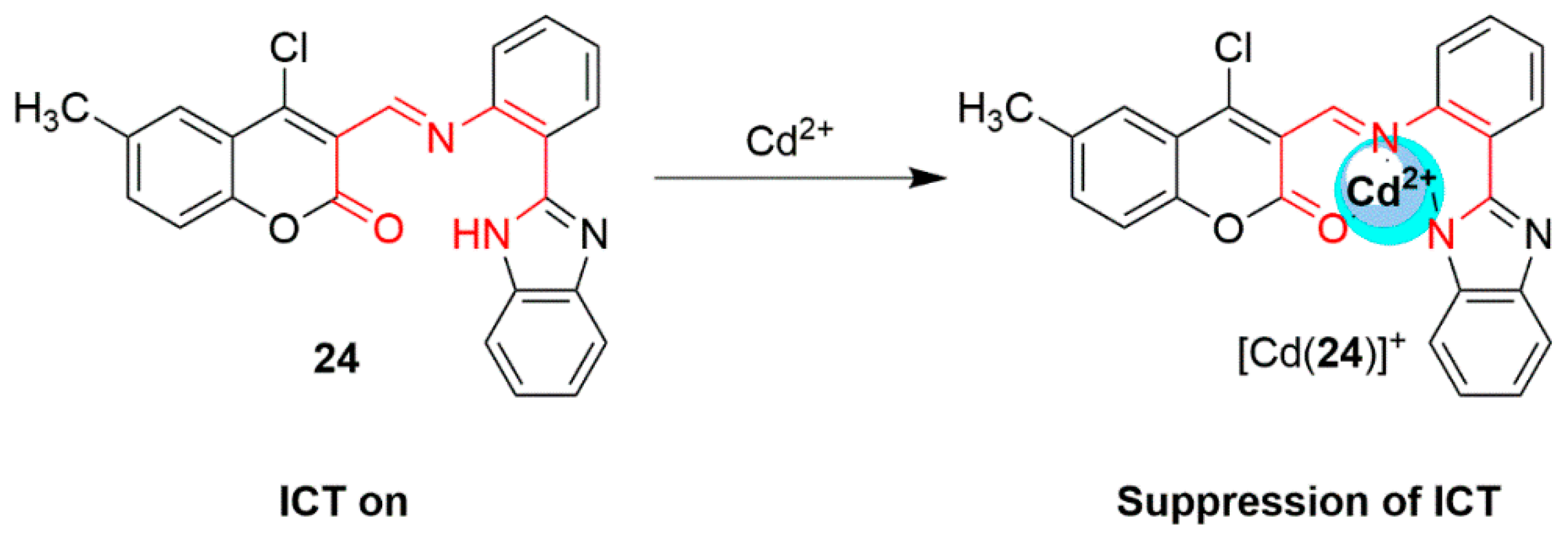
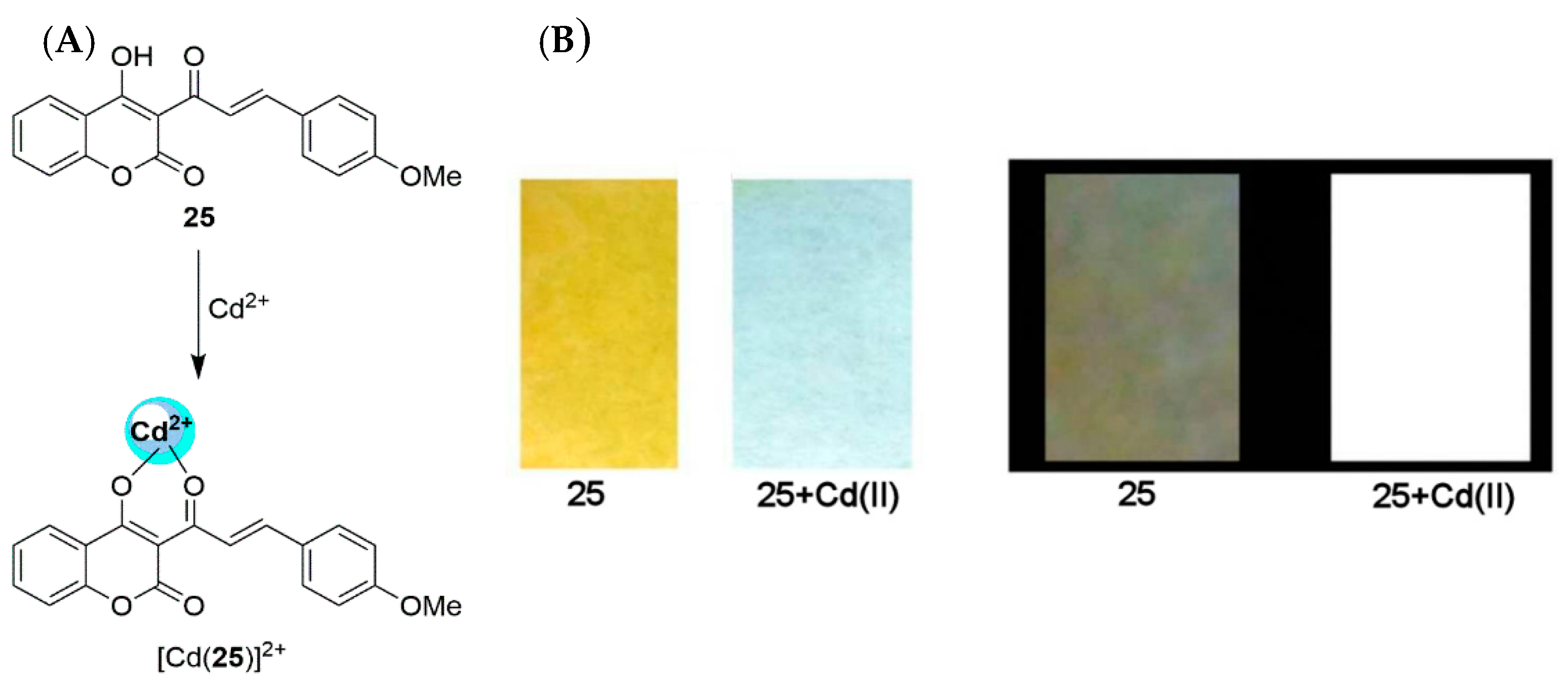
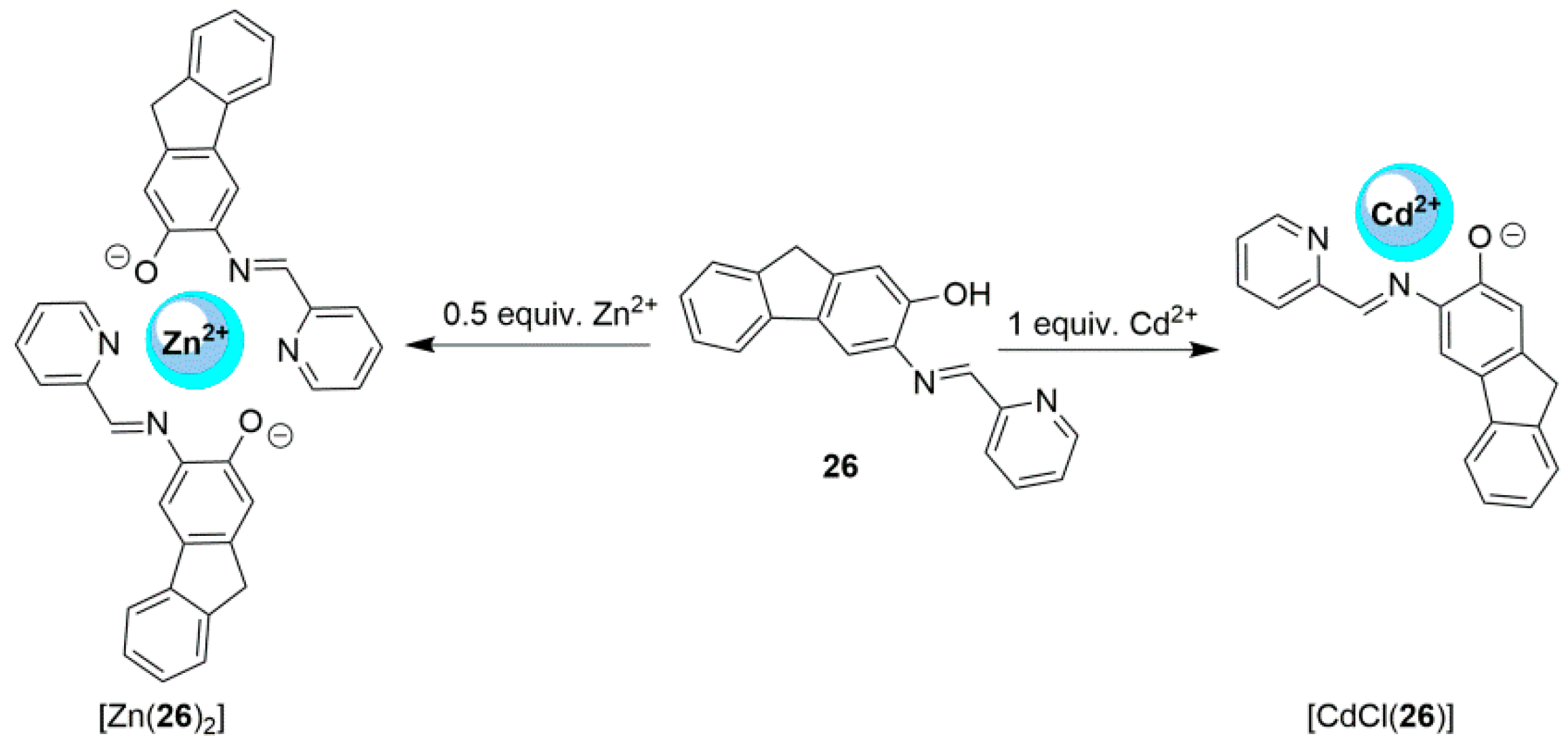
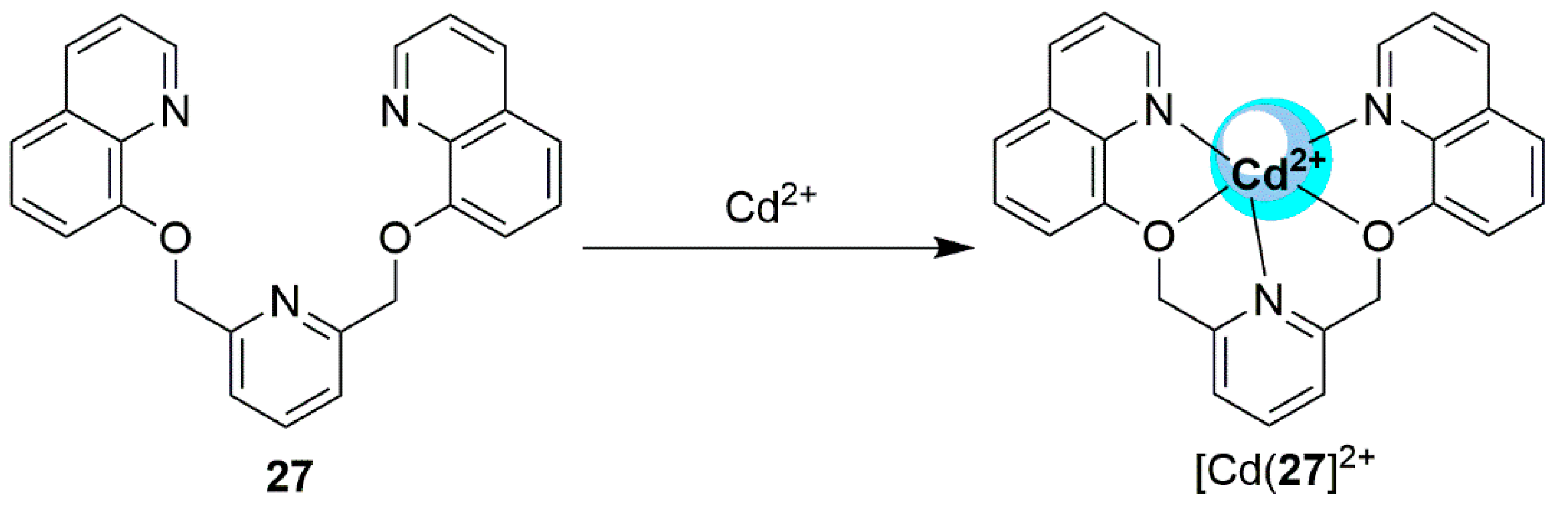
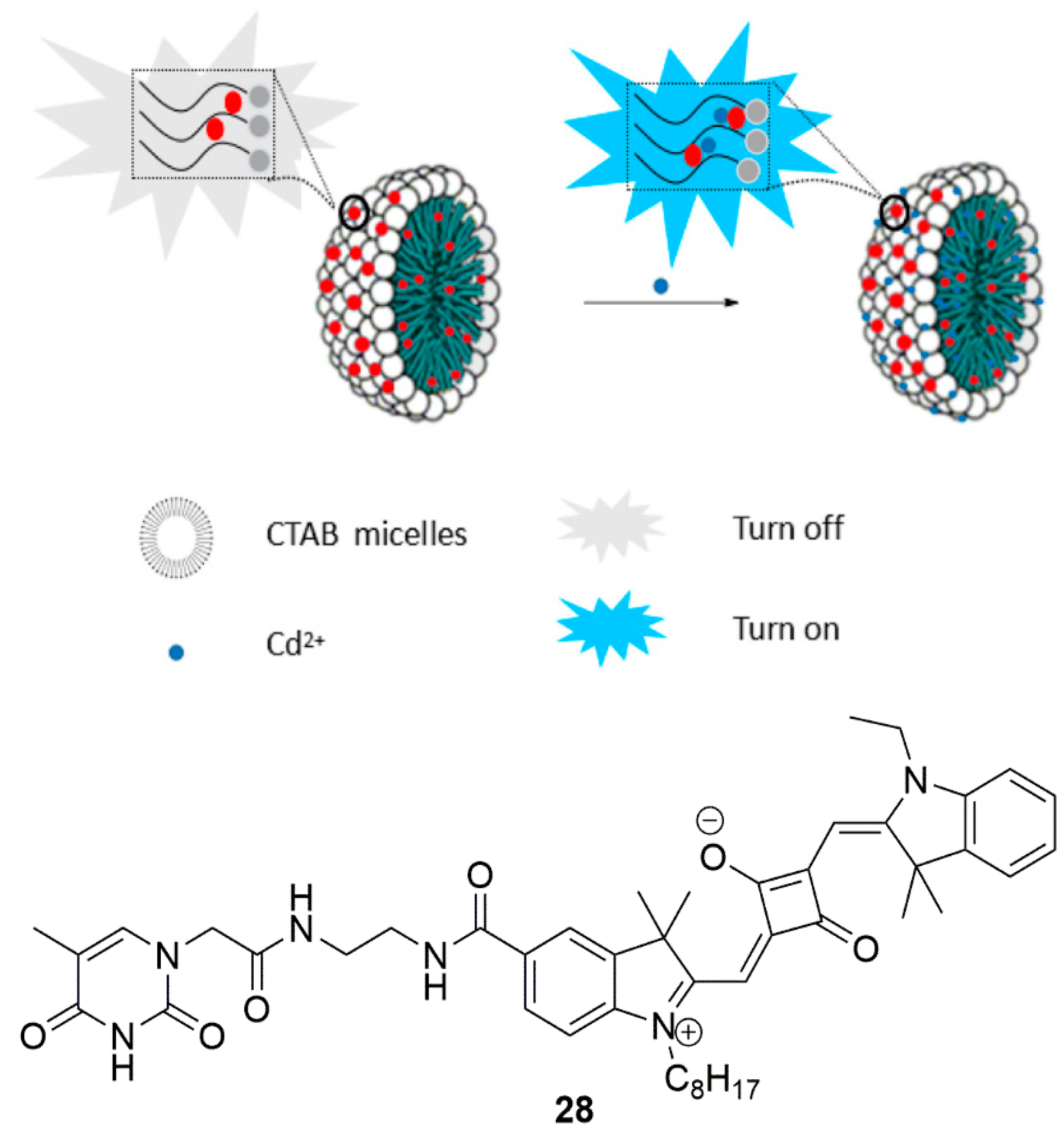
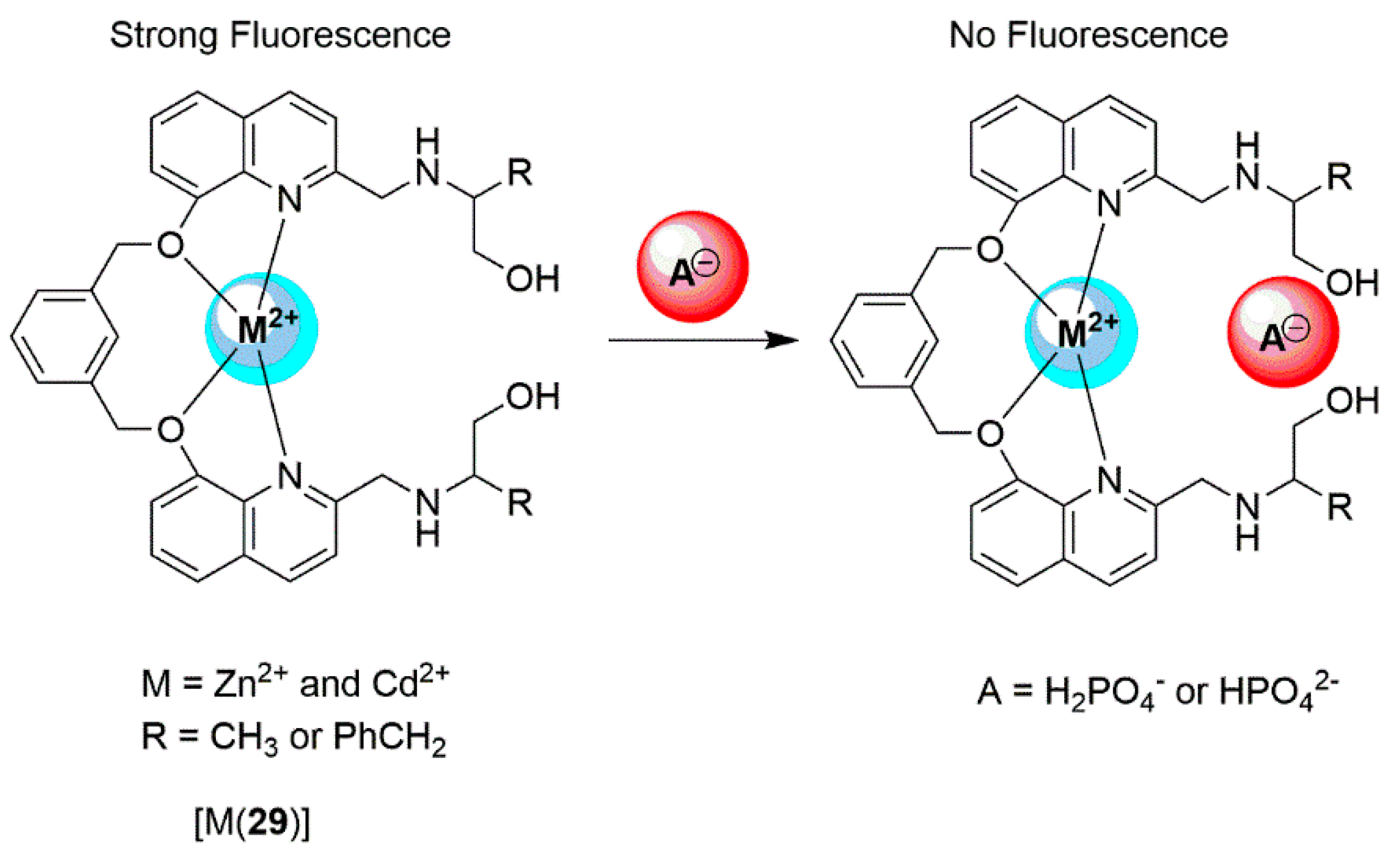
2.2. Cadmium Probes
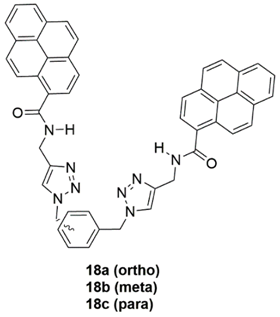
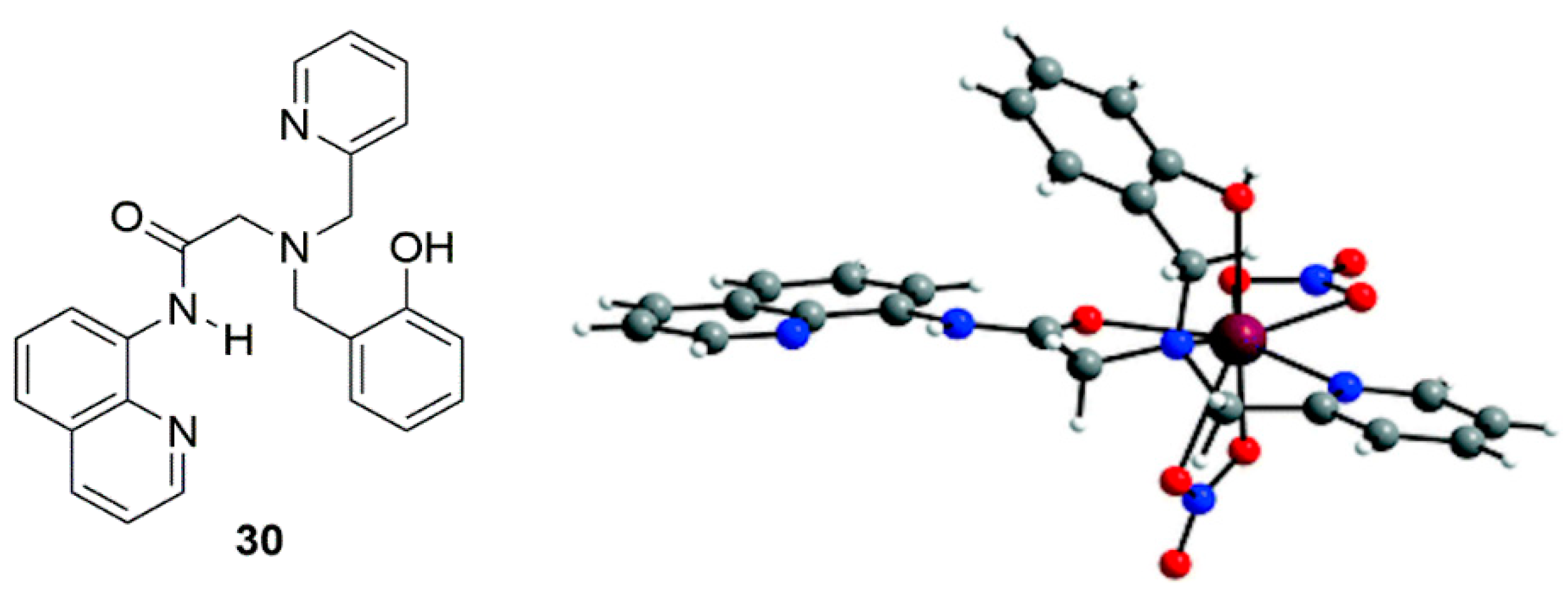
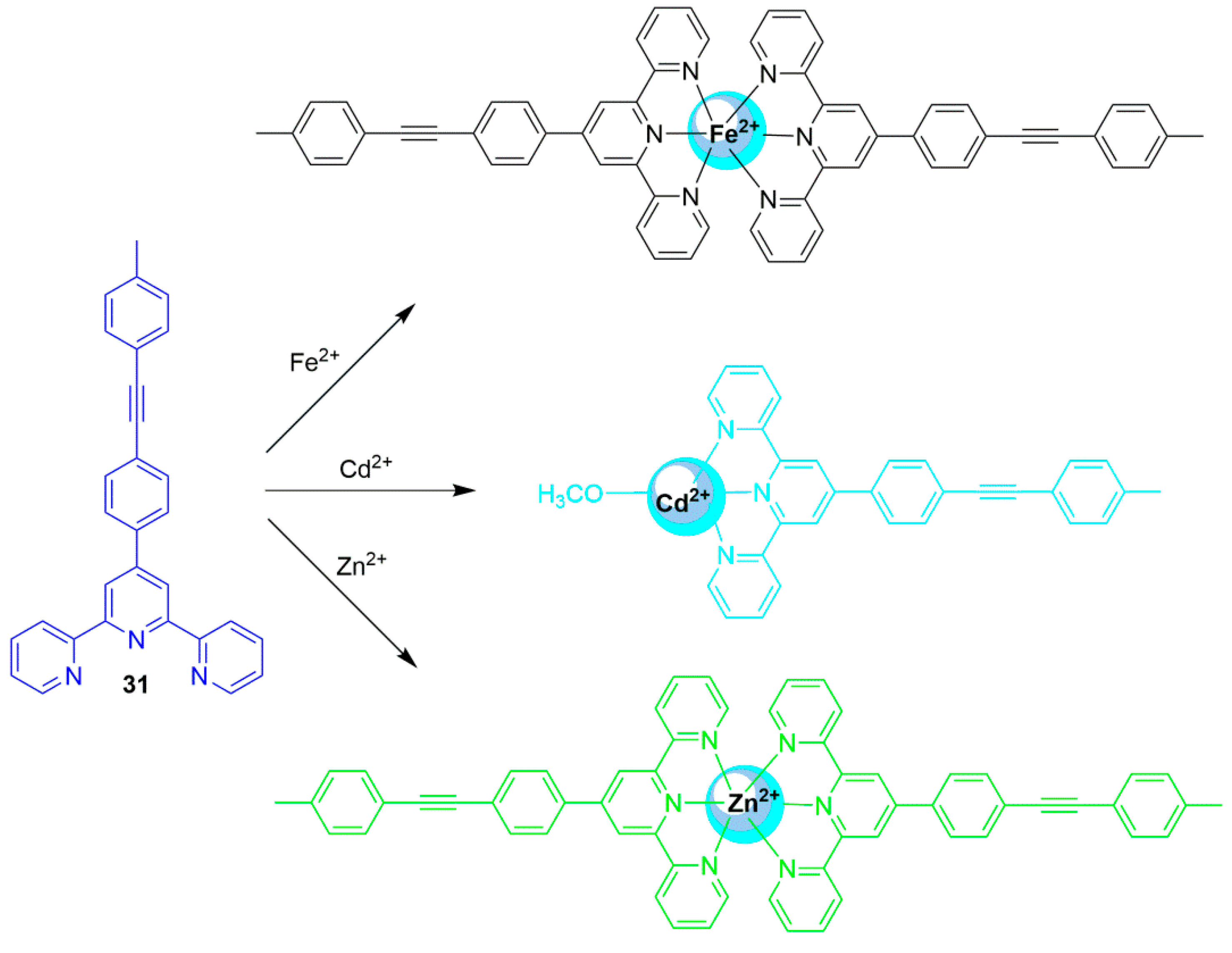
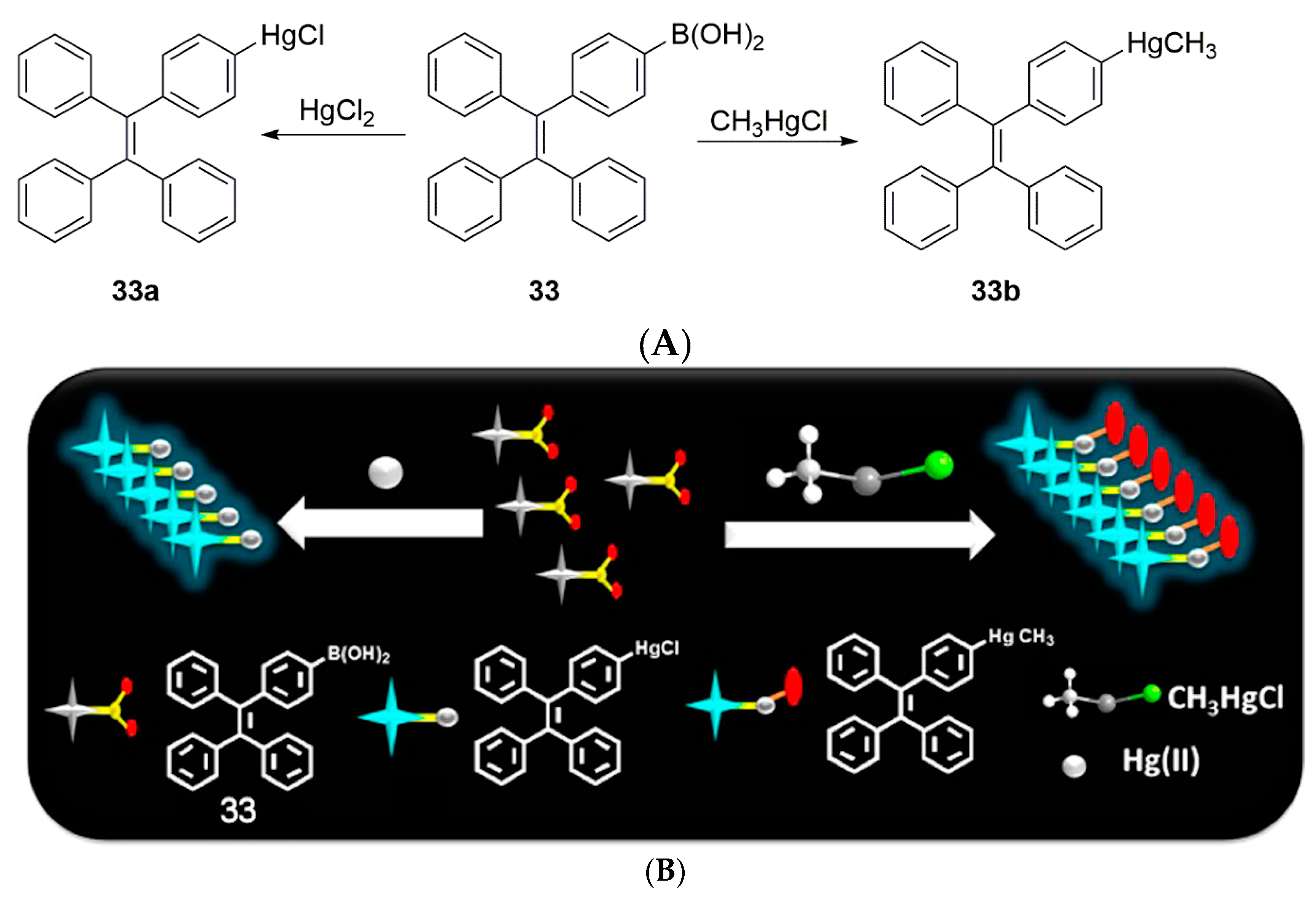
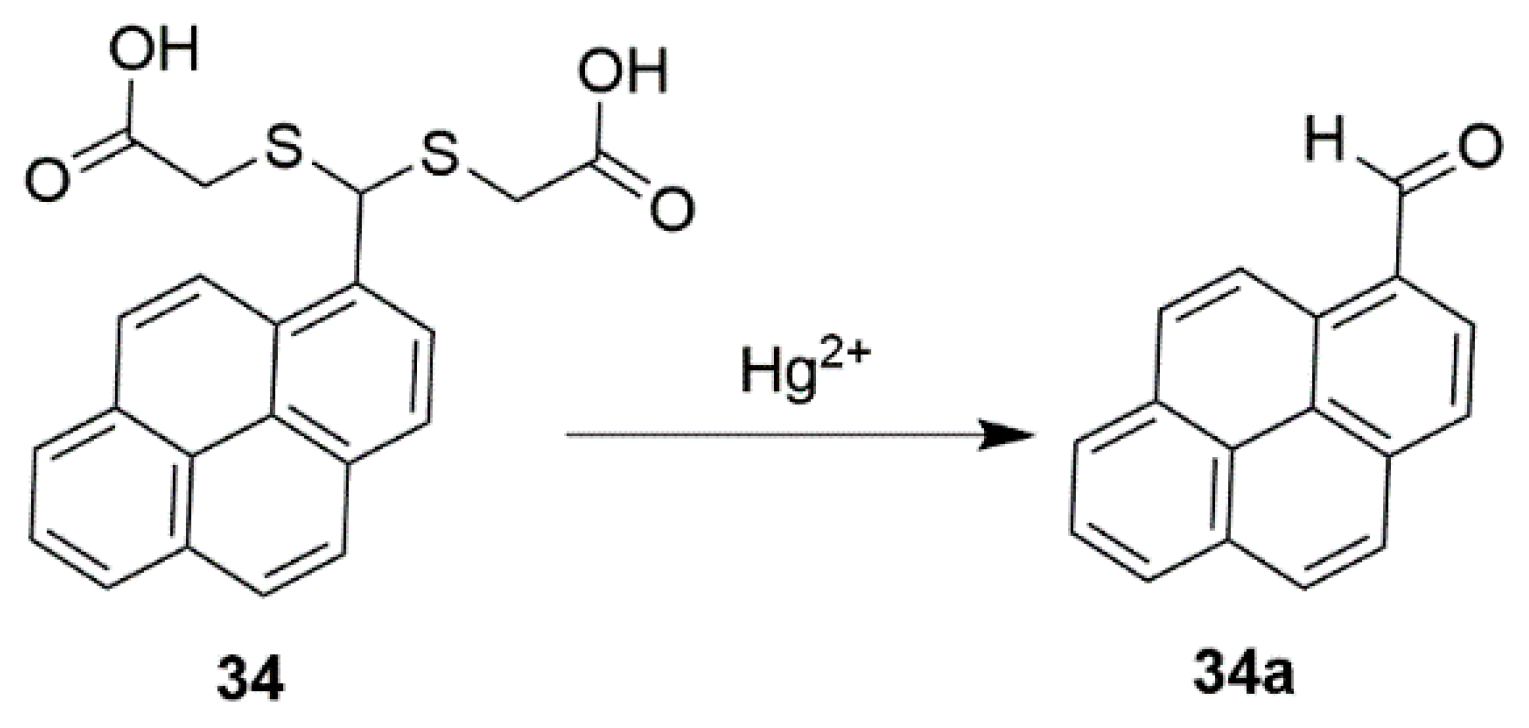
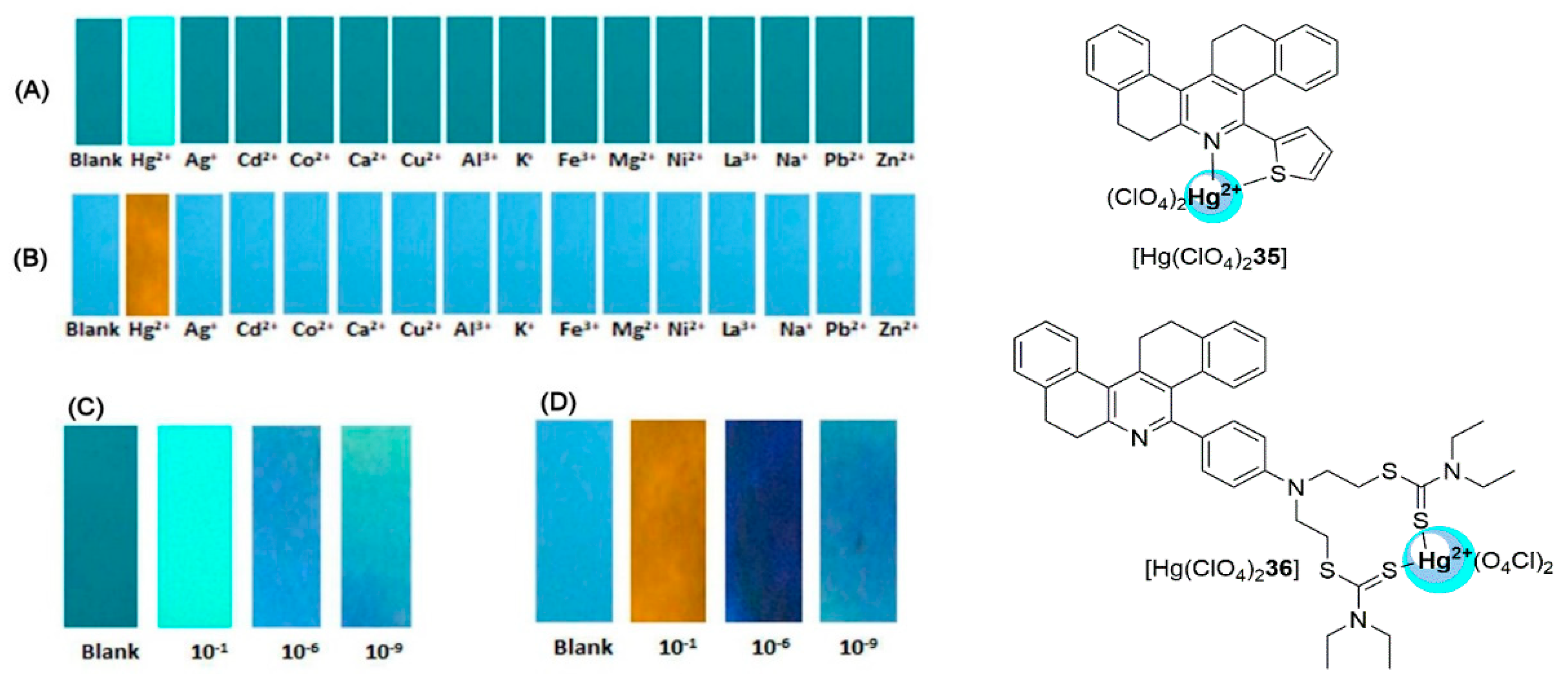
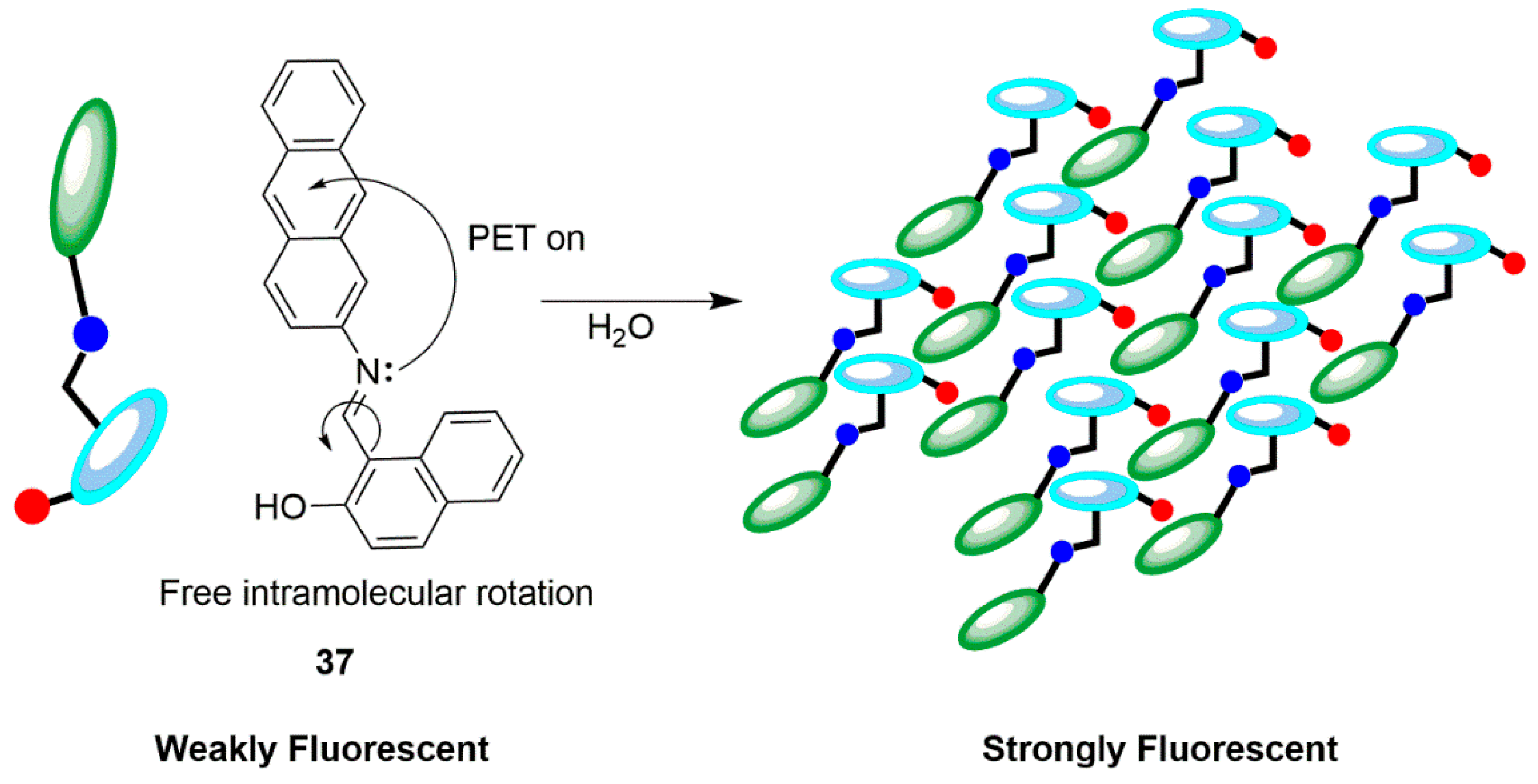
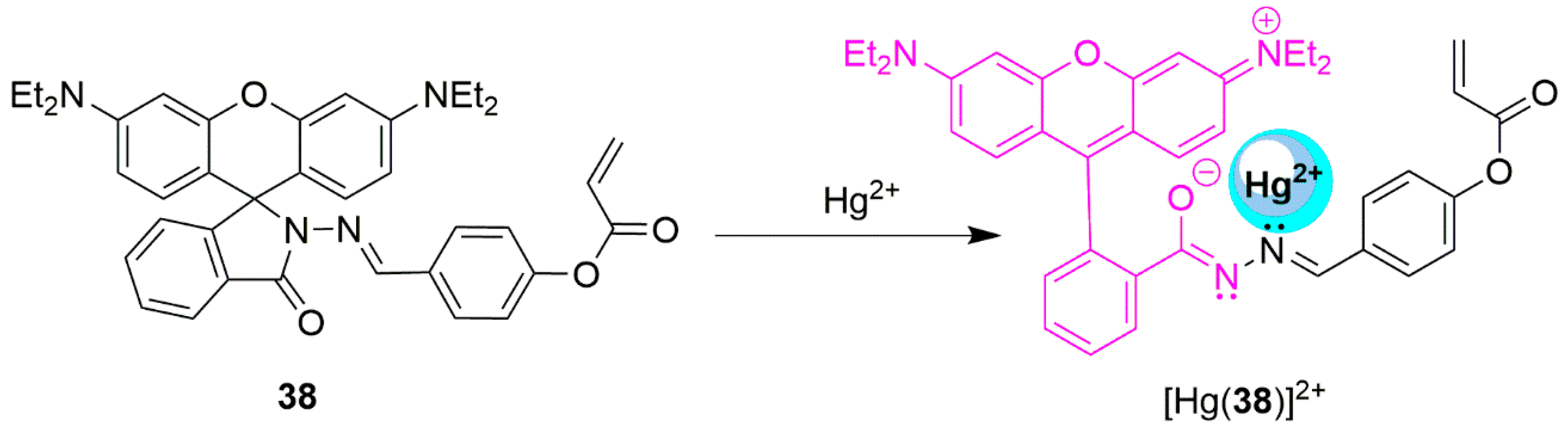
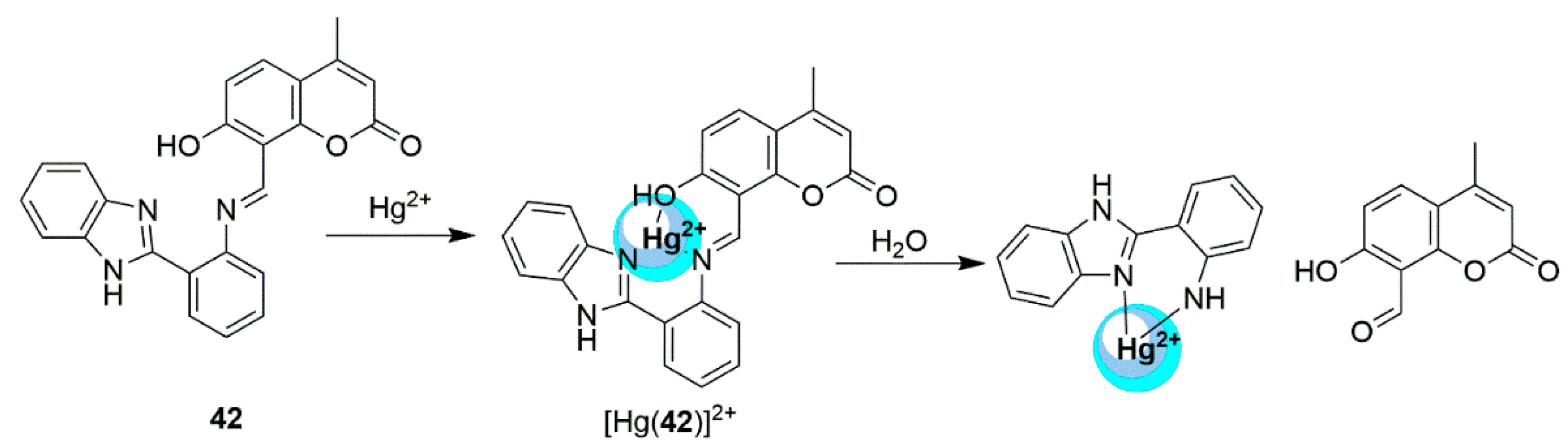
2.3. Mercury Probes
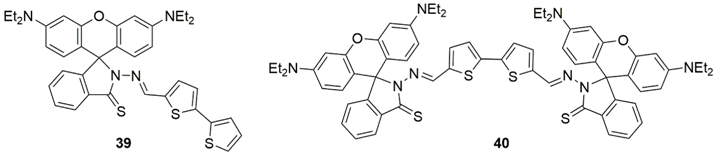
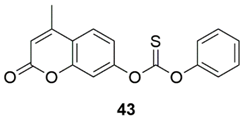
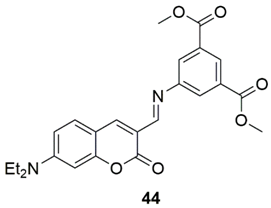
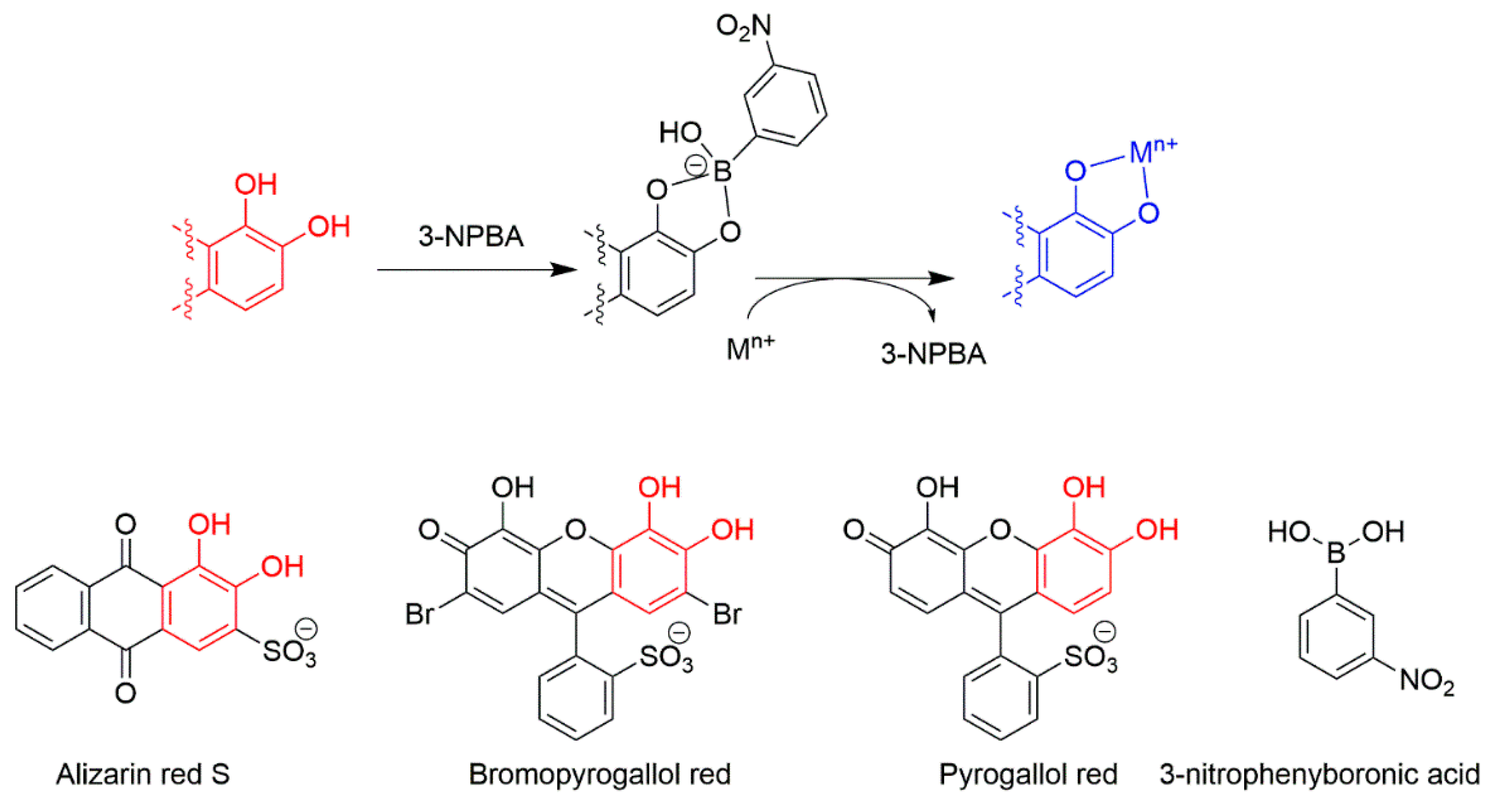
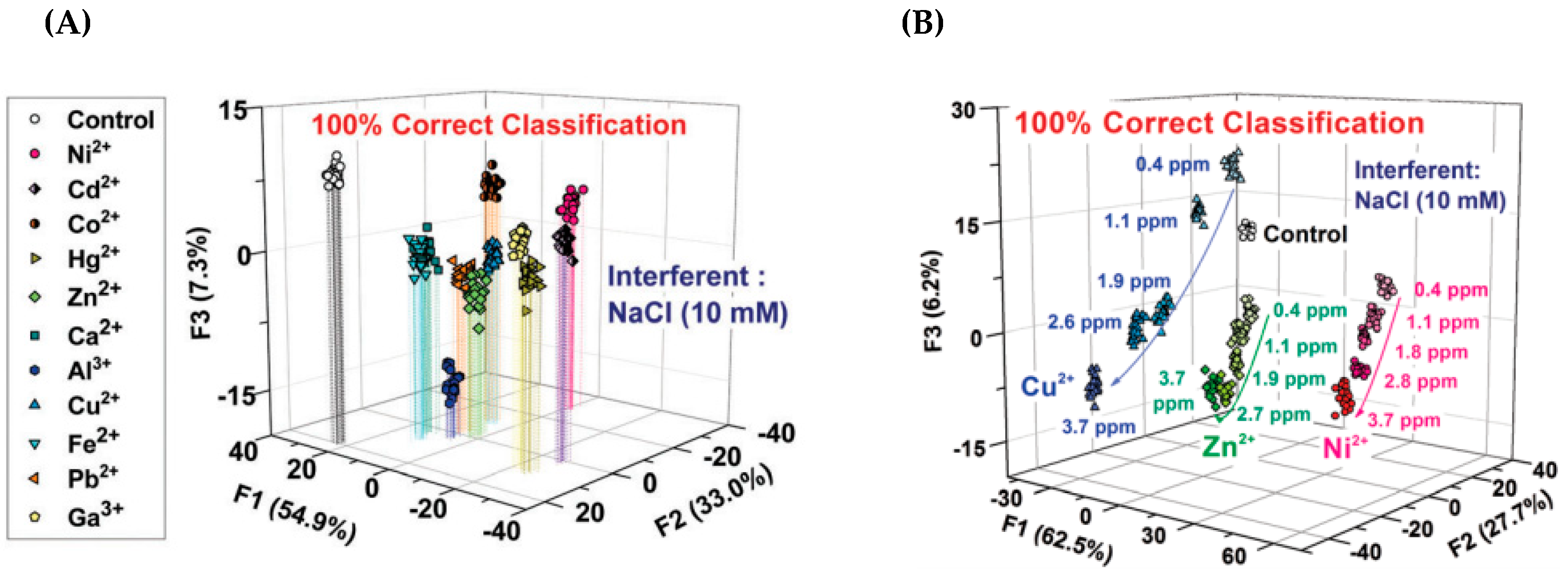
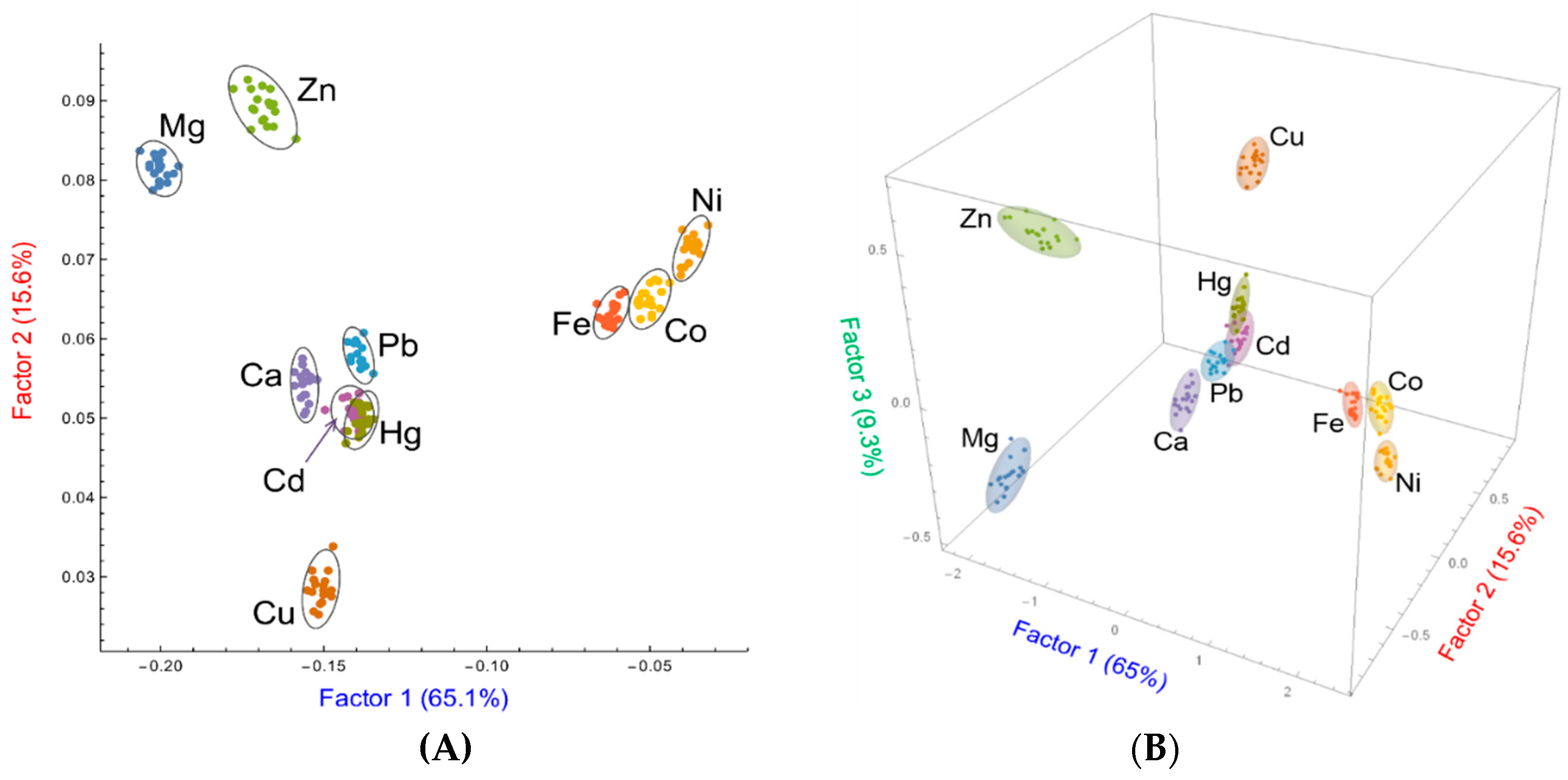
3. Multivariate Analysis

4. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eggins, B.R. Chemical Sensors and Biosensors; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Girigoswami, K.; Akhtar, N. Nanobiosensors and fluorescence based biosensors: An overview. Int. J. Nano Dimens. 2019, 10, 1–17. [Google Scholar]
- Xiong, S.; Deng, Y.; Zhou, Y.; Gong, D.; Xu, Y.; Yang, L.; Chen, H.; Chen, L.; Song, T.; Luo, A. Current progress in biosensors for organophosphorus pesticides based on enzyme functionalized nanostructures: A review. Anal. Methods 2018, 10, 5468–5479. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, J.; Zhang, P.; Zhang, Y.; Miao, Y.; Gao, S.; Deng, Y.; Geng, L. Recent advances in microfluidic chip integrated electronic biosensors for multiplexed detection. Biosens. Bioelectron. 2018, 121, 272–280. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens. Bioelectron. 2019, 124–125, 205–215. [Google Scholar] [CrossRef]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef]
- Cheng, F.; Tang, X.L.; Kardashliev, T. Transcription factor-based biosensors in high-throughput screening: Advances and applications. Biotechnol. J. 2018, 13, e1700648. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.M.; Koudelkova, Z.; Sedlackova, E.; Richtera, L.; Adam, V. Electrochemical sensors and biosensors for determination of mercury ions. J. Electrochem. Soc. 2018, 165, B824–B834. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.; Zhuang, R.; Demirci, U.; Asghar, W. Paper-based analytical devices for clinical diagnosis: Recent advances in the fabrication techniques and sensing mechanisms. Expert Rev. Mol. Diagn. 2017, 17, 351–366. [Google Scholar] [CrossRef]
- Saidur, M.; Aziz, A.A.; Basirun, W. Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A review. Biosens. Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, Y.; Dong, H.; Liu, K.; He, N. Progress on sensors based on nanomaterials for rapid detection of heavy metal ions. Sci. China Chem. 2017, 60, 329–337. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, V.S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef]
- National Research Council. Expanding the Vision of Sensor Materials; National Academemis Press: Washington, DC, USA, 1995; Chapter 6; pp. 73–88. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. IUPAC Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 2014. [Google Scholar]
- Macielag, M.J. Antibiotic Discovery and Development; Springer: Berlin/Heidelberg, Germany, 2012; pp. 793–820. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Phan, M.-H.; Peng, H.-X. Giant magnetoimpedance materials: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 323–420. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Huang, N.-J.; Yu, Z.-R.; Wang, P.-H.; Zhang, Z.-H.; Xia, Q.-Q.; Gong, L.-X.; Li, S.-N.; Zhao, L. Temperature-triggered sensitive resistance transition of graphene oxide wide-ribbons wrapped sponge for fire ultrafast detecting and early warning. J. Hazard. Mater. 2019, 363, 286–294. [Google Scholar] [CrossRef]
- Kobayashi, M. A perspective on steep-subthreshold-slope negative-capacitance field-effect transistor. Appl. Phys. Express 2018, 11, 110101. [Google Scholar] [CrossRef]
- Li, C.; Tan, Q.; Jia, P.; Zhang, W.; Liu, J.; Xue, C.; Xiong, J. Review of research status and development trends of wireless passive LC resonant sensors for harsh environments. Sensors 2015, 15, 13097–13109. [Google Scholar] [CrossRef]
- Balandin, A.A. Low-frequency 1/f noise in graphene devices. Nat. Nanotechnol. 2013, 8, 549–555. [Google Scholar] [CrossRef]
- Suárez, P.L.; García-Cortés, M.; Fernández-Argüelles, M.T.; Encinar, J.R.; Valledor, M.; Ferrero, F.J.; Campo, J.C.; Costa-Fernández, J.M. Functionalized phosphorescent nanoparticles in (bio)chemical sensing and imaging—A review. Anal. Chim. Acta 2019, 1046, 16–31. [Google Scholar] [CrossRef]
- Darr, J.A.; Poliakoff, M. New directions in inorganic and metal-organic coordination chemistry in supercritical fluids. Chem. Rev. 1999, 99, 495–542. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Fisher, A.S.; Hinds, M.W.; Lancaster, S.; Marshall, J. Atomic spectrometry update. Review of advances in the analysis of metals, chemicals and materials. J. Anal. Atom. Spectrom. 2013, 28, 1814–1869. [Google Scholar] [CrossRef]
- Shazzo, Y.K.; Karpov, Y.A. Laser sampling in inductively coupled plasma mass spectrometry in the inorganic analysis of solid samples: Elemental fractionation as the main source of errors. J. Anal. Chem. 2016, 71, 1069–1080. [Google Scholar] [CrossRef]
- Chen, S.-H.; Li, Y.-X.; Li, P.-H.; Xiao, X.-Y.; Jiang, M.; Li, S.-S.; Zhou, W.-Y.; Yang, M.; Huang, X.-J.; Liu, W.-Q. Electrochemical spectral methods for trace detection of heavy metals: A review. TrAC Trends Anal. Chem. 2018, 106, 139–150. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar]
- West, M.; Ellis, A.T.; Potts, P.J.; Streli, C.; Vanhoof, C.; Wobrauschek, P. 2016 Atomic Spectrometry update—A review of advances in X-ray fluorescence spectrometry and its applications. J. Anal. Atom. Spectrom. 2016, 31, 1706–1755. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.N.; Gunnlaugsson, T.; Huxley, A.J.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Kang, J.; Huo, F.; Zhang, Y.; Chao, J.; Glass, T.E.; Yin, C. A novel near-infrared ratiometric fluorescent probe for cyanide and its bioimaging applications. Spectrochim. Acta Part A 2019, 209, 95–99. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Khademabbasi, K. Heavy atom quenching of CdS quantum dots photoluminescence: Evidences for electron transfer mechanism. J. Lumin. 2018, 204, 130–134. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Yu, J.; Yersin, H. Blue-light emission of Cu(I) complexes and singlet harvesting. Inorg. Chem. 2011, 50, 8293–8301. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Birks, J. Excimers. Rep. Prog. Phys. 1975, 38, 903–974. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, Y.; Zhao, X.; Wang, L.; Ma, S.; Han, X.; Xu, B.; Tian, W.; Gao, H. Highly efficient far red/near-infrared solid fluorophores: Aggregation-induced emission, intramolecular charge transfer, twisted molecular conformation, and bioimaging applications. Angew. Chem. Int. Ed. 2016, 55, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W. Coordination and organometallic compounds as anion receptors and sensors. Chem. Soc. Rev. 2009, 38, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Fabbrizzi, L.; Mosca, L. Anion recognition by hydrogen bonding: Urea-based receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. [Google Scholar] [CrossRef]
- Hua, Y.; Flood, A.H. Click chemistry generates privileged CH hydrogen-bonding triazoles: The latest addition to anion supramolecular chemistry. Chen. Soc. Rev. 2010, 39, 1262–1271. [Google Scholar] [CrossRef]
- Dougherty, D.A. The cation−π interaction. Acc. Chem. Res. 2012, 46, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, F.; Dong, S.; Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular polymeric materials via cyclodextrin–guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Howe, E.N.; Wu, X. Anion receptor chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef]
- Schmidt, B.V.; Barner-Kowollik, C. Dynamic macromolecular material design—The versatility of cyclodextrin-based host–guest chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Howe, E.N.; Gale, P.A. Supramolecular transmembrane anion transport: New assays and insights. Acc. Chem. Res. 2018, 51, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, D.; Yang, X.-J.; Wu, B. Anion coordination chemistry: From recognition to supramolecular assembly. Coord. Chem. Rev. 2019, 378, 415–444. [Google Scholar] [CrossRef]
- Wallace, K.J.; Johnson, A.D.; Jones, W.S.; Manandhar, E. Chemodosimeters and chemoreactands for sensing ferric ions. Supramol. Chem. 2018, 30, 353–383. [Google Scholar] [CrossRef]
- Manandhar, E.; Wallace, K.J. Host–guest chemistry of pyrene-based molecular receptors. Inorg. Chim. Acta 2012, 381, 15–43. [Google Scholar] [CrossRef]
- McEwen, J.J.; Wallace, K.J. Squaraine dyes in molecular recognition and self-assembly. Chem. Commun. 2009, 42, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.J. Molecular dyes used for the detection of biological and environmental heavy metals: Highlights from 2004 to 2008. Supramol. Chem. 2009, 21, 89–102. [Google Scholar] [CrossRef]
- Michalke, B.; Willkommen, D.; Drobyshev, E.; Solovyev, N. The importance of speciation analysis in neurodegeneration research. TrAC Trends Anal. Chem. 2018, 104, 160–170. [Google Scholar] [CrossRef]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer‘s, Parkinson’s and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, X.; Boezen, H.M.; Huo, X. Children with health impairments by heavy metals in an e-waste recycling area. Chemosphere 2016, 148, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.-F. Target or barrier? The cell wall of early-and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Sundseth, K.; Pacyna, J.; Pacyna, E.; Pirrone, N.; Thorne, R. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pei, R.; Zhao, T.; Dyer, D.J. Gold Nanoparticle Assemblies by Metal Ion−Pyridine Complexation and Their Rectified Quantized Charging in Aqueous Solutions. J. Chem. Phys. B 2002, 106, 1903–1908. [Google Scholar] [CrossRef]
- McCarroll, M.E.; Shi, Y.; Harris, S.; Puli, S.; Kimaru, I.; Xu, R.; Wang, L.; Dyer, D.J. Computational prediction and experimental evaluation of a photoinduced electron-transfer sensor. J. Chem. Phys. B 2006, 110, 22991–22994. [Google Scholar] [CrossRef] [PubMed]
- Hudson, G.A.; Cheng, L.; Yu, J.; Yan, Y.; Dyer, D.J.; McCarroll, M.E.; Wang, L. Computational studies on response and binding selectivity of fluorescence sensors. J. Phys. Chem. B 2009, 114, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Lin, Z.; Voorhis, T.V. Fluorescence quenching by photoinduced electron transfer in the Zn2+ sensor Zinpyr-1: A computational investigation. J. Chem. Phys. B 2010, 114, 10427–10434. [Google Scholar] [CrossRef]
- Venkataraman, D.; Du, Y.; Wilson, S.R.; Hirsch, K.A.; Zhang, P.; Moore, J.S. A coordination geometry table of the d-block elements and their ions. J. Chem. Educ. 1997, 74, 915–918. [Google Scholar] [CrossRef]
- Ebnou, F.; M’Haiham, M.; Ebeid, K.; Carpenter-Warren, C.L.; Slawin, A.M.; Woollins, J.D.; Dhia, M.T.B.; Sanhoury, M.A. Synthesis, characterization, and structural properties of mercury (II), cadmium (II) and zinc (II) tripiperidinophosphine chalcogenide complexes. Polyhedron 2019, 159, 206–211. [Google Scholar] [CrossRef]
- Rajput, G.; Yadav, M.K.; Drew, M.G.; Singh, N. Influence of the ligand frameworks on the coordination environment and properties of new phenylmercury (ii) β-oxodithioester complexes. Dalton Trans. 2015, 44, 5909–5916. [Google Scholar] [CrossRef]
- Ghosh, D.C.; Gupta, K. A new scale of electronegativity of 54 elements of periodic table based on polarizability of atoms. J. Theor. Comput. Chem. 2006, 5, 895–911. [Google Scholar] [CrossRef]
- Crichton, R. Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function; Academic Press: Cambridge, MA, USA, 2019; Chapter 12; pp. 339–362. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, R.; Tang, Y.; Wang, S. Cadmium–zinc exchange and their binary relationship in the structure of Zn-related proteins: A mini review. Metallomics 2014, 6, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Saito, M.A.; George, G.N.; Pickering, I.J.; Prince, R.C.; Morel, F.M. Biochemistry: A cadmium enzyme from a marine diatom. Nature 2005, 435, 42. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Morel, F.M. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.; Esrafilzadeh, D.; Barrow, S.; Dickey, M.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.; Koch, C. Preparation of beta brass by mechanical alloying of elemental copper and zinc. Scr. Metall. 1986, 20, 669–672. [Google Scholar] [CrossRef]
- Marshall, S.; Marshall, G. Dental amalgam: The materials. Adv. Dent. Res. 1992, 6, 94–99. [Google Scholar] [CrossRef]
- Shi, G.L.; Li, D.J.; Wang, Y.F.; Liu, C.H.; Hu, Z.B.; Lou, L.Q.; Rengel, Z.; Cai, Q.S. Accumulation and distribution of arsenic and cadmium in winter wheat (Triticum aestivum L.) at different developmental stages. Sci. Total Environ. 2019, 667, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Tsakiris, K.A. Saint Ioannis Lampadistis, the first possible case of blindness due to organic mercury poisoning in history. J. Med. Biogr. 2018, 26, 207–210. [Google Scholar] [CrossRef]
- Langford, N.; Ferner, R. Toxicity of mercury. J. Hum. Hypertens. 1999, 13, 651–656. [Google Scholar] [CrossRef]
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Kainz, M.; Telmer, K.; Mazumder, A. Bioaccumulation patterns of methyl mercury and essential fatty acids in lacustrine planktonic food webs and fish. Sci. Total Environ. 2006, 368, 271–282. [Google Scholar] [CrossRef] [PubMed]
- EPA United States Environmental Protection Agencey. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganic (accessed on 24 April 2019).
- Guidelines for Drinking-Water Quality. Available online: https://www.who.int/water_sanitation_health/dwq/gdwq0506.pdf (accessed on 24 April 2019).
- Levaot, N.; Hershfinkel, M. How cellular Zn2+ signaling drives physiological functions. Cell Calcium 2018, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Matsui, C.; Takatani-Nakase, T.; Hatano, Y.; Kawahara, S.; Nakase, I.; Takahashi, K. Zinc and its transporter ZIP6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Lett. 2017, 591, 3348–3359. [Google Scholar] [CrossRef]
- Malm, J.; Hellman, J.; Hogg, P.; Lilja, H. Enzymatic action of prostate-specific antigen (PSA or hK3): Substrate specificity and regulation by Zn2+, a tight-binding inhibitor. Prostate 2000, 45, 132–139. [Google Scholar]
- Weiss, J.H.; Sensi, S.L.; Koh, J.Y. Zn2+: A novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000, 21, 395–401. [Google Scholar] [CrossRef]
- Tomat, E.; Lippard, S.J. Imaging mobile zinc in biology. Curr. Opin. Chem. Biol. 2010, 14, 225–230. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Bertoni-Freddari, C.; Marcellini, F.; Malavolta, M. Brain, aging and neurodegeneration: Role of zinc ion availability. Prog. Neurobiol. 2005, 75, 367–390. [Google Scholar] [CrossRef]
- Leinweber, B.; Barofsky, E.; Barofsky, D.F.; Ermilov, V.; Nylin, K.; Beckman, J.S. Aggregation of ALS mutant superoxide dismutase expressed in Escherichia coli. Free Radic. Biol. Med. 2004, 36, 911–918. [Google Scholar] [CrossRef]
- Woo, H.; You, Y.; Kim, T.; Jhon, G.-J.; Nam, W. Fluorescence ratiometric zinc sensors based on controlled energy transfer. J. Mater. Chem. 2012, 22, 17100–17112. [Google Scholar] [CrossRef]
- Kwon, J.E.; Lee, S.; You, Y.; Baek, K.-H.; Ohkubo, K.; Cho, J.; Fukuzumi, S.; Shin, I.; Park, S.Y.; Nam, W. Fluorescent zinc sensor with minimized proton-induced interferences: Photophysical mechanism for fluorescence turn-on response and detection of endogenous free zinc ions. Inorg. Chem. 2012, 51, 8760–8774. [Google Scholar] [CrossRef] [PubMed]
- Comby, S.; Tuck, S.A.; Truman, L.K.; Kotova, O.; Gunnlaugsson, T. New trick for an old ligand! The sensing of Zn(II) using a lanthanide based ternary Yb(III)-cyclen-8 hydroxyquinoline system as a dual emissive probe for displacement assay. Inorg. Chem. 2012, 51, 10158–10168. [Google Scholar] [CrossRef] [PubMed]
- Tsikalas, G.K.; Lazarou, P.; Klontzas, E.; Pergantis, S.A.; Spanopoulos, I.; Trikalitis, P.N.; Froudakis, G.E.; Katerinopoulos, H.E. A “turn-on”–turning-to-ratiometric sensor for zinc(II) ions in aqueous media. RSC Adv. 2014, 4, 693–696. [Google Scholar] [CrossRef]
- Badetti, E.; Wurst, K.; Licini, G.; Zonta, C. Multimetallic architectures from the self-assembly of amino acids and tris (2-pyridylmethyl) amine zinc(II) complexes: Circular dichroism enhancement by chromophores organization. Chem.-Eur. J. 2016, 22, 6515–6518. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ren, A.-M.; Zou, L.-Y.; Guo, J.-F.; Huang, S. A theoretical investigation of a series of novel two-photon zinc ion fluorescent probes based on bipyridine. J. Photochem. Photobiol. A 2017, 341, 20–30. [Google Scholar] [CrossRef]
- Panunzi, B.; Borbone, F.; Capobianco, A.; Concilio, S.; Diana, R.; Peluso, A.; Piotto, S.; Tuzi, A.; Velardo, A.; Caruso, U. Synthesis, spectroscopic properties and DFT calculations of a novel multipolar azo dye and its zinc(II) complex. Inorg. Chem. Commun. 2017, 84, 103–108. [Google Scholar] [CrossRef]
- Zhu, M.-Y.; Zhao, K.; Song, J.; Wang, C.-K. Responsive mechanism and molecular design of di-2-picolylamine-based two-photon fluorescent probes for zinc ions. Chin. Phys. B 2018, 27, 023302. [Google Scholar] [CrossRef]
- Xia, S.; Shen, J.; Wang, J.; Wang, H.; Fang, M.; Zhou, H.; Tanasova, M. Ratiometric fluorescent and colorimetric BODIPY-based sensor for zinc ions in solution and living cells. Sens. Actuators B Chem. 2018, 258, 1279–1286. [Google Scholar] [CrossRef]
- Zhao, K.; Song, J.; Zhu, M.-Y.; Zhang, H.; Wang, C.-K. Isomerism and coordination mode effects on two-photon absorption of tris (picolyl) amine-based fluorescent probes for zinc ions. Chin. Phys. B 2018, 27, 103301. [Google Scholar] [CrossRef]
- Oehme, I.; Wolfbeis, O.S. Optical sensors for determination of heavy metal ions. Microchim. Acta 1997, 126, 177–192. [Google Scholar] [CrossRef]
- Kimura, E.; Aoki, S. Zinc Biochemistry, Physiology, and Homeostasis; Springer: Berlin/Heidelberg, Germany, 2001; pp. 5–18. [Google Scholar]
- Jiang, P.; Guo, Z. Fluorescent detection of zinc in biological systems: Recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 2004, 248, 205–229. [Google Scholar] [CrossRef]
- Lim, N.C.; Schuster, J.V.; Porto, M.C.; Tanudra, M.A.; Yao, L.; Freake, H.C.; Brückner, C. Coumarin-based chemosensors for zinc(II): Toward the determination of the design algorithm for CHEF-type and ratiometric probes. Inorg. Chem. 2005, 44, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B. Studying zinc biology with fluorescence: ain’t we got fun? Curr. Opin. Chem. Biol. 2005, 9, 526–532. [Google Scholar] [CrossRef]
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signal. 2006, 8, 1419–1441. [Google Scholar] [CrossRef]
- Carol, P.; Sreejith, S.; Ajayaghosh, A. Ratiometric and near-infrared molecular probes for the detection and imaging of zinc Ions. Chem. Asian J. 2007, 2, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Canary, J.W. Tailoring tripodal ligands for zinc sensing. New J. Chem. 2007, 31, 1708–1718. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc. Chem. Res. 2008, 42, 193–203. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef]
- Sarkar, K.; Dhara, K.; Nandi, M.; Roy, P.; Bhaumik, A.; Banerjee, P. Selective zinc(II)-ion fluorescence sensing by a functionalized mesoporous material covalently grafted with a fluorescent chromophore and consequent biological applications. Adv. Funct. Mater. 2009, 19, 223–234. [Google Scholar] [CrossRef]
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef]
- Pluth, M.D.; Tomat, E.; Lippard, S.J. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu. Rev. Biochem. 2011, 80, 333–355. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.T. Fluorescent and Luminescent Probes for Biological Activity: A Practical Guide to Technology for Quantitative Real-Time Analysis; Academic Press: London, UK, 1999. [Google Scholar]
- Johnson, I. Fluorescent probes for living cells. Histochem. J. 1998, 30, 123–140. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Outten, C.E.; O’halloran, T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292, 2488–2492. [Google Scholar] [CrossRef]
- Assaf, S.; Chung, S.-H. Release of endogenous Zn2+ from brain tissue during activity. Nature 1984, 308, 734–736. [Google Scholar] [CrossRef]
- Zhang, L.; Clark, R.J.; Zhu, L. A heteroditopic fluoroionophoric platform for constructing fluorescent probes with large dynamic ranges for zinc ions. Chem. Eur. J. 2008, 14, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Pati, P.B.; Zade, S.S. Selective colorimetric and “turn-on” fluorimetric detection of cyanide using a chemodosimeter comprising salicylaldehyde and triphenylamine groups. Eur. J. Org. Chem. 2012, 6555–6561. [Google Scholar] [CrossRef]
- Chakraborty, B.; Halder, P.; Chakraborty, S.; Das, O.; Paria, S. Synthesis, characterization and emission study of zinc(II) and cobalt(II) complexes: Bis(bidentate) iminophenols as zinc(II) selective fluorescence probes. Inorg. Chim. Acta 2012, 387, 332–337. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mi, Q.L.; Wang, G.K.; Chen, J.H.; Zhang, J.F.; Zhao, Q.H.; Zhou, Y. 1,8-Naphthalimide-based ‘turn-on’ fluorescent sensor for the detection of zinc ion in aqueous media and its applications for bioimaging. Tetrahedron Lett. 2013, 54, 3353–3358. [Google Scholar] [CrossRef]
- Dugave, C.; Demange, L. Cis−trans isomerization of organic molecules and biomolecules: Implications and applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef]
- Hisamoto, H.; Tohma, H.; Yamada, T.; Yamauchi, K.-I.; Siswanta, D.; Yoshioka, N.; Suzuki, K. Molecular design, characterization, and application of multi-information dyes for multi-dimensional optical chemical sensing. Molecular design concepts of the dyes and their fundamental spectral characteristics. Anal. Chim. Acta 1998, 373, 271–289. [Google Scholar] [CrossRef]
- Natali, M.; Soldi, L.; Giordani, S. A photoswitchable Zn(II) selective spiropyran-based sensor. Tetrahedron 2010, 66, 7612–7617. [Google Scholar] [CrossRef]
- Luo, W.; Liu, M.; Yang, T.; Yang, X.; Wang, Y.; Xiang, H. Fluorescent ZnII Chemosensor Mediated by a 1,8-Naphthyridine Derivative and It’s Photophysical Properties. ChemistryOpen 2018, 7, 639–644. [Google Scholar] [CrossRef]
- Park, G.J.; Lee, M.M.; You, G.R.; Choi, Y.W.; Kim, C. A turn-on and reversible fluorescence sensor with high affinity to Zn2+ in aqueous solution. Tetrahedron Lett. 2014, 55, 2517–2522. [Google Scholar] [CrossRef]
- Park, G.J.; Na, Y.J.; Jo, H.Y.; Lee, S.A.; Kim, A.R.; Noh, I.; Kim, C. A single chemosensor for multiple analytes: Fluorogenic detection of Zn2+ and OAc− ions in aqueous solution, and an application to bioimaging. New J. Chem. 2014, 38, 2587–2594. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S. A novel “turn on” fluorescent sensor based on hydroxy-triphenylamine for Zn2+ and Cd2+ ions in MeCN. Sens. Actuators B Chem. 2013, 188, 1225–1229. [Google Scholar] [CrossRef]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for Zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Frederickson, C.J.; Bu, W.; Lippard, S.J. ZP4, an improved neuronal Zn2+ sensor of the Zinpyr family. J. Am. Chem. Soc. 2003, 125, 1778–1787. [Google Scholar] [CrossRef]
- Nolan, E.M.; Ryu, J.W.; Jaworski, J.; Feazell, R.P.; Sheng, M.; Lippard, S.J. Zinspy sensors with enhanced dynamic range for imaging neuronal cell zinc uptake and mobilization. J. Am. Chem. Soc. 2006, 128, 15517–15528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomat, E.; Nolan, E.M.; Jaworski, J.; Lippard, S.J. Organelle-specific zinc detection using zinpyr-labeled fusion proteins in live cells. J. Am. Chem. Soc. 2008, 130, 15776–15777. [Google Scholar] [CrossRef]
- Wong, B.A.; Friedle, S.; Lippard, S.J. Solution and fluorescence properties of symmetric dipicolylamine-containing dichlorofluorescein-based Zn2+ sensors. J. Am. Chem. Soc. 2009, 131, 7142–7152. [Google Scholar] [CrossRef]
- Du, P.; Lippard, S.J. A highly selective turn-on colorimetric, red fluorescent sensor for detecting mobile zinc in living cells. Inorg. Chem. 2010, 49, 10753–10755. [Google Scholar] [CrossRef]
- Sivaraman, G.; Anand, T.; Chellappa, D. Turn-on fluorescent chemosensor for Zn(II) via ring opening of rhodamine spirolactam and their live cell imaging. Analyst 2012, 137, 5881–5884. [Google Scholar] [CrossRef]
- Wong, B.A.; Friedle, S.; Lippard, S.J. Subtle modification of 2,2-dipicolylamine lowers the affinity and improves the turn-on of Zn(II)-selective fluorescent sensors. Inorg. Chem. 2009, 48, 7009–7011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steed, J.W.; Wallace, K.J. Advances in Supramolecular Chemistry; Gokel, G., Ed.; Cerberus: New York, NY, USA, 2003; Volume 9, pp. 221–262. [Google Scholar]
- Jung, J.M.; Kang, J.H.; Han, J.; Lee, H.; Lim, M.H.; Kim, K.-T.; Kim, C. A novel “off-on” type fluorescent chemosensor for detection of Zn2+ and its zinc complex for “on-off” fluorescent sensing of sulfide in aqueous solution, in vitro and in vivo. Sens. Actuators B Chem. 2018, 267, 58–69. [Google Scholar] [CrossRef]
- Ravitz, S.F. The solubilities and free energies of some metallic sulfides. J. Phys. Chem. 1936, 40, 61–70. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, C.; Liu, G.; Cui, S.; Pu, S. A highly selective and sensitive ratiometric fluorescent chemosensor for Zn2+ based on diarylethene with a benzyl-linked 8-aminoquinoline-2-aminomethylpyridine unit. Dyes Pigm. 2016, 126, 121–130. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, S.; Qiu, S.; Zhang, Z.; Pu, S. A highly sensitive fluorescent sensor for Zn2+ based on diarylethene with an imidazole unit. Spectrochim. Acta Part A 2018, 205, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Macias-Contreras, M.; Daykin, K.L.; Simmons, J.T.; Allen, J.R.; Hooper, Z.S.; Davidson, M.W.; Zhu, L. Progressive structural modification to a zinc-actuated photoinduced electron transfer (PeT) switch in the context of intracellular zinc imaging. Org. Biomol. Chem. 2017, 15, 9139–9148. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.T.; Allen, J.R.; Morris, D.R.; Clark, R.J.; Levenson, C.W.; Davidson, M.W.; Zhu, L. Integrated and passive 1,2,3-triazolyl groups in fluorescent indicators for zinc(II) ions: Thermodynamic and kinetic evaluations. Inorg. Chem. 2013, 52, 5838–5850. [Google Scholar] [CrossRef]
- Michaels, H.A.; Murphy, C.S.; Clark, R.J.; Davidson, M.W.; Zhu, L. 2-Anthryltriazolyl-containing multidentate ligands: Zinc-coordination mediated photophysical processes and potential in live-cell imaging applications. Inorg. Chem. 2010, 49, 4278–4287. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Clark, R.J.; Zhu, L. Highly sensitive fluorescent probes for zinc ion based on triazolyl-containing tetradentate coordination motifs. Org. Lett. 2007, 9, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.A.; Zavaleta, A.; Baron, D.E.; Allam, O.; Isidor, E.V.; Kashimura, N.; Percarpio, J.M. A fluorescent photoinduced electron transfer sensor for cations with an off-on-off proton switch. Tetrahedron Lett. 1997, 38, 2237–2240. [Google Scholar] [CrossRef]
- Walkup, G.K.; Burdette, S.C.; Lippard, S.J.; Tsien, R.Y. A new cell-permeable fluorescent probe for Zn2+. J. Am. Chem. Soc. 2000, 122, 5644–5645. [Google Scholar] [CrossRef]
- Gee, K.R.; Zhou, Z.-L.; Ton-That, D.; Sensi, S.; Weiss, J. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium 2002, 31, 245–251. [Google Scholar] [CrossRef]
- Yan, J.; Fan, L.; Qin, J.-C.; Li, C.-R.; Yang, Z.-Y. A novel and resumable Schiff-base fluorescent chemosensor for Zn(II). Tetrahedron Lett. 2016, 57, 2910–2914. [Google Scholar] [CrossRef]
- Zhang, G.; Li, H.; Bi, S.; Song, L.; Lu, Y.; Zhang, L.; Yu, J.; Wang, L. A new turn-on fluorescent chemosensor based on diketopyrrolopyrrole (DPP) for imaging Zn2+ in living cells. Analyst 2013, 138, 6163–6170. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, E.; Broome, J.H.; Myrick, J.; Lagrone, W.; Cragg, P.J.; Wallace, K.J. A pyrene-based fluorescent sensor for Zn2+ ions: A molecular ‘butterfly’. Chem. Commun. 2011, 47, 8796–8798. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, E.; Cragg, P.J.; Wallace, K.J. Detection of Zn(II) ions by fluorescent pyrene-derived molecular probes. Supramol. Chem. 2014, 26, 141–150. [Google Scholar] [CrossRef]
- Rosenthal, M.R. The myth of the non-coordinating anion. J. Chem. Educ. 1973, 50, 331–335. [Google Scholar] [CrossRef]
- MacAyeal, J.R. The comprehensive environmental response, compensation, and liability act: The correct paradigm of strict liability and the problem of individual causation. J. Environ. Law 1999, 18, 217–334. [Google Scholar]
- Khairy, M.; El-Safty, S.A.; Shenashen, M. Environmental remediation and monitoring of cadmium. TrAC Trends Anal. Chem. 2014, 62, 56–68. [Google Scholar] [CrossRef]
- Kimblin, C.; Bridgewater, B.M.; Churchill, D.G.; Hascall, T.; Parkin, G. Bis(mercaptoimidazolyl)(pyrazolyl) hydroborato complexes of Zinc, Cadmium, and Cobalt: Structural evidence for the enhanced tendency of Zinc in biological systems to adopt Tetrahedral M[S4] coordination. Inorg. Chem. 2000, 39, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; Singh, H.B.; Patel, R.P.; Butcher, R.J. Synthesis and structural characterization of monomeric selenolato complexes of zinc, cadmium, and mercury. Inorg. Chem. 1998, 37, 2663–2669. [Google Scholar] [CrossRef]
- Sigel, H.; Martin, R.B. The colourless ‘chameleon’or the peculiar properties of Zn2+ in complexes in solution. Quantification of equilibria involving a change of the coordination number of the metal ion. Chem. Soc. Rev. 1994, 23, 83–91. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, S.; Qiu, S.; Pu, S. A new fluorescence probe based on diarylethene with a benzothiazine unit for selective detection of Cd2+. Tetrahedron 2018, 74, 7431–7437. [Google Scholar] [CrossRef]
- Maniyazagan, M.; Mariadasse, R.; Jeyakanthan, J.; Lokanath, N.; Naveen, S.; Premkumar, K.; Muthuraja, P.; Manisankar, P.; Stalin, T. Rhodamine based “turn–on” molecular switch FRET–sensor for cadmium and sulfide ions and live cell imaging study. Sens. Actuators B Chem. 2017, 238, 565–577. [Google Scholar] [CrossRef]
- Aich, K.; Goswami, S.; Das, S.; Mukhopadhyay, C.D.; Quah, C.K.; Fun, H.-K. Cd2+ triggered the FRET “ON”: A new molecular switch for the ratiometric detection of Cd2+ with live-cell imaging and bound X-ray structure. Inorg. Chem. 2015, 54, 7309–7315. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, W.; Yu, L.; Wang, Y. Click synthesis of a novel triazole bridged AIE active cyclodextrin probe for specific detection of Cd2+. Chem. Commun. 2015, 51, 4298–4301. [Google Scholar] [CrossRef]
- Teolato, P.; Rampazzo, E.; Arduini, M.; Mancin, F.; Tecilla, P.; Tonellato, U. Silica nanoparticles for fluorescence sensing of Zn(II): Exploring the covalent strategy. Chemistry 2007, 13, 2238–2245. [Google Scholar] [CrossRef]
- Pal, P.; Rastogi, S.K.; Gibson, C.M.; Aston, D.E.; Branen, A.L.; Bitterwolf, T.E. Fluorescence sensing of zinc(II) using ordered mesoporous silica material (MCM-41) functionalized with N-(quinolin-8-yl)-2-[3-(triethoxysilyl) propylamino] acetamide. ACS Appl. Mater. Interfaces 2011, 3, 279–286. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.; Xu, Y.; Qian, X. A small molecular fluorescent sensor functionalized silica microsphere for detection and removal of mercury, cadmium, and lead ions in aqueous solutions. Sens. Actuators B Chem. 2015, 220, 762–771. [Google Scholar] [CrossRef]
- Huang, W.-B.; Gu, W.; Huang, H.-X.; Wang, J.-B.; Shen, W.-X.; Lv, Y.-Y.; Shen, J. A porphyrin-based fluorescent probe for optical detection of toxic Cd2+ ion in aqueous solution and living cells. Dyes Pigments 2017, 143, 427–435. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Iniya, M.; Jeyanthi, D.; Siva, A.; Chellappa, D. A new multifunctional benzimidazole tagged coumarin as ratiometric fluorophore for the detection of Cd2+/F− ions and imaging in live cells. Spectrochim. Acta Part A 2018, 205, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ahmed, N. A coumarin–chalcone hybrid used as a selective and sensitive colorimetric and turn-on fluorometric sensor for Cd2+ detection. New J. Chem. 2017, 41, 14746–14753. [Google Scholar]
- Roy, S.B.; Mondal, J.; Khuda-Bukhsh, A.R.; Rajak, K.K. A novel fluorene based “turn on” fluorescent sensor for the determination of zinc and cadmium: Experimental and theoretical studies along with live cell imaging. New J. Chem. 2016, 40, 9593–9608. [Google Scholar] [CrossRef]
- Xu, L.; He, M.-L.; Yang, H.-B.; Qian, X. A simple fluorescent probe for Cd2+ in aqueous solution with high selectivity and sensitivity. Dalton Trans. 2013, 42, 8218–8222. [Google Scholar] [CrossRef] [PubMed]
- Tuemmler, B.; Maass, G.; Voegtle, F.; Sieger, H.; Heimann, U.; Weber, E. Open-chain polyethers. Influence of aromatic donor end groups on thermodynamics and kinetics of alkali metal ion complex formation. J. Am. Chem. Soc. 1979, 101, 2588–2598. [Google Scholar] [CrossRef]
- Tuemmler, B.; Maass, G.; Weber, E.; Wehner, W.; Voegtle, F. Noncyclic crown-type polyethers, pyridinophane cryptands, and their alkali metal ion complexes: Synthesis, complex stability, and kinetics. J. Am. Chem. Soc. 1977, 99, 4683–4690. [Google Scholar] [CrossRef]
- Weber, V.E.; Vogtle, F. Kristalline 1:1-alkalimetallkomplexe nichtcyclischer neutralliganden. Tetrahedron Lett. 1975, 16, 2415–2418. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Xu, M.-M.; Jiang, C.; Wang, J.; Song, G.; Wang, Y. Turn on fluorescent detection for Cd2+ based on surfactant controlled squaraine aggregation. Spectrochim. Acta Part A 2019, 208, 236–242. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Fu, J.; Yao, K.; Xue, K.; Xu, K. Turn-on fluorescent sensor for zinc and cadmium ions based on quinolone and its sequential response to phosphate. J. Lumin. 2017, 186, 16–22. [Google Scholar] [CrossRef]
- Song, E.J.; Kang, J.; You, G.R.; Park, G.J.; Kim, Y.; Kim, S.-J.; Kim, C.; Harrison, R.G. A single molecule that acts as a fluorescence sensor for zinc and cadmium and a colorimetric sensor for cobalt. Dalton Trans. 2013, 42, 15514–15520. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Chen, X.-Z.; Liu, X.-Y.; Wang, M.; Liu, J.-J.; Gao, G.; Zhang, X.-Y.; Sun, R.-Z.; Hou, S.-C.; Wang, H.-M. A highly sensitive multifunctional sensor based on phenylene-acetylene for colorimetric detection of Fe2+ and ratiometric fluorescent detection of Cd2+ and Zn2+. Sens. Actuators B Chem. 2018, 273, 1077–1084. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.G.; Han, L.; Luo, H.Q.; Li, N.B. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper(II) and pyrophosphate ion. Sens. Actuators B Chem. 2019, 283, 215–221. [Google Scholar] [CrossRef]
- Plácido, J.; Bustamante-López, S.; Meissner, K.; Kelly, D.; Kelly, S. Microalgae biochar-derived carbon dots and their application in heavy metal sensing in aqueous systems. Sci. Total Environ. 2019, 656, 531–539. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Zhou, Z.; Li, Y.; Tang, S.; Xiao, W.; Hu, M.; Peng, C.; Chen, Y.; Gu, B. A dual colorimetric and near-infrared fluorescent turn-on probe for Hg2+ detection and its applications. Dyes Pigments 2019, 163, 118–125. [Google Scholar] [CrossRef]
- Amanulla, B.; Perumal, K.N.; Ramaraj, S.K. Chitosan functionalized gold nanoparticles assembled on sulphur doped graphitic carbon nitride as a new platform for colorimetric detection of trace Hg2+. Sens. Actuators B Chem. 2019, 281, 281–287. [Google Scholar] [CrossRef]
- Manivannan, S.; Seo, Y.; Kang, D.-K.; Kim, K. Colorimetric and optical Hg(II) ion sensor developed with conjugates of M13-bacteriophage and silver nanoparticles. New J. Chem. 2018, 42, 20007–20014. [Google Scholar] [CrossRef]
- Bahta, M.; Ahmed, N. Design and synthesis of 1,4-benzothiazine hydrazide as selective and sensitive colorimetric and turn-on fluorometric sensor for Hg2+ detection in aqueous medium. J. Photochem. Photobiol. A 2018, 357, 41–48. [Google Scholar] [CrossRef]
- Chatterjee, A.; Banerjee, M.; Khandare, D.G.; Gawas, R.U.; Mascarenhas, S.C.; Ganguly, A.; Gupta, R.; Joshi, H. Aggregation-induced emission-based chemodosimeter approach for selective sensing and imaging of Hg(II) and methylmercury species. Anal. Chem. 2017, 89, 12698–12704. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, T.; Ou, Z.; Cai, W.; Yang, G.; Li, Y.; Xu, M.; Li, Q. Highly sensitive and selective turn-on fluorescent chemosensors for Hg2+ based on thioacetal modified pyrene. Talanta 2018, 178, 663–669. [Google Scholar] [CrossRef]
- Nakanishi, T.; Suzuki, M.; Saimoto, A.; Kabasawa, T. Structural considerations of NK109, an antitumor benzo[c]phenanthridine alkaloid. J. Nat. Prod. 1999, 62, 864–867. [Google Scholar] [CrossRef]
- Takao, N.; Kamigauchi, M.; Okada, M. Biosynthesis of benzo[c]phenanthridine alkaloids sanguinarine, chelirubine and macarpine. Helv. Chim. Acta 1983, 66, 473–484. [Google Scholar] [CrossRef]
- Ponram, M.; Balijapalli, U.; Sambath, B.; Iyer, S.K.; Venkatachalapathy, B.; Cingaram, R.; Sundaramurthy, K.N. Development of paper-based chemosensor for the detection of mercury ions using mono-and tetra-sulfur bearing phenanthridines. New J. Chem. 2018, 42, 8530–8536. [Google Scholar] [CrossRef]
- Shyamal, M.; Maity, S.; Maity, A.; Maity, R.; Roy, S.; Misra, A. Aggregation induced emission based “turn-off” fluorescent chemosensor for selective and swift sensing of mercury(II) ions in water. Sens. Actuators B Chem. 2018, 263, 347–359. [Google Scholar] [CrossRef]
- Rurack, K. Flipping the light switch ‘ON’—The design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spectrochim. Acta Part A 2001, 57, 2161–2195. [Google Scholar] [CrossRef]
- Shimizu, Y.; Azumi, T. Mechanism of external heavy atom effect on intersystem crossing in fluid solutions. Analysis based on fluorescence decay data. J. Phys. Chem. 1982, 86, 22–26. [Google Scholar] [CrossRef]
- Fang, Y.; Li, X.; Li, J.-Y.; Wang, G.-Y.; Zhou, Y.; Xu, N.-Z.; Hu, Y.; Yao, C. Thiooxo-Rhodamine B hydrazone derivatives bearing bithiophene group as fluorescent chemosensors for detecting mercury(II) in aqueous media and living HeLa cells. Sens. Actuators B Chem. 2018, 255, 1182–1190. [Google Scholar] [CrossRef]
- Jones, M.M.; Vaughn, W.K. HSAB theory and acute metal ion toxicity and detoxification processes. J. Inorg. Nucl. Chem. 1978, 40, 2081–2088. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 1968, 45, 643–648. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Shu, T.; Yang, Z.; Cen, Z.; Deng, X.; Deng, Y.; Dong, C.; Yu, Y. A novel ratiometric fluorescent probe based on a BODIPY derivative for Cu2+ detection in aqueous solution. Anal. Methods 2018, 10, 5755–5762. [Google Scholar] [CrossRef]
- Sun, W.; Chen, R.; Cheng, X.; Marin, L. Bodipy-based chemosensors for highly sensitive and selective detection of Hg2+ ions. New J. Chem. 2018, 42, 19224–19231. [Google Scholar] [CrossRef]
- Zhou, B.; Qin, S.; Chen, B.; Han, Y. A new BODIPY-based fluorescent “turn-on” probe for highly selective and rapid detection of mercury ions. Tetrahedron Lett. 2018, 59, 4359–4363. [Google Scholar] [CrossRef]
- Gu, Z.; Cheng, H.; Shen, X.; He, T.; Jiang, K.; Qiu, H.; Zhang, Q.; Yin, S. A BODIPY derivative for colorimetric fluorescence sensing of Hg2+, Pb2+ and Cu2+ ions and its application in logic gates. Spectrochim. Acta Part A 2018, 203, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, C.; Peng, S.; Chen, H. A fluorescent and colorimetric probe enables simultaneous differential detection of Hg2+ and Cu2+ by two different mechanisms. Sens. Actuators B Chem. 2017, 238, 455–461. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Hou, H.-N.; Yang, W.-N.; Hu, S.-L. A new coumarin-carbonothioate-based turn-on fluorescent chemodosimeter for selective detection of Hg2+. Inorg. Chim. Acta 2018, 471, 705–708. [Google Scholar] [CrossRef]
- Patai, S. Chemistry of the Carbon-Nitrogen Double Bond; John Wiley and Sons: Chichester, UK, 1970. [Google Scholar]
- Machado, V.G.; Nascimento, M.; Rezende, M. Metal ion catalysis in the hydrolysis of imines. J. Braz. Chem. Soc. 1993, 4, 76–79. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, X.; Zhou, L.; He, H.; Zhou, P.; Duan, C. A schiff-base dual emission ratiometric fluorescent chemosensor for Hg2+ ions and its application in cellular imaging. Sens. Actuators B Chem. 2017, 247, 950–956. [Google Scholar] [CrossRef]
- Smith, D.G.; Topolnicki, I.L.; Zwicker, V.E.; Jolliffe, K.A.; New, E.J. Fluorescent sensing arrays for cations and anions. Analyst 2017, 142, 3549–3563. [Google Scholar] [CrossRef]
- Sasaki, Y.; Minamiki, T.; Tokito, S.; Minami, T. A molecular self-assembled colourimetric chemosensor array for simultaneous detection of metal ions in water. Chem. Commun. 2017, 53, 6561–6564. [Google Scholar] [CrossRef]
- Mallet, A.M.; Davis, A.B.; Davis, D.R.; Panella, J.; Wallace, K.J.; Bonizzoni, M. A cross reactive sensor array to probe divalent metal ions. Chem. Commun. 2015, 51, 16948–16951. [Google Scholar] [CrossRef]
- Jing, X.-Y.; Tang, Y.-Y.; Zhang, D. A Fourier–LDA approach for image recognition. Pattern Recognit. 2005, 38, 453–457. [Google Scholar] [CrossRef]
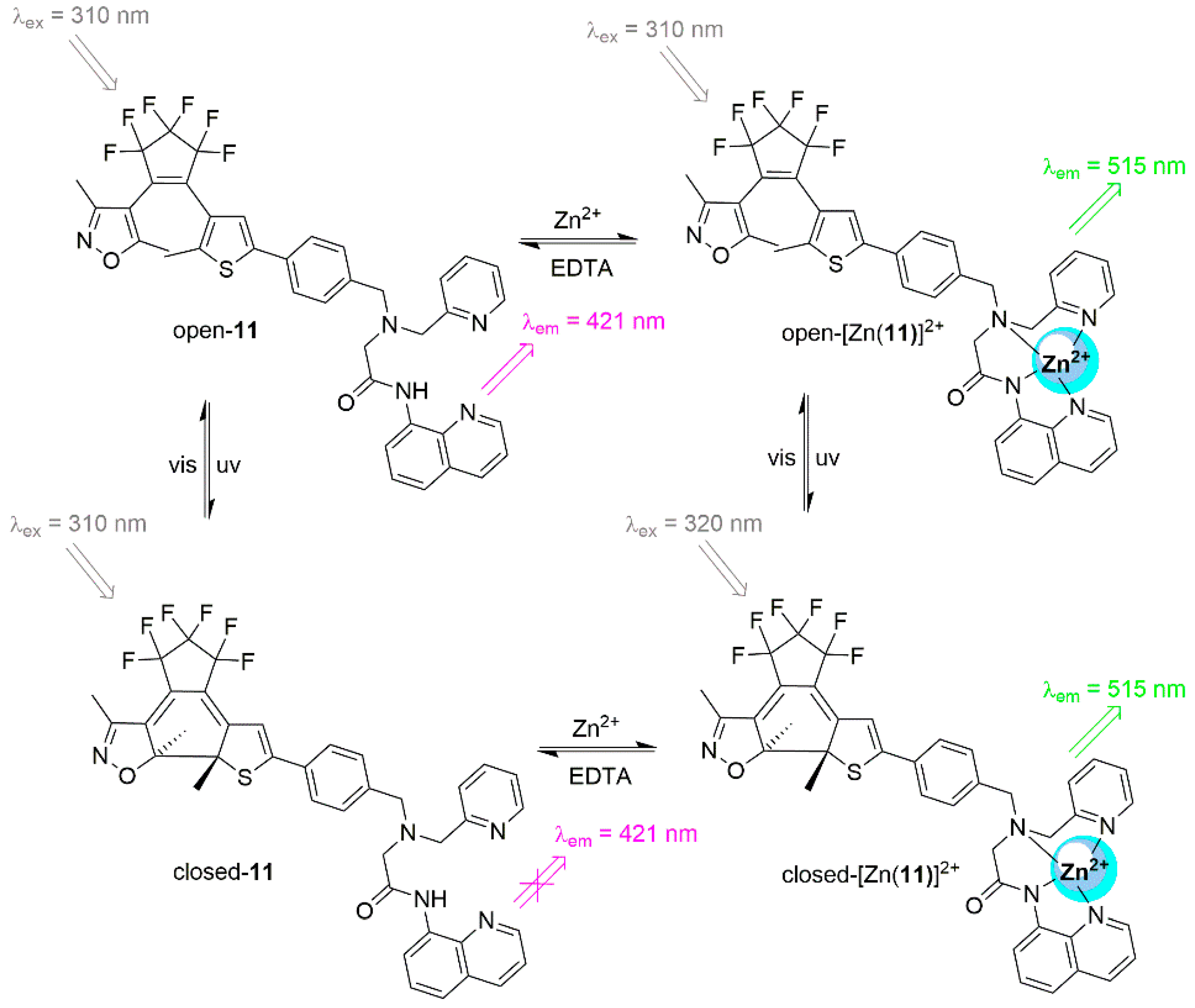
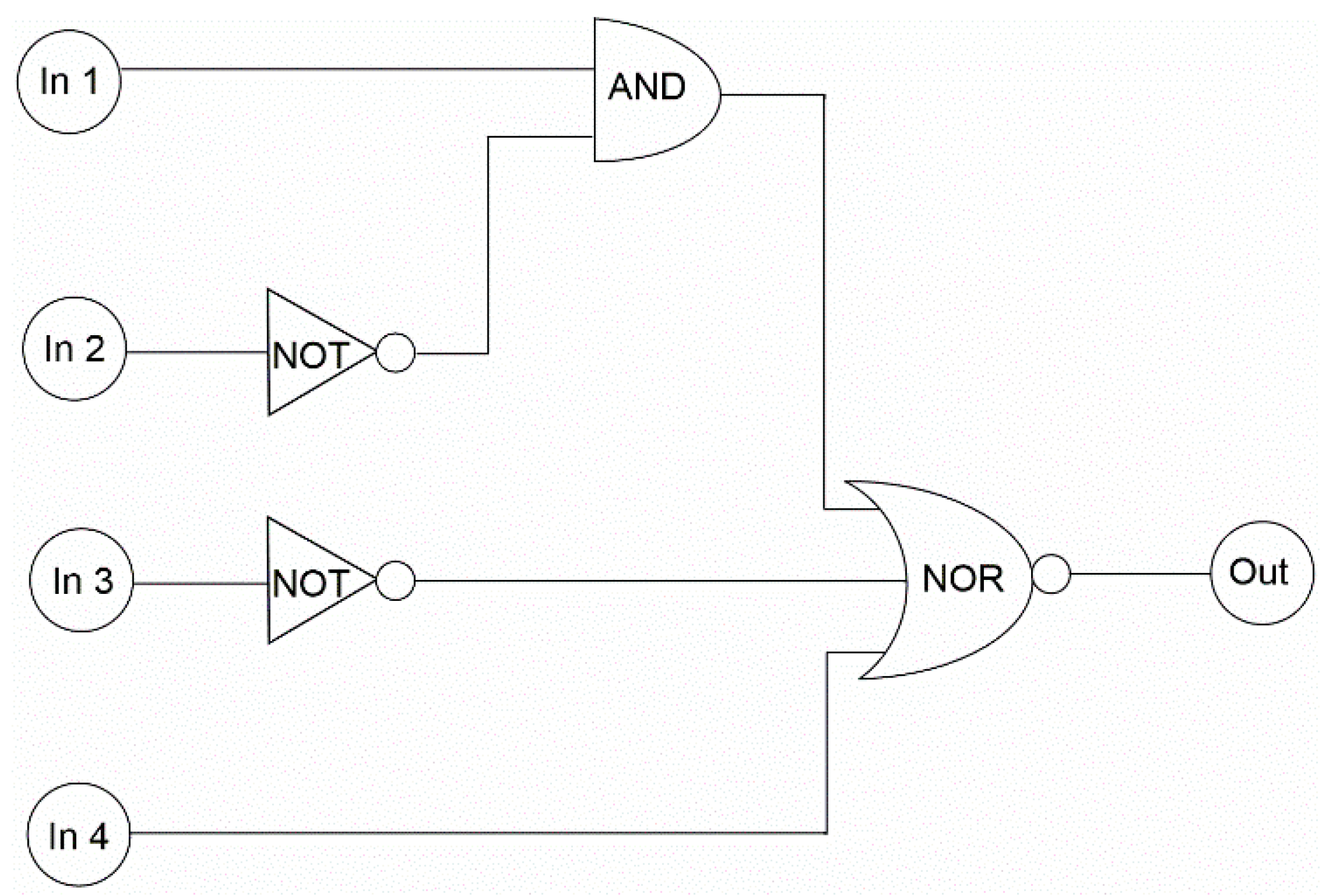
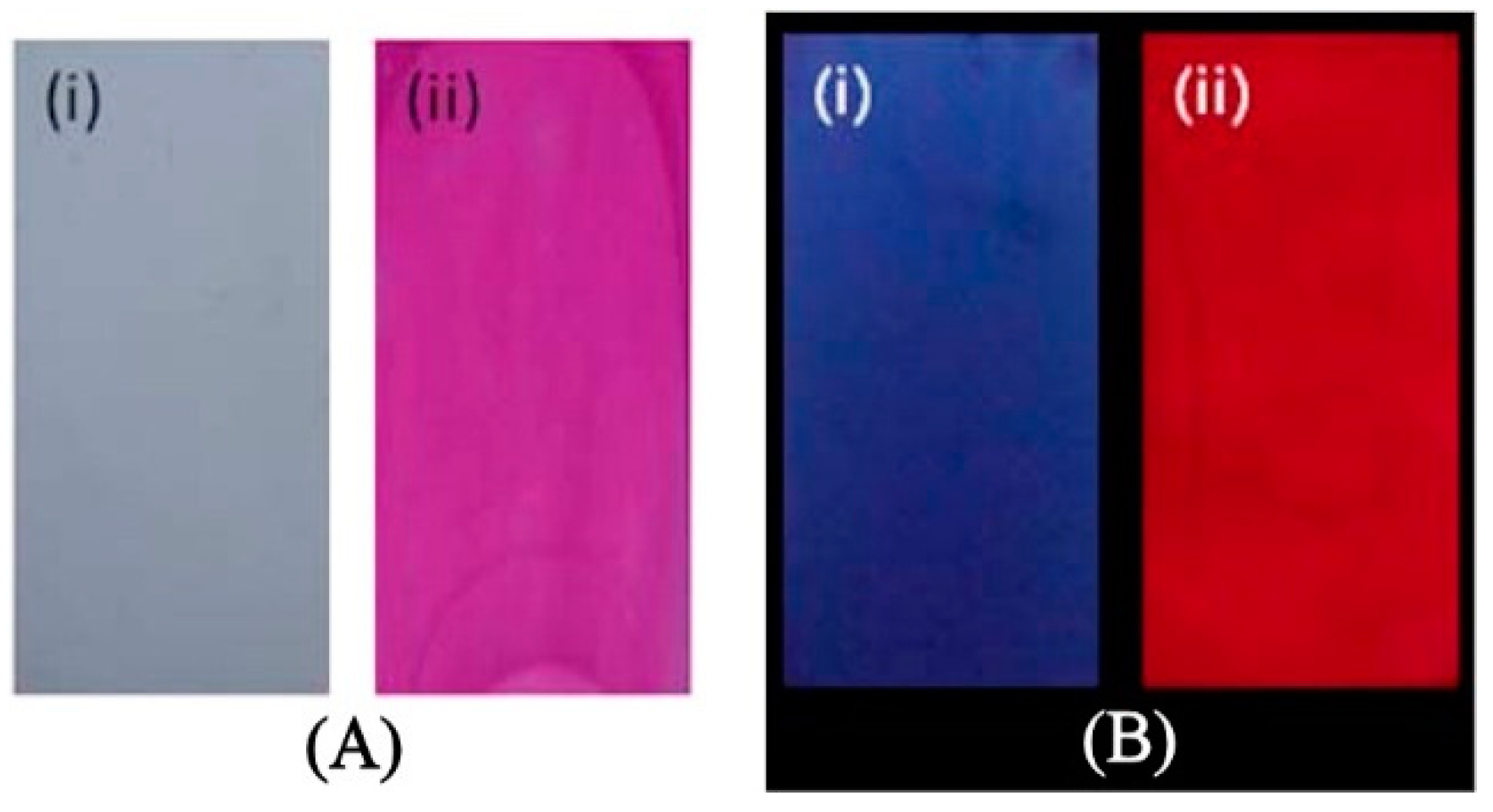


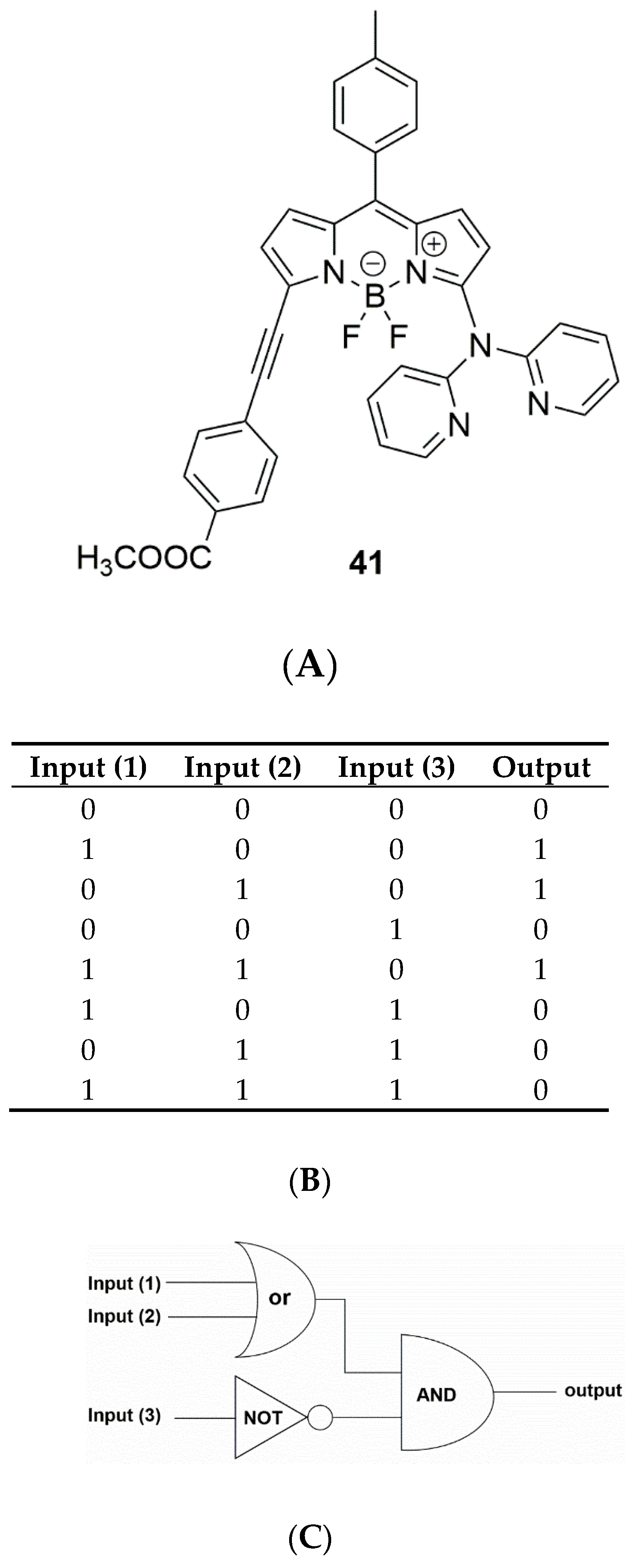
| Input | Output * | |||
|---|---|---|---|---|
| In (1) | In (2) | In (3) | In (4) | λem = 515 nm |
| UV | Vis | Zn2+ | EDTA | |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | 0 |
| 0 | 0 | 1 | 0 | 1 |
| 0 | 0 | 0 | 1 | 0 |
| 1 | 1 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 |
| 1 | 0 | 0 | 1 | 0 |
| 0 | 1 | 1 | 0 | 1 |
| 0 | 1 | 0 | 1 | 0 |
| 0 | 0 | 1 | 1 | 0 |
| 1 | 1 | 1 | 0 | 1 |
| 1 | 1 | 0 | 1 | 0 |
| 1 | 0 | 1 | 1 | 0 |
| 0 | 1 | 1 | 1 | 0 |
| 1 | 1 | 1 | 1 | 0 |
| Sample | Amount of Cd2+ in mol·L−1) | |
|---|---|---|
| Fluorometric with Probe 25 | AAS | |
| Lipstick | 2.07 × 10−6 | 1.96 × 10−6 |
| Hair color | 1.12 × 10−6 | 1.06 × 10−6 |
| Makeup cream | 7.92 × 10−6 | 7.20 × 10−6 |
| (A) | (B) | (C) | ||||||
|---|---|---|---|---|---|---|---|---|
| Input | Output * | Input | Output * | Input | Output * | |||
| Zn2+ | Cd2+ | λem 485 or 495 nm | Zn2+ | EDTA | λem 485 nm | Cd2+ | S2− | λem 495 nm |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.D.; Curtis, R.M.; Wallace, K.J. Low Molecular Weight Fluorescent Probes (LMFPs) to Detect the Group 12 Metal Triad. Chemosensors 2019, 7, 22. https://doi.org/10.3390/chemosensors7020022
Johnson AD, Curtis RM, Wallace KJ. Low Molecular Weight Fluorescent Probes (LMFPs) to Detect the Group 12 Metal Triad. Chemosensors. 2019; 7(2):22. https://doi.org/10.3390/chemosensors7020022
Chicago/Turabian StyleJohnson, Ashley D., Rose M. Curtis, and Karl J. Wallace. 2019. "Low Molecular Weight Fluorescent Probes (LMFPs) to Detect the Group 12 Metal Triad" Chemosensors 7, no. 2: 22. https://doi.org/10.3390/chemosensors7020022
APA StyleJohnson, A. D., Curtis, R. M., & Wallace, K. J. (2019). Low Molecular Weight Fluorescent Probes (LMFPs) to Detect the Group 12 Metal Triad. Chemosensors, 7(2), 22. https://doi.org/10.3390/chemosensors7020022