Optical Detection of Bromide Ions Using Pt(II)-5,10,15,20-Tetra-(4-methoxy-phenyl)-porphyrin
Abstract
1. Introduction
2. Materials and Methods
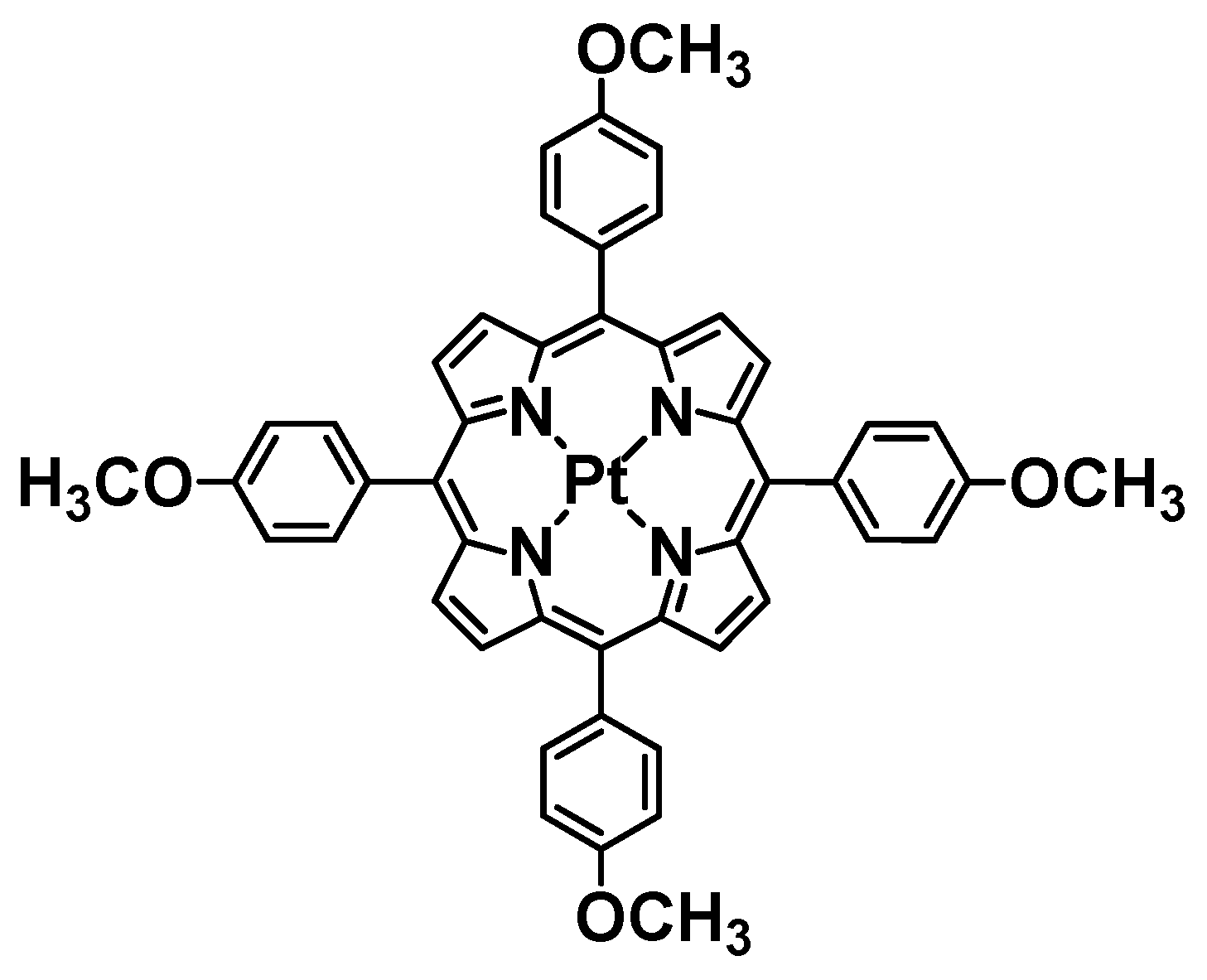
2.1. Materials
2.2. UV-Visible Spectral Studies
2.3. Atomic Force Microscopy (AFM) Imaging
3. Results and Discussion
3.1. Investigation of the Stability of PtTMeOPP Solution in THF in Different pH Media
3.1.1. Acidic Media
3.1.2. Basic Media
3.1.3. Phosphate Buffered Solution
3.2. AFM Studies Concerning the Aggregation Properties of PtTMeOPP at the Interface of Different Solvents/Air on Silica Plates
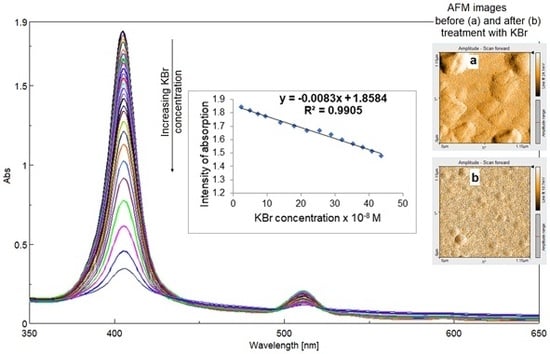
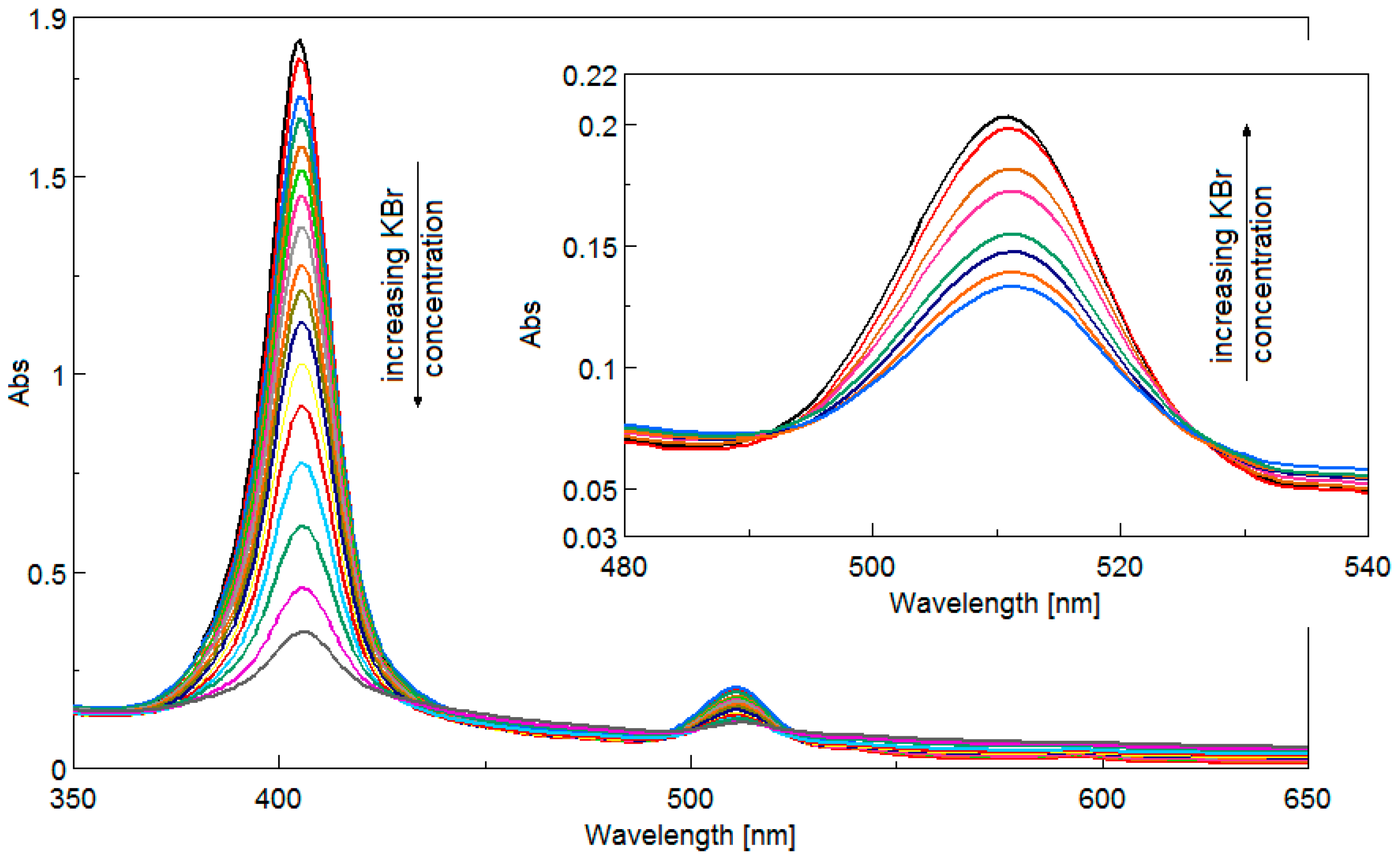
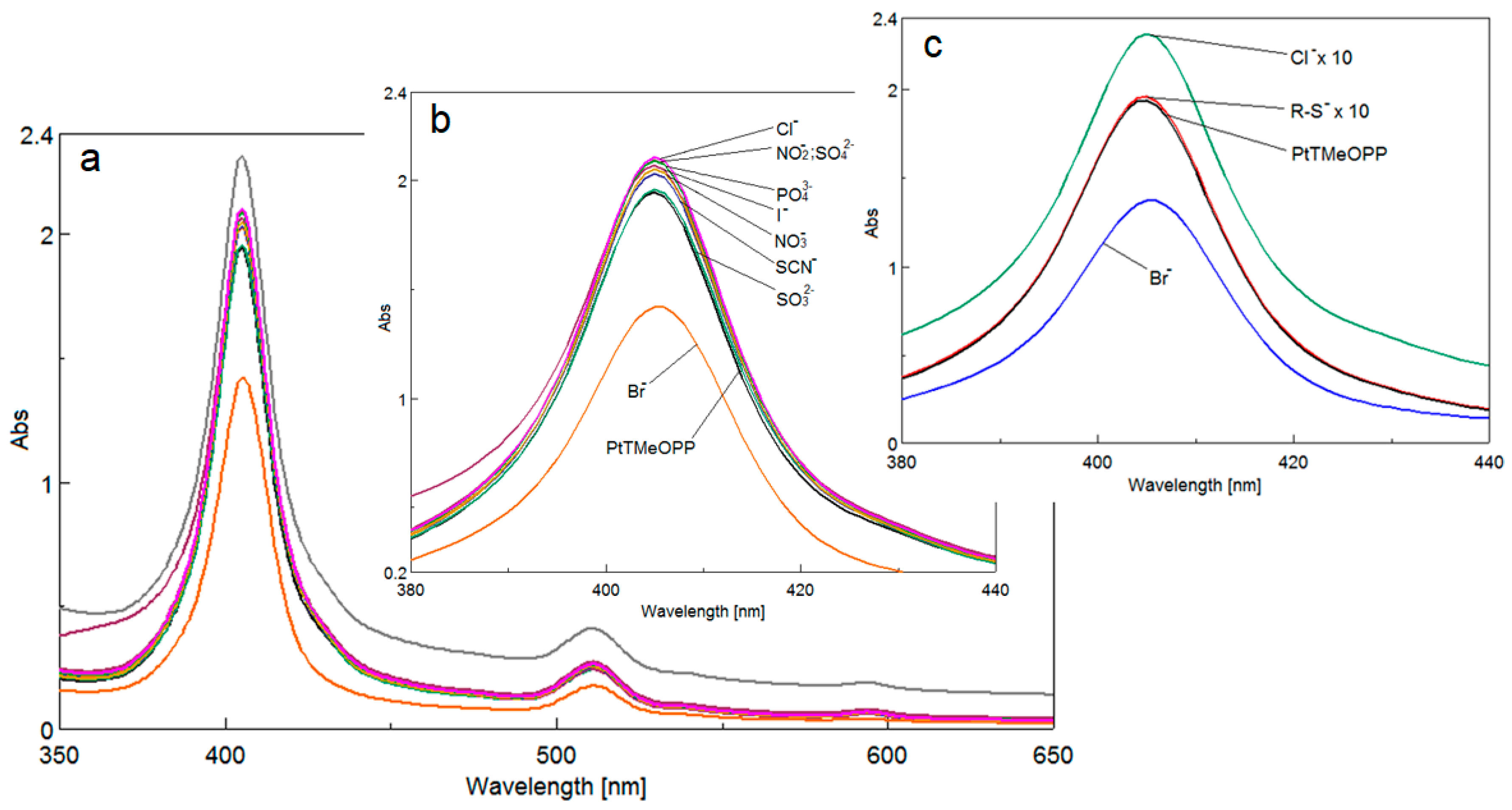
3.3. Detection of Bromide Ions
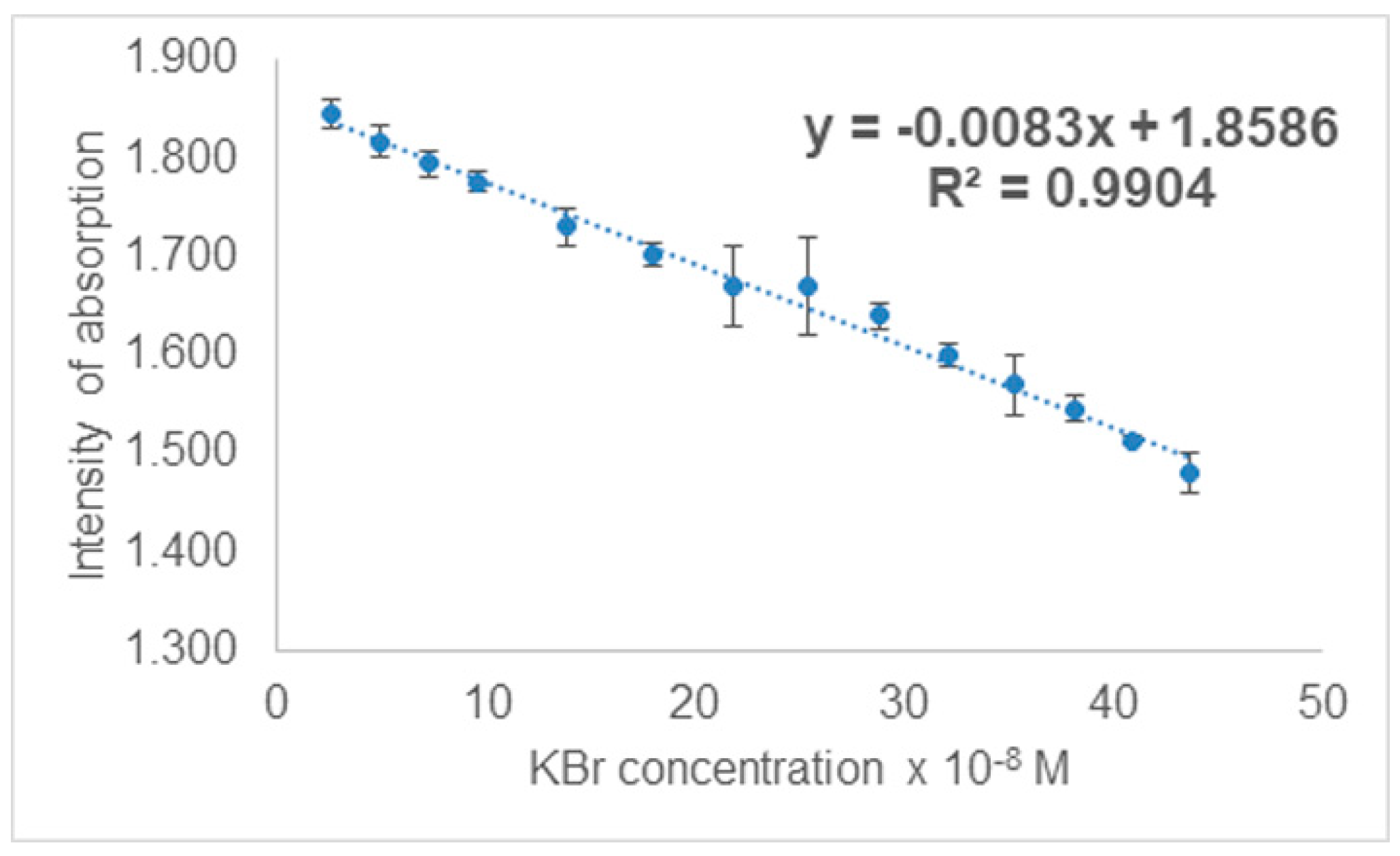
3.3.1. UV-Vis Spectrophotometric Investigations
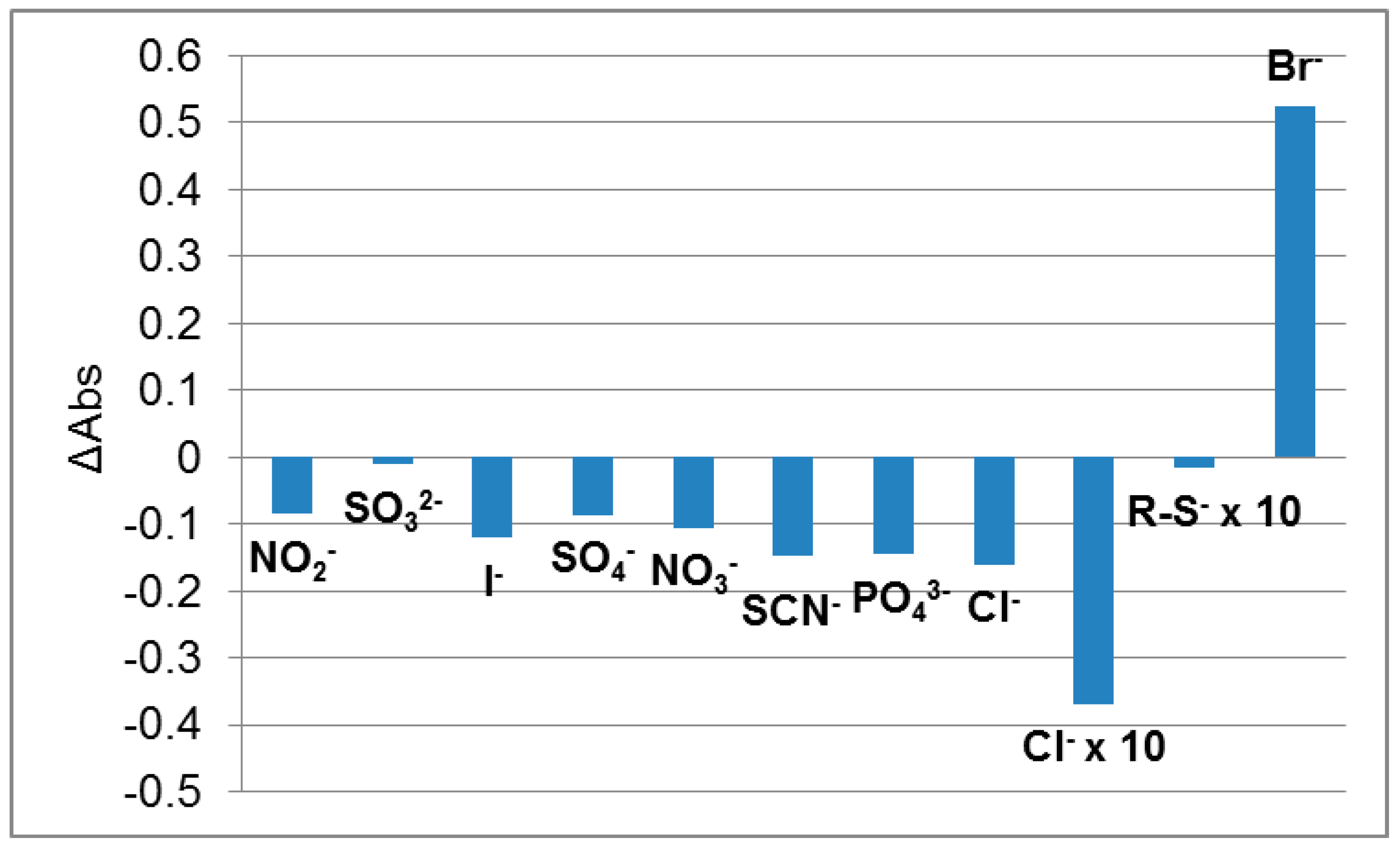
3.3.2. The Study of Interfering Ions
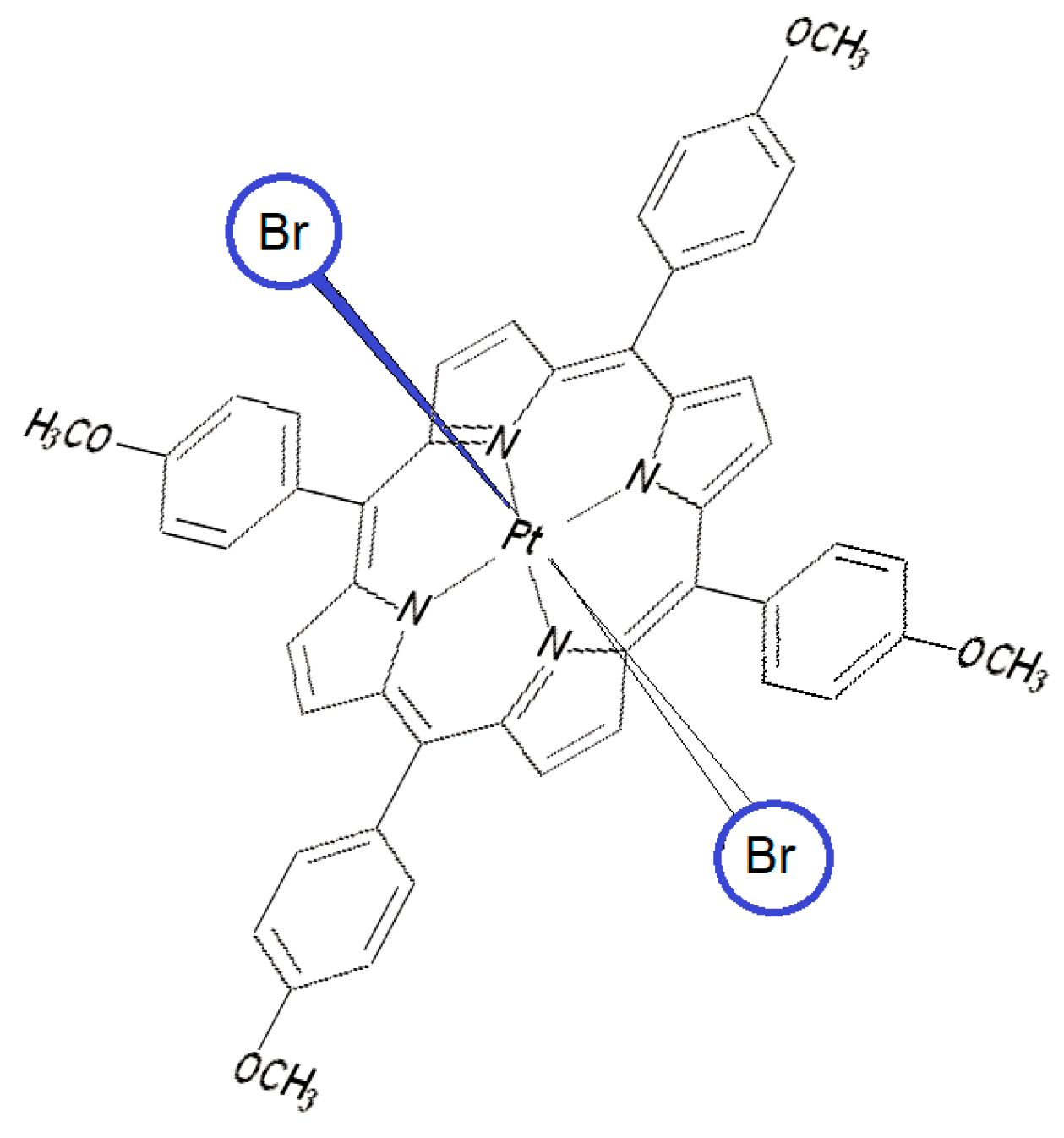
3.3.3. Mechanism of Detection
3.3.4. The Influence of the Ionic Strength
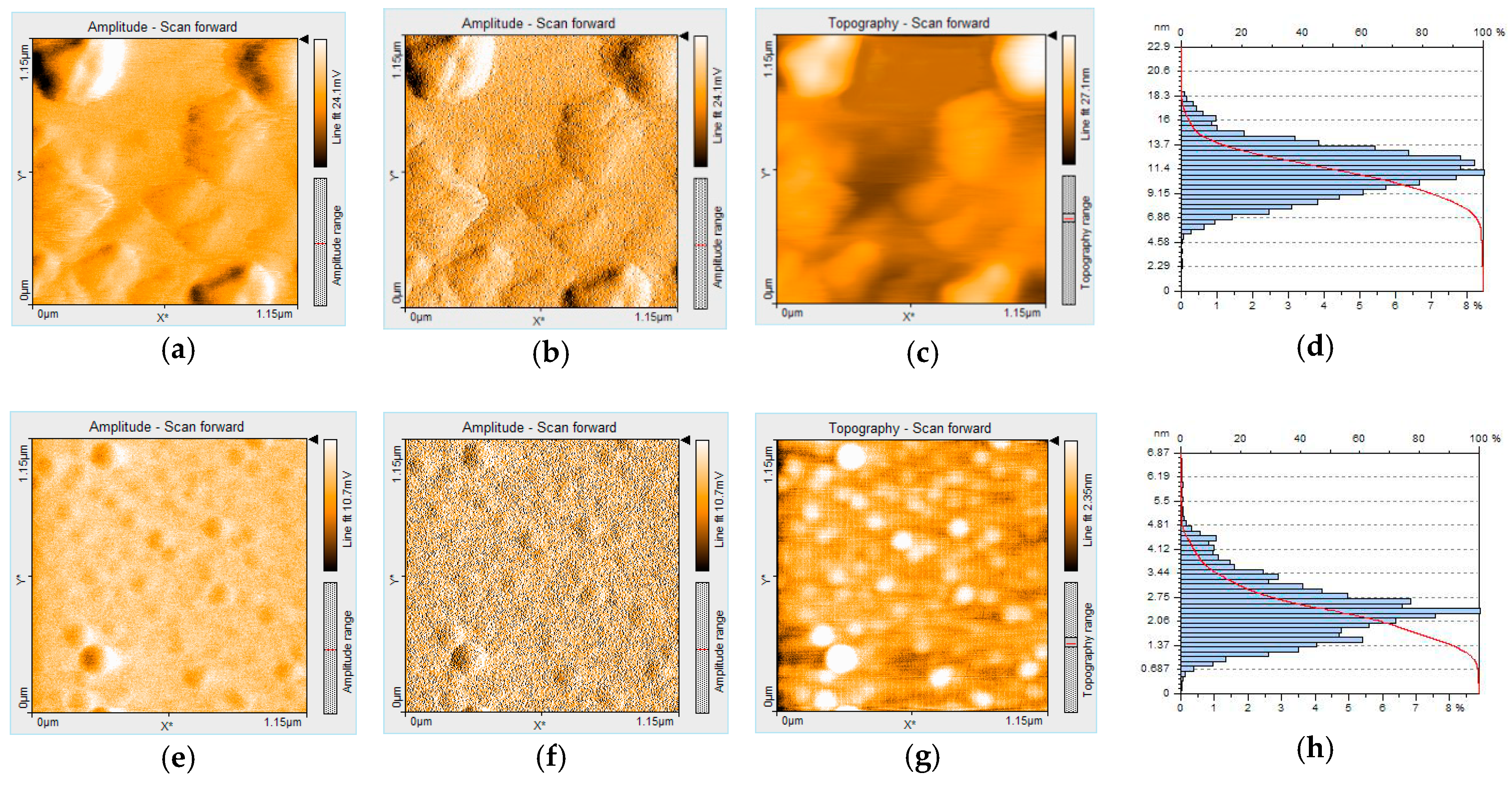
3.3.5. Morphological Modifications after Bromide Exposure
3.3.6. Real Sample Testing
3.3.7. Sample Preparations of Onion Extracts and Bromide Ions Detection and Validation by Potentiometric Titration
3.3.8. The Validation of Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McCall, A.S.; Cummings, C.F.; Bhave, G.; Vanacore, R.; Page-McCaw, A.; Hudson, B.G. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 2014, 157, 1380–1392. [Google Scholar] [CrossRef]
- Olszowy, H.A.; Rossiter, J.; Hegarty, J.; Geoghegan, P.; Haswell-Elkins, M. Background levels of bromide in human blood. J. Anal. Toxicol. 1998, 22, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, S. Metabolism of bromide and its interference with the metabolism of iodine. Physiol. Res. 2004, 53, 81–90. [Google Scholar]
- Baker, C.; Kahn, S.E.; Bermes, E.W. Effect of bromide and iodide on chloride methodologies in plasma or serum. Ann. Clin. Lab. Sci. 1980, 10, 523–528. [Google Scholar] [PubMed]
- Frances, C.; Hoizey, G.; Lamiable, D.; Millart, H.; Trenque, T. Bromism from daily over intake of bromide salt. J. Toxicol. Clin. Toxicol. 2003, 41, 181–183. [Google Scholar] [CrossRef]
- Plaisen, S.; Cheewasedtham, W.; Rujiralai, T. Robust colorimetric detection based on the anti-aggregation of gold nanoparticles for bromide in rice samples. RSC Adv. 2018, 8, 21566–21576. [Google Scholar] [CrossRef]
- Kou, L.; Liang, R. Detection of bromide ions in water samples with a nanomolar detection limit using a potentiometric ion-selective electrode. Int. J. Electrochem. Sci. 2019, 14, 1601–1609. [Google Scholar] [CrossRef]
- Nguyen, T.K.D.; Ludwig, R. Quantitative determination of bromine and iodine in food samples using ICP-MS. Anal. Sci. 2014, 30, 1089–1092. [Google Scholar] [CrossRef]
- La Rosa Novo, D.; Eisenhardt Mello, J.; Soares Rondan, F.; Schneider Henn, A.; Azevedo Mello, P.; Foster Mesko, M. Bromine and iodine determination in human saliva: Challenges in the development of an accurate method. Talanta 2019, 191, 415–421. [Google Scholar] [CrossRef]
- McCullough, T.; Allaire, P.N.; Boettcher, B.R. Detection of chloride, bromide, and iodide following sodium fusion. J. Chem. Educ. 1970, 4, 217. [Google Scholar] [CrossRef]
- Koga, M.; Hara, K.; Hori, H.; Kodama, Y.; Okubo, T. Determination of bromide ion concentration in urine using a head-space gas chromatography and an ion chromatography. J. UOEH 1991, 13, 19–24. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Zheng, X. Simultaneous analysis of iodate, iodide, bromate and bromide by ion chromatography with ultraviolet detection. Chin. J. Chromatogr. 2014, 32, 299–303. [Google Scholar] [CrossRef]
- ISO 10304-1:2007. Water Quality-Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate, 2nd ed.; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Sakai, H.; Fujiwara, T.; Kumamaru, T. Determination of inorganic anions in water samples by ion-exchange chromatography with chemiluminescence detection based on the neutralization reaction of nitric acid and potassium hydroxide. Anal. Chim. Acta 1996, 331, 239–244. [Google Scholar] [CrossRef]
- Borges, E.P.; Reis, B.F. Automatic flow analysis procedure for the determination of bromide in L-alanine by chemiluminescence detection. J. Braz. Chem. Soc. 2007, 18, 1336–1341. [Google Scholar] [CrossRef][Green Version]
- Sheinin, V.B.; Ratkova, E.L.; Mamardashvili, N.Z. pH-Dependent porphyrin-based receptor for bromide-ions selective binding. J. Porphyr. Phthalocyanines 2008, 12, 1211–1219. [Google Scholar] [CrossRef]
- Qu, F.; Li, N.B.; Luo, H.Q. Polyethyleneimine-Templated Ag Nanoclusters: A New Fluorescent and Colorimetric Platform for Sensitive and Selective Sensing Halide Ions and High Disturbance-Tolerant Recognitions of Iodide and Bromide in Coexistence with Chloride under Condition of High Ionic Strength. Anal. Chem. 2012, 84, 10373–10379. [Google Scholar] [CrossRef]
- Takahashi, M.; Kinoshita, H.; Nishiguchi, M.; Nishio, H. Bromide detection in blood using energy dispersive X-ray fluorescence; a chemical marker supportive of drowning in seawater. Leg. Med. 2010, 12, 132–136. [Google Scholar] [CrossRef]
- Mutic, J.J.; Nikolic-Mandic, S.D.; Lolic, A.D.; Manojlovic, D.D. Determination of iodide and bromide by flow methods with amperometric detection. J. Anal. Chem. 2008, 63, 907–913. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, G.; Plesu, N.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric sensors for iodide and bromide based on Pt(II)-porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef] [PubMed]
- Basel, C.L.; Defreese, J.D.; Whittemore, D.O. Interferences in automated phenol red method for determination of bromide in water. Anal. Chim. 1982, 54, 2090–2094. [Google Scholar] [CrossRef]
- Baso-Cejas, E.; Brito, G.; Dıaz, C.; Pena-Mendez, E.M. Determination of inorganic bromide content in several vegetable foods. Bull. Environ. Contam Toxicol. 2007, 78, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-Ray structure elucidation of a Pt-metalloporphyrin and its application for obtaining sensitive AuNPs-plasmonic hybrids capable to detect triiodide anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations. Pure Appl. Chem. 2002, 74, 923–994. [Google Scholar] [CrossRef]
- Sanders, J.K.M.; Bampos, N.; Clyde-Watson, Z.; Garling, S.L.; Hawley, J.C.; Kim, H.J.; Mak, C.C.; Webb, S.J. Axial Coordination Chemistry of Metalloporphyrins. In The Porphyrin Handbook; Inorganic, Organometallic and Coordination Chemistry; Kadis, K., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 3, Chapter 15; p. 4. ISBN 0-12-393200-3. [Google Scholar]
- Kruk, M.M.; Starukhin, A.S.; Mamardashvili, N.Z.; Mamardashvili, G.M.; Ivanova, Y.B.; Maltseva, O.V. Tetrapyrrolic Compounds as Hosts for Binding of Halides and Alkali Metal Cations. J. Porphyr. Phthalocyanines 2009, 13, 1148–1158. [Google Scholar] [CrossRef]
- Coury, L.A.; McGeoch, J.E.M.; Guidotti, G.; Brodsky, J.L. The yeast Saccharomyces cerevisiae does not sequester chloride but can express a functional mammalian chloride channel. FEMS Microbiol. Lett. 1999, 179, 327–332. [Google Scholar] [CrossRef][Green Version]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Bajić, A.; Zhang, C.; Song, X.; Carroll, S.L.; Cai, Z.L.; Tang, M.; Xue, M.; Cheng, N.; et al. Quantitative real-time imaging of glutathione. Nat. Commun. 2017, 8, 16087. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D’Amico, A. Porphyrin-Based Nanostructures for Sensing Applications. J. Sens. 2009, 2009, 856053. [Google Scholar] [CrossRef]
- Kielmann, M.; Prior, C.; Senge, M.O. Porphyrins in troubled times: A spotlight on porphyrins and their metal complexes for explosives testing and CBRN defense. New J. Chem. 2018, 42, 7529–7550. [Google Scholar] [CrossRef]
- Masih, D.; Aly, S.M.; Alarousu, E.; Mohammed, O.F. Photoinduced triplet-state electron transfer of platinum porphyrin: A one-step direct method for sensing iodide with an unprecedented detection limit. J. Mater. Chem. A 2015, 3, 6733–6738. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Garrity, P.; Ehrlich, B.; Davis, C.B.; Gibbs, E.J.; Orloff, G.; Giartosio, A.; Turano, C. The influence of ionic strength on the binding of a water-soluble porphyrin to nucleic acids. Nucleic Acids Res. 1986, 14, 5919–5931. [Google Scholar] [CrossRef]
- Adotey, D.K.; Serfor-Armah, Y.; Fianko, J.R.; Yeboah, P.O. Essential elements content in core vegetables grown and consumed in Ghana by instrumental neutron activation analysis. Afr. J. Food Sci. 2009, 3, 243–249. [Google Scholar]
- Galdon, B.R.; Gonzalez, R.O.; Rodrıguez, E.R.; Romero, C.D. Comparison of mineral and trace element contents in onion cultivars (Allium cepa L.). J. Sci. Food Agric. 2008, 88, 1554–1561. [Google Scholar] [CrossRef]
- Dudley, H.C. Bromide content of fruits and vegetables. Ind. Eng. Chem. Anal. Ed. 1939, 11, 259–261. [Google Scholar] [CrossRef]
- Dudley, H.C.; Miller, J.W.; Neal, P.A.; Sayers, R.R. Studies on Foodstuffs Fumigated with Methyl Bromide. Publ. Health Rep. (1896–1970) 1940, 55, 2251. [Google Scholar] [CrossRef]
- Pflaum, R.T.; Frohliger, J.O.; Berge, D.G. The Direct Potentiometric Determination of Bromide Ion. Anal. Chem. 1962, 34, 1812–1814. [Google Scholar] [CrossRef]
- Agarwal, S. Potentiometric Titration of Chloride and Bromide in the Presence of Each Other. Metrohm Application Bulletin. No. 27/2e. Available online: https://www.metrohm.com/en-sg/applications/AB-027 (accessed on 2 April 2019).
| Detection Method | Sensitive Compound/Sample Preparations | Detection Limit/Sample | Advantages | Draw-Backs | Reference |
|---|---|---|---|---|---|
| Inductively coupled plasma mass spectrometry | 1717 mg/L/from blood serum | Very expensive | [5] | ||
| Inductively coupled plasma mass spectrometry | 0.68 ng/g/from different vegetables | Accurate | Very expensive | [8] | |
| Inductively coupled plasma mass spectrometry | Microwave-induced combustion and microwave-assisted alkaline dissolution were considered suitable for sample preparations of Br and I in saliva by ICP-MS | 0.052 μg mL−1/from human saliva | Microwave-assisted alkaline dissolution is more efficient for sample preparation | [9] | |
| Colorimetric detection | AuNPs aggregation capacity in the presence of Br− prevented by Cr3+ | 3.12−5.21 mg kg−1/from rice | Robust, selective, precise | [6] | |
| Thin layer chromatography | Metallic sodium for sodium fusion with halide ions | Unprecise/from synthetic solutions | Obsolete and dangerous | [10] | |
| Head-space gas chromatography (GC) and ion chromatography coupled with a conductive detector | 7.7 mg/L/from human urine | Accurate | Expensive | [11] | |
| Ion chromatography with ultraviolet detection | Extraction in ionic liquids | 0.16 mg/L/from underground water | No influence of other halide ions | [12] | |
| Liquid chromatography of ions | 0.05 mg/l/from tap water | It represents the standard method last reviewed and confirmed in 2016 | [13] | ||
| Ion exchange coupled with chemo-luminescence | 100 ng/mL/from river water, ground water | Rapid, inexpensive and simple | [14] | ||
| Automatic flow analysis, chemo-luminescence | Luminol, T-chromate | 8.9 μg L−1/from L-alanine | Precise | Expensive | [15] |
| Spectrophotometry | Deprotonated form of 2, 8, 12, 18-tetramethyl-3,7,13,17-tetrabutyl-H21, H23-porphine | 3 × 10−8 M/from synthetic solutions | Sensitive, inexpensive | [16] | |
| Spectrophotometry | Polyethyleneimine-capped Ag nanoclusters as fluorescent and colorimetric platform | 0.1−14 μM/from water | Simple, rapid, reliable, inexpensive | [17] | |
| Energy dispersive X-ray fluorescence spectrometry | 19.7 μg/mL/from drowned bodies | Indicator for drowning in seawater | Low sensitivity, long time, tedious | [18] | |
| Flow injection analysis coupled with amperometric detection | Platinum electrode | 50.0 nM/from water, pharmaceutical preparations and biological material | Fast, accurate | [19] | |
| Potentiometry | Meso-tetraphenylporphyrin manganese(III)-chloride | 0.010–1.0 μM/from water, synthetic solutions | Efficient | [7] | |
| Potentiometry | Pt(II) 5,10,15,20-tetra(4-methoxy-phenyl)-porphyrin as ionophore, dioctylphtalate (DOP) as plasticizer, polyvinylchloride (PVC) as support and tridodeocylmethylammonium chloride (TDMACl) as additive | 8 × 10−6 M/from synthetic solutions and pharmaceuticals | Fast, sensitive and reliable | [20] | |
| Segmented flow analysis in automated Phenol red method | Oxidation of bromide to bromine by chloramine-T, followed by electrophilic substitution of the bromine on phenol red (PR) to produce bromophenol blue (BPB) | 10 μg/L/from water | No interference from chloride | [21] | |
| Phenol red spectrophotometry | 3.63–19.02 mg kg–1/from capsicum, potatoes and fungi | Fast, suitable for routine determinations | [22] |
| Sample | UV-Vis Detection (mg/Kg) | Found by Potentiometry (mg/Kg) |
|---|---|---|
| Garden onion (Romania, Cenad) | 97.27 ± 0.6 | |
| Garden Bio Onion (Romania, Malovat) | 82.17 ± 0.9 | 77.95 ± 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascu, A.; Plesu, N.; Anghel, D.; Birdeanu, M.; Vlascici, D.; Fagadar-Cosma, E. Optical Detection of Bromide Ions Using Pt(II)-5,10,15,20-Tetra-(4-methoxy-phenyl)-porphyrin. Chemosensors 2019, 7, 21. https://doi.org/10.3390/chemosensors7020021
Lascu A, Plesu N, Anghel D, Birdeanu M, Vlascici D, Fagadar-Cosma E. Optical Detection of Bromide Ions Using Pt(II)-5,10,15,20-Tetra-(4-methoxy-phenyl)-porphyrin. Chemosensors. 2019; 7(2):21. https://doi.org/10.3390/chemosensors7020021
Chicago/Turabian StyleLascu, Anca, Nicoleta Plesu, Diana Anghel, Mihaela Birdeanu, Dana Vlascici, and Eugenia Fagadar-Cosma. 2019. "Optical Detection of Bromide Ions Using Pt(II)-5,10,15,20-Tetra-(4-methoxy-phenyl)-porphyrin" Chemosensors 7, no. 2: 21. https://doi.org/10.3390/chemosensors7020021
APA StyleLascu, A., Plesu, N., Anghel, D., Birdeanu, M., Vlascici, D., & Fagadar-Cosma, E. (2019). Optical Detection of Bromide Ions Using Pt(II)-5,10,15,20-Tetra-(4-methoxy-phenyl)-porphyrin. Chemosensors, 7(2), 21. https://doi.org/10.3390/chemosensors7020021