Handheld Enzymatic Luminescent Biosensor for Rapid Detection of Heavy Metals in Water Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biosensor Fabrication
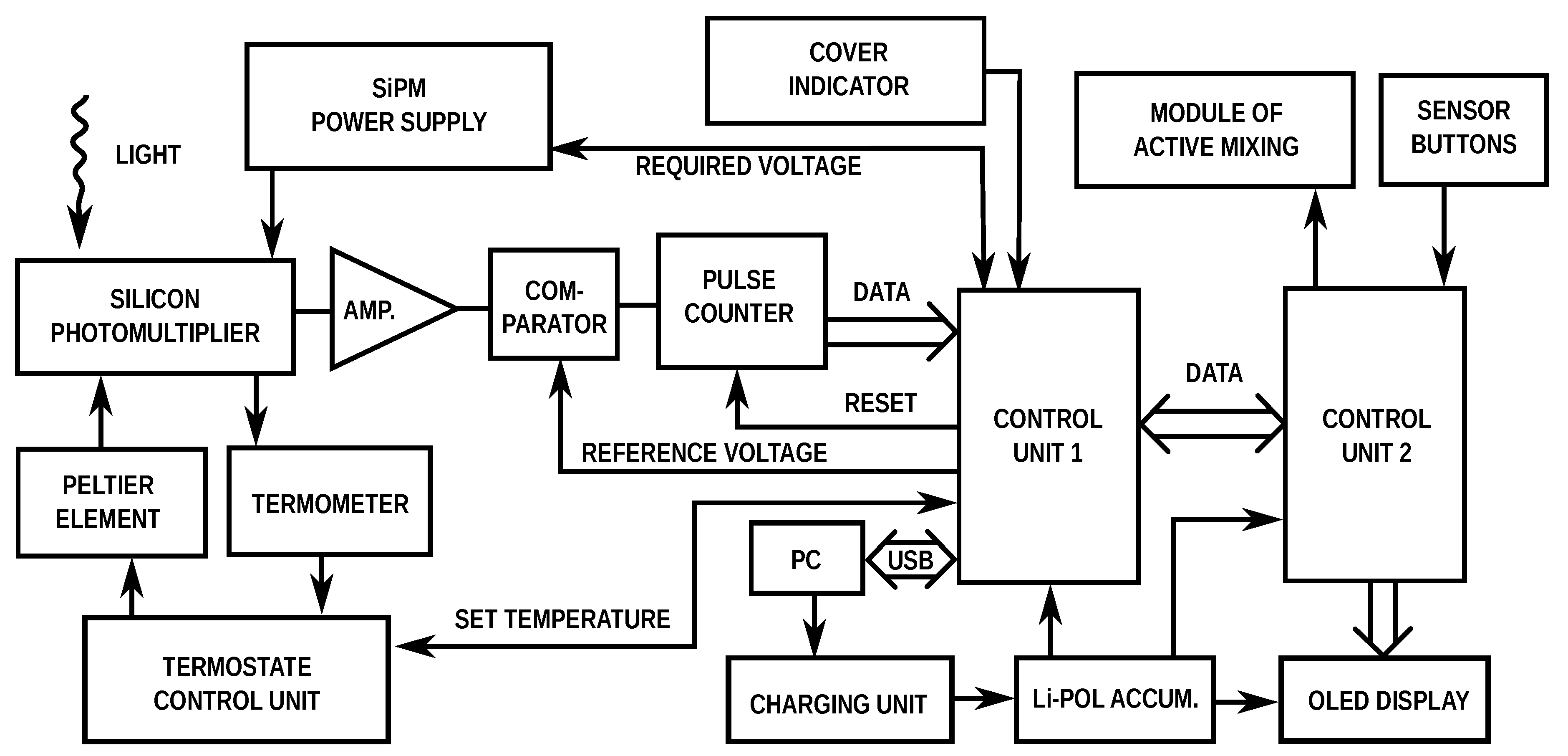
2.3. Signal Detection
2.4. Testing Procedure
3. Results and Discussion
3.1. Design of Portable Luminometer
3.2. Optimization of Microfluidic Chip Composition and Storage Conditions
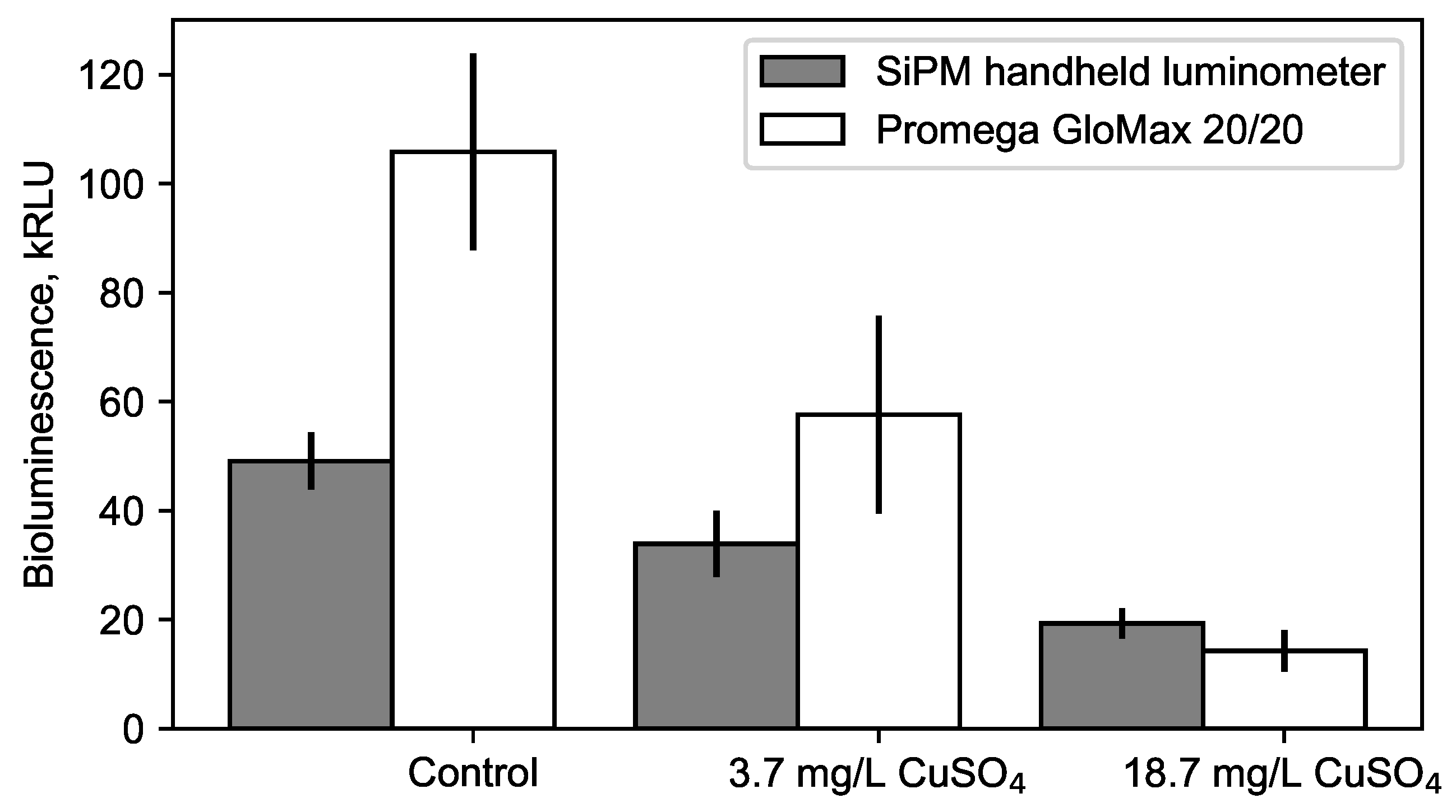
3.3. Sensitivity of the Portable Luminometer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Red+Luc | NAD(P)H:FMN-oxidoreductase and Luciferase; |
| PMT | Photomultiplier Tube; |
| SiPM | Silicon Photomultiplier; |
| FMN | Flavin Mononucleotide; |
| NADH | Nicotinamide Adenine Dinucleotide; |
| CAD | Computer-aided Design. |
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Marco, M.P.; Barceló, D. Biosensors for environmental monitoring: A global perspective. Talanta 2005, 65, 291–297. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Nagel, B.; Dellweg, H.; Gierasch, L. Glossary for chemists of terms used in biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Michelini, E.; Cevenini, L.; Calabretta, M.M.; Spinozzi, S.; Camborata, C.; Roda, A. Field-deployable whole-cell bioluminescent biosensors: So near and yet so far. Anal. Bioanal. Chem. 2013, 405, 6155–6163. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Taylor, J. Quality Assurance of Chemical Measurements; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Ligler, F.S. Perspective on optical biosensors and integrated sensor systems. Anal. Chem. 2008, 81, 519–526. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M.; Michelini, E.; Mirasoli, M. Bioluminescence in analytical chemistry and in vivo imaging. TrAC Trends Anal. Chem. 2009, 28, 307–322. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.; Kondik, A.; Kratasyuk, V. Bioluminescent enzymatic rapid assay of water integral toxicity. Environ. Monit. Assess. 2013, 185, 5909–5916. [Google Scholar] [CrossRef]
- Esimbekova, E.; Kratasyuk, V.; Shimomura, O. Application of Enzyme Bioluminescence in Ecology. In Bioluminescence: Fundamentals and Applications in Biotechnology—Volume 1; Springer: Berlin/Heidelberg, Germany, 2014; Volume 144, pp. 67–109. [Google Scholar]
- Hastings, J.W.; Gibson, Q.H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J. Biol. Chem. 1963, 238, 2537–2554. [Google Scholar] [PubMed]
- Hastings, J.W.; Riley, W.H.; Massa, J. The purification, properties, and chemiluminescent quantum yield of bacterial luciferase. J. Biol. Chem. 1965, 240, 1473–1481. [Google Scholar]
- Petushkov, V.; Kratasyuk, G.; Rodionova, N.; Fish, A.; Belobrov, P. Two-enzyme NADH:FMN-oxidoreductase-luciferase system from luminescent bacteria. Biochem. Acad. Sci. USSR 1984, 49, 93–604. [Google Scholar]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef]
- Fiorini, G.S.; Chiu, D.T. Disposable microfluidic devices: Fabrication, function, and application. BioTechniques 2005, 38, 429–446. [Google Scholar] [CrossRef]
- Kuo, J.S.; Chiu, D.T. Disposable microfluidic substrates: Transitioning from the research laboratory into the clinic. Lab Chip 2011, 11, 2656–2665. [Google Scholar] [CrossRef]
- Lukyanenko, K.; Denisov, I.; Yakimov, A.; Esimbekova, E.; Belousov, K.; Bukatin, A.; Kukhtevich, I.; Sorokin, V.; Evstrapov, A.; Belobrov, P. Analytical Enzymatic Reactions in Microfluidic Chips. Appl. Biochem. Microbiol. 2017, 53, 775–780. [Google Scholar] [CrossRef]
- Lukyanenko, K.A.; Belousov, K.I.; Denisov, I.A.; Yakimov, A.S.; Esimbekova, E.N.; Bukatin, A.S.; Evstrapov, A.A.; Belobrov, P.I. Active mixing of immobilised enzymatic system in microfluidic chip. Micro Nano Lett. 2017, 12, 377–381. [Google Scholar] [CrossRef]
- Denisov, I.; Lukyanenko, K.; Yakimov, A.; Kukhtevich, I.; Esimbekova, E.; Belobrov, P. Disposable luciferase-based microfluidic chip for rapid assay of water pollution. Luminescence 2018, 33, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Dolgoshein, B.; Balagura, V.; Buzhan, P.; Danilov, M.; Filatov, L.; Garutti, E.; Groll, M.; Ilyin, A.; Kantserov, V.; Kaplin, V.; et al. Status report on silicon photomultiplier development and its applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2006, 563, 368–376. [Google Scholar] [CrossRef]
- Kovaltchouk, V.; Lolos, G.; Papandreou, Z.; Wolbaum, K. Comparison of a silicon photomultiplier to a traditional vacuum photomultiplier. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2005, 538, 408–415. [Google Scholar] [CrossRef]
- Pasquardini, L.; Pancheri, L.; Potrich, C.; Ferri, A.; Piemonte, C.; Lunelli, L.; Napione, L.; Comunanza, V.; Alvaro, M.; Vanzetti, L.; et al. SPAD aptasensor for the detection of circulating protein biomarkers. Biosens. Bioelectron. 2015, 68, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Hui, Z.; Yu-Jin, Q.; Cui-Lan, Z. Design and development of compact readout electronics with silicon photomultiplier array for a compact imaging detector. Chin. Phys. C 2012, 36, 973. [Google Scholar]
- Wu, J.; Liu, X.; Wang, L.; Dong, L.; Pu, Q. An economical fluorescence detector for lab-on-a-chip devices with a light emitting photodiode and a low-cost avalanche photodiode. Analyst 2012, 137, 519–525. [Google Scholar] [CrossRef]
- Dinu, N.; Amara, Z.; Bazin, C.; Chaumat, V.; Cheikali, C.; Guilhem, G.; Puill, V.; Sylvia, C.; Vagnucci, J. Electro-optical characterization of SiPM: A comparative study. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2009, 610, 423–426. [Google Scholar] [CrossRef]
- Eckert, P.; Schultz-Coulon, H.C.; Shen, W.; Stamen, R.; Tadday, A. Characterisation studies of silicon photomultipliers. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 620, 217–226. [Google Scholar] [CrossRef]
- Li, H.; Lopes, N.; Moser, S.; Sayler, G.; Ripp, S. Silicon photomultiplier (SPM) detection of low-level bioluminescence for the development of deployable whole-cell biosensors: Possibilities and limitations. Biosens. Bioelectron. 2012, 33, 299–303. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.; Beebe, D.J.; Young, E.W. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef]
- Schagaev, I.; Kaegi-Trachsel, T. Programming Language for Safety Critical Systems. In Software Design for Resilient Computer Systems; Springer: Cham, Germany, 2016; pp. 159–182. [Google Scholar]
- Wojciechowski, J.R.; Shriver-Lake, L.C.; Yamaguchi, M.Y.; Fureder, E.; Pieler, R.; Schamesberger, M.; Winder, C.; Prall, H.J.; Sonnleitner, M.; Ligler, F.S. Organic photodiodes for biosensor miniaturization. Anal. Chem. 2009, 81, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Cevenini, L.; Calabretta, M.M.; Tarantino, G.; Michelini, E.; Roda, A. Smartphone-interfaced 3D printed toxicity biosensor integrating bioluminescent “sentinel cells”. Sens. Actuators B Chem. 2016, 225, 249–257. [Google Scholar] [CrossRef]
- Michelini, E.; Roda, A. Staying alive: New perspectives on cell immobilization for biosensing purposes. Anal. Bioanal. Chem. 2012, 402, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Date, A.; Pasini, P.; Daunert, S. Construction of spores for portable bacterial whole-cell biosensing systems. Anal. Chem. 2007, 79, 9391–9397. [Google Scholar] [CrossRef] [PubMed]
- Yagur-Kroll, S.; Schreuder, E.; Ingham, C.J.; Heideman, R.; Rosen, R.; Belkin, S. A miniature porous aluminum oxide-based flow-cell for online water quality monitoring using bacterial sensor cells. Biosens. Bioelectron. 2015, 64, 625–632. [Google Scholar] [CrossRef] [PubMed]
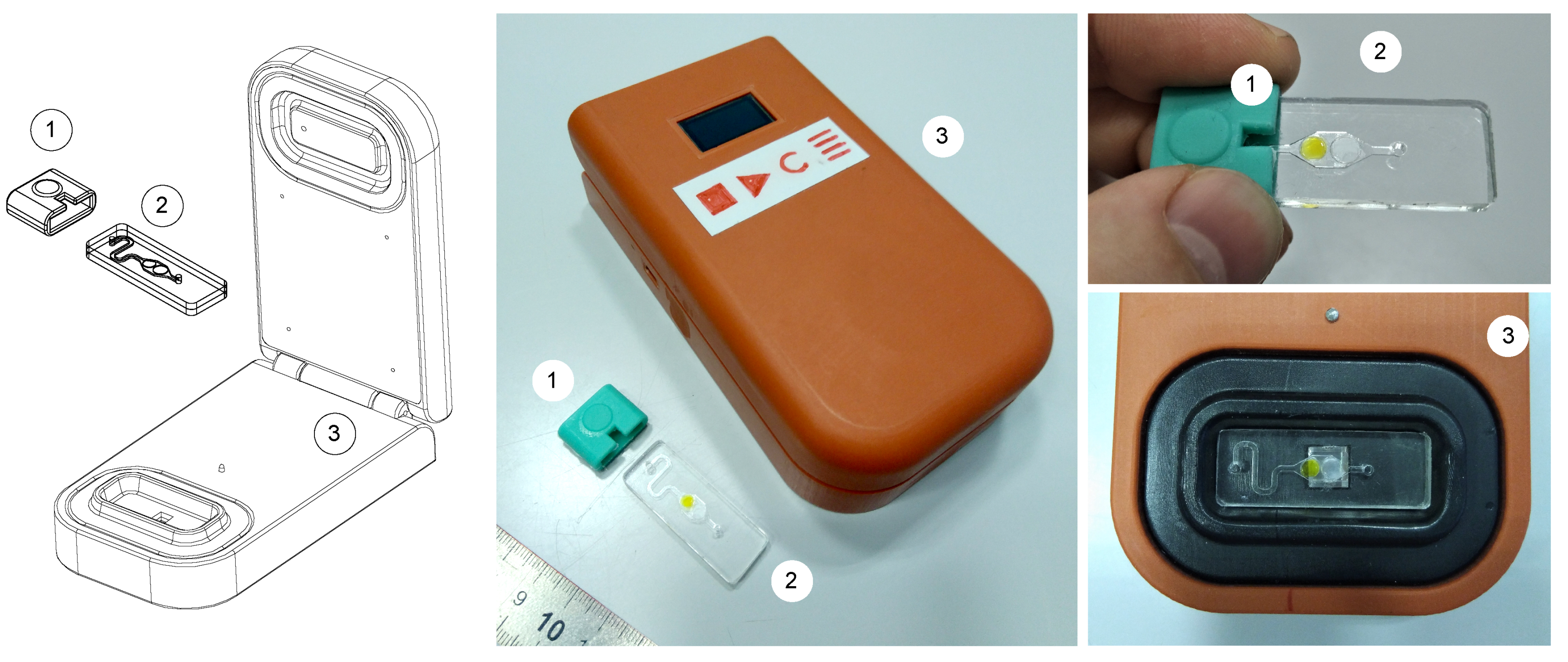

| [Aldehyde], % | Luciferase, g | Oxidoreductase, U | [NADH], mM | |
|---|---|---|---|---|
| No.1 | 0.00044 | 0.14 | 52.3 | 0.11 |
| No.2 | 0.00088 | 0.14 | 52.3 | 0.11 |
| No.3 | 0.00088 | 0.19 | 68.5 | 0.11 |
| No.4 | 0.00088 | 18.90 | 68.0 | 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukyanenko, K.A.; Denisov, I.A.; Sorokin, V.V.; Yakimov, A.S.; Esimbekova, E.N.; Belobrov, P.I. Handheld Enzymatic Luminescent Biosensor for Rapid Detection of Heavy Metals in Water Samples. Chemosensors 2019, 7, 16. https://doi.org/10.3390/chemosensors7010016
Lukyanenko KA, Denisov IA, Sorokin VV, Yakimov AS, Esimbekova EN, Belobrov PI. Handheld Enzymatic Luminescent Biosensor for Rapid Detection of Heavy Metals in Water Samples. Chemosensors. 2019; 7(1):16. https://doi.org/10.3390/chemosensors7010016
Chicago/Turabian StyleLukyanenko, Kirill A., Ivan A. Denisov, Vladimir V. Sorokin, Anton S. Yakimov, Elena N. Esimbekova, and Peter I. Belobrov. 2019. "Handheld Enzymatic Luminescent Biosensor for Rapid Detection of Heavy Metals in Water Samples" Chemosensors 7, no. 1: 16. https://doi.org/10.3390/chemosensors7010016
APA StyleLukyanenko, K. A., Denisov, I. A., Sorokin, V. V., Yakimov, A. S., Esimbekova, E. N., & Belobrov, P. I. (2019). Handheld Enzymatic Luminescent Biosensor for Rapid Detection of Heavy Metals in Water Samples. Chemosensors, 7(1), 16. https://doi.org/10.3390/chemosensors7010016









