Impedance Study of Dopamine Effects after Application on 2D and 3D Neuroblastoma Cell Cultures Developed on a 3D-Printed Well
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theory
2.2. Cells Preparation/ Immobilization
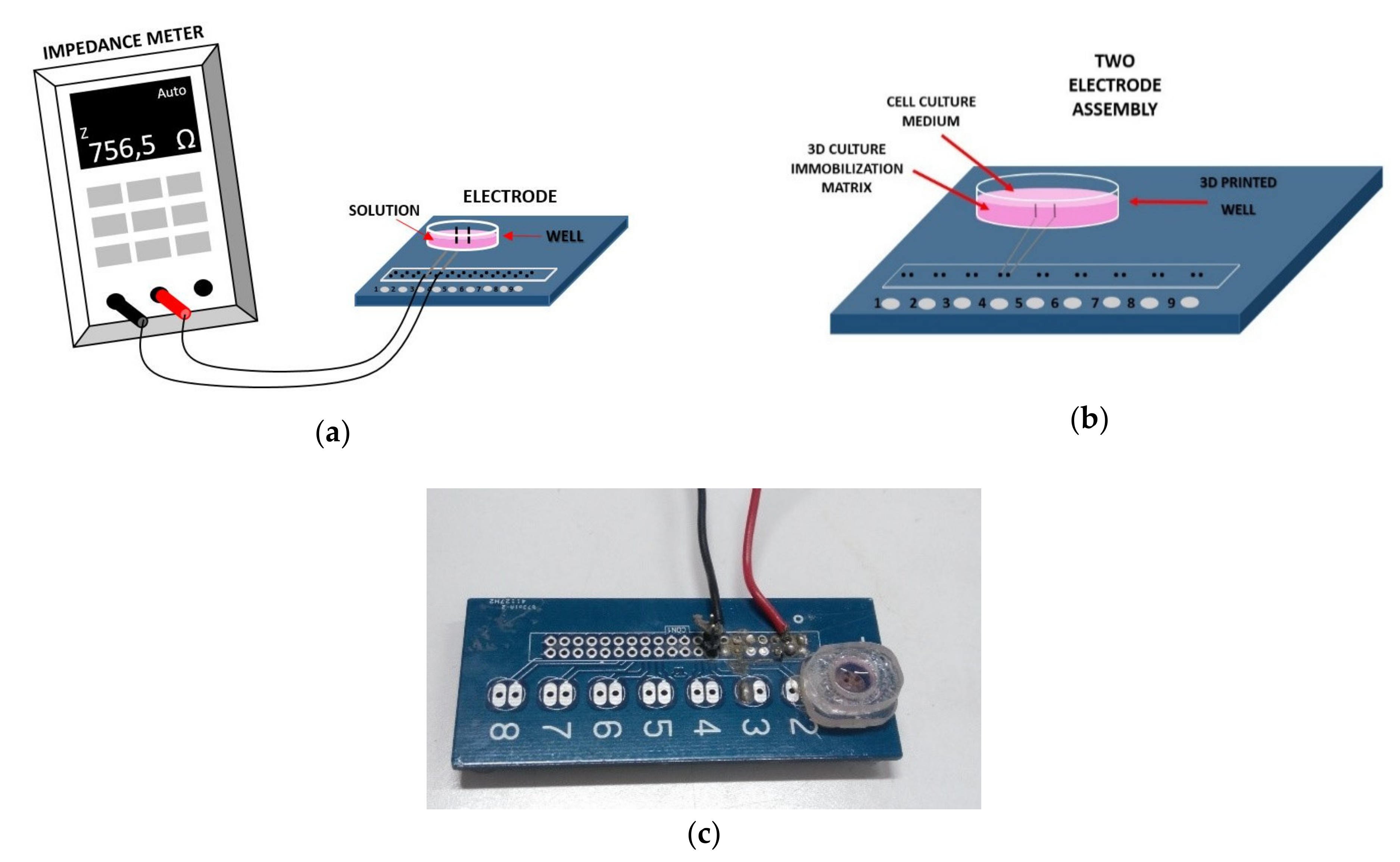
2.3. Experimental Setup
3. Results
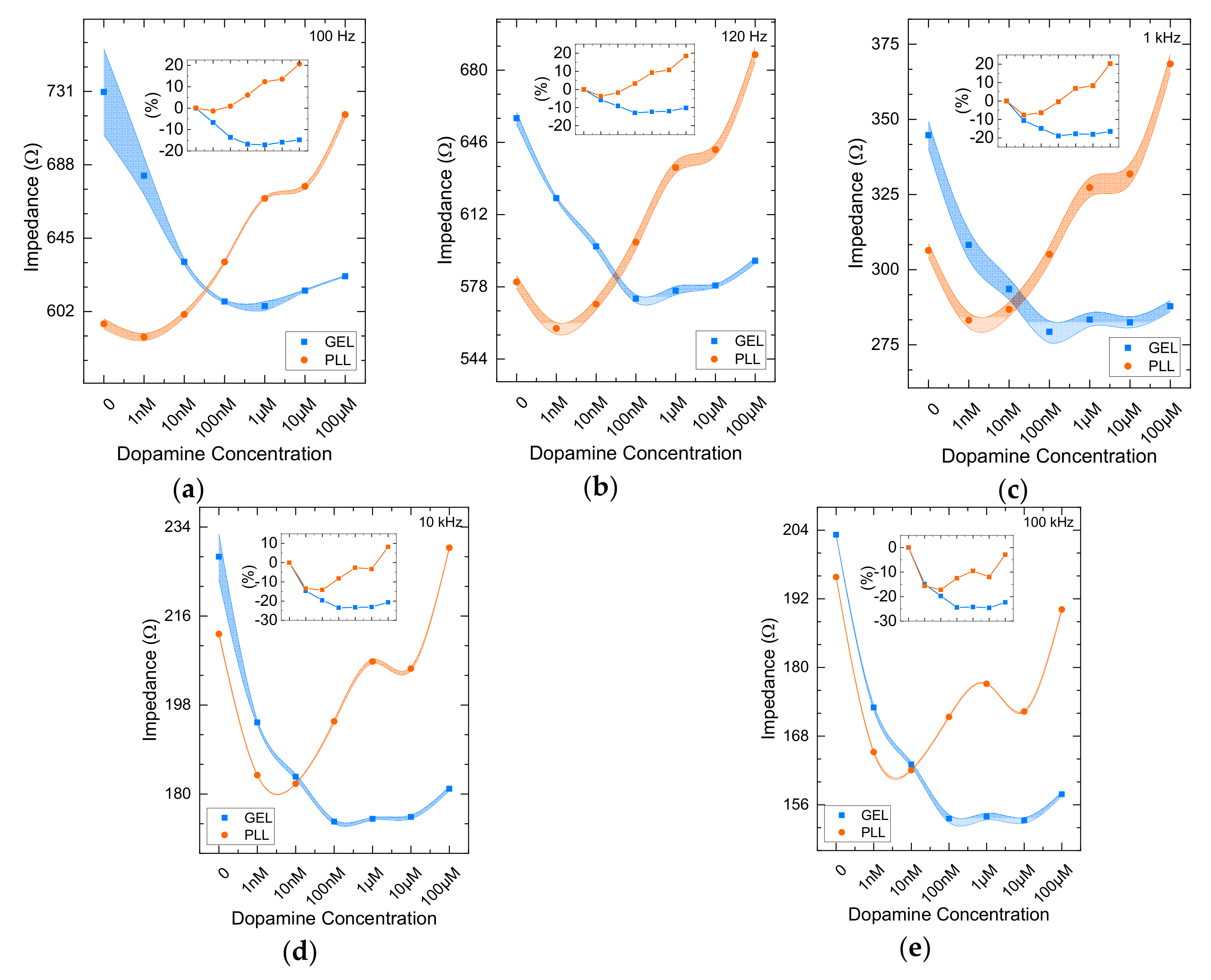
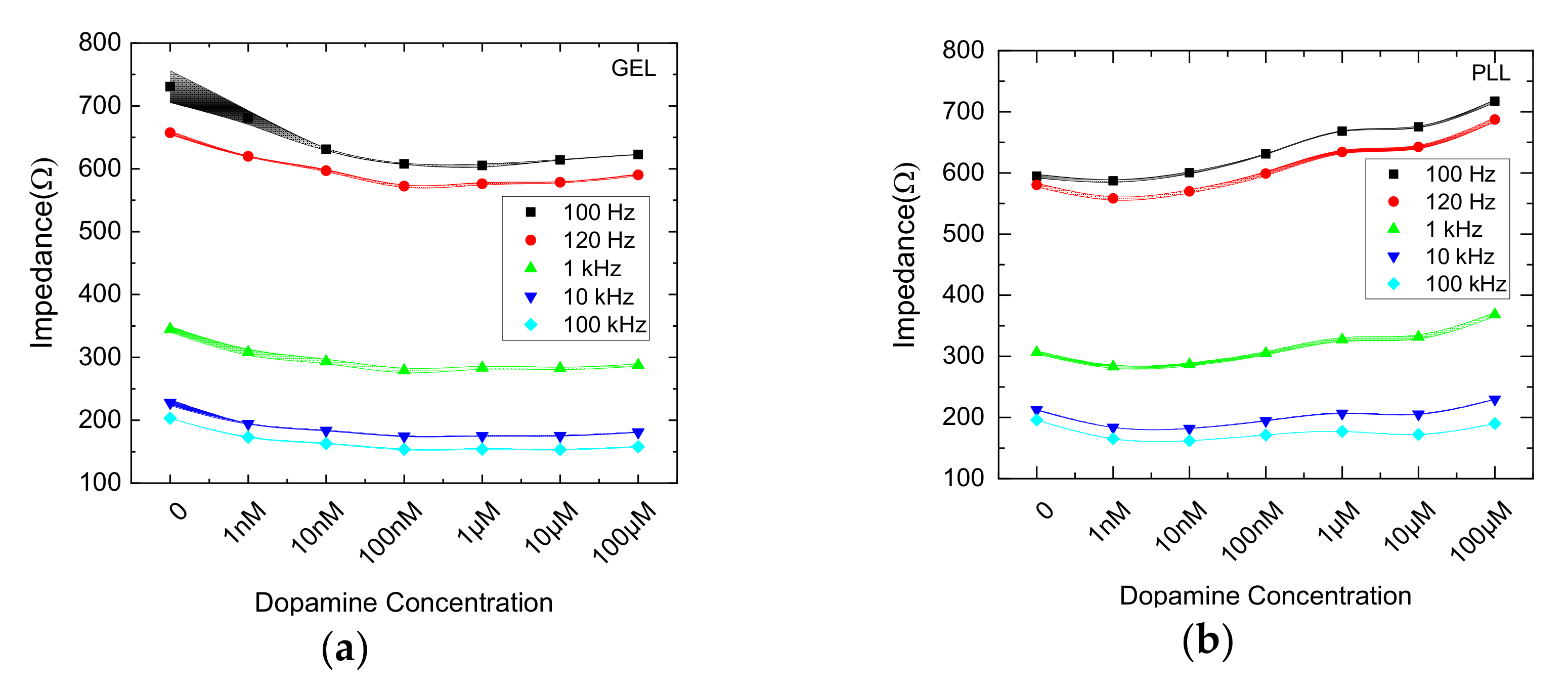
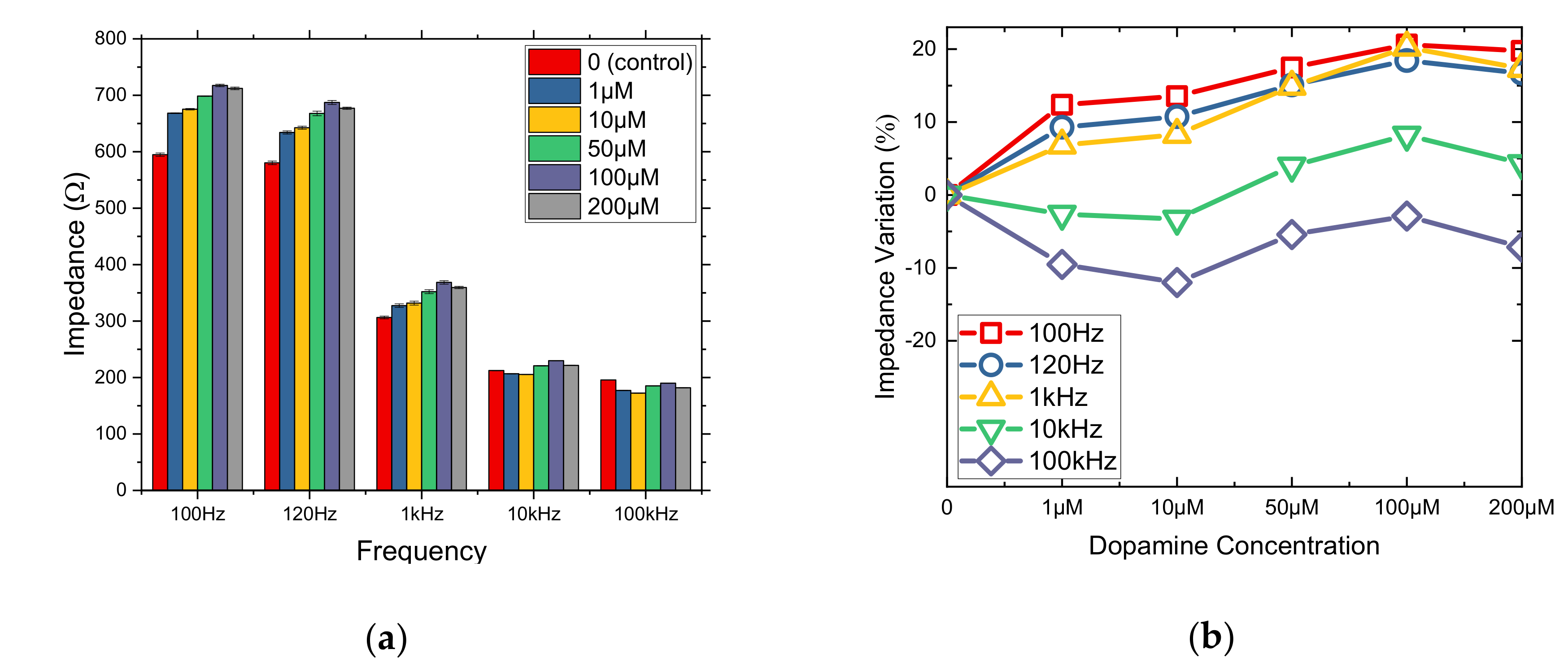
3.1. Impedance Comparative Results from 2D and 3D Cell Cultures
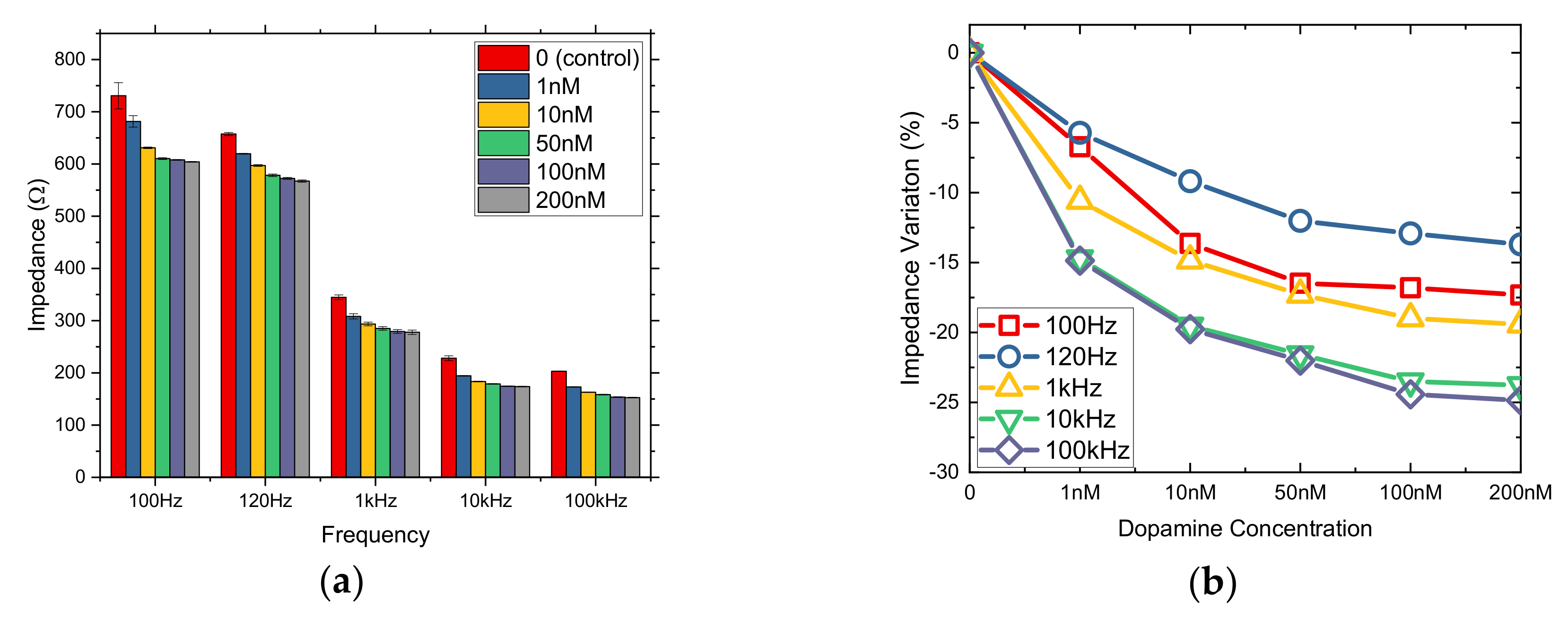
3.2. nM DA Effects on Cells Immobilized in Bactoagar Matrix
3.3. μM DA Effects on Cells Immobilized in PLL
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef]
- Tsai, S.L.; Wang, M.H. 24-h observation of a single HeLa cell by impedance measurement and numerical modeling. Sensor. Actuators B Chem. 2016, 229, 225–231. [Google Scholar] [CrossRef]
- Morgan, H.; Sun, T.; Holmes, D.; Gawad, S.; Green, N.G. Single cell dielectric spectroscopy. J. Phys. D Appl. Phys. 2007, 40, 61–70. [Google Scholar] [CrossRef]
- Valero, A.; Braschler, T.; Renaud, P. A unified approach to dielectric single cell analysis: Impedance and dielectrophoretic force spectroscopy. Lab. Chip. 2010, 10, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Noveletto, F.; Bertemes Filho, P.; Dutra, D.; Soares, A.V. Low-cost body impedance analyzer for healthcare applications. In Proceedings of the II Latin American Conference on Bioimpedance, Montevideo, Uruguay, 30 September–2 October 2015; pp. 56–59. [Google Scholar]
- Salvadore, G.; van der Veen, J.W.; Zhang, Y.; Marenco, S.; Machado-Vieira, R.; Baumann, J.; Ibrahim, L.A.; Luckenbaugh, D.A.; Shen, J.; Drevets, W.C.; et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int. J. Neuropsychop. 2012, 15, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Marc, D.T.; Ailts, J.W.; Campeau, D.C.A.; Bull, M.J.; Olson, K.L. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: Validity and clinical applicability. Neurosci. Biobehav. R. 2011, 35, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Nurullah, S.; Tague, S.E.; Lunte, C. Analysis of amino acid neurotransmitters from rat and mouse spinalcords by liquid chromatography with fluorescence detection. J. Pharmaceut. Biomed. 2015, 107, 217–222. [Google Scholar]
- Yoetz-Kopelman, T.; Dror, Y.; Shacham-Diamand, Y.; Freeman, A. Cells-on-Beads: A novel immobilization approach for the construction of whole-cell amperometric biosensors. Sensor. Actuators B Chem. 2016, 232, 758–764. [Google Scholar] [CrossRef]
- Asphahani, F.; Zhang, M. Cellular impedance biosensors for drug screening and toxin detection. Analyst 2007, 132, 835–841. [Google Scholar] [CrossRef]
- Wang, H.S.; Li, T.H.; Jia, W.L.; Xu, H.Y. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly(3-methylthiophene) modified electrode. Biosens. Bioelectron. 2006, 22, 664–669. [Google Scholar] [CrossRef]
- Song, W. Dopamine sensor based on molecularly imprinted electrosynthesized polymers. J. Solid State Electr. 2010, 14, 1909–1914. [Google Scholar] [CrossRef]
- Glezer, A.; Bronstein, M.D. Hyperprolactinemia. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M.J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Komathi, S.; Gopalan, A.; Lee, K.P. Nanomolar detection of dopamine at multi-walled carbon nanotube grafted silica network/gold nanoparticle functionalised nanocomposite electrodes. Analyst 2010, 135, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.S.; Agboolab, B.O.; Pillayc, J.; Ozoemena, K.I. Electrocatalytic detection of dopamine at single-walled carbon nanotubes–iron (III) oxide nanoparticles platform. Sensor. Actuators B Chem. 2010, 148, 93–102. [Google Scholar] [CrossRef]
- Wightman, R.M.; May, L.J.; Michael, A.C. Detection of dopamine dynamics in the brain. Anal. Chem. 1988, 60, 769–779. [Google Scholar] [CrossRef]
- Łuczak, T. Preparation and characterization of the dopamine film electrochemically deposited on a gold template and its applications for dopamine sensing in aqueous solution. Electrochim. Acta 2008, 53, 5725–5731. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Yanga, Z. Study of the sensitization of tetradecyl benzyl dimethyl ammonium chloride for spectrophotometric determination of dopamine hydrochloride using sodium 1,2-naphthoquinone-4-sulfonate as the chemical derivative chromogenic reagent. Anal. Chim. Acta 2007, 583, 147–152. [Google Scholar] [CrossRef]
- Muzzi, C.; Bertocci, E.; Terzuoli, L.; Porcelli, B.; Ciari, I.; Pagani, R.; Guerranti, R. Simultaneous determination of serum concentrations of levodopa, dopamine, 3-O-methyldopa and α-methyldopa by HPLC. Biomed. Pharmacother. 2008, 62, 253–258. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Lacroix, L.; Atkins, A.R.; Organ, A.J.; Murray, S.; West, A.; Shah, A.J. Development and application of a sensitive high performance ion-exchange chromatography method for the simultaneous measurement of dopamine, 5-hydroxytryptamine and norepinephrine in microdialysates from the rat brain. J. Neurosci Meth. 2001, 112, 135–144. [Google Scholar] [CrossRef]
- Jo, S.; Jeong, H.; Bae, S.R.; Jeon, S. Modified platinum electrode with phytic acid and single-walled carbon nanotube: Application to the selective determination of dopamine in the presence of ascorbic and uric acids. Microchem. J. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Henstridge, M.C.; Dickinson, E.J.F.; Aslanoglu, M.; Batchelor-McAuley, C.; Compton, R.G. Voltammetric selectivity conferred by the modification of electrodes using conductive porous layers or films: The oxidation of dopamine on glassy carbon electrodes modified with multiwalled carbon nanotubes. Sensor. Actuators B Chem. 2010, 145, 417–427. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem 2012, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Tung, S. Development and Applications of Portable Biosensors. J. Lab. Autom. 2015, 20, 365. [Google Scholar] [CrossRef] [PubMed]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-printed millifluidic and microfluidic ’lab on a chip’ reactionware devices. Lab. Chip 2012, 12, 183267–183271. [Google Scholar] [CrossRef] [PubMed]
- Waldbaur, A.; Rapp, H.; Lange, K.; Rapp, B.E. Let there be chip—towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Munshi, A.S.; Martin, R.S. Microchip-Based Electrochemical Detection using a 3-D Printed Wall-Jet Electrode Device. Analyst 2016, 141, 862–869. [Google Scholar] [CrossRef]
- Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y.; Kim, P. Soft lithography for microfluidics: A review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Liu, K.; Fan, Z.H. Thermoplastic microfluidic devices and their applications in protein and DNA analysis. Analyst 2011, 136, 1288–1297. [Google Scholar] [CrossRef]
- Becker, H.; Nevitt, M.; Gray, B.L. Selecting and designing with the right thermoplastic polymer for your microfluidic chip: A close look into cyclo-olefin polymer. Microfluid. BioMEMS Med. Microsyst. XI 2013, 8615, 86150F. [Google Scholar]
- Bhattacharyya, A.; Klapperich, C.M. Thermoplastic microfluidic device for on-chip purification of nucleic acids for disposable diagnostics. Anal. Chem. 2006, 78, 788–792. [Google Scholar] [CrossRef]
- Mehta, G.; Lee, J.; Cha, W.; Tung, Y.C.; Linderman, J.J.; Takayama, S. Hard top soft bottom microfluidic devices for cell culture and chemical analysis. Anal. Chem. 2009, 81, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Mirabella, F.; D’Urso, A.; Randazzo, R.; Purrello, R.; Fragala, M.E. Spontaneous deposition of polylysine on surfaces: Role of the secondary structure to optimize noncovalent strategies. J. Colloid Interf. Sci. 2015, 437, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, J.; You, H. Photodegradation behavior and mechanism of poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) (PETG) random copolymers: Correlation with copolymer composition. RSC Adv. 2016, 6, 102778–102790. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J. Surface hydrophilic modification of acrylonitrile-butadiene-styrene terpolymer by poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate): Preparation, characterization, and properties studies. Appl. Surf. Sci. 2016, 388, 133–140. [Google Scholar] [CrossRef]
- Paivana, G.; Apostolou, T.; Kaltsas, G.; Kintzios, S. Study of the dopamine effect into cell solutions by impedance analysis in Conference on Bio-Medical Instrumentation and related Engineering and Physical Sciences, Athens. J. Phys. 2017, 931, 012010. [Google Scholar]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Albulbul, A. Evaluating major electrode types for idle biological signal measurements for modern medical technology. Bioengineering 2016, 3, 2. [Google Scholar] [CrossRef]
- Shavali, S.; Sens, D.A. Synergistic neurotoxic effects of arsenic and dopamine in human dopaminergic neuroblastoma SH-SY5Y cells. Toxicol. Sci. 2008, 102, 254–261. [Google Scholar] [CrossRef]
- Yang, L.; Dai, M.; Xu, C.; Zhang, G.; Li, W.; Fu, F.; Shi, X.; Dong, X. The frequency spectral properties of electrode-skin contact impedance on human head and its frequency-dependent effects on frequency-difference eit in stroke detection from 10 Hz to 1 MHz. PloS ONE 2017, 12, e0170563. [Google Scholar] [CrossRef]
- Becchi, M.; Avendano, C.; Strigazzi, A.; Barbero, G. Impedance spectroscopy of water solutions: The Role of Ions at the liquid-electrode interface. J. Phys. Chem. B 2005, 109, 23444–23449. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, T.; Moschopoulou, G.; Kolotourou, E.; Kintzios, S. Assessment of in vitro dopamine-neuroblastoma cell interactions with a bioelectric biosensor: perspective for a novel in vitro functional assay for dopamine agonist/antagonist activity. Talanta 2017, 170, 69–73. [Google Scholar] [CrossRef] [PubMed]
| Frequency | R2 | |
|---|---|---|
| GEL | PLL | |
| 100 Hz | 0.9882 | 0.9649 |
| 120 Hz | 0.9892 | 0.9492 |
| 1 kHz | 0.9685 | 0.9306 |
| 10 kHz | 0.9627 | 0.7987 |
| 100 kHz | 0.9672 | 0.6571 |
| Frequency | R2 | |
|---|---|---|
| GEL (nM) | PLL (μM) | |
| 100 Hz | 0.921 | 0.9468 |
| 120 Hz | 0.997 | 0.9612 |
| 1 kHz | 0.98 | 0.9358 |
| 10 kHz | 0.9608 | 0.5526 |
| 100 kHz | 0.9632 | 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paivana, G.; Apostolou, T.; Mavrikou, S.; Barmpakos, D.; Kaltsas, G.; Kintzios, S. Impedance Study of Dopamine Effects after Application on 2D and 3D Neuroblastoma Cell Cultures Developed on a 3D-Printed Well. Chemosensors 2019, 7, 6. https://doi.org/10.3390/chemosensors7010006
Paivana G, Apostolou T, Mavrikou S, Barmpakos D, Kaltsas G, Kintzios S. Impedance Study of Dopamine Effects after Application on 2D and 3D Neuroblastoma Cell Cultures Developed on a 3D-Printed Well. Chemosensors. 2019; 7(1):6. https://doi.org/10.3390/chemosensors7010006
Chicago/Turabian StylePaivana, Georgia, Theofylaktos Apostolou, Sophie Mavrikou, Dimitris Barmpakos, Grigoris Kaltsas, and Spyridon Kintzios. 2019. "Impedance Study of Dopamine Effects after Application on 2D and 3D Neuroblastoma Cell Cultures Developed on a 3D-Printed Well" Chemosensors 7, no. 1: 6. https://doi.org/10.3390/chemosensors7010006
APA StylePaivana, G., Apostolou, T., Mavrikou, S., Barmpakos, D., Kaltsas, G., & Kintzios, S. (2019). Impedance Study of Dopamine Effects after Application on 2D and 3D Neuroblastoma Cell Cultures Developed on a 3D-Printed Well. Chemosensors, 7(1), 6. https://doi.org/10.3390/chemosensors7010006









