Sensors Based on Amino Group Surface-Modified CNTs
Abstract
:1. Introduction
2. The Main Principles of the DFT Method
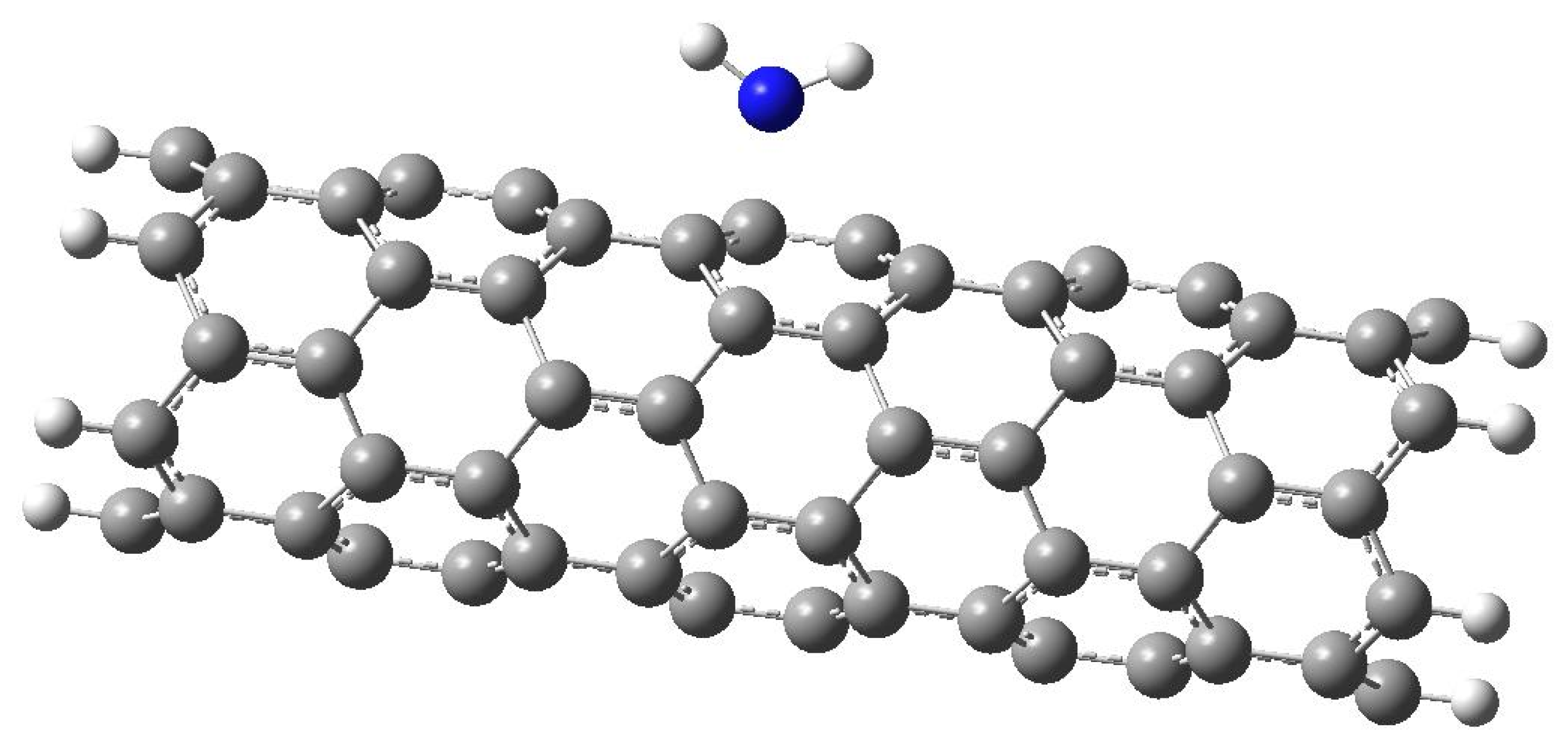
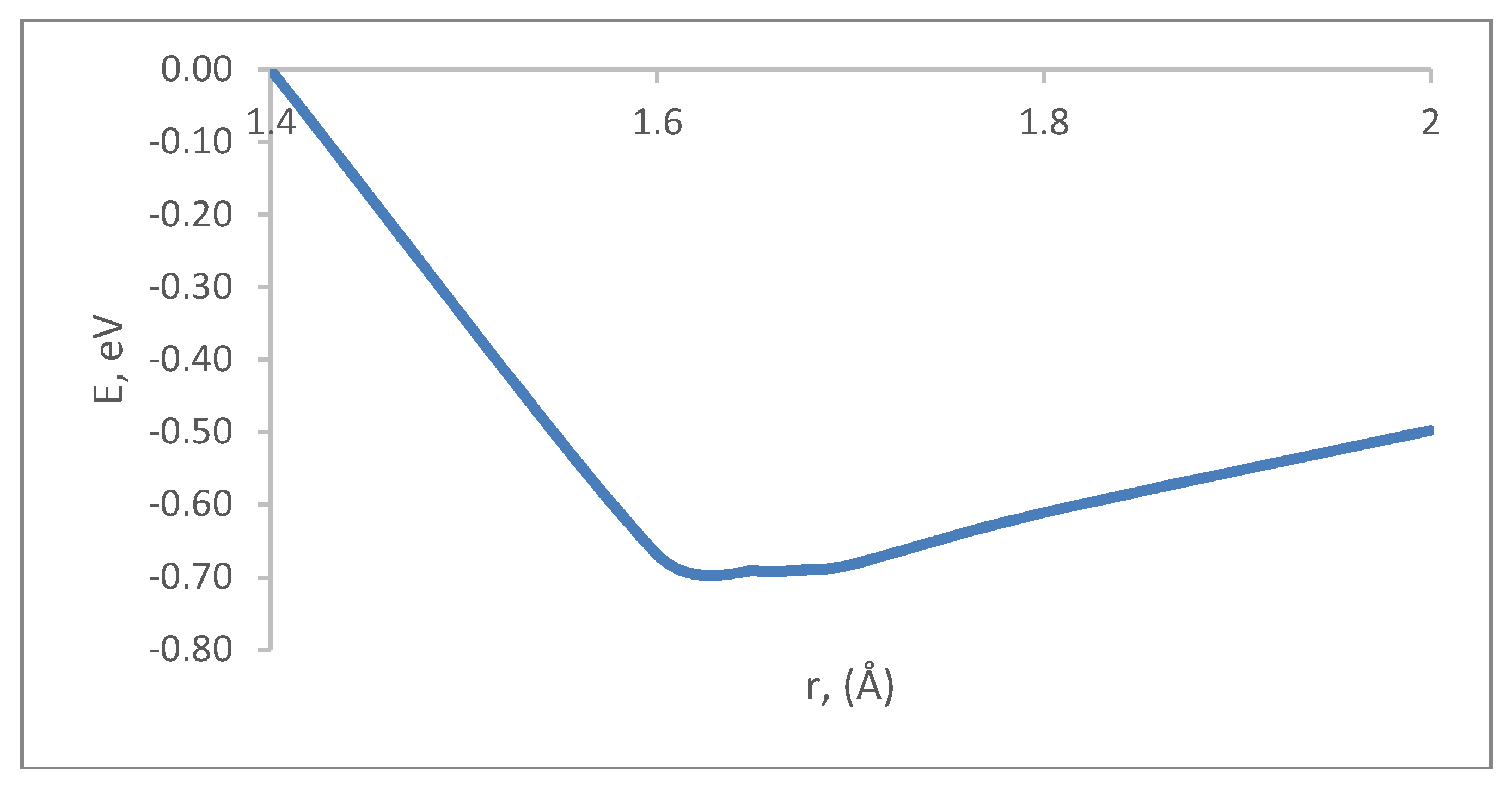
3. A Study of Carbon Nanotube Surface Modification by an NH2 Amino Group
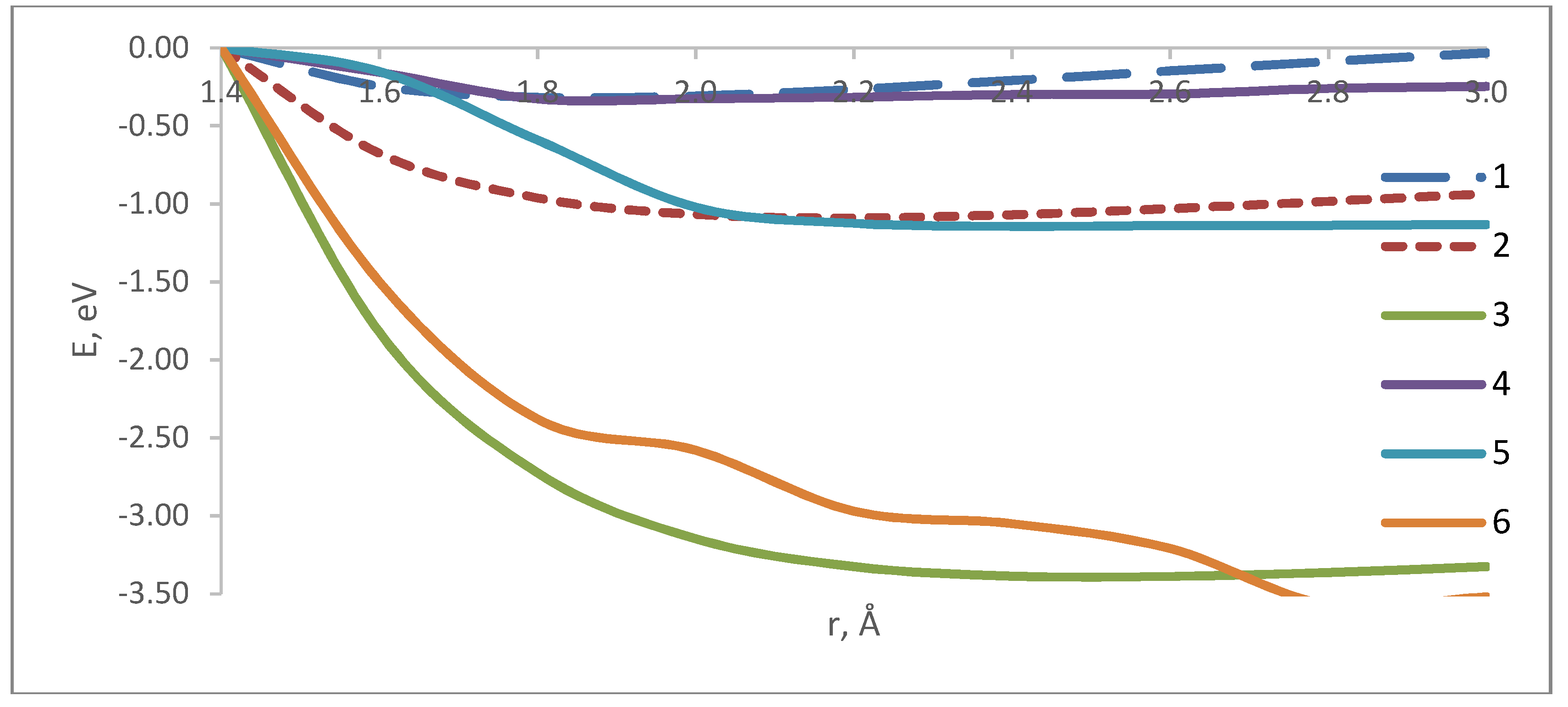
4. A Study of the Interaction Process between CNT–NH2 System and Atoms and Ions of Alkali Metals
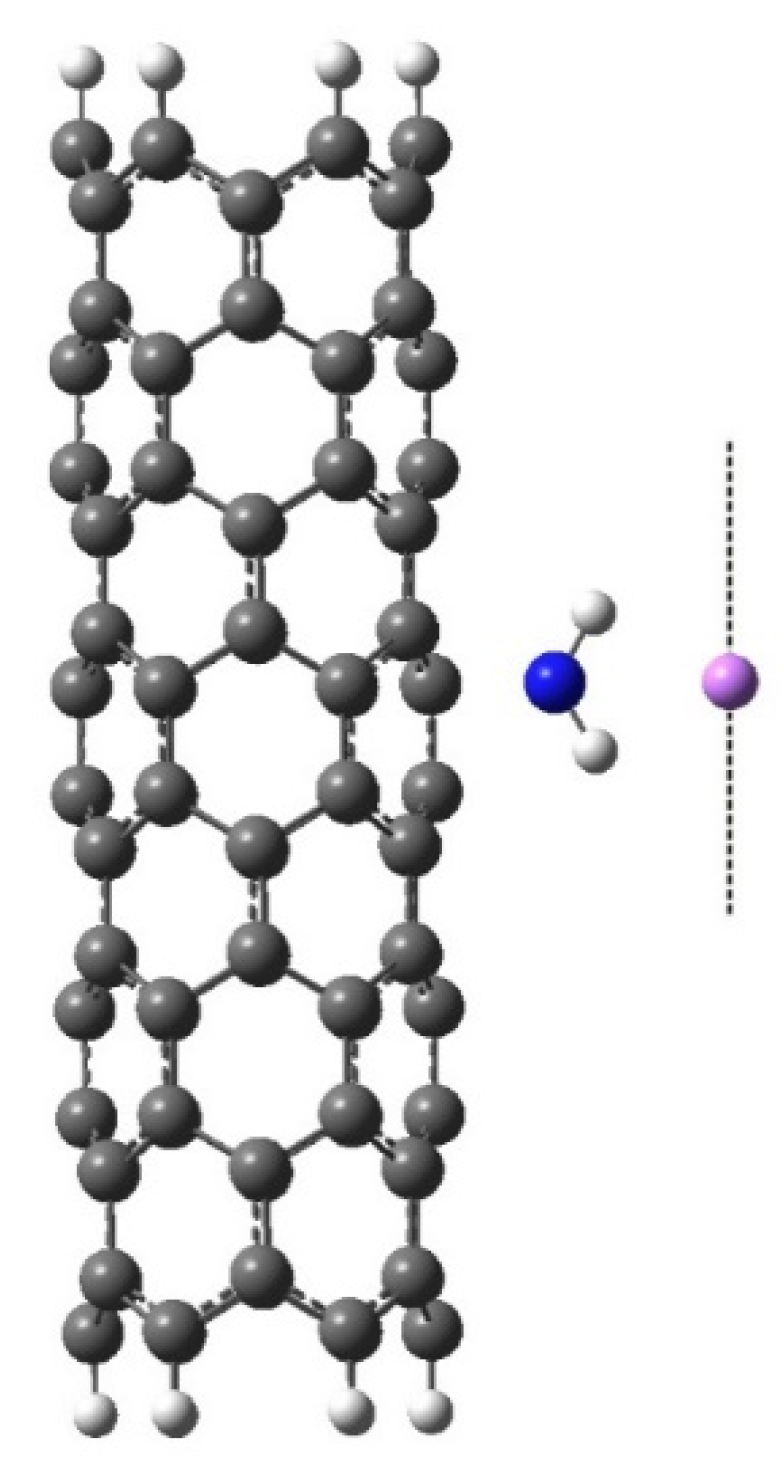
5. Modeling the Scanning Process of the Nanotube Surface That Contains Atoms and Ions of Alkali Metals
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Dresselhaus, G.; Avouris, P. Сarbon Nanotubes: Synthesis, Structure, Properties, and Application; Springer: Berlin/Heidelberg, Germany, 2001; p. 464. [Google Scholar]
- D’yachkov, P.N. Electron Properties and Applications of Nanotubes; Binom, Laboratoria Znanii: Moscow, Russia, 2010; p. 488. ISBN 978-5-9963-1096-8. [Google Scholar]
- Saito, R.; Dresselhaus, M.S.; Dresselhaus, G. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1999; p. 251. [Google Scholar]
- Harris, P.J.F. Carbon Nanotubes and Related Structures: New Materials of the XXI Century; TECHNOSHERA: Moscow, Russia, 2003; p. 336. ISBN 5-94836-013-X. [Google Scholar]
- Eletskii, A.V. Sorption properties of carbon nanostructures. Uspekhi Fizicheskikh Nauk. 2004, 47, 1191. [Google Scholar] [CrossRef]
- Zaporotskova, I.V. Carbon and Non-Carbon Nanomaterials and Composite Structures Based on Them: Structure and Electronic Properties; Izdatelstvo VolGU: Volgograd, Russia, 2009; p. 490. ISBN 978-5-9669-0644-3. [Google Scholar]
- Akhmadichina, K.F.; Bobrinetskii, I.I.; Komarov, I.A.; Malovichko, A.M.; Nevolin, V.K.; Petukhov, A.V.; Golovin, A.V.; Zalevskii, A.O. The flexible biological sensors based on carbon nanotubular films. Russ. Nanotechnol. 2013, 8, 721–726. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, W.H. Carbon Nanotubes as Active Components for Gas Sensors. J. Sens. 2009, 2009, 160698. [Google Scholar] [CrossRef]
- Farrera, C.; Torres Andón, F.; Feliu, N. Carbon Nanotubes as Optical Sensors in Biomedicine (Review). ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Cháfer, J.; Navarrete, E.; Noirfalise, X.; Umek, P.; Bittencourt, C.; Llobet, E. Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.; Ghica, M.E.; Brett, C.M.A. Gold nanoparticle decorated multiwalled carbon nanotube modified electrodes for the electrochemical determination of theophylline. Anal. Methods 2018, 10, 5634–5642. [Google Scholar] [CrossRef]
- Salvador, M.A.; Sousa, C.P.; Maciel, C.D.; Gomes, R.N.; Morais, S.; de Lima-Neto, P.; Coutinho-Neto, M.D.; Correia, A.N. Experimental and computational studies of the interactions between carbon nanotubes and ionic liquids used for detection of acetaminophen. Sens. Actuators B Chem. 2018, 277, 640–646. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [PubMed]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Sun, C.Q.; Fu, S.-Y.; Nie, Y.G. Dominance of broken bonds and unpaired nonbonding π-electrons in the band gap expansion and edge states generation in graphene nanoribbons. J. Phys. Chem. C 2008, 112, 18927–18934. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Polikarpova, N.P.; Vil’keeva, D.E. Sensor Activity of Carbon Nanotubes with a Boundary Functional Group. Nanosci. Nanotechnol. Lett. 2013, 5, 1169–1173. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Vilkeeva, D.E.; Polikarpova, N.P.; Polikarpov, D.I. Sensor properties of carboxylmodified carbon nanotubes. Nanosyst. Phys. Chem. Math. 2014, 5, 101–106. [Google Scholar]
- Tsai, T.-H.; Lin, K.-W.; Chen, H.-I.; Liu, I.-P.; Hung, C.-W.; Chen, L.-Y.; Tsai, Y.-Y.; Chen, T.-P.; Chu, K.-Y.; Liu, W.-C. Transient response of a transistor-based hydrogen sensor. Sens. Actuators B Chem. 2008, 129, 750–754. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Boroznin, S.V.; Zaporotskov, P.A. About Using Carbon Nanotubes with Amino Group Modification as Sensors. J. Nano Electron. Phys. 2015, 7, 04089. [Google Scholar]
- Zaporotskova, I.V.; Kozhitov, V.; Boroznina, N.P. Sensor Activity with Respect to Alkali Metals of a Carbon Nanotube Edge-Modified with Amino Group. Russ. J. Inorg. Chem. 2017, 62, 1458–1463. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; Pascoini, A.L.; Knupp, W.G.; Camps, I. Effects of surface functionalization on the electronic and structural properties of carbon nanotubes: A computational approach. Appl. Surf. Sci. 2017, 426, 781–787. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; Wiley-VCH: Weinheim, Germany, 2002; p. 306. [Google Scholar]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A.J. 6-31G* basis set for third-row atoms. Comp. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Pople, J.A. Quantum-chemical models. Uspekhi Fizicheskikh Nauk 2002, 172, 349–356. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Bincley, J.S.; DeFrees, D.J.; Pople, J.A.; Gordon, M.S.J. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Rassolov, V.; Pople, J.A. Gaussian-3 (G3) theory for molecules containing first and second-row atoms. J. Chem. Phys. 1998, 109, 7764–7776. [Google Scholar] [CrossRef]
- Chen, Z.; Nagase, S.; Hirsch, A.; Haddon, R.C.; Thiel, W.; Schleyer, P.R. Side-Wall Opening of Single-Walled Carbon Nanotubes (SWCNTs) by Chemical Modification: A Critical Theoretical Study. Angew. Chem. 2004, 116, 1578–1580. [Google Scholar] [CrossRef]
- Dinadayalane, T.C.; Leszczynski, J. Stone–Wales defects with two different orientations in (5, 5) single-walled carbon nanotubes: a theoretical study. Chem. Phys. Lett. 2007, 434, 86–91. [Google Scholar] [CrossRef]
- Budyka, M.F.; Zyubina, T.S.; Ryabenko, A.G.; Lin, S.H.; Mebel, A.M. Bond lengths and diameters of armchair single wall carbon nanotubes. Chem. Phys. Lett. 2005, 407, 266–271. [Google Scholar] [CrossRef]
- Bauschlicher, C.W.; Ricca, J.A. Binding of NH3 to graphite and to a (9, 0) carbon nanotube. Phys. Rev. B 2004, 70, 115409. [Google Scholar] [CrossRef]
- Walch, S.P. On the reaction of N and O atoms with carbon nanotubes. Chem. Phys. Lett. 2003, 374, 501–505. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. J. Chem. Phys. 1955, 23, 1833. [Google Scholar] [CrossRef]
- Chen, R.J.; Franklin, N.R.; Kong, J.; Cao, J.; Tombler, T.W.; Zhang, Y.; Dai, H. Molecular photodesorption from single-walled carbon nanotubes. Appl. Phys. Lett. 2001, 79, 2258–2260. [Google Scholar] [CrossRef]
- Nguyen, H.-Q.; Huh, J.-S. Behavior of single-walled carbon nanotube-based gas sensors at various temperatures of treatment and operation. Sens. Actuators B Chem. 2006, 117, 426–430. [Google Scholar] [CrossRef]
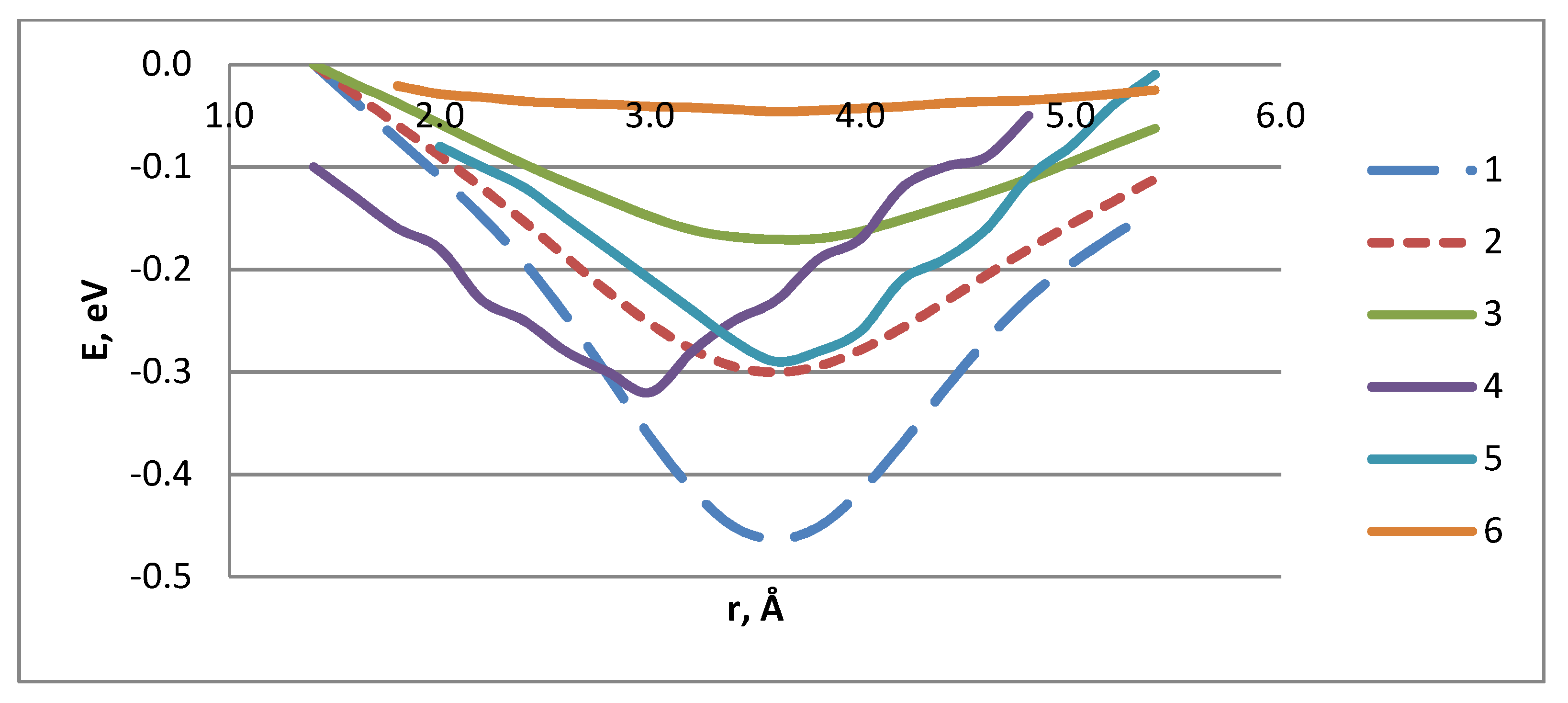
| Atomic Bonds | rint, Å | Eint, eV | Q |
|---|---|---|---|
| Li–H | 1.8 | −0.32 | +0.40 |
| Li+–H | 1.8 | −0.33 | +0.66 |
| Na–H | 2.2 | −0.41 | +0.52 |
| Na+–H | 2.2 | −1.12 | +0.79 |
| K–H | 2.6 | −0.66 | +0.73 |
| K+–H | 2.8 | −3.57 | +0.91 |
| Atomic Bonds | Es-int, eV |
|---|---|
| K–H | −0.46 |
| K+–H | −0.32 |
| Na–H | −0.30 |
| Na+–H | −0.29 |
| Li–H | −0.17 |
| Li+–H | −0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroznina, N.; Zaporotskova, I.; Boroznin, S.; Dryuchkov, E. Sensors Based on Amino Group Surface-Modified CNTs. Chemosensors 2019, 7, 11. https://doi.org/10.3390/chemosensors7010011
Boroznina N, Zaporotskova I, Boroznin S, Dryuchkov E. Sensors Based on Amino Group Surface-Modified CNTs. Chemosensors. 2019; 7(1):11. https://doi.org/10.3390/chemosensors7010011
Chicago/Turabian StyleBoroznina, Natalia, Irina Zaporotskova, Sergey Boroznin, and Evgeniy Dryuchkov. 2019. "Sensors Based on Amino Group Surface-Modified CNTs" Chemosensors 7, no. 1: 11. https://doi.org/10.3390/chemosensors7010011
APA StyleBoroznina, N., Zaporotskova, I., Boroznin, S., & Dryuchkov, E. (2019). Sensors Based on Amino Group Surface-Modified CNTs. Chemosensors, 7(1), 11. https://doi.org/10.3390/chemosensors7010011





